Abstract
Rationale/objectives
Heroin addiction is characterized by recurrent cycles of drug use, abstinence and relapse. It is likely that neurobiological changes during chronic heroin exposure persist across withdrawal and impact behavioral responses to re-exposure. We hypothesized that, after extended withdrawal, heroin-withdrawn rats would express behavioral tolerance and/or sensitization in response to heroin re-exposure and that these responses might be associated with altered mu-opioid receptor (MOPr) activity.
Methods
Male Fischer rats were exposed chronically to escalating doses of heroin (7.5–75mg/kg/day), experienced acute spontaneous withdrawal and extended (10-day) abstinence, and were re-exposed chronically to heroin. Homecage behaviors and locomotor activity in response to heroin, as well as somatic withdrawal signs, were recorded. Separate groups of rats were sacrificed after extended abstinence and MOPr expression and G-protein coupling were analyzed using [3H]DAMGO and [35S]GTPγS assays.
Results
The depth of behavioral stupor was lower during the initial days of heroin re-exposure compared to the initial days of the first exposure period. Behavioral responses (e.g., stereotypy) and locomotion were elevated in response to heroin re-exposure at low doses. Rats conditioned for heroin place preference during the chronic re-exposure period expressed heroin preference during acute withdrawal; this preference was stronger than rats conditioned during chronic heroin exposure that followed chronic saline and injection-free periods. Extended withdrawal was associated with increased MOPr expression in the caudate-putamen and frontal and cingulate cortices. No changes in G-protein coupling were identified.
Conclusions
Aspects of tolerance/sensitization to heroin are present even after extended abstinence and may be associated with altered MOPr density.
Keywords: opiate, behavior, rat, addiction, tolerance, sensitization, locomotor activity, conditioning, opioid receptor, autoradiography
INTRODUCTION
Heroin addiction is a chronic relapsing disorder characterized by cycles of escalating drug exposure, intermittent episodes of withdrawal with or without maintained abstinence, and acute or chronic relapse to drug use. Recurrent cycles of heroin use and abstinence are thought to cause neurobiological changes in brain regions associated with reward, motivation, stress, learning and executive function (Jentsch and Taylor 1999; Koob and Le Moal 2008; Kreek et al. 2009a,b; Le Merrer et al. 2009; Winstanley et al. 2010). Such changes are thought to persist across extended drug-free periods to alter an individual’s response to drug re-exposure and contribute to subsequent escalation of drug use (i.e., relapse-like behavior) (e.g., Dalley et al. 2005). However, the nature and persistence of behavioral responses upon heroin re-exposure after extended drug abstinence has not been explored using clinically relevant models of heroin addiction.
Recently, our laboratory developed a model in which rats are chronically exposed to intermittent, escalating doses of heroin, mimicking human-like patterns of opiate use (Zhou et al. 2006; Seip et al. 2012). In this model, male rats display rapid (within-24hr) development of tolerance to the sedative-like effects of heroin and parallel sensitization to somatic/motoric responses to heroin, including stereotypy, hyperactivity and pica (Seip et al. 2012). Importantly, rats also displayed severe somatic withdrawal once chronic heroin exposure was discontinued, indicating strong physical dependence. It is likely that aspects of tolerance and/or sensitization may still be evident upon heroin re-exposure after somatic withdrawal signs have dissipated and the individual has entered an extended period of drug abstinence. The present study assesses how behavioral responses to heroin change as re-exposure progresses to a chronic relapse-like state.
Although extensive work has demonstrated that repeated heroin or morphine is associated with analgesic tolerance (Duttaroy and Yoburn 1995; Kuribara 1996; Eitan et al. 2003; Contet et al. 2008) and/or locomotor sensitization (e.g., Vanderschuren et al. 1997; Eitan et al. 2003; Narita et al. 2005; Szumlinski et al. 2005; Paolone et al. 2007), these responses are typically induced using sub-chronic and/or constant doses of drug. To date, limited studies have explored the nature and persistence of tolerance and/or sensitization, measured behaviorally, across repeated cycles of chronic escalating-dose exposure, withdrawal and re-exposure. Further, the degree of incentive-motivational value attributed to heroin is thought to be driven, in part, by altered behavioral responses to heroin re-exposure. Limited work has measured heroin preference across chronic re-exposure but may offer insight into addiction progression and/or individual vulnerability to relapse.
Neurobiological changes are thought to alter behavioral responses to drug re-exposure but little is known about the extent to which endogenous opioid receptor systems are altered after extended drug abstinence and thus contribute to relapse vulnerability. Mu-opioid receptors (MOPrs) are the endogenous target of heroin’s bioactive metabolites (Inturrisi et al. 1983) and have been implicated in aspects of tolerance and physical dependence to heroin and morphine (Mucha and Kalant 1981; Johnson and Fleming 1989; Bailey and Connor 2005). The expression and function of MOPrs are altered after chronic exposure to heroin and morphine (Sim et al. 1996; Sim-Selley et al. 2000; Kruzich et al. 2003) and it is thought that such adaptive changes to MOPrs may also contribute to altered behavioral responses to opiate re-exposure (Bailey and Connor 2005; Martini and Whistler 2007). In the present study, we predicted that MOPr activity would be altered after extended heroin withdrawal, prior to heroin re-exposure. As MOPr expression is elevated in forebrain regions after extended withdrawal from a chronic binge-like pattern of cocaine exposure (Bailey et al. 2005), we measured MOPr expression and function (i.e., coupling to G-proteins) in select forebrain regions after extended heroin withdrawal. As MOPrs and kappa-opioid receptors (KOPrs) assert opposing forces on midbrain dopamine activity (Johnson and North 1992; Ford et al. 2007) and reward-related behavior (e.g., Bals-Kubik et al. 1993), KOPr function was also evaluated to contextualize MOPr data. To our knowledge, this is the first study to measure opioid receptor expression and function in behaviorally characterized heroin-exposed rodents after extended withdrawal.
METHODS
Animals
Adult male Fischer-344 rats (90–120 days old; Charles River, Wilmington MA) were housed in a stress-minimized, AAALAC-accredited animal facility at The Rockefeller University. Fischer rats were used for direct comparisons with our prior neurochemical and molecular studies (e.g., Bailey et al. 2005; Zhou et al. 2008). Rats were housed individually in standard clear cages with bedding, nest material and food/water ad libitum, weighed and handled daily, and adapted to a reverse 12h:12h light:dark cycle (lights off 0500h) for >1 week. Dim red lights allowed observation in dark periods. Animal care and experimental procedures were conducted according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council 2010) and approved by the Institutional Animal Care and Use Committee.
Schedules of chronic exposure to escalating doses of heroin
Heroin (diacetylmorphine; National Institute on Drug Abuse, Bethesda, MD) was dissolved in physiological saline (0.9%) solution.
Heroin/Heroin group
Rats (n=16) received three daily intraperitoneal injections of heroin for ten consecutive days, starting 4h into the dark cycle (0900h) and spaced 6hrs apart thereafter, in their homecage (Zhou et al., 2008; Seip et al. 2012). Doses of heroin were escalated every other day, from 2.5–25mg/kg/injection (7.5–75mg/kg/day), calculated based on daily weight (Table 1; see Seip et al. 2012). Experimenter-administered injections were used to regulate total drug exposure, approximate drug consumption patterns in human addicts (80–100mg/kg/day; E. Ducat, personal communication), and clarify molecular analyses in each rat. Four rats died after injection during this chronic exposure period (respiratory depression); two were sacrificed due to health concerns. On Day 11, heroin was withheld to allow spontaneous withdrawal; rats remained heroin-free for the subsequent ten days. On Day 21, rats were chronically re-exposed to heroin (n=10) for ten days, identical to the schedule described above, to allow direct comparisons of behavioral measures (below) recorded on each day. One rat died during the chronic re-exposure period. Control rats (Saline/Saline group, n=8) received saline vehicle on an identical schedule and thus remained heroin-naïve.
Table 1.
Schedule of chronic exposure to escalating doses of heroin, extended withdrawal and chronic re-exposure to escalating doses of heroin (Heroin/Heroin group)
| Dose (mg/kg) per injectiona | Chronic Exposure to Escalating-Dose Heroinb | Spontaneous Withdrawalc | Chronic Exposure to Escalating-Dose Heroin | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||
| Time(h) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Day 11–20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 |
| 0900 | 2.5 | 2.5 | 5 | 5 | 10 | 10 | 15 | 15 | 25 | 25 | -- | 2.5 | 2.5 | 5 | 5 | 10 | 10 | 15 | 15 | 25 | 25 | ** |
| 1300 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0 | 0* | 0 | 0* | 0 | 0* | 0 | 0* | 0 | 0* | |
| 1500 | 2.5 | 2.5 | 5 | 5 | 10 | 10 | 15 | 15 | 25 | 25 | -- | 2.5* | 2.5 | 5* | 5 | 10* | 10 | 15* | 15 | 25* | 25 | |
| 2100 | 2.5 | 2.5 | 5 | 5 | 10 | 10 | 15 | 15 | 25 | 25 | -- | 2.5 | 2.5 | 5 | 5 | 10 | 10 | 15 | 15 | 25 | 25 | |
| Total | 7.5 | 7.5 | 15 | 15 | 30 | 30 | 45 | 45 | 75 | 75 | -- | 7.5 | 7.5 | 15 | 15 | 30 | 30 | 45 | 45 | 75 | 75 | |
Administered via intraperitoneal injection by the experimenter. Injections of saline vehicle are denoted by 0.
