Abstract
Purpose
To examine longitudinal parent-reported social outcomes for children treated for pediatric embryonal brain tumors.
Patients and Methods
Patients (N = 220) were enrolled onto a multisite clinical treatment protocol. Parents completed the Child Behavior Checklist/6-18 at the time of their child's diagnosis and yearly thereafter. A generalized linear mixed effects model regression approach was used to examine longitudinal changes in parent ratings of social competence, social problems, and withdrawn/depressed behaviors with demographic and treatment factors as covariates.
Results
During the 5-year period following diagnosis and treatment, few patients were reported to have clinically elevated scores on measures of social functioning. Mean scores differed significantly from population norms, yet remained within the average range. Several factors associated with unfavorable patterns of change in social functioning were identified. Patients with high-risk treatment status had a greater increase in parent-reported social problems (P = .001) and withdrawn/depressed behaviors (P = .01) over time compared with average-risk patients. Patients with posterior fossa syndrome had greater parent-reported social problems over time (P = .03). Female patients showed higher withdrawn/depressed scores over time compared with male patients (P < .001). Patient intelligence, age at diagnosis, and parent education level also contributed to parent report of social functioning.
Conclusion
Results of this study largely suggest positive social adjustment several years after diagnosis and treatment of a pediatric embryonal tumor. However, several factors, including treatment risk status and posterior fossa syndrome, may be important precursors of long-term social outcomes. Future research is needed to elucidate the trajectory of social functioning as these patients transition into adulthood.
INTRODUCTION
Survivors of pediatric brain tumors are at particularly high risk for experiencing adverse effects related to their disease and treatments.1,2 Although substantial effort has been directed at characterizing medical and neurocognitive outcomes,1,3–6 considerably less attention has focused on behavioral and social consequences of treatment for childhood brain tumors. Although evidence suggests that deficits in social functioning represent a significant part of the morbidity experienced by these survivors,7 the nature and time course of these difficulties remain poorly understood.
Previous cross-sectional studies, using heterogeneous samples of brain tumor survivors, have reported that survivors have fewer close friendships8,9 and are socially isolated compared with peers.9 Survivors also demonstrate greater social problems10,11 and diminished social competence12,13 relative to normative samples. Compared with siblings, adolescent survivors are reported to have increased depression/anxiety and antisocial behaviors, as well as reduced social competence.14 In a rare longitudinal study of 53 patients treated with cranial radiation therapy for posterior fossa tumors, Mabbott et al15 reported a progressive decline in social functioning with increasing time from diagnosis. However, because of the limited number of behavioral observations, these investigators were unable to examine the associations between multiple predictor variables and social outcomes in their longitudinal models.
Neurocognitive deficits have been proposed as a potential cause of social problems, and associations between intelligence quotient and social problems as well as reduced social competence have been reported.7,11 The extent to which intellectual ability may be associated with change in social functioning over time has not yet been investigated. Research has begun to suggest that established predictors of neurocognitive outcomes such as cranial radiation therapy,15,16 patient sex,17 age at diagnosis,18 posterior fossa syndrome,19 and time since diagnosis11,12,15 may contribute to social outcomes. However, results are mixed and inconsistent findings may be related to the use of heterogeneous samples and variable timing of evaluations. The study of homogeneous groups of patients may allow for improved investigation of risk factors with known effects on neurocognitive processes, including dose of craniospinal irradiation. Therefore, we sought to prospectively investigate longitudinal patterns of social functioning in patients treated for childhood embryonal tumors from the time of diagnosis onward.
PATIENTS AND METHODS
Patient Population
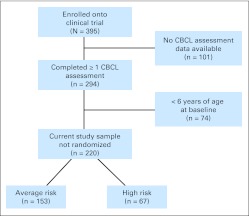
Study participants were recruited from an institutional review board–approved clinical trial for patients newly diagnosed with an embryonal tumor. Patients were enrolled onto a multisite treatment study at one of nine institutions and were included in our study if their parents completed a behavioral questionnaire that included indices of social functioning. The study protocol did not require that the same caregiver report on child behavior at each assessment time point. The current sample included 220 patients (Fig 1), resulting in 750 observations (mean, 3.4; standard deviation [SD], 1.8 observations per patient) over a 5-year follow-up period. Details regarding participant attrition are provided in the Appendix (online only).
