Abstract
A connection between pain and depression has long been recognized in the clinical setting; however, its mechanism remains unclear. In this study, we showed that mechanical hyperalgesia induced by unilateral temporomandibular joint (TMJ) inflammation was exacerbated in Wistar-Kyoto (WKY) rats with genetically predisposed depressive behavior. Reciprocally, TMJ inflammation enhanced depressive behavior such that a lower nociceptive threshold correlated with a higher score of depressive behavior in the same WKY rats. As compared with Wistar rats, WKY rats exhibited a lower plasma melatonin level, downregulation of the melatonin MT1 receptor, but upregulation of the NR1 subunit of the NMDA receptor in the ipsilateral trigeminal subnucleus caudalis (Sp5C). Intracisternal administration of 6-chloromelatonin (250μg, twice daily × 7 days) concurrently attenuated mechanical hyperalgesia and depressive behavior in WKY rats as well as downregulated the NR1 expression in the ipsilateral Sp5C. In patch-clamp recordings, melatonin dose-dependently decreased NMDA-induced currents in spinal cord dorsal horn substantia gelatinosa neurons. These results demonstrate a reciprocal relationship between TMJ inflammation-induced mechanical hyperalgesia and depressive behavior and suggest that the central melatoninergic system, through modulation of the NMDA receptor expression and activity, may play a role in the mechanisms of the comorbidity between pain and depression.
Keywords: Trigeminal pain, Hyperalgesia, Melatonin, Depression, WKY rat, Inflammation, Temporomandibular joint
Introduction
Previous studies have consistently shown a high degree of comorbidity between depression and chronic pain [1, 3, 6, 11, 33, 39, 51, 55, 82]. The prevalence of pain in subjects with depression, as well as that of depression in subjects with pain, is higher than in the cohort with either condition alone [6]. Moreover, pain and depression often share similar predictors and appear to exacerbate each other in the clinical setting [7, 13, 24, 32, 33, 44, 52, 82]. Despite this apparent connection between clinical pain and depression, the exact mechanism of their interaction remains unclear.
We have shown in a previous study that a reciprocal relationship between allodynia and depressive behavior is present in rats with combined sciatic nerve injury and depression [97]. Recent studies have indicated that inflammatory pathways may play a role in the interaction between pain and depression [11, 59, 83]. For example, animals with spared nerve injury showed increased gene expression of interleukin-1beta (IL-1β) within the frontal cortex, and exposure to stress for two weeks before spared nerve injury exacerbated mechanical allodynia and depressive behavior [59]. Moreover, the corticosteroid synthesis inhibitor metyrapone given before exposure to stress prevented exacerbation of mechanical allodynia in rats with spared nerve injury, whereas administration of an IL-1 receptor antagonist diminished the effect of nociception on depressive behavior in the same study [59]. These findings suggest that the mechanisms of interaction between pain and depression could be examined using animal models with combined nociceptive and depressive conditions.
Melatonin is a hormone secreted mainly by the pineal gland, which regulates important biological functions including circadian rhythms, sleep, and mood [12, 23, 57, 64, 70, 79, 85, 87, 96]. It has been shown that melatonin produces a transient antinociceptive effect in rats and mice [62, 80, 94, 95] modulates lipopolysaccharide-induced hyperalgesia [70], and interacts with opioid antinociception [28, 41, 65, 70, 75]. In clinical studies, melatonin has been reported to reduce cluster headache, irritable bowel syndrome, and fibromyalgia [14, 40, 76]. We have also shown that the plasma melatonin level and the spinal melatonin receptor expression were altered in rats with sciatic nerve injury and depressive behavior [97], supporting a role for endogenous melatonin in this process.
Using a rat model of temporomandibular joint (TMJ) inflammation in WKY rats (a variant of Wistar rats) with genetically predisposed depressive behavior, as well as age-matched Wistar rats, we aimed to examine 1) differences in TMJ inflammation-induced mechanical hyperalgesia and depressive behavior between WKY and Wistar rats, 2) plasma melatonin level and expression of the melatonin MT1 receptor and the NR1 subunit of the NMDA receptor in the Sp5C, and 3) the effect of melatonin or its analog on mechanical hyperalgesia, depressive behavior, NMDA receptor expression, and NMDA-induced current in spinal cord dorsal horn substantia gelatinosa neurons.
Materials and Methods
Drugs
Complete Freund’s adjuvant (CFA), incomplete Freund’s adjuvant (IFA), melatonin (N-acetyl-5-methoxytryptamine), 6-chloromelatonin (N-[2(6-chloro-5-methoxy-1H-indol-3-yl)ethyl] acetamide), NMDA, glycine, tetrodotoxin (TTX), strychnine, bicuculline methchloride, a-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), D-(−)-2-amino-5-phosphonopentanoic acid (d-AP5), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo [f] quinoxaline -7-sulfonamide (NBQX) were all purchased from Sigma-Aldrich (St. Louis, MO, US). Luzindole (MT1&MT2 antagonist) and prazosin hydrochloride (MT3 antagonist) were purchased from Tocris (Ellisville, MI).
Animals and trigeminal inflammation model
Male Wistar and WKY rats (Charles River Lab, Wilmington, MA, 220–260g at the beginning of experiment) were used. The experimental protocol was approved by our Institutional Animal Care and Use Committee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were housed under controlled temperature (21°C±2°C), relative humidity (50%±10%) and artificial lighting (12/12 h light/dark cycle, lights on at 7 A.M.). All animals had ad libitum access to distilled water and a standard rat diet. The nomenclature from the rat’s brain atlas by Paxinos and Watson [66] was used for describing trigeminal nuclei.
Persistent TMJ inflammation was induced by injecting CFA (as a suspension in 1:1 oil/saline ratio and a total volume of 50μl) into one TMJ capsule under pentobarbital sodium (50mg/kg, i.p.) anesthesia. TMJ was identified by palpation and the injection was given by advancing a 22-gauge needle inferior to the posterior border of the zygomatic arch until it reached the mandibular condyle. Since a small amount of CFA out-flew into surrounding tissues including muscles during the injection, TMJ inflammation involved both the TMJ capsule and surrounding tissues. Previous studies have shown that this dose of CFA injected into the TMJ produces persistent hyperalgesia [61, 88] and elevates Fos-like immunoreactivity in the lower trigeminal complex [98]. For control, 50 μl of IFA was injected according to the same protocol used for the CFA injection.
For intracisternal drug administration, a 7-cm piece of PE10 tube was prepared, creating a circle in the proximal part of the tubing to be used to secure the catheter to subcutaneous tissues, and sterilized in 70% ethanol overnight before surgery. Under pentobarbital anesthesia, a rat was fixed to a stereotaxic apparatus and a small cut was made to expose the cisterna magna. The PE10 tubing was inserted cephalically (about 3cm) and secured to the skull by using 3-0 silk suture around the circle of the tubing. Those rats exhibiting postoperative neurological deficits (e.g., paralysis) or distress (poor grooming or eating) were excluded from the experiments as previously described [54]. 6-chloromelatonin (a melatonin analog) was diluted in 5% ethanol to the final concentration of 25mg/ml. Ten μl of this solution (250 μg 6-chloromelatonin), followed by 10 μL saline flush, was given twice daily via the catheter. This 5% ethanol solution was used as vehicle control. Motor activities were observed in rat’s home cage on each day of behavioral test including gate and exploratory activities in order to assess the effect of an agent or vehicle on rat’s motor function.
Assessment of mechanical hyperalgesia
To assess mechanical hyperalgesia, a rat was gently held in a hand of an experimenter. The level tip of a digital algometer (Wagner Instruments, Greenwich, CT), producing a linear increase of mechanical force, was firmly pressed against the TMJ region ipsilateral and contralateral to the CFA or IFA injection. The stimulation was exerted to an area of about 0.75cm2 that included TMJ and the sounding tissue. We did not use von Frey filaments because the stimulation from a von Frey filament focuses on only a tiny point of the skin surface. Given that TMJ-related nociception in this model affects both TMJ and surrounding tissues, we opted to use the algometer instead of von Frey filaments. Nociceptive threshold (g/mm2) was determined when the rat either vocalized or withdrew (sudden and vigorous turning of its head) from the stimulation. Two trials were made for each rat with a 10min interval and the values were averaged from two trials. The mechanical hyperalgesia test took place between 9 and 10 AM, before the first daily intrathecal injection.