A separate group of rats (Saline/Heroin) received saline injections, rather than heroin injections, on an identical schedule on Days 1–10, followed by an injection-free period from Days 11–20. Heroin and saline injections, as well as timing of conditioning sessions, occurring on Days 21–30 were identical to those received by the Heroin/Heroin group.
Autoradiographic analyses were performed on rats that received chronic heroin exposure (Days 1–10) and extended withdrawal (Days 11–20), when they were sacrificed.
Injection was administered immediately before the rat was placed in the associated conditioning chamber of the place preference apparatus.
Conditioned place preference testing
Saline/Heroin group
Rats (n=12) received injections of saline vehicle for ten consecutive days on a schedule identical to the Heroin/Heroin group. On Day 11, rats entered an injection-free period for ten days and, on Day 21, were chronically exposed to heroin for ten days, on a schedule identical to the Heroin/Heroin group (Table 1). One rat died after injection and one was sacrificed due to health concerns. Control rats (Saline/Saline group, n=7) received saline vehicle on an identical schedule and thus remained heroin-naïve.
Heroin/Withdrawal group
For autoradiographic endpoints, a third group of rats was exposed chronically to escalating doses of heroin (n=12) or vehicle (n=8) and then experienced withdrawal for ten days, identical to Heroin/Heroin rats, before being sacrificed at 1000h on Day 21.
Behavioral observations
To minimize stress and disruption, observations were performed while rats were in their homecage. The observer was blind to condition.
Heroin exposure
Observations were performed as described in Seip et al. (2012). Briefly, behaviors were recorded during visual checks once per minute for 40min after the first daily injection (0900h). Normal behaviors included: explore, rear, corporal groom, gnaw object, nest-build, walk, eat/drink, quiet wakefulness, and apparent sleep. Heroin-induced behaviors included: pica (chewing/eating bedding), stereotypy of head/forelimbs, aberrant grooming (plucking/pulling at fur or digits) and hyperactivity (hops/darts). A point was awarded for each response present during each check and points were totaled across the 40min session. Rats expressed these responses more frequently when stupor was mild (rated 1–2, see below) or absent, consistent with earlier reports (e.g., Havemann and Kuschinsky 1982). The depth of behavioral stupor was rated on a graded scale based on volitional movement, orienting response, muscle rigidity, and stance (1= little volitional movement, slowed orienting response, little/no muscle rigidity, upright stance; 5= no orienting/startle response, severe muscle rigidity, prone position); regardless of depth, stupor almost always coincided with exophthalmos. Duration of stupor was consistent with previous work by our laboratory (Seip et al. 2012) and by others (e.g., Havemann and Kuschinsky 1982; Mayo-Michelson and Young 1992).
Withdrawal
Somatic withdrawal signs were observed for 5min, starting at 0900h. The number of wet shakes, hops/darts, paw tremors, facial fasciculations or teeth chatters, and swallowing movements, as well as the presence of ptosis, piloerection, erection, abnormal posture, and a loss of ≥1% body weight, were recorded for each rat (Gellert and Holtzman 1978; Langerman et al. 2001). A point was given for each present sign and added to the total number of observed signs for a daily score.
Place preference
Apparatus
The place preference apparatus (Med Associates, St Albans, VT) consisted of two equal-sized chambers (27.5cm W × 21cm L × 20.5cm H) connected to a small central chamber with grey walls and solid grey flooring through manually operated doors. One side chamber contained white walls and mesh flooring and the other side chamber had black walls and bar flooring. Time spent in each chamber was recorded by infrared photobeams traversing the chamber floors and locomotor activity was calculated as breaks in new infrared beams, denoting movement.
Pre-conditioning
Briefly, rats were placed in the apparatus for 30min (Day 20). Most rats did not express a pre-existing preference; if a pre-existing chamber preference was noted, heroin injections were assigned to the opposite chamber.
Conditioning phase
The conditioning procedure was designed to allow contextual learning to occur during the chronic heroin exposure period, mimicking drug-associated learning patterns that occur in human addiction. Starting on Day 21, rats received their 2nd regularly scheduled heroin injection (at 1500h) before being confined to one side chamber for 30min. These sessions occurred every other day for a total of five sessions (Days 21, 23, 25, 27, 29). As heroin doses were increased on these days (Table 1), rats learned to associate this chamber with escalating doses of heroin. After each session, rats were returned to their homecage. Controls received vehicle injections. On alternate days (Days 22, 24, 26, 28, 30), rats were injected with saline vehicle (at 1300h) and were confined to the other side chamber for 30min. For details, see Seip et al. (2012).
Post-conditioning test
Rats were tested 12h after their final heroin injection (0900 of Day 31). Rats had free access to all chambers for 30min, and time spent in the heroin-versus vehicle-paired chamber was compared. Preference was expressed in a drug-free state, extending existing re-exposure studies in which conditioning occurs during withdrawal or preference is reinstated by drug priming (e.g., Harris and Aston-Jones 2000; Ribeiro Do Couto et al. 2003).
Sacrifice and tissue preservation
On Day 20, separate groups of heroin- (n=8) and vehicle-treated (n=5) rats that experienced extended heroin withdrawal were lightly anaesthetized using isoflurane and decapitated in an unconscious state. Brains were rapidly removed, frozen in dry ice, and stored at −80ºC until sectioned. Coronal sections (20 μm thick) were cut at −13ºC, thaw-mounted, and stored at −80ºC until processed. Each slide contained triplicate or quadruplicate sections from a single rat.
In situ autoradiography
Mu-opioid receptor expression
Brain sections of heroin- or vehicle-treated rats that experienced extended heroin withdrawal were processed in parallel to determine total and nonspecific binding of mu-opioid receptors (MOPrs) using [3H]DAMGO (D-Ala2, N-Me-Phe4, Gly-ol5-enkephalin), as previously described (e.g., Unterwald et al. 1998) and performed in our laboratory (Bailey et al. 2005; Zhang et al. 2009). Briefly, slide-mounted brain sections were pre-incubated for 30min in 50mM Tris-HCl, pH 7.4, at room temperature. Sections were incubated in 5nM [3H]DAMGO for 60min at 4ºC to identify total radioligand binding to MOPrs and then washed in 50mM Tris-HCl (6 × 20sec) at 4ºC and dried under a stream of cool air. Non-specific binding was determined in the presence of 10 μM naloxone. Slides were apposed to 3H-sensitive Kodak BioMax MR Film (Sigma-Aldrich, St. Louis, MO) with 3H microscale standards and stored in the dark for seven weeks. Films were developed using Kodak GBX developer and fixative.
Agonist-stimulated GTPγS binding
Brain sections of heroin- (n=6) and vehicle-treated (n=5) rats, adjacent to those used to identify MOPr expression, were processed for G-protein activation in response to selective MOPr or KOPr agonists, using a well-established in situ [35S]GTPγS binding protocol (e.g., Sim et al. 1996; Schroeder et al. 2003; Piras et al. 2010). Briefly, sections were thawed at room temperature for 5min, incubated in assay buffer (50mM Tris HCl, 3mM MgCl2, 0.2mM EGTA, and 100mM NaCl; pH 7.4) for 10min at 25ºC, and pretreated in assay buffer + 2mM GDP for 15min at 25ºC. Slides containing adjacent sections were then incubated for 2h at 25ºC in one of the following conditions: basal, agonist-stimulated, non-specific or total binding. Basal binding was assessed by incubating slides in assay buffer + 2mM GDP + 0.04nM [35S]GTPγS. Agonist-stimulated binding was assessed by incubating slides in assay buffer + 2mM GDP + 0.04nM [35S]GTPγS + either 3 μM DAMGO for mu-agonist stimulated binding or 10 μM U-69,593 for kappa-agonist stimulated binding. Non-specific binding was assessed by incubating slides in assay buffer + 2mM GDP + 0.04nM [35S]GTPγS + 10 μM GTPγS. Total binding was assessed by incubating slides in assay buffer + 0.04nM [35S]GTPγS. After the 2hr incubation, slides were washed in ice-cold 50mM Tris-HCl (2 × 2min) and dH20 (1 × 30sec) at 4ºC and promptly dried under a stream of cool air. Slides were apposed to 35S-sensitive film (Kodak BioMax MR Film; Sigma-Aldrich) with 14C microscale standards, stored in the dark for 48hr, and developed as above.
