Abstract
One of the most common types of epilepsy in adults is temporal lobe epilepsy. Temporal lobe epilepsy is often resistant to pharmacological treatment, requiring urgent understanding of its molecular and cellular mechanisms. It is generally accepted that an imbalance between excitatory and inhibitory inputs is related to epileptogenesis. We have recently identified that fibroblast growth factor (FGF) 7 is critical for inhibitory synapse formation in the developing hippocampus. Remarkably, FGF7 knockout mice are prone to epileptic seizures induced by chemical kindling (Terauchi et al., 2010). Here we show that FGF7 knockout mice exhibit epileptogenesis-related changes in the hippocampus even without kindling induction. FGF7 knockout mice show mossy fiber sprouting and enhanced dentate neurogenesis by 2 months of age, without apparent spontaneous seizures. These results suggest that FGF7-deficiency impairs inhibitory synapse formation, which results in mossy fiber sprouting and enhanced neurogenesis during development, making FGF7 knockout mice vulnerable to epilepsy.
Keywords: Hippocampus, Fibroblast growth factor 7, Inhibitory synapse formation, Mossy fiber sprouting, Neurogenesis, Epilepsy
Introduction
Epilepsy is one of the most prevalent neurological disorders. Recent figures estimate that 0.4–1% of the population suffers from the disorder (WHO, 2009). The most common type of intractable epilepsy in adults is temporal lobe epilepsy (TLE). The abnormality in TLE patients often stems from mesial temporal structures such as the hippocampus and amygdala. Unfortunately, TLE is often resistant to pharmacological treatment (~30%). Therefore, a better understanding of the molecular and cellular mechanisms of TLE is urgently needed.
It is generally accepted that an imbalance between excitatory and inhibitory inputs is closely related to epileptogenesis (McNamara, 1994; Coulter, 2001; Morimoto et al., 2004; Rakhade and Jensen, 2009). We have recently found that fibroblast growth factor (FGF) 7 is critical for inhibitory synapse formation in the hippocampus as a target-derived presynaptic organizer (Terauchi et al., 2010). The differentiation of inhibitory nerve terminals on dendrites of CA3 pyramidal neurons, by which FGF7 is highly expressed, is specifically impaired in mutants lacking FGF7. Remarkably, its synaptogenic effects have life-long impacts on brain function: FGF7 knockout (KO) mice are prone to epileptic seizures induced by chemical kindling in adults (Terauchi et al., 2010). Thus, FGF7 is an attractive target for designing new strategies for treatment and prevention of TLE. An important next question is the cellular basis of their seizure phenotype.
Although several mechanisms have been proposed that might be involved in epileptogenesis, three mechanisms have widely attracted particular interest and are supported by various experiments: loss of hilar cells, enhanced dentate neurogenesis, and the formation of mossy fiber sprouting (MFS) (Dudek and Sutula, 2007; Parent, 2007). Loss of hilar cells may alter physiological properties of the dentate network (Dudek and Sutula, 2007; Jiao and Nadler, 2007). Enhanced neurogenesis may lead to aberrant integration of dentate granule cells (DGCs), including abnormal axonal and dendritic reorganization and ectopic DGCs (Parent et al., 2006; Walter et al., 2007; Jessberger et al., 2007; Kron et al, 2010). MFS may contribute to recurrent excitation of the DGCs (Sutula et al., 1989; Koyama and Ikegaya, 2004; Morimoto et al., 2004). Indeed, these changes are common features in TLE patients and epileptic animal models.
Here we examine the possibility that FGF7KO mice exhibit these three epileptogenesis-related changes in the hippocampus during development to become prone to epilepsy. We show that FGF7-deficiency, which impairs inhibitory synapse formation, results in enhanced neurogenesis and MFS during development even without kindling induction. These results suggest that FGF7-deficiency has significant impacts on seizure vulnerability as a result of epileptogenesis-related changes during development and open a possibility that activation of FGF7 may be able to lessen the vulnerability to epilepsy.
Results
FGF7KO mice show MFS even without kindling induction
In humans and experimental TLE, mossy fibers from DGCs often abnormally innervate and synapse onto neighboring DGCs (mossy fiber sprouting; MFS). It is proposed that these abnormal synapses create recurrent excitation of DGCs, which may cause recurrent seizures and contribute to epileptogenesis. Since FGF7KO mice are prone to epilepsy, we examined whether MFS occurs in FGF7KO mice with or without kindling induction. For kindling induction, 5 month old mice were repeatedly injected with pentylenetetrazole (PTZ), a GABA receptor antagonist, until they show epileptic seizures ("kindled"; ~2 months for wild type and ~1 month for FGF7KO mice). Control animals received PBS for the same period of time.