Forced swimming test
The forced swim test was performed to assess depressive behavior according to the method originally described by Porsolt et al [68, 69] and used in our previous study [97]. One day prior to the test, a rat was placed for conditioning for 5min (pretest session) in a clear plastic tank (45×35×60cm) containing 30cm of water (24±0.5 °C). The next day, each rat was tested under the same condition for 5min (test session). Both WKY and Wistar rats underwent the same test procedure. Following each session, a rat was removed from water tank, dried with paper towel, placed in a warm cage for 10min, and then returned to its home cage. Each test session was recorded with a stopwatch. A rat was judged to be non-swimming when no effort was made to escape from the tank (floating passively). The total duration of immobility (non-swimming) within a 5-min session was recorded as immobility score (in second) and compared among groups [17, 45, 97]. All sessions were observed by the same experimenter blinded to the group assignment in order to minimize between-session variation. All swimming test sessions took place between 2 and 4 PM before the second daily intrathecal injection.
ELISA
Blood samples were taken in the afternoon to minimize natural variation of the serum melatonin level. The blood was allowed to clot for 60min in dark cold room (4°C), and then centrifuged at 1000rpm for 5min. The serum was obtained, wrapped with aluminum foil for photo protection and stored at −80 °C until use. The melatonin ELISA kit (catalog #RE 54021) was purchased from IBL (Immuno-Biological Laboratories, Minneapolis, MN). A standard curve was generated using the reagent supplied in the kit. Experimental samples were measured according to the manual provided in the kit. Fifty μl of the standard agent or the extracted sample from each rat was first put into individual plate wells, followed by adding 50μL each of melatonin biotin and antiserum. The plate was well shaken, sealed with adhesive foil, and incubated overnight in a dark cold room. The plate was then washed three times with an assay buffer and 150μl of an enzyme conjugate supplied in the kit was added. The plate was again incubated for 120min at room temperature. After three washes with the assay buffer, 200μl of para-nitrophenyl phosphates (PNPP) substrate solution was added, followed by incubation for 30min at room temperature. The reaction was stopped by adding 50μl of PNPP solution into wells. Absorbance values were obtained using a microplate reader (Bio-TeK Instruments Winooski, VT) at 405nm wavelength. The serum melatonin concentration was calculated based on the standard curve and presented as pg/ml.
Western blot
Trigeminal (Sp5C) tissue, separated by each side, was homogenized in a buffer (20mM Tris-HCl, pH 7.5, 150mM NaCl, 1mM EDTA, 1mM EGTA, 2.5mM Sodium pyrophosphate, 1mM β-Glycerophosphate, 1mM Sodium Orthovanadate, 0.01% Tirton-X100, 0.01% NP-40) containing a mixture of proteinase inhibitors (Sigma). Tissue homogenates were centrifuged at 7,000rpm for 10min at 4 °C. Protein samples (50μg) were loaded and separated on SD S-PAGE gel (12%) and transferred to polyvinylidene difluoride filters (Millipore, Bedford, MA). Filters were blocked with 5% non-fat dry milk and subsequently incubated with a primary antibody, MTR-1A (MT1, 1: 500, rabbit anti-rat polyclonal, 1:500, Abbiotec, San Diego, CA), overnight in a cold room and then 1hr at room temperature with HRP-conjugated secondary antibody (Donkey anti-rabbit 1:4000; Santa Cruz). ECL solution was used for visualization and blots were exposed onto a hyperfilm (Amersham) for 1–10min. The blots were then incubated in a stripping buffer (67.5mM Tris, pH 6.8, 2% SDS, and 0.7% β-mercaptoethanol) for 1–15min at room temperature and reprobed with an NR1 antibody (1:500, mouse monoclonal; United States Biological) under the same incubation condition. With a similar procedure, a monoclonal mouse anti-β-actin antibody (1:12,000; Abcam) was used for loading control. Samples from each rat were probed in triplicates. The density of each blot was measured with Adobe PhotoShop and normalized against a corresponding β-actin blot. Differences in the normalized band density among different groups were compared using Graphpad Instat software.
Immunohistochemistry
Rats were anesthetized with sodium pentobarbital (50mg/kg, i.p.) and transcardially perfused with 200 ml of saline followed by 200–300ml of ice-cold 4% paraformaldehyde in 0.1M phosphate buffer (PB). Brains were harvested, post-fixed for 4hr, and kept in 30% sucrose in 0.1M PB until sunk to the bottom. Brain tissues from all rats were cut, processed and immunostained using the same procedure. Immunohistochemical staining was used to detect MT1 (1: 500, rabbit anti-rat polyclonal, 1:500, Abbiotec, San Diego, CA) and NR1 (1:250, mouse monoclonal; United States Biological). Briefly, brain sections were blocked with 1% BSA, 3% donkey serum in 0.3% Triton for 1hr at room temperature and incubated overnight at 4 °C with a primary antibody. For control, a primary antibody was omitted or antigen absorption was used. Sections were then incubated for 1hr at room temperature with a corresponding DyLight 488- or DyLight 594-conjugated secondary antibody (1:300; Jackson Immunoresearch, West Grove, PA). For double staining, a second primary antibody was added using the same procedure. Sections were mounted onto slides, analyzed using a fluorescence microscope (Olympus, Japan), recorded using a digital camera, and processed using Adobe Photoshop.
Patch-clamp recording
Substantia gelatinosa (lamina II) of the spinal cord dorsal horn has a similar anatomical structure as the trigeminal dorsal horn, which has been implicated in nociceptive transmission because both Aδ and C primary nociceptive afferent fibers are heavily projected onto this region [37, 42, 93]. The upper cervical spinal cord is the continuation of the elongated part of trigeminal nuclei. Since activation of NMDA receptor plays a crucial role in the mechanisms of peripheral and central sensitization [15, 20, 53, 67], we used slices taken from the upper cervical spinal cord as a surrogate model of the trigeminal dorsal horn to examine whether melatonin would regulate NMDA-induced current in substantia gelatinosa neurons.
Spinal cord slices were prepared from postnatal 4~6 weeks old WKY or Wistar rats. Under sodium pentobarbital anesthesia (50mg/kg, i.p.), a portion of cervical spinal cord (C1–C5) was rapidly removed and placed in chilled, oxygenated artificial cerebrospinal fluid (ACSF) containing (mM): 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 2 MgCl2, and 25 d-glucose. Transverse slices (400μm) were cut on a vibratome (Vibratome, IL) and incubated in the oxygenated ACSF at 35°C for 30min and then at room temperature for 1hr before recording. Melatonin, luzindole, prazosin, d-AP5, or NBQX was first dissolved in DMSO and then diluted with artificial cerebrospinal fluid (ACSF) to their final concentrations. The final concentration of DMSO in a solution was less than 0.1%. Accordingly, a corresponding concentration of DMSO or ACSF was used as vehicle.
For whole-cell patch clamp recording, a slice was placed in a recording chamber mounted onto the stage of an upright microscope (Olympus, Japan) and perfused with ACSF (2~3ml/min). Substantia gelatinosa was clearly visible as a translucent band across the spinal cord dorsal horn under a low magnification microscopic view. Patch pipettes, with resistance of 3–5MΩ, were made from thick-walled, borosilicate, glass-capillary tubing (1.5mm outer diameter). The internal solution contained the following (mM): 140 K-gluconate, 2 MgCl2, 1 CaCl2, 11 EGTA, 10 HEPES, 5 Mg-ATP, and 0.5 Na-GTP, pH 7.3. Only neurons that had an apparent resting membrane potential more negative than −50mV and produced normal action potentials after injection of depolarizing currents (25–200pA in 25pA steps for 1s) were further investigated [30, 74].
NMDA currents were evoked by ejecting 50μM NMDA plus 10μM glycine in Mg2+-free ACSF for 30s at a holding potential of −70mV (Stoelting, WI). One μM strychnine, 10μM bicuculline, 10μM NBQX, and 1μM TTX were added to the bathing solution to block glycine receptors, GABAA receptors, AMPA receptors and voltage-gated sodium channels, respectively. Once baseline NMDA current was acquired, melatonin (0.1, 1, 5mM) in the presence or absence of a melatonin receptor antagonist, was bath-applied for 5min. Recordings were made using an Axopatch 700B amplifier (Molecular Devices, CA). Signals were filtered at 2kHz, digitized at 10kHz, acquired using a personal computer and pClamp software (version 9.2; Molecular Devices, CA), and stored for later analysis. Input resistance was monitored throughout the experiments. The mean series resistance was 25~35Ω and the current noise was ~10pA. All experiments were performed at room temperature (22~25°C).