Quantitative analysis
Analysis of brain sections was performed as described previously (Zhang et al. 2009) using a computerized image processing system (MCID, Imaging Research, St. Catharines, Ontario). For receptor binding, optical densities produced on each film by the 3H microscale standard allowed the conversion of optical density into fmol of radioligand bound per mg of wet-mass tissue equivalent (fmol/mg). For GTPγS binding, optical densities were quantified against the 14C microscale standards and expressed as percent increase from basal [35S]GTPγS binding. Brain structures were identified using a standard rat atlas (Paxinos and Watson 1986). For each structure, optical densities were obtained from both hemispheres, averaged, and corrected for non-specific binding and background film density. Values from three to ten sections per rat were averaged to generate a representative region-specific value in that rat. Stringent criteria (i.e., densities deviating more than two standard deviations from the mean) were used to omit rare outliers. Mean optical densities were calculated for each group (n=4–8 rats).
Statistics
Statistical analyses were performed using Statistica (v5.5) and a significance level of P<0.05, as previously described (Seip et al. 2012). ANOVAs were followed with a Newman-Keuls post-hoc test or planned comparisons between treatment groups and/or across days. All t-tests were two-tailed and were preceded by a Levine’s test for homogeneity of variance. Non-parametric tests were used as needed. Body weights across chronic exposure (Days 1–10), withdrawal (Days 11–20) and re-exposure (Days 21–30) were each compared using a two-way ANOVA (day as repeated measure). Weights of Heroin/Heroin rats during each heroin exposure period were compared using a two-way ANOVA (exposure phase and day as repeated measure). The percentages of body weight lost during heroin exposures in Heroin/Heroin rats and during heroin exposure in Saline/Heroin rats were compared using a repeated-measures and an independent t-test, respectively. Withdrawal scores were compared in the Heroin/Heroin group using a two-way ANOVA (day as repeated measure). Data from the Saline/Saline groups did not differ on any behavioral measure and were pooled for presentation and analyses. Behavioral stupor scores, averaged across each session, were compared across heroin exposure periods in Heroin/Heroin rats using a three-way ANOVA (exposure period, dose and repeated days at each dose as repeated measures) and were compared between the re-exposure period of the Heroin/Heroin rats and the initial (only) exposure period of Saline/Heroin rats using a three-way ANOVA (dose and repeated days at each dose as repeated measures). T-tests were used to compare the total number of heroin-induced behavioral responses that occurred during the first day of heroin exposure/re-exposure. Locomotor activity (total number of new beam breaks recorded across each session) recorded during each set of conditioning sessions was compared across all three treatment groups using a two-way ANOVA (session as repeated measure). Locomotor data saved correctly for only three Saline/Heroin subjects after the fourth saline conditioning session so this session was omitted from analyses. Times spent in the heroin- and vehicle-paired chamber during the post-conditioning session were compared across all three treatment groups using a two-way ANOVA (chamber as repeated measure). Unpaired t-tests were used to compare autoradiographic data (optical densities) in each brain region of vehicle- and heroin-treated rats.
RESULTS
Body weight
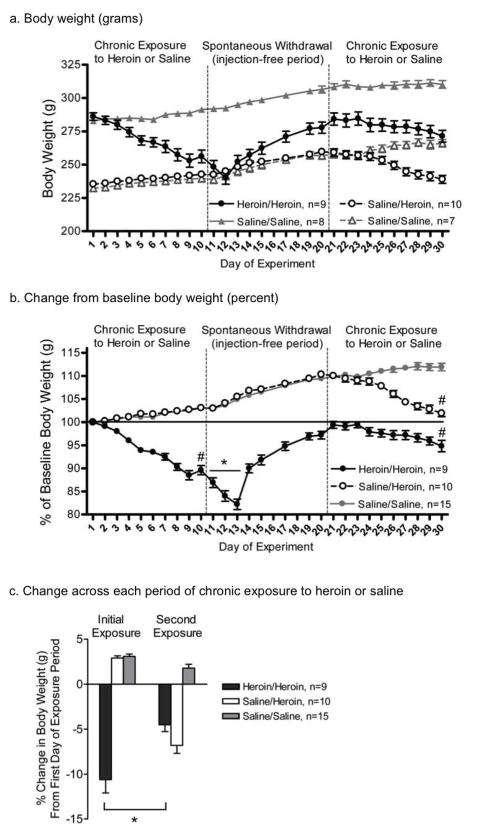
Weight loss is common in response to experimenter-administered heroin (Weber et al. 2004; Seip et al. 2012) but the extent of weight loss across repeated cycles of chronic heroin exposure has remained unclear. Body weights are shown in grams (Fig. 1a) and calculated as the percent of baseline (Day 1) weight (Fig. 1b). There were significant interactions between treatment and day across all three periods [exposure, F(27,297)=3.22; withdrawal, F(21,231)=23.79; re-exposure, F(27,270)=23.17; all P<0.01]. In the Heroin/Heroin group, rats’ weight dropped significantly across the first two days of acute spontaneous withdrawal [Day 11 versus 13, F(1,33)=22.99, P<0.01]. All rats lost weight between the first and last day of heroin exposure [Heroin/Heroin: F(1,33)=319.54, P<0.01; Saline/Heroin: F(1,30)=119.28, P<0.01] or heroin re-exposure [Heroin/Heroin: F(1,30)=38.56, P<0.01]. Heroin/Heroin rats also lost a significantly greater percentage of body weight during the initial heroin exposure period compared to the second heroin re-exposure period [t(8)=5.43, P<0.01] (Fig. 1c); neither percentage of body weight lost differed from the percentage lost by Saline/Heroin rats during heroin exposure. Rats used for autoradiographic analyses did not differ from the Heroin/Heroin group (data not shown).
Fig. 1.
Body weights of rats across an addiction-like cycle of heroin exposure. All data are presented as mean ± S.E.M. (a) Body weight, in grams, of rats exposed and re-exposed to escalating doses of heroin (Heroin/Heroin, black circles) or saline (Saline/Saline, solid grey triangles) or rats first exposed to saline and then escalating doses of heroin (Saline/Heroin, white circles) or saline (Saline/Saline, open grey triangles). (b) Percentage of baseline (Day 1) weight of rats exposed to escalating doses of heroin (Heroin/Heroin, black circles; Saline/Heroin, white circles) or saline (pooled Saline/Saline groups, grey circles). * P<0.05 within-group (Heroin/Heroin) comparison between Days 11 and 13; # P<0.01 within-group comparison between the first and final day of the chronic heroin exposure/re-exposure period (c) Change in weight across each 10-day period of chronic exposure to saline and/or escalating doses of heroin. * P<0.05 within-group comparison
Withdrawal signs
Classic signs of somatic withdrawal were observed in the Heroin/Heroin group across the extended withdrawal period. Withdrawal scores of heroin- and saline-treated rats differed by treatment and withdrawal day [main effects: Treatment, F(1,22)=82.86; Day, F(5,110)=8.01; Treatment × Day interaction: F(5,110)=8.01; all P<0.01] (Fig. 2). Scores were highest 36hrs after heroin exposure was discontinued, compared to all other days [Day 12: F(1,22)=8.09, P<0.01].
Fig. 2.