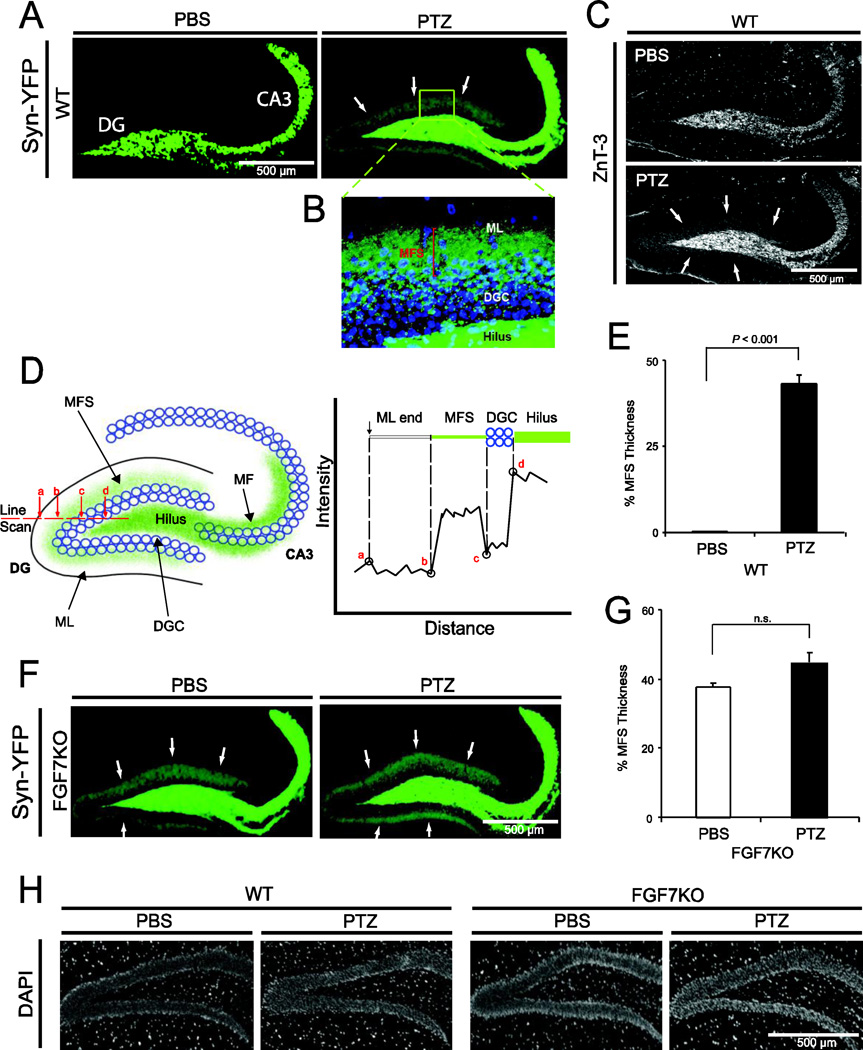
To visualize MFS, we used a synaptophysin-YFP transgenic mouse line, in which a synaptophysin-YFP fusion protein is expressed in mossy fibers in the hippocampus (Fig. 1). In PBS-injected wild type (WT) synaptophysin-YFP mice, mossy fibers from the dentate gyrus (DG) projected to the hilus and CA3 region of the hippocampus (Fig. 1A). By contrast, upon chemical kindling with repeated injections of PTZ, they displayed robust MFS in the DG. MFS was observed mostly in the inner molecular layer (Fig. 1B), which is consistent with previous reports with Timm staining (Tack and Nadler, 1985; Sutula et al., 1988, 1989). PTZ-injected mice also showed suprapyramidal and infrapyramidal MFS in CA3 (Fig. 1A). To confirm that synaptophysin-YFP labeling in the dentate inner molecular layer represent MFS, we stained sections for ZnT-3, a zinc transporter that is abundant in the zinc-enriched mossy fibers (Wenzel et al., 1997). ZnT-3 staining showed the same labeling pattern as synaptophysin-YFP in PBS- and PTZ-treated WT mice (Fig. 1C), indicating that synaptophysin-YFP fluorescence in this mouse line is an accurate method of detecting MFS. In order to quantitatively evaluate MFS, we measured the thickness of the MFS area using the linescan intensity (Fig. 1D). PTZ injected WT mice showed 43.2 ± 1.0% thickness of MFS, whereas the PBS controls did not exhibit any MFS (Fig. 1E).
Figure 1. FGF7KO mice show MFS without kindling induction.
A synaptophysin-YFP (Syn-YFP) transgenic mouse line in which mossy fibers (MFs) express Syn-YFP in the hippocampus was used to visualize the MF terminals. WT and FGF7KO mice were mated with the Syn-YFP transgenic mice and subjected to the PTZ-kindling protocol at 5 months of age. Control mice received PBS.