Statistical analysis
Data from behavioral tests were analyzed by using two-way ANOVA, followed by post-hoc tests, to compare across various groups and time points. Differences were considered to be statistically significant at the level of α =0.05. The Spearman’s correlation coefficient analysis was used to examine a correlative relationship between mechanical nociceptive threshold and depressive behavior score (immobility time in the forced swimming test).
Results
Exacerbation of depressive behavior in WKY rats after TMJ inflammation
Baseline immobility scores obtained using the forced swimming test were significantly higher in WKY than Wistar rats (Fig. 1A, F=9.904, df =27, p<0.01), indicating the presence of a basal level of depressive behavior. While the immobility score was not changed in Wistar rats receiving CFA or IFA on day 7 (Fig. 1A, p>0.05), the immobility score was significantly higher on day 7 in WKY rats receiving CFA when compared with both WKY rats receiving IFA or Wistar rats receiving CFA (Fig. 1A, p<0.05). The data indicates that TMJ inflammation exacerbated depressive behavior in WKY rats with genetically predisposed depressive behavior.
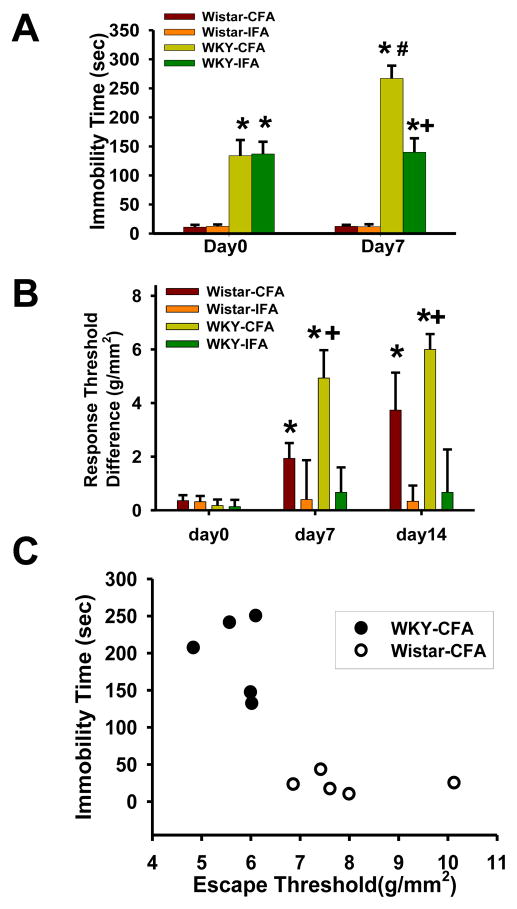
Fig. 1. Exacerbated mechanical hyperalgesia and depressive behavior in WKY rats.
A. WKY rats showed a prolonged immobility time in a 5-min forced swimming test on day 0 (baseline) and day 7 after CFA or IFA injection into the right TMJ. *, p<0.05 as compared to Wistar rats on the same day (n=5 for each group); #, p<0.05 as compared with WKY rats with inflammation on day 0; +, p<0.05 as compared with WKY rats with TMJ inflammation. B. Exacerbated mechanical hyperalgesia at the ipsilateral TMJ site in WKY rats when tested on day 7 and 14 after CFA injection. *, p<0.05 as compared with day 0; +, p<0.05 as compared with Wistar-CFA rats tested on the same day. C. The immobility time was inversely related to nociceptive threshold after the CFA injection in WKY rats, as compared with Wistar rats.
Exacerbation of mechanical hyperalgesia in WKY rats after TMJ inflammation
Difference scores (the side of TMJ without CFA injection minus the side of TMJ injected with CFA) did not differ at baseline between WKY and Wistar rats (Fig. 1B, day 0, p>0.05, n=6), indicating that the presence of predisposed depressive behavior in WKY rats did not change their baseline nociceptive threshold to mechanical stimulation. When examined 7 and 14 days after TMJ inflammation, both WKY and Wistar rats showed a lower nociceptive threshold to mechanical stimulation in the ipsilateral, but not contralateral, TMJ region, as compared with their corresponding baseline (Fig. 1B, F=15.278, df=41, p<0.05). However, mechanical hyperalgesia was exacerbated in WKY rats with TMJ inflammation such that mechanical nociceptive threshold was significantly lower in these WKY rats with TMJ inflammation than that of their counterpart Wistar rats (Fig. 1B, p<0.01, n=5). These results indicate that mechanical hyperalgesia was exacerbated in WKY rats following TMJ inflammation as compared with Wistar rats.
A correlative relationship between mechanical hyperalgesia and depressive behavior was examined by using the Spearman’s rank analysis. This analysis showed that a longer immobility time in the forced swimming test (in seconds) inversely correlated with a lower nociceptive threshold (escape threshold in g/mm2) in WKY rats (Fig. 1C; R= −0.2533, n=5; Y=464.693-0.1868X) but not in Wistar rats (Fig. 1C; R= −0.0792, n =5; Y=614.895-0.6057X), indicating a reciprocal, albeit weak, relationship between mechanical hyperalgesia and depressive behavior in WKY rats.
Lower plasma melatonin concentration in WKY rats
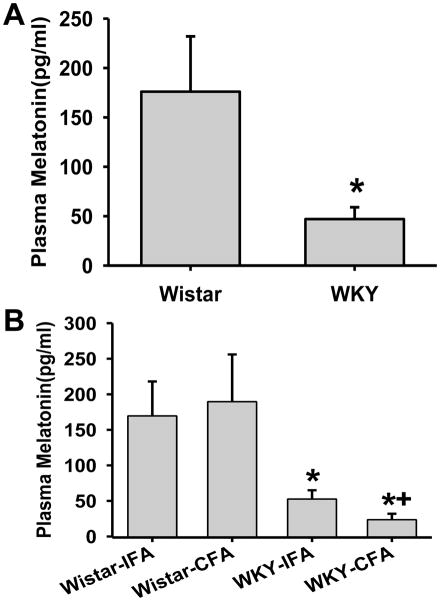
Baseline plasma melatonin concentration assayed by ELISA was significantly lower in WKY rats than Wistar rats in the absence of TMJ inflammation (Fig. 2A, p<0.05, n=12/group). The plasma melatonin concentration in WKY rats was further reduced at 7 days after TMJ inflammation, as compared to both Wistar rats with TMJ inflammation and WKY rats injected with IFA (Fig. 2B, p<0.05, n=12/group). These results indicate that WKY rats had a lower melatonin level than Wistar rats both before and after TMJ inflammation.
Fig. 2. Plasma melatonin level before and after TMJ inflammation.
A. The plasma melatonin level as detected by ELISA in Wistar and WKY rats before TMJ inflammation. *, p<0.05 as compared with Wistar rats. B. The Plasma melatonin level after TMJ inflammation. In both A&B, plasma was collected at 5 days after CFA or IFA injection. *, p<0.05 as compared with Wistar rats; +, p<0.05 as compared with the WKY-IFA group.