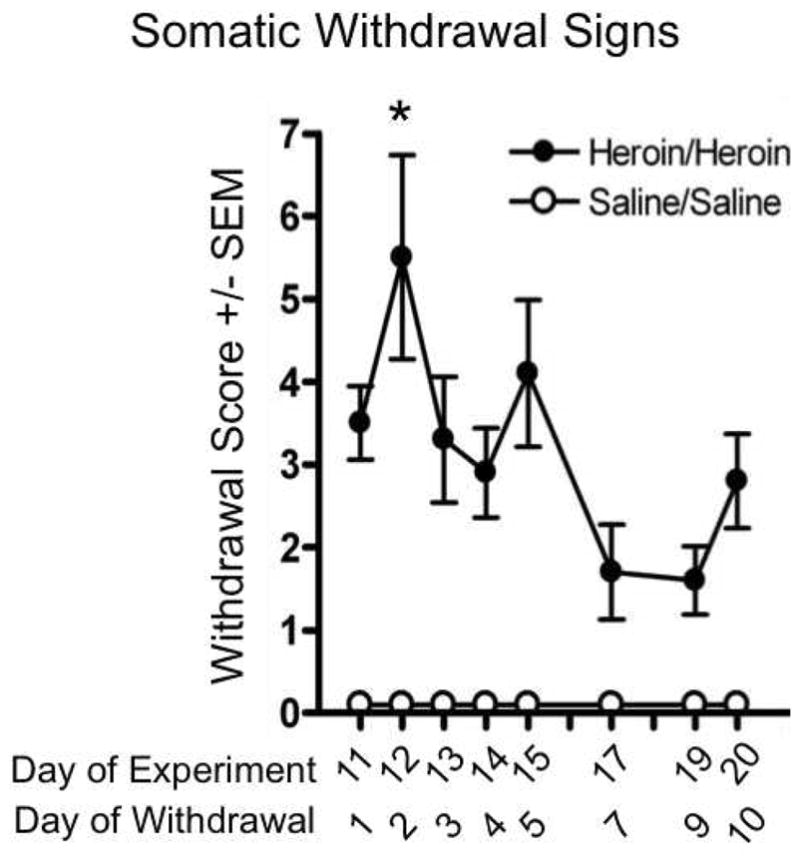
Somatic withdrawal signs observed across the extended (10-day) period of spontaneous withdrawal. Rats were exposed chronically to escalating doses of heroin before heroin injections were discontinued; withdrawal signs were observed in the homecage for ten days (Heroin/Heroin, black circles). Control rats received saline injections on an identical schedule before entering an injection-free period for ten days (Saline/Saline, open circles). * P<0.05 between Day 12 and all other withdrawal days
Heroin-induced behaviors
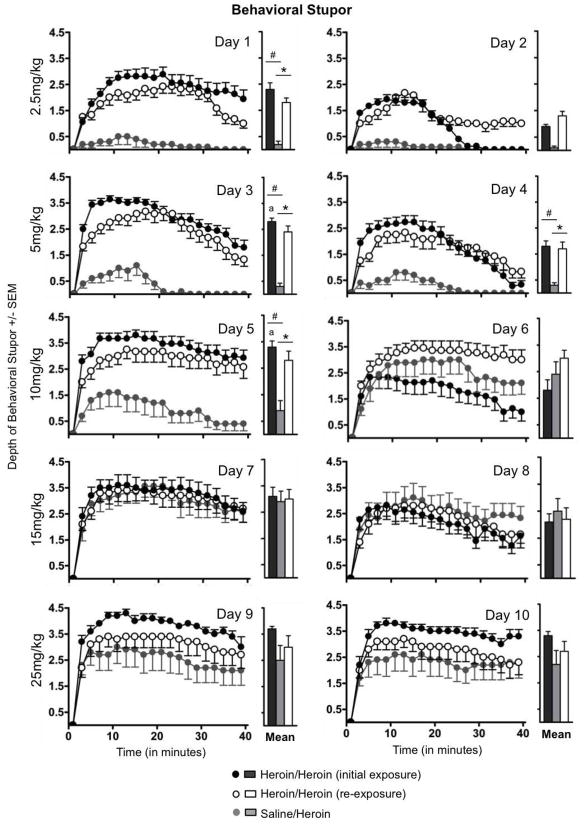
Behavioral stupor
Rats expressed different patterns of behavioral stupor over time in response to the same dose administered during chronic heroin exposure versus re-exposure (Fig 3). The depth of behavioral stupor expressed by rats in the Heroin/Heroin group was affected by the heroin exposure period (i.e., exposure versus re-exposure), dose, and repeated days at each dose [interactions: exposure × dose, F(4,32)=5.54, P<0.01; exposure × repeated days at each dose, F(1,8)= 25.29, P<0.01; exposure × dose × repeated days at each dose, F(4,32)=3.07, P<0.05]. These rats expressed less stupor on Days 1, 3, 4 and 5 of the chronic heroin re-exposure period compared to the same days of the initial chronic heroin exposure period (all P<0.05). On Day 2, there was a qualitative difference in the persistence of stupor in response to the initial heroin exposure in Heroin/Heroin rats only; however, this difference was not reflected in a significant difference in the means. During chronic exposure, stupor increased when the dose was escalated from 2.5–5mg/kg (between Days 2–3) and from 5–10mg/kg (between Days 4–5) (both P<0.05); these increases were not seen at higher doses or during re-exposure. Stupor expressed by Heroin/Heroin rats during re-exposure also differed significantly from stupor expressed by Saline/Heroin rats [main effect of treatment group, F(1,17)=25.77, P<0.01; main effect of dose, F(4,68)=26.78, P<0.01; treatment group x dose interaction, F(4,68)=4.69, P<0.01]. As in the within-group comparisons, above, Heroin/Heroin rats also expressed less stupor on Days 1, 3, 4 and 5 of re-exposure than did Saline/Heroin rats during their initial (only) exposure [Day 1, F(1,17)=48.53; Day 3, F(1,17)=37.69; Day 4, F(1,17)=15.53; Day 5, F(1,17)=8.20; all P<0.01].
Fig. 3.
Behavioral stupor in response to escalating doses of heroin, recorded across daily (40-min) observation sessions. Stupor was recorded in response to escalating doses of heroin administered during chronic exposure (Heroin/Heroin, black circles; Saline/Heroin, white circles) and, following an extended withdrawal period, during chronic re-exposure (Heroin/Heroin, grey circles). The daily mean, averaged across the entire observation session, is shown to the right of each expanded dataset. * P<0.05, between-group comparisons; # P<0.05, within-group comparisons; a P<0.05, within-group comparison to exposure data on the previous day. Data are presented as mean ± S.E.M.
Somatic/behavioral responses
Rats expressed more pica, stereotypy, hyperactivity and aberrant grooming behaviors in response to heroin re-exposure after extended withdrawal than during their initial heroin exposures (Fig. 4). These behavioral responses were more prevalent on rats’ first day of heroin re-exposure compared to their first day of exposure [t(9)=7.84, P<0.01] and compared to the responses expressed by Saline/Heroin rats on their first day of heroin exposure [t(20)=4.56, P<0.01], even though the dose (2.5mg/kg) was the same. Compared to Heroin/Heroin rats, the Saline/Heroin group showed more aberrant grooming during their first exposure to heroin; when these behaviors were omitted, the frequency of pica, stereotypy and hyperactivity was identical between groups (Fig. 4, inset).
Fig. 4.
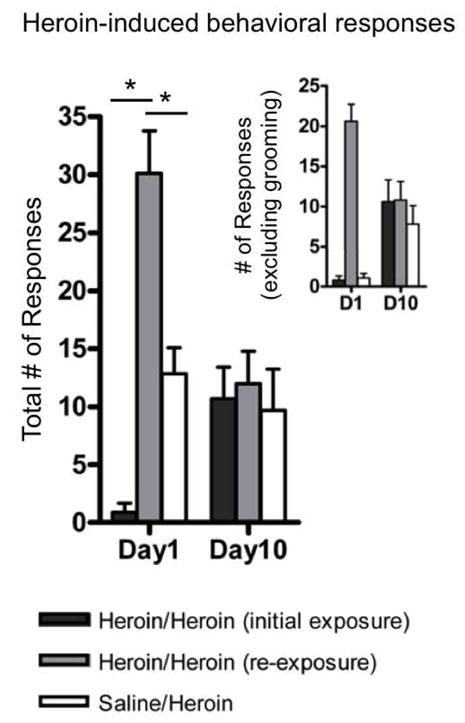
Total number of heroin-induced somatic responses, including hyperactivity, stereotypy, pica and aberrant grooming, recorded during the 40-min observation session on the first and final day of the chronic heroin exposure period. Responses to the same dose administered on Day 1 (2.5mg/kg) and Day 10 (25mg/kg) of the heroin exposure period were recorded in Heroin/Heroin rats during chronic exposure (dark grey bars) and, following an extended withdrawal period, during chronic re-exposure (light grey bars), and were recorded in Saline/Heroin rats during chronic exposure (white bars). Inset: Heroin-induced somatic responses, excluding aberrant grooming. All data are presented as mean ± S.E.M. * P<0.05 for within- and between-group comparisons
Locomotor activity
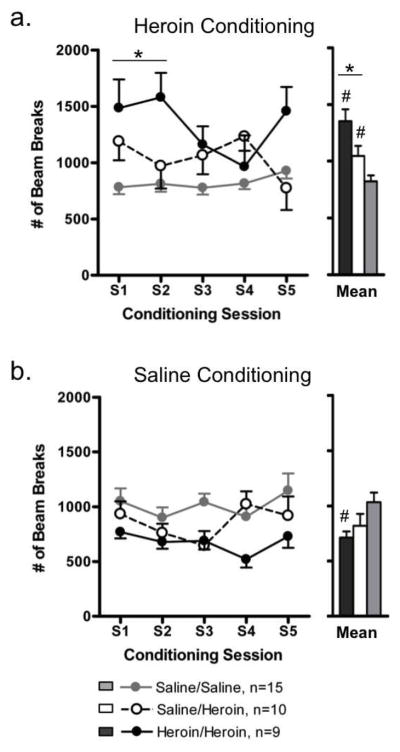
Heroin conditioning
Locomotor activity during heroin conditioning sessions differed significantly between groups [main effect of treatment group: F(2,31)=11.82, P<0.01; treatment group x session interaction; F(8,124)=2.17, P<0.05] (Fig. 5a). Locomotor activity across all five session was elevated above control levels in both Heroin/Heroin rats [F(1,31)=23.57, P<0.01] and Saline/Heroin rats [F(1,31)=4.49, P<0.05]. Locomotor activity was also higher in Heroin/Heroin rats than in Saline/Heroin rats across all five conditioning sessions [F(1,31)=6.61, P<0.05] and, more specifically, during Sessions 1–2 [F(1,31)=7.69, P<0.01].