Fig 1.
Flow diagram of study participation. CBCL, Child Behavior Checklist.
Patients were treated with postsurgical risk-adapted craniospinal irradiation (CSI) followed by 4 cycles of chemotherapy (cyclophosphamide, cisplatin, vincristine) with stem cell support. Average-risk patients (n = 153) received 23.4 Gy CSI, three-dimensional conformal boost to the primary site to 55.8 Gy. High-risk patients (n = 67) received 36 to 39.6 Gy CSI and three-dimensional conformal boost to the primary site to 55.8 to 59.4 Gy. The median age at diagnosis was 10 years and patients were an average of 3.6 years from diagnosis at their most recent follow-up.
Social Outcomes
Parents completed the Child Behavior Checklist (CBCL/6-18)20 at the time of their child's diagnosis and yearly thereafter, providing longitudinal measures of social functioning. The CBCL/6-18 has twenty competence items covering child activities, social relations, and school performance, as well as 118 items describing specific behavioral and emotional problems. In our study, only the social competence, social problems, and withdrawn/depressed scales were examined. The social competence scale consists of items assessing peer relations (eg, frequency of contact with friends, involvement in extracurricular activities) whereas the social problems scale includes indicators of negative social interactions (eg, gets teased, doesn't get along with others). The withdrawn/depressed scale includes items that reflect depressive symptoms and socially withdrawn behavior (eg, prefers to be alone, withdrawn, shy/timid). The CBCL/6-18 was developed with normative sampling, and scores for each scale are age and sex adjusted. The CBCL/6-18 is characterized by strong reliability and validity and is widely used in the assessment of child behavior.20,21 Because the younger version, the CBCL/11/2-5, does not provide the social indices examined in this study, patients younger than 6 years at diagnosis were not included in analysis of these outcomes.
Covariates
Demographic and socioeconomic variables used in the analyses included patient sex and parent education. Clinical variables included patient age at diagnosis, patient treatment risk status, posterior fossa syndrome (PFS) after surgery (yes/no), and the number of years on study (ie, time from diagnosis). Patient general intellectual ability (GIA) was assessed following diagnosis, using the Woodcock Johnson Tests of Cognitive Abilities.22 The overall intelligence composite was obtained by administering the standard battery of tests, which consists of seven subtests measuring comprehension knowledge, visual spatial thinking, processing speed, memory, and auditory processing. The Woodcock Johnson Tests of Cognitive Abilities is a standardized assessment battery with normative data from the United States and provides age-adjusted standard scores (mean, 100; SD, 15).
Statistical Analysis
A generalized linear mixed effects model (GLMM) regression approach was used to examine longitudinal changes in parent ratings of social competence, social problems, and withdrawn/depressed behaviors.15,23 Because social problems and withdrawn/depressed T scores were truncated at 50, a normal distribution could not be assumed. In addition, as all T scores assumed only integer values, each outcome was modeled as an integer response using Poisson distribution by employing a log-link function in PROC NLMIXED SAS version 9.2 (SAS Institute, Cary, NC). T scores rather than raw scores were used to allow for clinical comparison with age- and sex-adjusted standardized normative data. For each model, intercepts represent the estimated baseline functioning and slopes characterize changes in functioning over time. The effect of covariates on the estimated intercept and slope for each behavioral scale was investigated. The best fitting and most parsimonious multivariate model was constructed considering all covariates, including their interaction with time, using a backward-selection method.
RESULTS
Clinical characteristics of the patients are listed in Table 1. With the exception of treatment risk status, patients with only baseline data do not differ significantly from those with baseline and 3-year data (Table 2; 5-year comparison data are listed in Appendix Table A1 [online-only]). At the time of diagnosis, parent-reported social competence (mean, 49.9; SD, 9.1), social problems (mean, 53.5; SD, 4.7), and withdrawn/depressed (mean, 56.0; SD, 7.3) scores fell in the average range. Mean scores remained in the average range across all assessment time points, though scores differed significantly from population norms (Table 3). Few patients' scores fell in the clinical range, although the proportion exceeding clinical significance differed from the expected proportion of 2% at several assessments. Appendix Tables A2 and A3 provide scores over time by treatment risk status and PFS.
Table 1.