Effect of intracisternal 6-chloromelatonin on mechanical hyperalgesia and depressive behavior in WKY rats
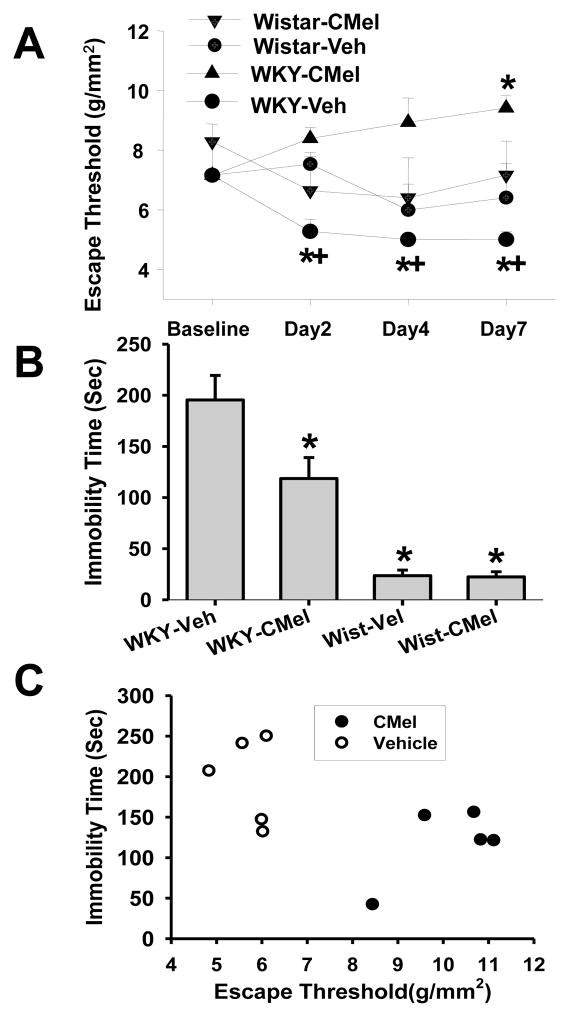
Since depressive behavior correlated with exacerbated mechanical hyperalgesia in WKY rats with a lower plasma melatonin level, we examined whether 6-chloromelatonin (a melatonin analog) would improve both mechanical hyperalgesia and depressive behavior in WKY rats. Accordingly, 6-chloromelatonin (250μg) or vehicle was administered, via an intracisternal catheter, twice daily for six consecutive days beginning immediately after the CFA injection into unilateral TMJ (n=5/group). When tested on day 2, 4 and 7 after TMJ inflammation, mechanical hyperalgesia was significantly improved in WKY rats treated with 6-chloromelatonin, without motor impairment (e.g., gait change), as compared with those rats treated with vehicle (Fig. 3A; F=21.494, df=27, p<0.05).
Fig. 3. Effect of 6-chloromelatonin.
A. 6-Chloromelatonin (C-Mel; 250μg) or vehicle (Vel) was injected intracisternally (twice daily × 6 days) in Wistar and WKY rats (n=5/group) beginning immediately after CFA injection into unilateral TMJ. 6-chloromelatonin prevented exacerbation of mechanical hyperalgesia in WKY rats. * p<0.01, as compared with baseline; +, p<0.001, as compared with WKY rats treated with vehicle and tested at the same time point. B. The same 6-Chloromelatonin treatment also improved depressive behavior (forced swimming test) in WKY rats with TMJ inflammation on day 7. * p<0.05, as compared with WKY-Vehicle group. C. The 6-Chloromelatonin treatment reversed the relationship between mechanical hyperalgesia and depressive behavior as demonstrated in WKY rats treated with vehicle (see Fig. 1C).
Intracisternal 6-chloromelatonin administration also improved depressive behavior in WKY rats such that the duration of immobility in the forced swimming test was significantly reduced when tested on day 7 after TMJ inflammation, as compared with vehicle-treated WKY rats (Fig. 3B, p<0.05, n=5/group). In contrast, intracisternal administration of 6-chloromelatonin did not change the immobility time in the forced swimming test (Fig. 3B, n=5), nor did it alter baseline nociceptive threshold (data not shown), in Wistar rats. Moreover, the correlative relationship between mechanical hyperalgesia and depressive behavior in WKY rats with TMJ inflammation shown in Figure 1C was reversed after the intracisternal 6-chloromelatonin administration (Fig. 3C; n=5/group; WKY rats treated with 6-chloromelatonin: R=0.6738, n =5; Y=615.478+1.2201X; WKY rats treated with vehicle: R=0.0642, n =5; Y=399.544+0.0763X). Collectively, the results indicate that intracisternal administration of a melatonin analog concurrently improve both mechanical hyperalgesia and depressive behavior in WKY rats.
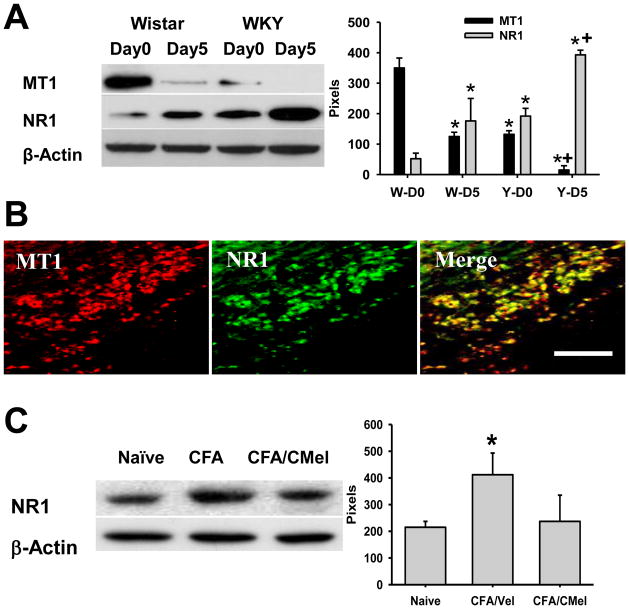
Modulation of MT1 and NR1 expression in Sp5C by TMJ inflammation and 6-chloromelatonin
As compared with its corresponding baseline, expression of MT1 (Western blot) in the ipsilateral trigeminal Sp5C was downregulated (Fig. 4A, F=5.995, df=15, p< 0.05), while NR1 expression was upregulated (Fig. 4A, p< 0.05, n=5), in both Wistar and WKY rats when examined at 5 days after TMJ inflammation. MT1 and NR1 were also colocalized in Sp5C (Fig. 4B). The same intracisternal 6-chloromelatonin administration (250μg, twice daily × 6 days) that concurrently improved mechanical hyperalgesia and depressive behavior in WKY rats also reversed the upregulated expression of NR1 in the ipsilateral Sp5C (Fig. 4C, p> 0.05, n=5), indicating that 6-chloromelatonin also regulated the NR1 expression in Sp5C in WKY rats.
Fig. 4. Expression of MT1 and NR1 in SP5C.
A. Expression of MT1 and NR1 in the ipsilateral trigeminal Sp5C, as detected by Western blot before and after TMJ inflammation. W: Wistar rats; Y: WKY rats. D0, D5: before or 5 days after the CFA injection into the right TMJ. * p<0.05 as compared with D0 of Wistar rats, + p<0.05 as compared with D0 of WKY rats. B. Immunostaining results showing co-localization of MT1 and NR1 in Sp5C after TMJ inflammation. Bar, 100μm. C. Intracisternal administration of 6-Chloromelatonin (CMel; 250μg) but not vehicle (Vel), twice daily × 6 days, in WKY rats downregualted the expression of NR1 in the ipsilateral Sp5C.
Inhibition by melatonin of NMDA-induced current in substantia gelatinosa neurons
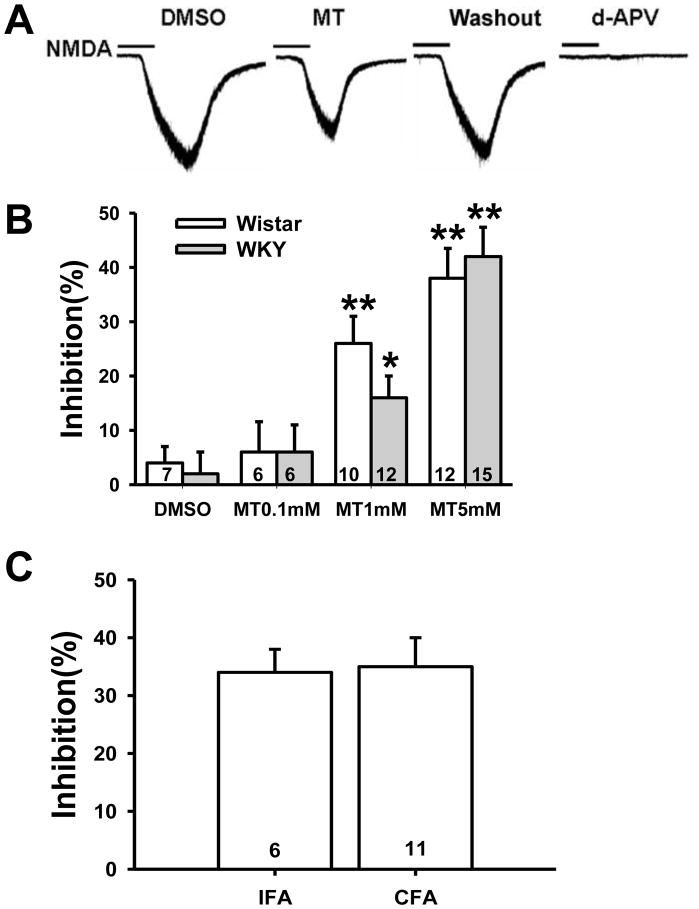
NMDA-induced current in spinal cord dorsal horn substantia gelatinosa neurons was evoked by ejecting 50μM NMDA plus 10μM glycine in Mg2+-free ACSF for 30s at a holding potential of −70mV. The amplitude of NMDA-induced currents ranged from 50pA to 500pA and blocked by 0.1mM d-AP5 (Fig. 5A). Exposure to melatonin (5mM) for 5min markedly decreased NMDA-induced current in substantia gelatinosa neurons of WKY rats (Fig. 5A&B; p< 0.01), which returned to the baseline after melatonin was washed out for 8min (Fig. 5A). Exposure to vehicle (DMSO) for 5min had no effect on NMDA-induced current (n=8, p>0.05, Fig. 5A&B). The melatonin effect on NMDA-induced current was dose-dependent (Fig. 5B) such that 0.1mM melatonin had no significant effect (n=6, p>0.05), 1mM melatonin decreased about 16% (n=12, p<0.05), and 5 mM melatonin had the maximal effect of nearly 40% inhibition (n=15, p<0.01). A similar dose-response effect of melatonin on NMDA-induced current also was demonstrated in substantia gelatinosa neurons of Wistar rats (Fig 5B).