Fig. 5.
Locomotor activity recorded during place preference conditioning sessions with escalating doses of heroin (a) or saline (b) during the chronic heroin re-exposure period (Heroin/Heroin, black circles) or the chronic heroin exposure period (Saline/Heroin, white circles). Data from control rats conditioned with saline during chronic saline exposure or re-exposure were pooled (Saline/Saline, grey circles). The mean, averaged across all conditioning sessions, is shown to the right of each expanded dataset. All data are presented as mean ± S.E.M. * P<0.05, comparisons between Heroin/Heroin and Saline/Heroin groups; # P<0.05, compared to Saline/Saline control group
Saline conditioning
Locomotor activity during saline conditioning sessions also differed significantly between groups [main effect of treatment group: F(2,31)=3.68, P<0.05] (Fig. 5b). Locomotor activity across all five sessions was lower than control levels in Heroin/Heroin rats [F(1,31)=6.32, P<0.05] but not Saline/Heroin rats; activity levels in Heroin/Heroin and Saline/Heroin groups did not differ.
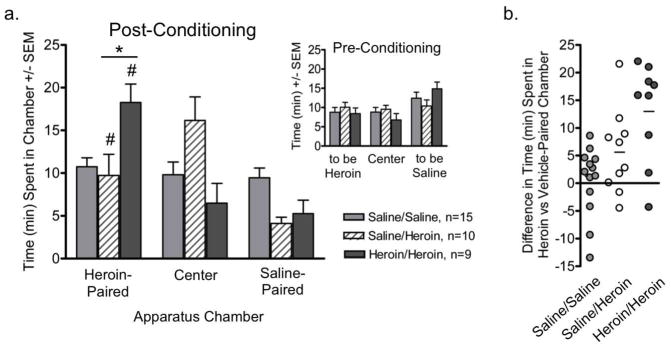
Heroin place preference
Rats learned to associate injections of escalating doses of heroin or vehicle with unique conditioning chambers while being chronically exposed (Saline/Heroin) or re-exposed (Heroin/Heroin) to escalating-dose heroin in their homecage. The amount of time that Saline/Heroin, Heroin/Heroin and Saline/Saline rats spent in each of the three chambers during the postconditioning session differed significantly from each other [main effect of chamber: F(2,62)=7.29, P<0.01; group × chamber interaction: F(4,62)=5.33, P<0.01]. Both groups conditioned with escalating doses of heroin spent more time in the heroin- versus vehicle-paired chamber [Heroin/Heroin: F(1,31)=27.18, P<0.01; Saline/Heroin: F(1,31)=5.64, P<0.05]. Heroin/Heroin rats spent more time in the heroin-paired chamber than did Saline/Heroin rats [F(1,31)=9.42, P<0.01]. It is of interest that Saline/Heroin rats spent the majority of this session in the neutral center chamber. The strength of heroin preference varied between individual rats (Fig. 6b) and the variability in strength of heroin preference was comparable in Heroin/Heroin and Saline/Heroin groups.
Fig. 6.
Conditioned place preference for a heroin-paired chamber expressed by rats during acute (12hr) withdrawal. (a) Time spent in each chamber during the post-conditioning session by rats conditioned with escalating doses of heroin during the chronic heroin re-exposure period (Heroin/Heroin, dark grey bars) or the initial chronic heroin exposure period (Saline/Heroin, striped bars); control rats were conditioned with saline (Saline/Saline, light grey bars). Data are presented as mean ± S.E.M. Inset: Time spent in each chamber during the pre-conditioning session. (b) Strength of preference for the heroin-paired chamber expressed by individual rats conditioned with escalating doses of heroin during chronic heroin re-exposure period (Heroin/Heroin, dark grey circles), during the initial chronic heroin exposure period (Saline/Heroin, white circles); control rats conditioned with saline were pooled (Saline/Saline, light grey circles). Line denotes group mean. * P<0.05, comparisons between Heroin/Heroin and Saline/Heroin groups; # P<0.05, compared to time spent by the Saline/Saline group in that chamber
Mu-opioid receptor autoradiography
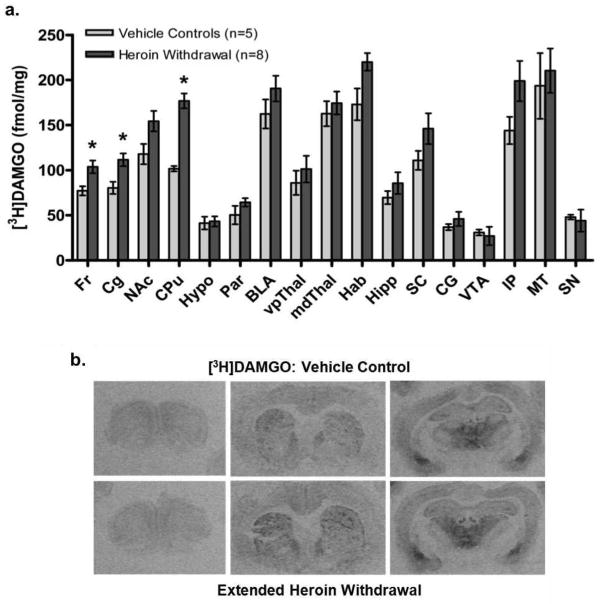
To explore potential receptor-based changes that might underlie altered behavioral responses to heroin re-exposure after extended withdrawal, the distribution and density of MOPrs was identified using [3H]DAMGO (4nM) in coronal brain sections of rats given chronic heroin (n=6–8) or vehicle (n=4–5) exposure and sacrificed after extended (10-day) withdrawal. The quantitative distribution of MOPrs was similar to previous studies (e.g., Bailey et al. 2005). Unpaired t-tests revealed increased [3H]DAMGO binding in the frontal cortex [Fr, t(10)=2.82, P<0.05], cingulate cortex [Cg, t(9)=3.14, P<0.05] and caudate-putamen [CPu, t(10)=6.96, P<0.01] of heroin-withdrawn rats compared to controls (Fig. 7). A trend toward increased [3H]DAMGO binding was also identified in the nucleus accumbens [NAc, t(11)=2.16, P=0.054] and habenula [t(6)=2.36, P=0.057] of heroin-withdrawn rats.
Fig. 7.
Expression of mu-opioid receptors after extended withdrawal from chronic escalating-dose heroin, using quantitative receptor autoradiography. (a) Specific [3H]DAMGO binding to mu-opioid receptors in brain regions from heroin- and vehicle-treated rats. Data are presented as mean ± S.E.M. of specific fmol/mg of tissue. * P<0.05, unpaired two-tailed t-tests. Fr, frontal cortex; Cg, cingulate cortex; NAc, nucleus accumbens; CPu, caudate-putamen; Hypo, hypothalamus; Par, parietal cortex; BLA, basolateral amygdala; vpThal, ventroposterior thalamic nuclei; mdThal, mediodorsal thalamic nuclei; Hab, habenula; Hipp, hippocampus; SC, superior colliculus; CG, central grey; VTA, ventral tegmental area; IP, interpeduncular nuclei; MT, medial terminal nucleus of the accessory optic tract; SN, substantia nigra. (b) Computer-enhanced autoradiograms of coronal brain sections from representative heroin- or vehicle-treated rats following extended (10-day) withdrawal. Sections are shown at the level of the frontal cortex (Bregma 3.70mm), caudate-putamen (1.00mm) and thalamus (−3.14mm). Sections from heroin- and vehicle-treated rats were processed in parallel for [3H]DAMGO binding.
Agonist-stimulated GTPγS binding
Select sections adjacent to those processed for MOPr autoradiography were processed for mu- and kappa-agonist stimulated G-protein activation, using [35S]GTPγS binding (Online Resource 1).
Mu-agonist-stimulated binding
DAMGO-stimulated [35S]GTPγS binding was analyzed in three regions showing elevated MOPr expression (CPu, Fr, Cg) and in one region showing no change in MOPr expression (parietal cortex). Elevated [35S]GTPγS binding was seen in these regions after incubation with DAMGO (e.g., Schroeder et al. 2003) but these binding levels did not differ between saline- and heroin-withdrawn rats in any region analyzed.