A, Visualization of MFs and MFS in Syn-YFP WT mice. Supragranular MFS in the DG, indicated with arrows, as well as suprapyramidal and infrapyramidal MFS (enhanced Syn-YFP signals in CA3) are present in PTZ-injected kindled mice.
B, Higher magnification of MFS in PTZ-kindled Syn-YFP mice. Pictured area corresponds to the boxed area in (A). Synaptophysin-YFP is in green; DAPI staining is in blue, which shows the location of dentate granule cells (DGCs). MFS was detected mainly in the inner molecular layer (ML) of the DG and a part of the DGC layer, which is consistent with previous reports on MFS.
C, ZnT-3 immunostaining of PBS- and PTZ-injected WT mice. PBS-injected WT mice did not show MFS while PTZ-injected WT mice showed MFS (arrows indicate supragranular MFS; enhanced ZnT-3 signals in CA3 indicate suprapyramidal and infrapyramidal MFS) as observed with Syn-YFP fluorescence in (A).
D, Schematic drawing of MFS in the hippocampus and a diagram of the linescan intensity of Syn-YFP fluorescence. Thickness of MFS was measured using the intensity of a line scan. The MFS thickness (%) was calculated using the formula (distance between b and c)/(distance between a and d).
E, Quantification of the MFS thickness in PBS- or PTZ-treated WT mice. Quantification was performed as described in (D). P value by Student's t test is indicated.
F, MFS in PBS- or PTZ-treated FGF7KO mice. MFS was observed in both PBS- and PTZ-injected FGF7KO mice (arrows).
G, Quantification of the MFS thickness in PBS- or PTZ-injected FGF7KO mice. Bars are mean ± SEM. Data are from 5–13 mice per condition. n.s.: not significant.
H, Normal cytoarchitectural structures in the DG of PBS or PTZ injected WT or FGF7KO mice as assessed by DAPI staining.
To investigate whether MFS is induced in FGF7KO mice, we mated FGF7KO mice with the synaptophysin-YFP transgenic mice. Kindled FGF7KO mice displayed MFS (Fig. 1F, G, PTZ), which had a similar thickness to that of WT mice (WT, 43.2%; FGF7KO, 45.6%; Fig. 1E, G). Remarkably, MFS is also noted in PBS-injected FGF7KO mice (Fig. 1F, G), indicating that FGF7-deficiency leads to MFS even without kindling induction during development. We did not detect apparent changes in cytoarchitectural structures in the DG of these mice as assessed by DAPI staining (Fig. 1H).
MFS appears in FGF7KO mice by 2 months of age
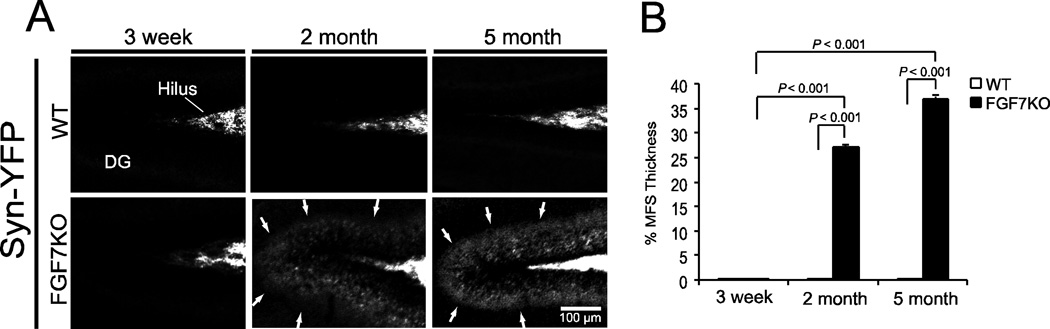
Since FGF7KO mice exhibit MFS without kindling induction in adults (~6 months old; Fig. 1F, G), we further examined the development of MFS in non-kindled FGF7KO mice. WT and FGF7KO mice were mated with synaptophysin-YFP mice and their hippocampal sections were examined at different ages during development: at 3 weeks (P21), 2 months (P60), and 5 months (P150) old (Fig. 2). In FGF7KO mice, MFS was not obvious at P21 but was clearly visible by P60, and became thicker by P150. WT mice did not show MFS between P21 and P150. Quantification of developmental MFS indicated that FGF7KO mice developed marked MFS by P60, which increased by P150. Thus, FGF7KO mice display MFS even without the kindlinginduced seizures, with developmental onset between P21 and P60.
Figure 2. MFS appears in FGF7KO mice by 2 months of age.
WT and FGF7KO mice were mated with Syn-YFP mice and their hippocampal sections were examined at 3 weeks, 2 months, or 5 months of age.