Fig. 5. Inhibition of NMDA-induced current by melatonin.
A. Representative current traces showing NMDA-induced current in a cervical spinal cord dorsal horn substantia gelatinosa neuron of a WKY rat. NMDA-induced current was decreased by melatonin (5mM in the recording chamber for 5min) and recovered 8min after the melatonin washout. NMDA-induced current was completely blocked by 0.1mM d-AP5. B. The inhibitory effect of melatonin on NMDA-induced current was concentration-dependent. MT, melatonin. *p<0.05, **p<0.01 versus vehicle (DMSO). C. At a concentration of 5mM, melatonin significantly inhibited NMDA-induced current recorded from WKY rats received the CFA or IFA injection. Data are shown as mean ± s.e.m. Numbers in the column bars represent cells recorded in each group.
Since melatonin at 5mM had the maximal inhibition on NMDA-induced current in both WKY and Wistar rats, we examined whether melatonin at this concentration would change NMDA-induced currents in WKY rats at 7 days after TMJ inflammation. The results showed that melatonin also significantly blocked NMDA-induced current (about 35%) in WKY rats with TMJ inflammation (Fig. 5C; n=11), similar to its effect on WKY rats receiving the IFA injection (Fig. 5C; n=6).
Effect of melatonin receptor antagonists on NMDA-induced current
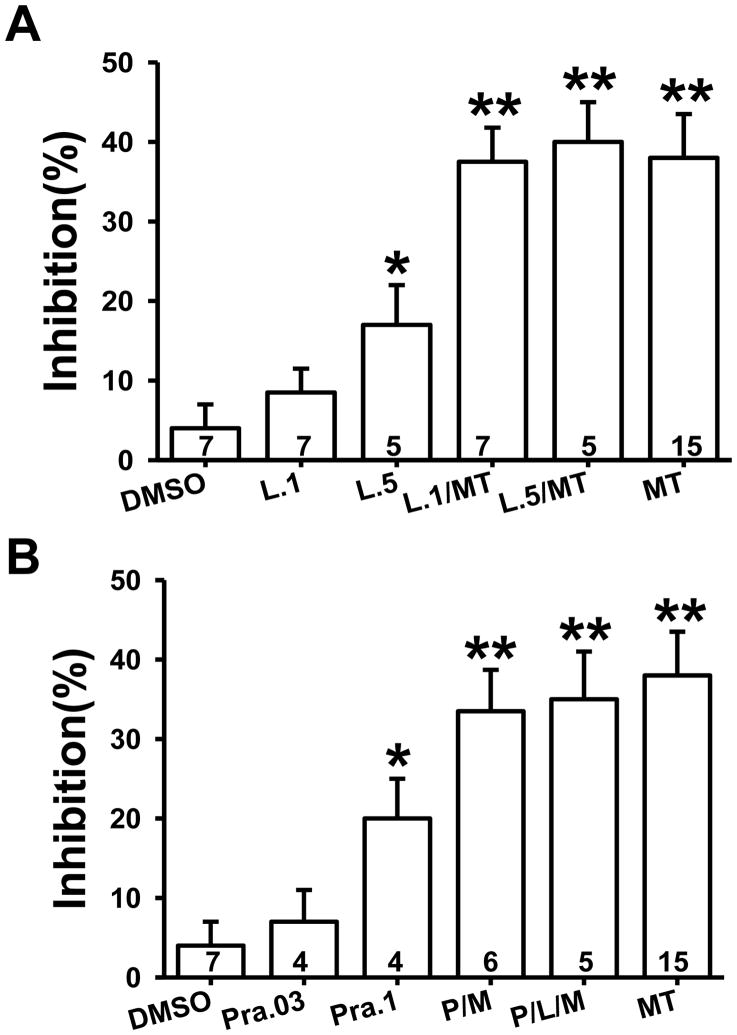
To examine which melatonin receptors were involved in the melatonin effect on NMDA-induced current, luzindole (a competitive melatonin MT1/MT2 receptor antagonist) and prazosin (an MT3 and alpha-1 adrenoreceptor antagonist) were used (Fig. 6). Luzindole at 0.1mM did not significantly change baseline NMDA current (Fig. 6A, n=7). However, co-perfusion of luzindole (0.1mM) with melatonin (5mM) for 5min did not prevent the inhibition of NMDA-induced current (Fig. 6A, n=7) induced by 5mM melatonin alone (Fig. 6A, n=15). At a higher concentration (0.5mM), luzindole itself inhibited NMDA current (Fig. 6A, n=5; p<0.05), but again did not diminish the inhibitory effect of 5 mM melatonin on NMDA-induced current (Fig. 6A, n=5).
Fig. 6. Melatonin receptor antagonists and the melatonin effect on NMDA-induced current.
A. 0.1mM and 0.5mM luzindole did not affect the inhibitory effect of melatonin (5mM) on NMDA-induced current. B. Neither 0.1 mM prazosin nor 0.1 mM prazosin plus 0.1 mM luzindole blocked the inhibitory effect of melatonin (5mM) on NMDA-induced current. Data are shown as mean±S.E. Numbers in the column bars represent cells recorded in each group. M or MT: melatonin; L: luzindole; P or Pra-.03, .1, and .5: prazosin concentrations at 0.03, 0.1, and 0.5mM. *p < 0.05, **p < 0.01 as compared to vehicle.
Prazosin (0.1mM) by itself also inhibited NMDA-induced current (Fig. 6B, n=4) but did not block the effect of 5mM melatonin on NMDA-induced current (Fig. 6B, n=6). Furthermore, a combination of perfusion with 0.1mM luzindole, 0.1mM prazosin, and 5 mM melatonin also failed to block the melatonin effect on NMDA-induced current (Fig. 6B, n=5). These results indicate that the inhibitory effect of melatonin on NMDA-induced current could not be directly blocked by melatonin receptor antagonists tested under the current experimental condition.
Discussion
In this study, we demonstrated that 1) WKY rats exhibited baseline depressive behavior as compared to age-matched Wistar rats; 2) the presence of depressive behavior in WKY rats exacerbated mechanical hyperalgesia induced by TMJ inflammation; 3) TMJ inflammation worsened depressive behavior in WKY rats; 4) WKY rats also exhibited a lower plasma melatonin level at the baseline, which was further reduced by TMJ inflammation; 5) intracisternal administration of 6-chloromelatonin (a melatonin analog) concurrently improved mechanical hyperalgesia and depressive behavior in WKY rats; 6) the expression of the MT1 receptor was downregulated, whereas the expression of the NR1 subunit was upregulated, in the ipsilateral Sp5C of WKY rats; and 7) in association with its behavioral effect, melatonin or its analog prevented the upregulation of NR1 expression and dose-dependently inhibited NMDA-induced current in spinal cord dorsal horn substantia gelatinosa neurons. These results support a reciprocal relationship between depressive behavior and TMJ inflammation-induced mechanical hyperalgesia in WKY rats and suggest that the central melatoninergic system, via regulation of the NMDA receptor expression and activity, plays a significant role in the mechanisms of comorbidity between pain and depression.