Kappa-agonist-stimulated binding
U-69,593-stimulated [35S]GTPγS binding was analyzed in the CPu, NAc, frontal and cingulate cortices and claustrum, which express relatively high KOPr levels (Zukin et al. 1988; Unterwald et al., 1991). U-69,593 was associated with elevated [35S]GTPγS binding in these regions but, again, binding did not differ between saline- and heroin-withdrawn rats.
DISCUSSION
The present study reveals profound behavioral differences in response to repeated chronic exposures to escalating doses of heroin, using a rodent model designed to mimic the chronic relapsing nature of human addiction. While repeated heroin or morphine administration is associated with tolerance and/or sensitization to select drug effects that can occur rapidly (Seip et al. 2012) and persist over prolonged periods of drug abstinence (Babbini et al. 1975; Bartoletti et al. 1983), to our knowledge this is the first study to systematically explore the nature and persistence of altered behavioral responses to chronic drug re-exposure after an extended drug-free period, which have been thought to contribute to escalating drug use and relapse-like behavior (e.g., Dalley et al. 2005).
First, heroin-treated rats lost less weight when chronically re-exposed to escalating-dose heroin than they did in their initial chronic exposure period. While regulation of feeding behavior may have been altered (Glass et al. 1999; Beckman et al. 2009; see Bodnar 2011), it is more likely that heroin re-exposed rats lost less weight due, in part, to injection stress across the first exposure period, given the comparable weight loss in Saline/Heroin rats (Izumi et al., 1997). As in human populations, a degree of mortality was also observed in response to escalating-dose heroin exposure. Respiratory depression is a major cause of overdose death in heroin users (Grigorakos et al. 2010; Warner-Smith et al. 2001) and in prescription opiate abuse (Centers for Disease Control and Prevention 2012), and appears to account for 8–25% mortality using this schedule of chronic heroin exposure in rodents. The lower mortality rate during re-exposure may have been impacted by the overdose death of the most sensitive rats during the first chronic exposure period and/or the development of tolerance to these respiratory effects in the remaining rats (Roerig et al., 1987). In self-administration studies, Fischer rats escalate their heroin dose less rapidly than do Lewis rats (Picetti et al. 2011) and self-administer less morphine, heroin’s primary bioactive metabolite, than do Lewis rats (Martin et al., 1999; Garcia-Lecumberri et al., 2011), suggesting that Fischer rats may not tolerate high opiate doses or rapid dose escalation well. The relatively drug-resistant phenotype of this strain suggests that environmental and drug-induced effects must act in concert to drive rats’ preference for drug and propensity for relapse-like behavior, as seen in human populations (Kreek et al., 2005).
The fact that the depth of behavioral stupor was reduced upon re-exposure to low to moderate heroin doses suggests that tolerance to sedation-like behavioral effects persists across extended periods of drug abstinence. These results extend our previous findings that revealed instances of rapid (within-24hr) tolerance to heroin (Seip et al. 2012) and suggest that even multiple (4–5) days of chronic intermittent heroin re-exposure are associated with altered behavioral responses in drug-abstinent rats. Based on the route of administration used in the present study, we predict that plasma and brain levels of heroin’s bioactive metabolites peak 5–15min post-injection (Djurendic-Brenesel et al. 2010). While peak plasma levels also coincide with peak euphorigenic effects of opiate drugs (Comer et al. 2009) and tolerance also develops to these effects in humans (Haertzen and Hooks 1969; Mirin et al. 1976), the relationship between sedation and euphoria in rodents remains unclear.
In contrast to tolerance-like responses upon heroin re-exposure, somatic responses to heroin (e.g., stereotypy) were most frequent in heroin-withdrawn rats upon re-exposure to the lowest heroin dose (2.5mg/kg) used in this study. These results are consistent with the two-phase response to opiate drugs discussed elsewhere (e.g., Havemann and Kuschinsky 1982). While Saline/Heroin rats also expressed substantially fewer of these responses than did Heroin/Heroin rats during re-exposure, the Saline/Heroin group did show elevated aberrant grooming behavior in response to their first drug injection, which may point to a role of mild injection-related stress (Izumi et al., 1997; Spangler et al., 1997; Zhou et al., unpublished data) impacting initial drug response. Locomotor activity was not elevated above control levels in the Saline/Heroin group but was elevated in Heroin/Heroin rats upon re-exposure to low heroin doses. These findings are consistent with data that acute re-exposure to low-dose heroin after a heroin-free period produces psychomotor sensitization (e.g., D’Este et al., 2002). While differences in drug dose, administration schedule, and test environment limit our ability to make direct comparisons across datasets (e.g., Badiani et al. 2000; D’Este et al., 2002), it is also possible that the relative novelty of the conditioning chamber unmasked motoric sensitization in the Heroin/Heroin group after extended withdrawal (Paolone et al., 2007). Locomotion was also elevated above control levels in the Saline/Heroin group, suggesting that the drug, novel context of the conditioning chamber, or both may be contributing to motor responses. Although motor sensitization is not seen in human opiate addicts, it remains possible that molecular changes that occur across the addiction-like trajectory in humans and rodents may not manifest in analogous behavioral responses.
Rats also learned to associate escalating doses of heroin with a unique chamber while being exposed (Saline/Heroin) or re-exposed (Heroin/Heroin) to escalating doses of heroin in the homecage. When conditioned during heroin re-exposure, Heroin/Heroin rats expressed strong preference for the heroin-paired chamber, indicating that positive incentive value was attributed to heroin re-exposure. Further studies are needed to explore how the behavioral differences observed during the early phase of heroin exposure/re-exposure might impact the incentive value attributed to the initial phase of heroin re-exposure and the extent to which positive and negative reinforcement drive this process. Clinical reports suggest that heroin retains unequivocal hedonic value across repeated exposures (Mirin et al. 1976), but negative reinforcement processes associated with physical dependence and the aversive state of withdrawal are also thought to contribute to continued drug use (Kreek and Koob, 1998; Koob and Le Moal 2008). The relative contributions of positive and negative reinforcement to drug preference cannot be determined easily in place conditioning studies.
Rats conditioned during the initial heroin exposure period (Saline/Heroin) also preferred the heroin-over the saline-paired chamber. It is important to note that the magnitude of this preference was considerably less than Heroin/Heroin rats conditioned during re-exposure and that rats spent much of their time in the smaller, central chamber, suggesting that the heroin-paired chamber may have had limited value to this group. The magnitude of this heroin preference, measured 12hr after rats’ final heroin injection, was also less dramatic than that expressed by rats conditioned during chronic heroin exposure but tested 36hrs after their final heroin injection (Seip et al. 2012), possibly due to the timing of testing (i.e., during the onset versus peak of acute withdrawal) or the history of injections that preceded heroin exposure. Saline injections are a mild stressor to male Fischer rats (Izumi et al. 1997; Spangler et al. 1997; Zhou et al., unpublished data) and, as Fischer rats display a large and persistent hypothalamus-pituitary-adrenal response to severe restraint stress (Dhabhar et al., 1997), it is possible that mild injection-related stress impacted this group’s subsequent response to chronic heroin exposure (Kreek and Koob 1998; Koob and Kreek 2007).
While bioaminergic changes have been associated with affective and somatic components of opiate exposure and/or withdrawal (Diana et al. 1999; Delfs et al. 2000; Harris and Aston-Jones 2000; Georges et al. 2006; Goeldner et al. 2011), limited work has explored how MOPrs, the target of heroin’s bioactive metabolites (Inturrisi et al. 1983), are altered after extended drug abstinence. MOPrs mediate aspects of reward and motivation (Contet et al. 2004; Le Merrer et al. 2009), locomotor activation (e.g., Zhang et al. 2009) and neuroendocrine responses to opiate drugs (Kreek 2008; Kreek and LaForge 2007). Despite compelling behavioral data to suggest that MOPrs mediate key aspects of opiate dependence-related processes (Matthes et al. 1996; Negus and Rice 2009), inconsistent findings have pointed to increases, decreases or a lack of change in MOPr mRNA (Brodsky et al. 1995; Castelli et al. 1997; Zhou et al. 2006) and receptor density (Ulibarri et al. 1987; Bhargava and Gulati 1990; Sim-Selley et al. 2000; Bailey et al. 2010) after chronic exposure to heroin or morphine. These discrepancies are likely due to differences in drug, dose, administration schedule, radioligand used, and timing of tissue collection after the final drug exposure. Using a schedule of chronic escalating-dose exposure to morphine, similar to the schedule used in the present study, we reported that MOPr mRNA was unchanged after 14 days of morphine exposure but was elevated in the CPu and NAc during acute spontaneous withdrawal (Zhou et al. 2006). It is likely that a withdrawal-induced increase in MOPr mRNA would result in elevated receptor protein levels after 7–14 days, based on a time course described in response to cocaine (Unterwald et al. 1994).