Patient and Clinical Characteristics (N = 220)
| Characteristic | No. of Patients | % |
|---|---|---|
| Age at diagnosis, years | ||
| Mean | 10.7 | |
| SD | 3.6 | |
| Range | 5.8-21.6 | |
| Time since diagnosis, years* | ||
| Mean | 3.6 | |
| SD | 2.1 | |
| Range | 0.2-8.3 | |
| Current age, years* | ||
| Mean | 14.3 | |
| SD | 4.1 | |
| Range | 7.1-26.3 | |
| Baseline general intellectual ability | ||
| Mean | 99.3 | |
| SD | 18.7 | |
| Range | 50-154 | |
| Parent education, years | ||
| Mean | 13.8 | |
| SD | 2.5 | |
| Range | 3-20 | |
| Patient sex | ||
| Male | 129 | 58.6 |
| Female | 91 | 41.4 |
| Parent sex | ||
| Male | 19 | 9.9 |
| Female | 173 | 90.1 |
| Risk status | ||
| Average | 153 | 69.6 |
| High | 67 | 30.5 |
| Posterior fossa syndrome | ||
| Yes | 41 | 18.6 |
| No | 179 | 81.4 |
| Diagnosis | ||
| Medulloblastoma | 174 | 79.1 |
| Primitive neuroectodermal tumor | 34 | 15.5 |
| Atypical teratoid rhabdoid tumor | 12 | 5.5 |
Abbreviation: SD, standard deviation.
At most recent follow-up.
Table 2.
Comparison of Patients With and Without Year 3 Data
| Characteristic | Baseline Only |
Baseline and Year 3 |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Sex* | .35 | ||||
| Male | 21 | 51.2 | 67 | 60.9 | |
| Female | 20 | 48.8 | 43 | 39.1 | |
| PFS* | .81 | ||||
| Yes | 6 | 14.6 | 20 | 18.2 | |
| No | 35 | 85.4 | 90 | 81.8 | |
| Risk* | .002 | ||||
| High | 19 | 46.3 | 22 | 20.0 | |
| Average | 22 | 53.7 | 88 | 80.0 | |
| Age at baseline† | |||||
| Mean | 11.1 | 11.1 | .99 | ||
| SD | 3.7 | 3.7 | |||
| Age at diagnosis† | .97 | ||||
| Mean | 11.0 | 11.0 | |||
| SD | 3.7 | 3.7 | |||
| Social competence† | .95 | ||||
| Mean | 50.2 | 50.3 | |||
| SD | 8.6 | 9.7 | |||
| Withdrawn/depressed† | .12 | ||||
| Mean | 54.0 | 56.3 | |||
| SD | 5.6 | 7.5 | |||
| Social problems† | .27 | ||||
| Mean | 52.7 | 53.7 | |||
| SD | 3.8 | 4.9 | |||
NOTE. Bold font indicates significance.
Abbreviations: PFS, posterior fossa syndrome; SD, standard deviation.
Fisher's exact test for equality of proportions.
t test for equality of means.
Table 3.
Parent-Reported Social Outcomes by Time on Study
| Year | Social Competence* |
Social Problems† |
Withdrawn/Depressed† |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | P‡ | No. of Patients§ | %§ | P‖ | No. | Mean | SD | P‡ | No. of Patients | % | P‖ | No. | Mean | SD | P‡ | No. of Patients | % | P‖ | |
| Baseline | 168 | 49.9 | 9.1 | .94 | 3 | 1.8 | 1.0 | 169 | 53.5 | 4.7 | < .001 | 3 | 1.8 | 1.0 | 169 | 56.0 | 7.3 | < .001 | 10 | 5.9 | .002 |
| 1 | 135 | 44.8 | 9.0 | < .001 | 9 | 6.7 | .002 | 140 | 54.8 | 5.7 | < .001 | 4 | 2.9 | .37 | 140 | 57.2 | 8.2 | < .001 | 15 | 10.7 | < .001 |
| 2 | 63 | 46.5 | 9.0 | .003 | 3 | 4.8 | .13 | 62 | 55.5 | 6.4 | < .001 | 2 | 3.2 | .35 | 62 | 56.5 | 6.9 | < .001 | 3 | 4.8 | .13 |
| 3 | 75 | 45.5 | 9.2 | < .001 | 3 | 4.0 | .19 | 76 | 56.4 | 7.2 | < .001 | 5 | 6.6 | .02 | 76 | 57.1 | 7.8 | < .001 | 6 | 7.9 | .004 |
| 4 | 41 | 47.3 | 9.1 | .07 | 1 | 2.4 | .56 | 41 | 56.0 | 6.6 | < .001 | 3 | 7.3 | .05 | 41 | 55.4 | 7.3 | < .001 | 3 | 7.3 | .05 |
| 5 | 33 | 45.9 | 10.3 | .03 | 3 | 9.1 | .03 | 33 | 57.4 | 8.0 | < .001 | 4 | 12.1 | .004 | 33 | 57.0 | 7.1 | < .001 | 1 | 3.0 | .49 |
NOTE. Bold font indicates significance.