It has long been recognized that comorbid depression is associated with pain complaints [5, 24, 43, 60], which complicates clinical diagnosis and treatment of chronic pain [39]. Depression has been shown to result in decreased pain threshold and increased analgesic requirement [16, 31, 38, 46, 47]. Use of antidepressant therapy in combination with psychotherapy program substantially improved depression as well as produced a moderate reduction in pain severity and disability [36]. This finding is consistent with clinical observations that antidepressants such as duloxetine and venlafaxine are beneficial in the treatment of both depression and chronic pain [8, 25, 39]. On the other hand, chronic pain is also closely associated with psychological and psychiatric comorbidities including depression [4, 10, 24, 32, 33, 39, 43, 60]. Consistently, attenuation of pain symptoms often leads to significant improvement in a variety of psychological outcome measures including depression [2, 18, 58] and a higher remission rate of comorbid symptoms [25].
To date, several hypotheses have been proposed regarding the relationship between pain and depression [9, 19, 26], although these hypotheses do not necessarily address the underlying cellular mechanisms of such comorbidity. Recent studies have indicated that multiple anatomical structures are activated and/or altered in response to both depression and pain [22, 59, 73, 78, 81, 84]. In addition, depression and pain may share common biological pathways, neurotransmitters, and neurobiological mechanisms [4, 5, 29, 39, 73] and are associated with common psychological alterations [73]. Of interest is that depression in the context of chronic pain does not appear to be restricted to specific types of pain [55] or their detection [34]. Indeed, temporomandibular disorder is a common stress-related condition with comorbidities including depression [6, 35, 56]. The present study further supports the notion that the comorbid condition of TMJ inflammation-induced nociception and depression can be modeled and assessed in rodents utilizing behavioral tests. This extends our earlier observation in rats with combined depressive behavior and chronic constriction sciatic nerve injury [97] as well as observations from other investigators in rats with spared nerve injury [59]. Of note, although a correlative relationship between nociceptive and depressive behavior was demonstrated in our experiments, the number of animals in each group is relatively low and further confirmation of this relationship requires additional experiments by using perhaps a larger sample size in each group.
Melatonin (N-acetyl-5-methoxytryptamine) has immunomodulatory effects including anti-inflammatory action. Melatonin protects tissue during inflammatory processes by its ability to 1) directly scavenge toxic free radicals [72]; 2) prevent translocation of nuclear factor-kappa B (NF-κB) to the nucleus and its binding to DNA thereby reducing the upregulation of pro-inflammatory cytokines such as IL-1 and TNF-α [27, 71]; and/or 3) inhibit the production of adhesion molecules that promote the attachment of leukocytes to endothelial cells, deterring transendothelial cell migration and edema [48–50]. Recently, melatonin analogs have been shown to have the antidepressant property [21]. 6-choloromelatonin is a melatonin analog, which has a higher affinity than melatonin at melatonin receptors [92]. Moreover, melatonin receptors are located in various brain regions [57, 77, 86, 96]. Preclinical studies have shown that knockout of MT1 receptors increased [91], whereas a melatonin analog decreased, the immobility time in the forced swimming test [63]. In the present study, we found that the expression of MT1 receptors in the ipsilateral Sp5C was downregulated both at the baseline and after TMJ inflammation in WKY rats, in addition to a lower melatonin lever in these same WKY rats. Consistently, 6-chloromelatonin was effective in improving both mechanical hyperalgesia and depressive behavior in WKY rats.
The present data also suggest that the behavioral effect of melatonin is likely to be mediated through its regulatory mechanism on NMDA receptors. Our previous study showed that 1) upregulation of the NR1 subunit plays a role in the mechanisms of inflammatory and neuropathic pain [88, 89]; 2) intraperitoneal co-administration of melatonin (30mg/kg) and dextromethorphan (15mg/kg) effectively reversed both thermal hyperalgesia and mechanical allodynia in WKY rats with chronic constriction sciatic nerve injury, although each individual dose alone had no effect on nociceptive behaviors [90]; and 3) administration of melatonin into the anterior cingulate cortex contralateral to peripheral nerve injury prevented the exacerbation of mechanical allodynia with a concurrent improvement of depressive behavior in WKY rats [97]. In this study, expression of the NR1 subunit in Sp5C was downregulated following intracisternal administration of 6-chloromelatonin, an intervention that also improved nociceptive and depressive behavior. Patch-clamp recordings further demonstrate that melatonin dose- dependently inhibited NMDA-induced current in spinal cord dorsal horn substantia gelatinosa neurons. Of interest, blockade of three known melatonin receptor subtypes (MT1, MT2, and/or MT3), alone or in combination, under our experimental conditions did not prevent the effects of melatonin on NMDA-induced current. Since melatonin produces an inhibitory effect on neuronal excitability, whereas activation of NMDA receptors requires partial depolarization of neuronal cells, a possible mechanism of the observed melatonin effect may be related to its overall inhibitory effect on neuronal cell membrane. However, the exact mechanism of these findings remains to be investigated in future studies, including the use of a broader dose range to rule out the non-specific effect of MT receptor antagonists.
In summary, the current data indicate a correlative and reciprocal relationship between depression and pain in a rat model of combined TMJ inflammation and genetically predisposed depressive behavior. The improvement by melatonin of both mechanical hyperalgesia and depressive behavior lends support to a functional role for the central melatoninergic system in this process. Future studies should elucidate the cellular mechanism of melatonin actions, including its regulation of NMDA receptors, and explore the relationship between a melatonin deficiency state and chronic pain in the clinical setting.
Summary.
The melatonin system dysfunction and the up regulated NMDA receptors in trigeminal nuclei might be involved in trigeminal pain and depression comorbidity.
Acknowledgments
This work was supported by US PHS RO1 grants DE18538 and DE18214.
Footnotes
There is no Conflict of Interest (COI) in this project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altindag O, Gur A, Altindag A. The relationship between clinical parameters and depression level in patients with myofascial pain syndrome. Pain Med. 2008;9:161–5. doi: 10.1111/j.1526-4637.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 2.Arnold LM, Meyers AL, Sunderajan P, Montano CB, Kass E, Trivedi M, Wohlreich MM. Ann The effect of pain on outcomes in a trial of duloxetine treatment of major depressive disorder. Clin Psychiatry. 2008;20:187–93. doi: 10.1080/10401230802435609. [DOI] [PubMed] [Google Scholar]
- 3.Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, Kraemer HC, Dea R, Robinson R, Hayward C. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68:262–8. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 4.Bailey KP. Physical symptoms comorbid with depression and the new antidepressant duloxetine. J Psychosoc Nurs Ment Health Serv. 2003;41:13–8. doi: 10.3928/0279-3695-20031201-08. [DOI] [PubMed] [Google Scholar]
- 5.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and Pain Comorbidity; a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 6.Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blinded study Cephalalgia. 2008;28:832–41. doi: 10.1111/j.1468-2982.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 7.Barry DT, Beitel M, Garnet B, Joshi D, Rosenblum A, Schottenfeld RS. Relations among psychopathology, substance use, and physical pain experiences in methadone-maintained patients. J Clin Psychiatry. 2009;70:1213–8. doi: 10.4088/JCP.08m04367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begré S, Traber M, Gerber M, von Känel R. Change in pain severity with open label venlafaxine use in patients with a depressive symptomatology: an observational study in primary care. Eur Psychiatry. 2008;23:178–86. doi: 10.1016/j.eurpsy.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: Coincidence or consequence? J Neuroendocrinol. 2001;13:1009–23. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonnewyn A, Katona C, Bruffaerts R, Haro JM, de Graaf R, Alonso J, Demyttenaere K. Pain and depression in older people: comorbidity and patterns of help seeking. J Affect Disord. 2009;117:193–6. doi: 10.1016/j.jad.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Bruce TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep. 2008;10:258–64. doi: 10.1007/s11920-008-0042-1. [DOI] [PubMed] [Google Scholar]