A, FGF7KO mice showed MFS at the ages of 2 and 5 months (arrows). WT mice did not exhibit any MFS during this period.
B, Quantification of the MFS thickness in WT and FGF7KO mice during development. MFS in FGF7KO mice became evident by 2 months, and its thickness increased by 5 months of age. Bars are mean ± SEM. Data are from 3–5 mice per condition. P values by ANOVA followed by Turkey’s test are indicated.
Hilar cell death is not increased in FGF7KO mice
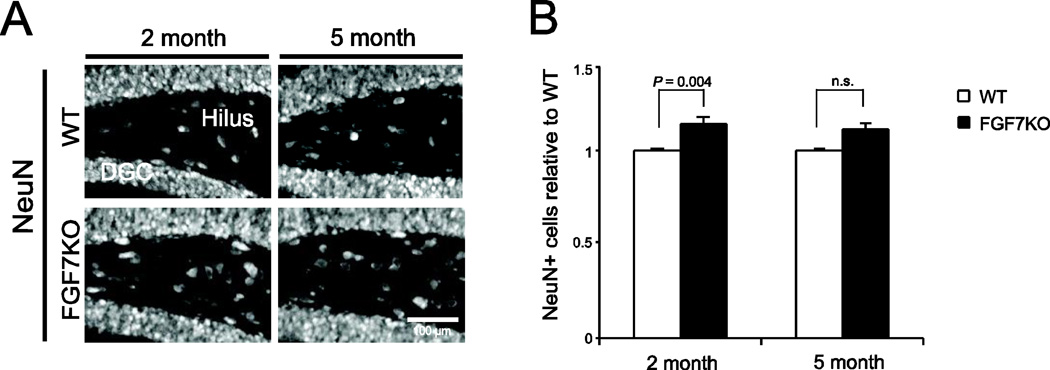
Hilar cell death is another pathological event that may contribute to epileptogenesis. To determine whether hilar cell death increases in FGF7KO mice during development, we stained hippocampal sections from WT and FGF7KO mice at 2 and 5 months of age for NeuN, a marker for mature neurons. Hilar cell death was assessed by quantifying the numbers of NeuN immunoreactive hilar neurons. The number of NeuN-positive (i.e. surviving) cells in the hilus of FGF7KO mice was similar to that of WT mice at 5 months and slightly increased at 2 months (Fig. 3). This result suggests that hilar cell death is not increased in FGF7KO mice compared to WT mice during development. This result also implies that MFS may be induced without apparent hilar cell death.
Figure 3. Hilar cell death does not increase in FGF7KO mice.
Region-matched sagittal hippocampal sections were prepared from WT and FGF7KO mice at 2 months and 5 months of age and immunostained for NeuN.
A, NeuN immunoreactivity in the dentate granule cell (DGC) layer and hilus.
B, Quantification of the number of NeuN-positive (surviving) cells in the hilus. The number of NeuN-positive cells is similar or slightly greater in FGF7KO mice than in WT mice. Bars are mean ± SEM. Data are from 6 sections/mouse from 3 mice per condition. Student's t-test P values are indicated. n.s.: not significant.
Neurogenesis is enhanced in FGF7KO mice during development
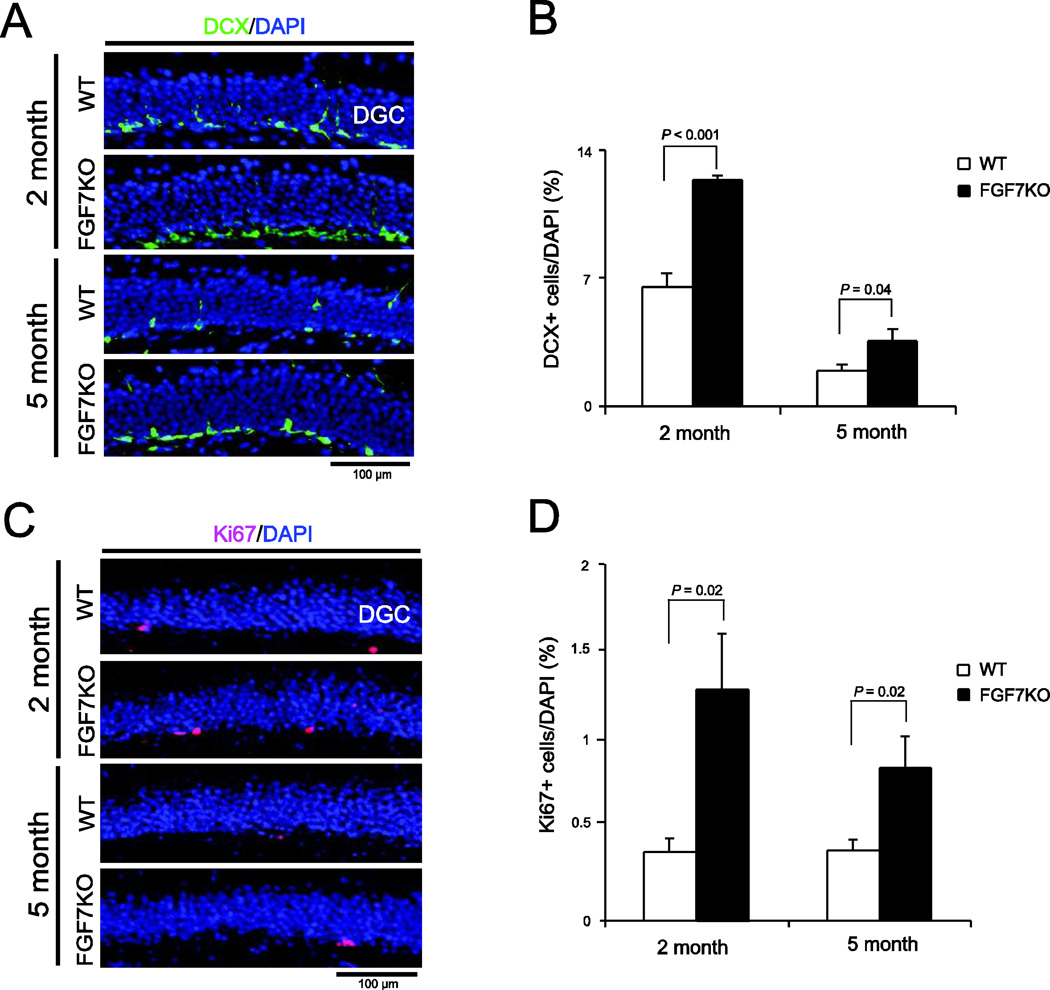
Increased production of DGCs and their aberrant integration is also suggested to play important roles in epileptogenesis. Hence, we next examined the degree of dentate neurogenesis in WT and FGF7KO mice by staining hippocampal sections for doublecortin (DCX), a marker for immature neurons, and Ki67, a proliferating cell marker. FGF7KO mice had a much greater number of DCX-positive cells in the DG than WT mice at both 2 and 5 months of age (Fig. 4A, B). Furthermore, there