In the present study, MOPr density was elevated in specific forebrain regions (e.g., CPu, frontal and cingulate cortices) after an extended withdrawal period and once most classic withdrawal signs had dissipated. These findings offer a potential mechanism by which behavioral responses differ between initial and repeated drug exposures, extending others’ work (Mucha and Kalant 1981; Werling et al. 1989; Bohn et al. 2000). The regional specificity of altered MOPrs corresponds to changes identified after 14-day withdrawal from chronic “binge” cocaine (Bailey et al. 2005), indicating that MOPr expression is altered after extended withdrawal from both psychostimulant and opiate drugs. Regions showing the greatest elevations in MOPr expression (e.g., CPu) have been implicated in motoric sensitization to heroin and morphine (Erdtmann-Vourliotis et al. 1999; D’Este et al. 2002; Taracha et al. 2008) and it is plausible that elevated MOPr expression in these regions mediates aspects of tolerance and/or sensitization, measured behaviorally, to heroin re-exposure.
Importantly, both morphine and heroin can initiate rapid MOPr desensitization via uncoupling receptors from their G-protein effectors (Sim et al. 1996; Sim-Selley et al. 2000; Borgland et al. 2003; Maher et al. 2005), as opposed to MOPr internalization (Keith et al. 1996; Sternini et al. 1996; Borgland et al. 2003), and may contribute to drug tolerance (Bohn et al. 2000; Sim-Selley et al. 2000; Bailey and Connor, 2005). While GTPγS binding and receptor expression cannot be compared directly, due to signal amplification, it is possible that increased MOPr expression would not be associated with increased MOPr function (Maher et al., 2000). We did not identify altered DAMGO-stimulated [35S]GTPγS binding, or altered U-69,593-stimulated [35S]GTPγS binding, in select forebrain regions of heroin-treated rats after extended withdrawal. Given the emerging role of dysregulated KOPr/dynorphin activity in opiate and psychostimulant dependence (Tejada et al. 2012) and acute cocaine withdrawal (Piras et al., 2010), further studies are needed to identify changes to the endogenous opioid systems across periods of extended drug abstinence and re-exposure.
In conclusion, this is the first study to use a clinically relevant model of heroin addiction to examine the nature and persistence of altered behavioral responses to heroin re-exposure after extended withdrawal. These tolerance- and sensitization-like behavioral responses are suggestive of underlying neurobiological changes, including altered MOPr function, that may contribute to continued drug use and relapse vulnerability and offer new directions for future studies.
Supplementary Material
Acknowledgments
This research was supported by NIH-NIDA P60-DA05130-25 to M.J.K., and the Dorothea Dix Postdoctoral Fellowship and NIH-NIDA 5 F32-DA030831-02 to K.M.S-C. Diacetylmorphine hydrochloride was generously provided by the NIH-NIDA Division of Drug Supply and Analytical Services. The authors have no conflicts of interest, financial or otherwise, pertaining to any aspect of the work reported in this manuscript. All experiments described herein comply with the current laws of the country in which they were performed. The authors thank Drs. Ellen Unterwald and Alexis Bailey for their advice on imaging analyses and protocols.
References
- Babbini M, Gaiardi M, Bartoletti M. Persistence of chronic morphine effects upon activity in rats 8 months after ceasing the treatment. Neuropharmacology. 1975;14:611–614. doi: 10.1016/0028-3908(75)90129-x. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Robinson TE. Modulation of morphine sensitization in the rat by contextual stimuli. Psychopharmacology (Berl) 2000;151:273–282. doi: 10.1007/s002130000447. [DOI] [PubMed] [Google Scholar]
- Bailey A, Gianotti R, Ho A, Kreek MJ. Persistent upregulation of mu-opioid, but not adenosine, receptors in brains of long-term withdrawn escalating dose “binge” cocaine-treated rats. Synapse. 2005;57:160–166. doi: 10.1002/syn.20168. [DOI] [PubMed] [Google Scholar]
- Bailey A, Metaxas A, Al-Hasani R, Keyworth HL, Forster DM, Kitchen I. Mouse strain differences in locomotor, sensitisation and rewarding effect of heroin; association with alterations in MOP-r activation and dopamine transporter binding. Eur J Neurosci. 2010;31:742–753. doi: 10.1111/j.1460-9568.2010.07104.x. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M. Long-term sensitization to the excitatory effects of morphine. A motility study in post-dependent rats. Neuropharmacology. 1983;22:1193–1196. doi: 10.1016/0028-3908(83)90080-1. [DOI] [PubMed] [Google Scholar]
- Beckman TR, Shi Q, Levine AS, Billington CJ. Amygdalar opioids modulate hypothalamic melanocortin-induced anorexia. Physiol Behav. 2009;96:568–573. doi: 10.1016/j.physbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava HN, Gulati A. Down-regulation of brain and spinal cord mu-opiate receptors in morphine tolerant-dependent rats. Eur J Neurosci. 1990;190:305–311. doi: 10.1016/0014-2999(90)94194-3. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2010. Peptides. 2011;32:2522–2552. doi: 10.1016/j.peptides.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Brodsky M, Elliott K, Hynansky A, Inturrisi CE. CNS levels of mu opioid receptor (MOR-1) mRNA during chronic treatment with morphine or naltrexone. Brain Res Bull. 1995;38:135–141. doi: 10.1016/0361-9230(95)00079-t. [DOI] [PubMed] [Google Scholar]
- Castelli MP, Melis M, Mameli M, Fadda P, Diaz G, Gessa GL. Chronic morphine and naltrexone fail to modify mu-opioid receptor mRNA levels in the rat brain. Brain Res Bull. 1997;45:149–153. doi: 10.1016/s0169-328x(96)00305-1. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10–13. [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Sullivan MA, Vosburg SK, Saccone PA, Foltin RW. Relationship between rate of infusion and reinforcing strength of oxycodone in humans. J Opioid Manag. 2009;5:203–212. doi: 10.5055/jom.2009.0022. [DOI] [PubMed] [Google Scholar]
- Contet C, Filliol D, Matifas A, Kieffer BL. Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology. 2008;54:475–486. doi: 10.1016/j.neuropharm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Pena Y, Theobald DEH, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- D’Este L, Scontrini A, Casini A, Pontieri FE, Renda TG. Heroin sensitization as mapped by c-Fos immunoreactivity in the rat striatum. Brain Res. 2002;933:144–149. doi: 10.1016/s0006-8993(02)02312-0. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress -- comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- Diana M, Muntoni AL, Pistis M, Melis M, Gessa GL. Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J Neurosci. 1999;11:1037–1041. doi: 10.1046/j.1460-9568.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- Djurendic-Brenesel M, Mimica-Dukic N, Piilija V, Tasic M. Gender-related differences in the pharmacokinetics of opiates. Forensic Sci Int. 2010;194:28–33. doi: 10.1016/j.forsciint.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn BC. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, Polakiewicz R, Evans CJ. Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci. 2003;23:8360–8369. doi: 10.1523/JNEUROSCI.23-23-08360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdtmann-Vourlioti M, Mayer P, Linke R, Reiechert U, Hollt V. Long-lasting sensitization towards morphine in motoric and limbic areas as determined by c-fos expression in rat brain. Mol Brain Res. 1999;72:1–16. doi: 10.1016/s0169-328x(99)00184-9. [DOI] [PubMed] [Google Scholar]
- Ford CP, Beckstead MJ, Williams JT. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J Neurophysiol. 2007;97:883–891. doi: 10.1152/jn.00963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lecumberri C, Torres I, Martin S, Crespo JA, Miguens M, Nicanor C, Higuera-Matas A, Ambrosio E. Strain differences in the dose–response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J Psychopharmacol. 2011;25:783–791. doi: 10.1177/0269881110367444. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther. 1978;205:536–546. [PubMed] [Google Scholar]
- Georges F, Le Moine C, Aston-Jones G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006;26:5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorakos L, Sakagianni K, Tsigou E, Apostolakos G, Nikolopoulos G, Veldekis D. Outcome of acute heroin overdose requiring intensive care unit admission. J Opioid Manag. 2010;6:227–231. doi: 10.5055/jom.2010.0021. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Hooks NT. Changes in personality and subjective experience associated with the chronic administration and withdrawal of opiates. J Nerv Men Dis. 1969;148:606–614. doi: 10.1097/00005053-196906000-00004. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology. 2000;24:75–85. doi: 10.1016/S0893-133X(00)00184-6. [DOI] [PubMed] [Google Scholar]