- 12.Brzezinski A. Melatonin in humans. New Eng J Med. 1997;336:186–95. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 13.Chou KL. Reciprocal relationship between pain and depression in older adults: evidence from the English Longitudinal Study of Ageing. J Affect Disord. 2007;102:115–23. doi: 10.1016/j.jad.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Citera G, Arias MA, Maldonado-Cocco JA, Lazaro MA, Rosemffet MG, Brusco LI, Scheines EJ, Cardinalli DP. The effect of melatonin in patients with fibromyalgia: a pilot study. Clin Rheumatol. 2000;19:9–13. doi: 10.1007/s100670050003. [DOI] [PubMed] [Google Scholar]
- 15.D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 16.de Zwaan M, Biener D, Bach M, Wiesnagrotzki S, Stacher G. Pain sensitivity, alexithymia, and depression in patients with eating disorders: are they related? J Psychosom Res. 1996;41:65–70. doi: 10.1016/0022-3999(96)00088-8. [DOI] [PubMed] [Google Scholar]
- 17.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;21:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 18.Dobscha SK, Corson K, Perrin NA, Hanson GC, Leibowitz RQ, Doak MN, Dickinson KC, Sullivan MD, Gerrity MS. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA. 2009;301:1242–52. doi: 10.1001/jama.2009.377. [DOI] [PubMed] [Google Scholar]
- 19.Dohrenwend BP, Raphael KG, Marbach JJ, Gallagher RM. Why is depression comorbid with chronic myofascial face pain? A family study test of alternative hypotheses. Pain. 1999;83:183–92. doi: 10.1016/s0304-3959(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 20.Doubell TP, Mannion RJ, Woolf CJ. The dorsal horn: state-dependent sensory processing, plasticity and the generation of pain. In: Wall PD, Melzack R, editors. Textbook of pain. London: Churchill Livingstone; 1999. pp. 165–81. [Google Scholar]
- 21.Dubovsky SL, Warren C. Agomelatine, a melatonin agonist with antidepressant properties. Expert Opin Investig Drugs. 2009;18:1533–40. doi: 10.1517/13543780903292634. [DOI] [PubMed] [Google Scholar]
- 22.Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7:544–55. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- 23.El-Shenawy SM, Abdel-Salam OM, Baiuomy AR, El-Batran S, Arbid MS. Studies on the anti-inflammatory and anti-nociceptive effects of melatonin in the rat. Pharmacol Res. 2002;46:235–43. doi: 10.1016/s1043-6618(02)00094-4. [DOI] [PubMed] [Google Scholar]
- 24.Emptage NP, Sturm R, Robinson RL. Depression and comorbid pain as predictors of disability, employment, insurance status, and health care costs. Psychiatr Serv. 2005;56:468–74. doi: 10.1176/appi.ps.56.4.468. [DOI] [PubMed] [Google Scholar]
- 25.Fava M, Mallinckrodt CH, Detke MJ, Watkin JG, Wohlreich MM. The effect of duloxetine on painful physical symptoms in depressed patients: do improvements in these symptoms result in higher remission rates? J Clin Psychiatry. 2004;65:521–30. doi: 10.4088/jcp.v65n0411. [DOI] [PubMed] [Google Scholar]
- 26.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain. 1997;13:116–37. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Gitto E, Reiter RJ, Sabatino G, Buonocore G, Romero C, Gitto P, Buggé C, Trimarchi G, Barberi I. Correlation among cytokines, bronchopulmonary dysplasia and modality of ventilation in preterm newborns: improvement with melatonin treatment. J Pineal Res. 2005;39:287–93. doi: 10.1111/j.1600-079X.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 28.Golombek DA, Escolar E, Burin LJ, De Brito Sanchez MG, Cardinali DP. Time-dependent melatonin analgesia in mice: inhibition by opiate or benzodiazepine antagonism. Eur J Pharmacol. 1991;194:25–30. doi: 10.1016/0014-2999(91)90119-b. [DOI] [PubMed] [Google Scholar]
- 29.Greden JF. Treating depression and pain. J Clin Psychiatry. 2009;70:e16. doi: 10.4088/jcp.8005cc3c. [DOI] [PubMed] [Google Scholar]
- 30.Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson KC, 2nd, St Onge EL. Antidepressant pharmacotherapy: considerations for the pain clinician. Pain Pract. 2003;3:135–43. doi: 10.1046/j.1533-2500.2003.03020.x. [DOI] [PubMed] [Google Scholar]
- 32.Jain R. The implications of pain and physical symptoms in depression. J Clin Psychiatry. 2009;70:e19. doi: 10.4088/jcp.ms8001tx2c. [DOI] [PubMed] [Google Scholar]
- 33.Karp JF, Scott J, Houck P, Reynolds CF, 3rd, Kupfer DJ, Frank E. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66:591–7. doi: 10.4088/jcp.v66n0508. [DOI] [PubMed] [Google Scholar]
- 34.Klauenberg S, Maier C, Assion HJ, Hoffmann A, Krumova EK, Magerl W, Scherens A, Treede RD, Juckel G. Depression and changed pain perception: hints for a central disinhibition mechanism. Pain. 2008;140:332–43. doi: 10.1016/j.pain.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Korszun A, Young EA, Singer K, Carlson NE, Brown MB, Crofford L. Basal circadian cortisol secretion in women with temporomandibular disorders. J Dent Res. 2002;81:279–83. doi: 10.1177/154405910208100411. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Bair MJ, Damush TM, Wu J, Hoke S, Sutherland J, Tu W. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: a randomized controlled trial. JAMA. 2009;301:2099–110. doi: 10.1001/jama.2009.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumazawa T, Perl ER. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978;177:417–34. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- 38.Leino P, Magni G. Depression and distress symptoms as predictors of low back pain, neck-shoulder pain, and other musculoskeletal morbidity: a 10 year follow–up of metal industry employees. Pain. 1993;53:89–94. doi: 10.1016/0304-3959(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 39.Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7:403–12. doi: 10.1007/s11940-005-0032-0. [DOI] [PubMed] [Google Scholar]
- 40.Leone M, D’Amico D, Moschiano F, Fraschini F, Bussone G. Melatonin versus placebo in the prophylaxis of cluster headache: a double-blind pilot study with parallel groups. Cephalalgia. 1996;16:494–6. doi: 10.1046/j.1468-2982.1996.1607494.x. [DOI] [PubMed] [Google Scholar]
- 41.Li SR, Wang T, Wang R, Dai X, Chen Q, Li RD. Melatonin enhances antinociceptive effects of delta-, but not mu-opioid agonist in mice. Brain Res. 2005;1043:132–8. doi: 10.1016/j.brainres.2005.02.067. [DOI] [PubMed] [Google Scholar]
- 42.Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–50. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- 43.Lin EHB, Katon W, Von Korff M, Tang L, Williams JW, Jr, Kroenke K, Hunkeler E, Harpole L, Hegel M, Arean P, Hoffing M, Della Penna R, Langston C, Unützer J. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290:2428–34. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 44.Livingston G, Watkin V, Milne B, Manela MV, Katona C. Who becomes depressed? The Islington community study of older people. J Affect Disord. 2000;58:125–33. doi: 10.1016/s0165-0327(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–9. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 46.Magni G, Marchetti M, Moreschi C, Merskey H, Luchini SR. Chronic musculoskeletal pain and depression symptoms in the National Health and Nutrition Examination. I. Epidemiologic follow-up study. Pain. 1993;53:163–8. doi: 10.1016/0304-3959(93)90076-2. [DOI] [PubMed] [Google Scholar]
- 47.Magni G, Moreschi C, Rigatti-Luchini S, Merskey H. Prospective study on the relationship between depression symptoms and chronic musculoskeletal pain. Pain. 1994;56:289–97. doi: 10.1016/0304-3959(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 48.Maldonado MD, Murillo-Cabezas F, Calvo JR. Effects of early melatonin administration on traumatized critical patients. C Med Psicosom. 2009;89:21–9. [Google Scholar]
- 49.Maldonado MD, Murillo-Cabezas F, Calvo JR, Lardone PJ, Tan DX, Guerrero JM, Reiter RJ. Melatonin as pharmacologic support in burn patients: a proposed solution to thermal injury-related lymphocytopenia and oxidative damage. Crit Care Med. 2007a;35:1177–85. doi: 10.1097/01.CCM.0000259380.52437.E9. [DOI] [PubMed] [Google Scholar]
- 50.Maldonado MD, Murillo-Cabezas F, Terron MP, Flores LJ, Tan DX, Manchester LC, Reiter RJ. The potencial of melatonin in reducing morbidity-mortality after craniocerebral trauma. J Pineal Res. 2007b;42:1–11. doi: 10.1111/j.1600-079X.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 51.Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci. 2009;14:5291–338. doi: 10.2741/3598. [DOI] [PubMed] [Google Scholar]