were significantly more Ki67-positive cells in the DG of FGF7KO mice compared to WT mice at both ages (Fig. 4C, D). These results suggest that dentate neurogenesis is significantly enhanced in the absence of FGF7 during development. Since MFS in the pilocarpine TLE model is closely related to neurogenesis (Kron et al, 2010), increased neurogenesis may play a role in developmental MFS in FGF7KO mice.
Figure 4. Neurogenesis is enhanced in FGF7KO mice.
Region-matched sagittal hippocampal sections were prepared from WT and FGF7KO mice at 2 months and 5 months of age and immunostained for DCX (A and B) or Ki67 (C and D; a marker of proliferating cells). The percentages of DCX positive DGCs (B) and Ki67 positive DGCs (D) in WT and FGF7KO mice were quantified and are shown. FGF7KO mice had more DCX and Ki67 positive cells compared to WT at both 2 and 5 months of age, indicating that neurogenesis is enhanced in FGF7KO mice. Bars are mean ± SEM. Data are from 4–6 sections/mouse from 3 mice per condition. Student's t-test P values are indicated.
No spontaneous seizures in FGF7KO mice
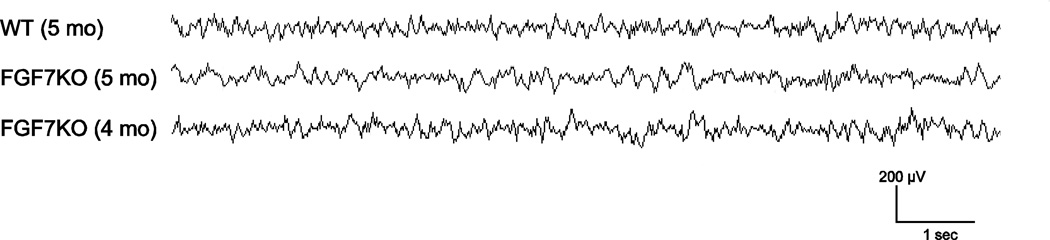
We found MFS and increased neurogenesis in FGF7KO mice during development. It is possible that these changes are caused by spontaneous seizures in FGF7KO mice. To examine whether FGF7KO mice exhibit spontaneous seizures, we monitored 5 month old WT and FGF7KO mice by continuous video and electroencephalographic (EEG) recording for 24 hours/day for 14 consecutive days. We did not detect any seizure activity in either WT or FGF7KO mice during the monitoring (Fig. 5), suggesting that FGF7KO mice do not develop spontaneous seizure at least by 5 months of age. We did not detect any seizure activity in 4 month old FGF7KO mice, either (Fig. 5). Although we cannot entirely exclude the presence of focal hippocampal seizures not detected by surface EEG, the lack of seizure activity and absence of interictal epileptiform discharges over two weeks of recording strongly support an absence of epilepsy in these mice. In addition, we have not observed any behavioral seizures or increased mortality at least by 5 months of age, further supporting a lack of epilepsy in FGF7KO mice. The data therefore suggest that the formation of MFS and enhanced neurogenesis in FGF7KO mice during development are not caused by spontaneous seizures, but rather directly by FGF7 deficiency.