- Havemann U, Kuschinsky K. Review. Neurochemical aspects of the opioid-induced ‘catatonia’. Neurochem Int. 1982;4:199–215. doi: 10.1016/0197-0186(82)90055-9. [DOI] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33:773–776. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- Izumi J, Washizuka M, Hayashi-Kuwabara Y, Yoshinaga K, Tanaka Y, Ikeda Y, Kiuchi Y, Oguchi K. Evidence for a depressive-like state induced by repeated saline injections in Fischer 344 rats. Pharmacol Biochem Behav. 1997;57:883–888. doi: 10.1016/s0091-3057(96)00455-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Fleming WW. Mechanisms of cellular adaptive sensitivity changes: applications to opioid tolerance and dependence. Pharmacol Rev. 1989;41:435–488. [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans C, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Ann Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Role of a functional human gene polymorphism in stress responsivity and addictions. Clin Pharmacol Ther. 2008;83:615–618. doi: 10.1038/clpt.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS. Stress responsivity, addiction, and a functional variant of the human mu-opioid receptor gene. Mol Interv. 2007;7:74–78. doi: 10.1124/mi.7.2.7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, Zhou Y, Butelman ER. Bidirectional translational research: Progress in understanding addictive diseases. Neuropharmacology. 2009a;56(Suppl 1):32–43. doi: 10.1016/j.neuropharm.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Zhou Y, Butelman ER, Levran O. Opiate and cocaine addiction: from bench to clinic and back to the bench. Curr Opin Pharmacol. 2009b;9:74–80. doi: 10.1016/j.coph.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Chen AC, Unterwald EM, Kreek MJ. Subject-regulated dosing alters morphine self-administration behavior and morphine-stimulated [35S]GTPgammaS binding. Synapse. 2003;47:243–249. doi: 10.1002/syn.10173. [DOI] [PubMed] [Google Scholar]
- Kuribara H. Effects of interdose interval on ambulatory sensitization to methamphetamine, cocaine and morphine in mice. Eur J Pharmacol. 1996;326:1–5. doi: 10.1016/s0014-2999(96)00635-8. [DOI] [PubMed] [Google Scholar]
- Langerman L, Piscoun B, Bansinath M, Shemesh Y, Turndorf H, Grant GJ. Quantifiable dose-dependent withdrawal after morphine discontinuation in a rat model. Pharmacol Biochem Behav. 2001;68:1–6. doi: 10.1016/s0091-3057(00)00442-1. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher CE, Martin TJ, Childers SR. Mechanisms of mu opioid receptor/G-protein desensitization in brain by chronic heroin administration. Life Sci. 2005;77:1140–1154. doi: 10.1016/j.lfs.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Maher CE, Selley DE, Childers SR. Relationship of mu opioid receptor binding to activation of G-proteins in specific rat brain regions. Biochem Pharmacol. 2000;59:1395–1401. doi: 10.1016/s0006-2952(00)00272-0. [DOI] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–355. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Mayo-Michelson L, Young GA. Effects of chronic morphine administration and naloxone on EEG, EEG power spectra, and associated behavior in two inbred rat strains. Pharmacol Biochem Behav. 1992;42:815–821. doi: 10.1016/0091-3057(92)90035-e. [DOI] [PubMed] [Google Scholar]
- Mirin SM, Meyer RE, McNamee HB. Psychopathology and mood during heroin use: acute vs chronic effects. Arch Gen Psychiatry. 1976;33:1503–1508. doi: 10.1001/archpsyc.1976.01770120107011. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Kalant H. Naloxone prevention of morphine LDR curve flattening associated with high-dose tolerance. Psychopharmacology (Berl) 1981;75:132–133. doi: 10.1007/BF00432174. [DOI] [PubMed] [Google Scholar]
- Narita M, Shibasaki M, Nagumo Y, Narita M, Yajima Y, Suzuki T. Implication of cyclin-dependent kinase 5 in the development of psychological dependence on and behavioral sensitization to morphine. J Neurochem. 2005;93:1463–1468. doi: 10.1111/j.1471-4159.2005.03136.x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; Washington D.C: 2010. [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs. food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Conversi D, Caprioli D, Bianco PD, Nencini P, Cabib S, Badiani A. Modulatory effect of environmental context and drug history on heroin-induced psychomotor activity and fos protein expression in the rat brain. Neuropsychopharmacology. 2007;32:2611–2623. doi: 10.1038/sj.npp.1301388. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. Academic Press; New York: 1986. [DOI] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology (Berl) 2012;220:163–172. doi: 10.1007/s00213-011-2464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras AP, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Acute withdrawal from chronic escalating-dose binge cocaine administration alters kappa opioid receptor stimulation of [35S] guanosine 5′-O-[gamma-thio]triphosphate acid binding in the rat ventral tegmental area. Neuroscience. 2010;169:751–757. doi: 10.1016/j.neuroscience.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Minarro J. Reinstatement of morphine-induced conditioned place preference in mice by priming injections. Neural Plast. 2003;10:279–290. doi: 10.1155/NP.2003.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerig SC, Fujimoto JM, Lange DG. Development of tolerance to respiratory depression in morphine- and etorphine-pellet-implanted mice. Brain Res. 1987;400:278–284. doi: 10.1016/0006-8993(87)90627-5. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Niculescu M, Unterwald EM. Cocaine alters mu but not delta or kappa opioid receptor-stimulated in situ [35S]GTPgammaS binding in rat brain. Synapse. 2003;47:26–32. doi: 10.1002/syn.10148. [DOI] [PubMed] [Google Scholar]
- Seip KM, Reed B, Ho A, Kreek MJ. Measuring the incentive value of escalating doses of heroin in heroin-dependent Fischer rats during acute spontaneous withdrawal. Psychopharmacology (Berl) 2012;219:59–72. doi: 10.1007/s00213-011-2380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes mu-opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci. 2000;20:4555–4562. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Behavioral stereotypies induced by ‘binge’ cocaine administration are independent of drug-induced increases in corticosterone levels. Behav Brain Res. 1997;86:201–204. doi: 10.1016/s0166-4328(96)02257-7. [DOI] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith DE, Jr, Bunnett NW, Von Zastrow M, Evans C, Brecha NC. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci U S A. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Frys KA, Middaugh LD. Genetic variation in heroin-induced changes in behaviour: effects of B6 strain dose on conditioned reward and locomotor sensitization in 129-B6 hybrid mice. Genes Brain Behav. 2005;4:324–336. doi: 10.1111/j.1601-183X.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Taracha E, Chrapusta SJ, Lehner M, Skorzewska A, Maciejak P, Szyndler J, Plaznik A. Morphine and methadone pre-exposures differently modify brain regional Fos protein expression and locomotor activity responses to morphine challenge in the rat. Drug Alcohol Depend. 2008;97:21–32. doi: 10.1016/j.drugalcdep.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Tejada HA, Shippenberg TS, Henriksson R. The dynorphin/kappa-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69:857–896. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulibarri I, Garcia-Sevilla JA, Ugedo L. Modulation of brain alpha 2-adrenoceptor and mu-opioid receptor densities during morphine dependence and spontaneous withdrawal in rats. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:530–537. doi: 10.1007/BF00169310. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Anton B, To T, Lam H, Evans CJ. Quantitative immunolocalization of mu opioid receptors: regulation by naltrexone. Neuroscience. 1998;85:897–905. doi: 10.1016/s0306-4522(97)00659-3. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Knapp C, Zukin RS. Neuroanatomical localization of kappa 1 and kappa 2 opioid receptors in rat and guinea pig brain. Brain Res. 1991;562:57–65. doi: 10.1016/0006-8993(91)91186-5. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther. 1994;270:1387–1396. [PubMed] [Google Scholar]
- Vanderschuren LJ, Tjon GH, Nestby P, Mulder AH, Schoffelmeer AN, De Vries TJ. Morphine-induced long-term sensitization to the locomotor effects of morphine and amphetamine depends on the temporal pattern of the pretreatment regimen. Psychopharmacology (Berl) 1997;131:115–122. doi: 10.1007/s002130050273. [DOI] [PubMed] [Google Scholar]
- Warner-Smith M, Darke S, Lynskey M, Hall W. Heroin overdose: causes and consequences. Addiction. 2001;96:1113–1125. doi: 10.1046/j.1360-0443.2001.96811135.x. [DOI] [PubMed] [Google Scholar]
- Weber RJ, Gomez-Flores R, Smith JE, Martin TJ. Immune, neuroendocrine, and somatic alterations in animal models of human heroin abuse. J Neuroimmunol. 2004;147:134–137. doi: 10.1016/j.jneuroim.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Werling LL, McMahon PN, Cox BM. Selective changes in mu opioid receptor properties induced by chronic morphine exposure. Proc Natl Acad Sci U S A. 1989;86:6393–6397. doi: 10.1073/pnas.86.16.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Landthaler M, Schlussman SD, Yuferov V, Ho A, Tuschl T, Kreek MJ. Mu opioid receptor knockdown in the substantia nigra/ventral tegmental area by synthetic small interfering RNA blocks the rewarding and locomotor effects of heroin. Neuroscience. 2009;158:474–483. doi: 10.1016/j.neuroscience.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain kappa opioid receptors: evidence for kappa 1 and kappa 2 opioid receptors. Proc Natl Acad Sci U S A. 1988;85:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.