- 52.Mantyselka PT, Turunen JHO, Ahonen RS, Kumpusalo EA. Chronic pain and poor self-rated health. JAMA. 2003;290:2435–42. doi: 10.1001/jama.290.18.2435. [DOI] [PubMed] [Google Scholar]
- 53.Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral neuropathy. Brain Res. 1992;598:271–8. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- 54.Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002;22:7650–61. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009;10:619–27. doi: 10.1016/j.jpain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Mongini F. Temporomandibular disorders and tension-type headache. Curr Pain Headache Rep. 2007;11:465–70. doi: 10.1007/s11916-007-0235-z. [DOI] [PubMed] [Google Scholar]
- 57.Morgan PJ, Barrett P, Howell HE, Helliwell R. Melatonin receptor: localization, molecular pharmacology and physiological significance. Neurochem Int. 1994;24:101–46. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 58.Mossey JM, Gallagher RM. The longitudinal occurrence and impact of comorbid chronic pain and chronic depression over two years in continuing care retirement community residents. Pain Med. 2004;5:335–48. doi: 10.1111/j.1526-4637.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- 59.Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010;15:404–14. doi: 10.1038/mp.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto K, Kimura A, Donishi T, Imbe H, Goda K, Kawanishi K, Tamai Y, Senba E. Persistent monoarthritis of the temporomandibular joint region enhances nocifensive behavior and lumbar spinal Fos expression after noxious stimulation to the hindpaw in rats. Exp Brain Res. 2006;170:358–67. doi: 10.1007/s00221-005-0218-4. [DOI] [PubMed] [Google Scholar]
- 62.Onal SA, Inalkac S, Kutlu S, Kelestimur H. Intrathecal melatonin increases the mechanical nociceptive threshold in the rat. Agri. 2004;16:35–40. [PubMed] [Google Scholar]
- 63.Overstreet DH, Pucilowski O, Retton MC, Delagrange P, Guardiola-Lemaitre B. Effects of melatonin receptor ligands on swim test immobility. Neuroreport. 1998;9:249–53. doi: 10.1097/00001756-199801260-00014. [DOI] [PubMed] [Google Scholar]
- 64.Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: nature’s most versatile biological signal? FEBS Journal. 2006;273:2813–38. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 65.Pang CS, Tsang SF, Yang JC. Effects of melatonin, morphine and diazepam on formalin-induced nociception in mice. Life Sci. 2001;68:943–51. doi: 10.1016/s0024-3205(00)00996-6. [DOI] [PubMed] [Google Scholar]
- 66.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; New York: 1997. [Google Scholar]
- 67.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–16. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 68.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats: a new model sensitive to antidepressant treatment. Eur J Pharmacol. 1978;47:379–91. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 69.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–32. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 70.Raghavendra V, Kulkarni SK. Possible mechanisms of action in melatonin reversal of morphine tolerance and dependence in mice. Eur J Pharmacol. 2000;409:279–89. doi: 10.1016/s0014-2999(00)00849-9. [DOI] [PubMed] [Google Scholar]
- 71.Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX. Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci. 2000a;917:376–86. doi: 10.1111/j.1749-6632.2000.tb05402.x. [DOI] [PubMed] [Google Scholar]
- 72.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review J Biomed Sci. 2000b;7:444–58. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 73.Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W. Depression and pain. Front Biosci. 2009;14:5031–51. doi: 10.2741/3585. [DOI] [PubMed] [Google Scholar]
- 74.Ruscheweyh R, Sandkühler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002;541:231–44. doi: 10.1113/jphysiol.2002.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shavali S, Ho B, Govitrapong P, Sawlom S, Ajjimaporn A, Klongpanichapak S, Ebadi M. Melatonin exerts its analgesic actions not by binding to opioid receptor subtypes but by increasing the release of beta-endorphin an endogenous opioid. Brain Res Bull. 2005;64:471–9. doi: 10.1016/j.brainresbull.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54:1402–7. doi: 10.1136/gut.2004.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stankov B, Cozzi B, Lucini V, Capsoni S, Fauteck J, Fumagalli P, Fraschini F. Localization and characterization of melatonin binding sites in the brain of the rabbit (Oryctolagus cuniculus) by autoradiography and in vitro ligand-receptor binding. Neurosci Lett. 1991;133:68–72. doi: 10.1016/0304-3940(91)90059-3. [DOI] [PubMed] [Google Scholar]
- 78.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Psychosom Med. 2008;70:338–44. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther. 1983;227:587–91. [PubMed] [Google Scholar]
- 80.Tu Y, Sun RQ, Willis WD. Effects of intrathecal injections of melatonin analogs on capsaicin-induced secondary mechanical allodynia and hyperalgesia in rats. Pain. 2004;109:340–50. doi: 10.1016/j.pain.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 81.Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C. Frontolimbic response to negative feedback in clinical depression. J Abnorm Psychol. 2003;112:667–78. doi: 10.1037/0021-843X.112.4.667. [DOI] [PubMed] [Google Scholar]
- 82.Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Can J Psychiatry. 2008;53:224–34. doi: 10.1177/070674370805300403. [DOI] [PubMed] [Google Scholar]
- 83.Uçeyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69:42–9. doi: 10.1212/01.wnl.0000265062.92340.a5. [DOI] [PubMed] [Google Scholar]
- 84.Valet M, Gündel H, Sprenger T, Sorg C, Mühlau M, Zimmer C, Henningsen P, Tölle TR. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosom Med. 2009;71:49–56. doi: 10.1097/PSY.0b013e31818d1e02. [DOI] [PubMed] [Google Scholar]
- 85.Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998;78:687–721. doi: 10.1152/physrev.1998.78.3.687. [DOI] [PubMed] [Google Scholar]
- 86.Vitte PA, Harthe C, Pevet P, Claustrat B. Brain autoradiographic study in the golden hamster after intracarotid injection of [14C]melatonin. Neurosci Lett. 1990;110:1–5. doi: 10.1016/0304-3940(90)90777-7. [DOI] [PubMed] [Google Scholar]
- 87.von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–62. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- 88.Wang S, Lim G, Mao J, Sung B, Mao J. Regulation of the trigeminal NR1 subunit expression induced by inflammation of the temporomandibular joint region in rats. Pain. 2009a;141:97–103. doi: 10.1016/j.pain.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci. 2004;24:8595–605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang S, Zhang L, Lim G, Sung B, Tian Y, Chou CW, Hernstadt H, Rusanescu G, Ma Y, Mao J. A combined effect of dextromethorphan and melatonin on neuropathic pain behavior in rats. Brain Res. 2009b;1288:42–9. doi: 10.1016/j.brainres.2009.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull. 2006;68:425–9. doi: 10.1016/j.brainresbull.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 92.Ying SW, Niles LP, Crocker C. Human malignant melanoma cells express high-affinity receptors for melatonin: antiproliferative effects of melatonin and 6-chloromelatonin. Eur J Pharmacol. 1993;246:89–96. doi: 10.1016/0922-4106(93)90084-m. [DOI] [PubMed] [Google Scholar]
- 93.Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- 94.Yu CX, Zhu CB, Xu SF, Cao XD, Wu GC. Selective MT(2) melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci Lett. 2000a;24(282):161–4. doi: 10.1016/s0304-3940(00)00883-1. [DOI] [PubMed] [Google Scholar]
- 95.Yu CX, Zhu CB, Xu SF, Cao XD, Wu GC. The analgesic effects of peripheral and central administration of melatonin in rats. Eur J Pharmacol. 2000b;403:49–53. doi: 10.1016/s0014-2999(00)00421-0. [DOI] [PubMed] [Google Scholar]
- 96.Zahn PK, Lansmann T, Berger E, Speckmann EJ, Musshoff U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: implications for pain modulation. J Pineal Res. 2003;35:24–31. doi: 10.1034/j.1600-079x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 97.Zeng Q, Wang S, Lim G, Yang L, Mao J, Sung B, Chang Y, Lim JA, Guo G, Mao J. Exacerbated mechanical allodynia in rats with depression-like behavior. Brain Res. 2008;1200:27–38. doi: 10.1016/j.brainres.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Q, Imbe H, Dubner R, Ren K. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J Comp Neurol. 1999;412:276–91. doi: 10.1002/(sici)1096-9861(19990920)412:2<276::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]