Figure 5. FGF7KO mice at 5 months of age do not exhibit apparent spontaneous seizures.
EEG was recorded from 5 month old WT and FGF7KO mice (n=3) for 1008 hours. Representative traces of EEG recordings are shown. WT and FGF7KO mice did not show evidence of electrographic or behavioral seizure activity. 4 month old FGF7KO mice did not show seizure activity, either.
Discussion
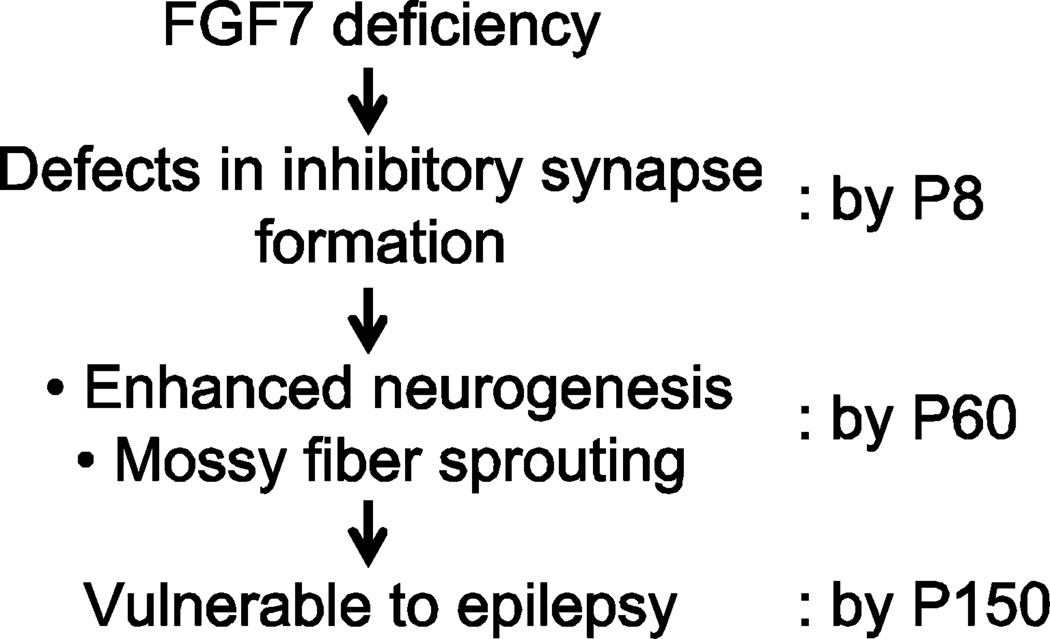
Here, we have analyzed whether epileptogenesis-related changes occur in the hippocampus of mice lacking FGF7, the presynaptic organizers critical to inhibitory synapse formation. We found that in FGF7KO mice, MFS arose and neurogenesis was enhanced even without kindling induction and in the absence of apparent spontaneous seizures. These results suggest that FGF7-deficiency, which impairs inhibitory synapse formation, results in enhanced neurogenesis and MFS during development, making FGF7KO mice vulnerable to epilepsy (Fig. 6). Synaptic defects appear as early as P8 (Terauchi et al., 2010). At that point, we did not detect significant changes in the pattern of mossy fiber projections (Terauchi et al., 2010). MFS and enhanced neurogenesis appear later, by P60. Importantly, FGF7KO mice did not show apparent spontaneous seizures at least at P150. Thus, the consequences of FGF7-deficiency are impaired inhibitory synapse formation followed by changes in dentate neurogenesis and MFS during development, which may make FGF7KO mice vulnerable to kindled seizures in adults. It is still controversial whether MFS plays critical roles in epileptogenesis (Tauck and Nadler, 1985; Buckmaster and Lew, 2011); however, computer simulations suggest that it is very likely that MFS contributes to seizure susceptibility (Morgan et al., 2007).
Figure 6. Proposed model for the roles of FGF7 in seizure vulnerability.
FGF7 deficiency leads to impaired inhibitory synapse formation in the hippocampus (by P8), which results in enhanced neurogenesis and mossy fiber sprouting by 2 months of age, making FGF7KO mice vulnerable to epilepsy.
What are the mechanisms underlying these consequences? One possible sequence is as follows: FGF7 deficiency leads to a decrease in CA3 inhibitory synapses (Terauchi et al., 2010) and results in the overexcitation of CA3 pyramidal neurons (but not severe enough to induce spontaneous seizures). This may send signals to DGCs to rewire, leading to MFS. Identification of signals within DGCs is an important next question, and we are currently identifying them using FGF7KO mice. There are various molecules that are implicated in dentate neurogenesis, including growth factors, neurotrophins, morphogens, and cytokines (Parent, 2007; Zhao et al., 2008). FGF7 deficiency might affect the expression and function of these molecules. As for MFS, PLC-β1KO mice are also reported to show MFS without kindling induction (Bohm et al., 2002). In addition, overexpression of GAP-43 can induce MFS (Aigner et al., 1995), and Wnt7 signaling appears to be involved in remodeling of large mossy fiber terminals (Gogolla et al., 2009). FGF7 signaling might interact with these molecules for the regulation of MFS. It is also possible that the impairment of the depolarizing action of GABA (Ben-Ari, 2002) may be involved in the FGF7KO phenotypes. Further investigation should reveal the molecular mechanisms underlying developmental changes related to seizure vulnerability caused by FGF7 deficiency and impaired GABAergic synapse formation.
An important implication of our study is that activation of FGF7 may have a potential to lessen vulnerability to epilepsy. Thus, our work may yield appropriate treatments for certain forms of epilepsy.
Experimental Methods
Animals
FGF7KO mice were obtained from the Jackson Laboratory (Guo et al., 1996). Synaptophysin-YFP transgenic mice, in which a synaptophysin-YFP fusion protein is expressed under the control of regulatory elements from the thy-1 gene, were described previously (Umemori et al., 2004). In the line of synaptophysin-YFP mice used in this study, synaptophysin-YFP is expressed by mossy fibers in the hippocampus. FGF7KO and synaptophysin-YFP mice were backcrossed with C57/BL6 mice for at least 5 generations to minimize background effects. FGF7KO mice were then mated with synaptophysin-YFP transgenic mice. Genotypes were verified by genomic PCR. Male mice were used in this study. FGF7+/− animals did not show any significant phenotypes in this study. All animal care and use was in accordance with the institutional guidelines and approved by the University Committee on Use and Care of Animals.
Kindling
Pentylenetetrazole (PTZ; 35 mg/kg), a GABA receptor antagonist, was injected every 48 hours to induce seizures. The mice (5 months old) were injected until they were kindled (~2 months for WT and ~1 month for FGF7KO mice). The mice were observed for an hour after each injection, and the maximum level of seizure within the hour was recorded. The seizure responses were noted on a scale of 0–5 as follows: Class 0: no seizure observed; Class 1: head nodding; Class 2: sporadic full-body shaking and spasms; Class 3: constant full-body spasms, jolts, and shrieking; Class 4: jolts, shrieking, jumping, and falling over; Class 5: violent convulsions, falling over, and death (Racine, 1972). Mice were defined as "kindled" when they displayed either one class 5 seizure or two successive class 4 seizures. Control animals received PBS.
Brain sectioning
Mice were perfused with 4% paraformaldehyde (PFA). Their brains were dissected and post-fixed in 4% PFA for 24 hours. Brains were then placed in 30% sucrose solution for at least 48 hours, frozen at −80°C and sectioned sagittally (20 µm thick) with a cryostat. Sections from the same region of the hippocampus were used in this study to compare between WT and FGF7KO mice in order to standardize quantifications of cell numbers. Distance from the midline of the brain was calculated by numbering the cryostat sections. Hippocampi in adult mice were between 1.8 mm and 4.2 mm from the midline. Sections between 2.5 mm and 3.0 mm from the midline were used for the study.
Immunohistochemistry
Sections were blocked in 2% BSA, 2% normal goat serum and 0.1% TritonX-100 for 1 hour, followed by incubation with primary antibodies overnight at 4°C. Secondary antibodies were applied for 1 hour at room temperature, and slides were mounted with p-phenylenediamine. Antibodies used were: NeuN (mouse monoclonal, MAB377; dilution 1:1000; Millipore), DCX (rabbit polyclonal, ab18723; dilution 1:200; Abcam), Ki67 (rabbit polyclonal, ab15580; dilution 1:100; Abcam), and ZnT-3 (rabbit polyclonal, 197002, dilution 1:200, Synaptic Systems). DAPI was added to each section as a nuclear stain. Stained sections were observed under the Olympus BX61 fluorescent microscope. Images were photographed with the F-View Soft Imaging System camera.
Cell death
Cell death in the hilus was evaluated by quantifying the number of NeuN-positive (i.e. surviving) cells. The number of NeuN-positive cells was quantified from the entire hilar region of each section (6 sections/mouse; 3 mice/condition).
Neurogenesis
Neurogenesis was assessed by quantifying the percentage of dentate granule cells (DGCs) that were positive for doublecortin (DCX), a marker for immature neurons, and for Ki67, a marker for proliferating cells. DCX and Ki67 positive cells were counted from the entire DG of each section (4–6 sections/mouse; 3 mice/condition) and divided by the total number of DGCs stained with DAPI.
Mossy fiber sprouting
Quantification of mossy fiber sprouting (MFS) in synaptophysin-YFP transgenic mice was performed using an intensity linescan (see Fig. 1D) with MetaMorph software. The thickness of MFS was measured via linescan intensity using minima and maxima to set the demarcation between the DGC layer, the molecular layer, and the beginning and end of the MFS (Fig. 1D). The % thickness of MFS was defined as [the distance between the beginning and end of the sprouted area] divided by [the distance between the granule cell layer and the distal edge of the outer molecular layer at the sulcus].
Electroencephalographic (EEG) recordings
Five month old WT and FGF7KO (n=3/group) mice were used for monitoring, 24 hours/day for 14 consecutive days (total of 1008 hours of recording). EEG hardware (amplifier) was from Ceegraph Vision and Bio-Logic. 6 electrodes were placed with mounting screw and socket (PlasticsOne, E363/20): two frontal, two temporal, one cerebellar, and one reference electrode in the sinus cavity. The sockets were fit into the multi-channel electrode pestal (PlasticsOne MS363) and connected to an electrode holder (MH363). The screws, wires, and the pedestal were covered with dental cement to make a head cap. Mice were recovered for 1–2 days before recordings started. Recordings were sampled at 400Hz and high-pass-filtered at 1Hz. Low-pass filtering was done at 70Hz. Simultaneous video was obtained using Zoom 260xDSP cameras (Shinet SN-ZS260IR) linked to a computer running Ceegraph Vision software.
Acknowledgements
This work was supported by the Ester A. & Joseph Klingenstein Fund, the March of Dimes Foundation, and NIH grant NS070005 (H.U.). We thank A. Terauchi and M. Zhang for technical assistance.
Abbreviations
- FGF
fibroblast growth factor
- TLE
temporal lobe epilepsy
- KO
knockout
- DG
dentate gyrus
- MFS
mossy fiber sprouting
- DGC
dentate granule cell
- WT
wild type
- YFP
yellow fluorescent protein
- PFA
paraformaldehyde
- DCX
doublecortin
- EEG
electroencephalographic
- PTZ
pentylenetetrazole
- ZnT
zinc transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Böhm D, Schwegler H, Kotthaus L, Nayernia K, Rickmann M, Köhler M, Rosenbusch J, Engel W, Flügge G, Burfeind P. Disruption of PLC-B1-Mediated Signal Transduction in Mutant Mice Causes Age-Dependant Hippocampal Mossy Fiber Sprouting and Neurodegeneration. Mol. Cell. Neurosci. 2002;21:584–601. doi: 10.1006/mcne.2002.1199. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J. Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA. Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int. Rev. Neurobiol. 2001;45:237–252. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog. Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J. Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp. Neurol. 2007;205:569–582. doi: 10.1016/j.expneurol.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. Mossy fiber sprouting as a potential therapeutic target for epilepsy. Curr. Neurovasc. Res. 2004;1:3–10. doi: 10.2174/1567202043480242. [DOI] [PubMed] [Google Scholar]
- Kron MM, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J. Neurosci. 2010;30:2051–2059. doi: 10.1523/JNEUROSCI.5655-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara JO. Cellular and molecular basis of epilepsy. J. Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RJ, Santhakumar V, Soltesz I. Modeling the dentate gyrus. Prog. Brain Res. 2007;163:639–658. doi: 10.1016/S0079-6123(07)63035-0. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog. Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog. Brain Res. 2007;163:529–540. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann. Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroenchephalogr. Clin. Neurophysiol. 1972;3:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Jensen FE. Epileptogenesis in the immature brain: emerging mechanisms. Nat. Rev. Neurol. 2009;5:380–391. doi: 10.1038/nrneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann. Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J. Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–787. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J. Neurosci. 2007;27:7541–7552. doi: 10.1523/JNEUROSCI.0431-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc. Natl. Acad. Sci. USA. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Epilepsy. 2009 URL: http://www.who.int/mediacentre/factsheets/fs999/en/index.html.
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]