Abbreviation: SD, standard deviation.
Average range defined as T scores ranging from 36-50. Clinically significant scores are defined as T scores ≤ 30.
Average range defined as T scores ranging from 50-64. Clinically significant scores defined as T scores ≥ 70.
t test for equality of means, with expected mean of 50.
No. of patients and corresponding % refer to those whose scores exceeded clinical significance.
Exact binomial test, with expected clinical proportion of 2%.
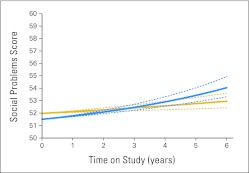
A positive time-by-risk interaction was observed for social problems suggesting that parent-reported social problems increased at a greater rate over time for high-risk compared with average-risk patients (P = .001; Fig 2). The interaction of time and risk status contributed to change in withdrawn/depressed scores, with high-risk patients demonstrating a greater increase in parent-reported withdrawn/depressed behaviors over time compared with average-risk patients (P = .01).
Fig 2.
Patient risk status and parent-reported social problems over time (T scores: mean, 50; standard deviation, 10). Lower social problems scores reflect better functioning. Solid gold line indicates average risk; dashed gold lines indicate 95% CI. Solid blue line indicates high risk; dashed blue lines indicate 95% CI.
Patients with PFS had greater parent-reported withdrawn/depressed behaviors (P = .002) and lower social competence (P = .04) at diagnosis. A negative time by PFS interaction revealed that parent-reported withdrawn/depressed behaviors decreased at a greater rate over time for patients with PFS (P = .002). A positive time by social problems interaction indicated that among patients with PFS, parent-reported social problems increased at a greater rate over time compared with patients without PFS (P = .03).
Baseline GIA was associated with parent report of social problems, such that patients with a higher baseline GIA score were reported to have fewer social problems at diagnosis (P = .005). In addition, a positive interaction of time and GIA was associated with social competence, indicating that patients with a higher baseline GIA score had higher social competence scores over time (P = .004).
At diagnosis, older patients had significantly higher social competence scores (P = .004) and fewer parent-reported social problems (P = .02) than younger patients. A time-by-sex interaction revealed that female patients were reported to have higher withdrawn/depressed scores over time compared with male patients (P < .001). Patients of parents with more years of completed education were reported to have higher social competence scores at baseline (P = .002) and greater decline in social competence scores over time (P = .008).
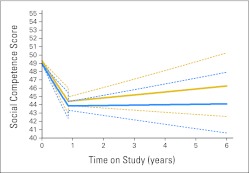
Visual inspection of the raw data revealed a trend for decline in social competence scores during the initial year after diagnosis, followed by a less distinct pattern of functioning beyond 1 year postdiagnosis. Using mean time until the first follow-up assessment (0.86 years), analysis of this trend using a discontinuous-slope GLMM revealed that the change in slope was significant (P < .001), with a significant negative slope between diagnosis and initial follow-up (P = .001) and a negative but nonsignificant slope after 1 year postdiagnosis. Figure 3 shows change in social competence over time by patient risk status using the discontinuous-slope GLMM.
Fig 3.
Patient risk status and parent-reported social competence before and after mean time until first follow-up (0.86 years) using a discontinuous-slope generalized linear mixed effects model (T scores: mean, 50; standard deviation, 10). Higher social competence scores reflect better functioning. Solid blue line indicates high risk; dashed blue lines indicate 95% CI. Solid gold line indicates average risk; dashed gold lines indicate 95% CI.
Impact of Long-Term Observations
Because the study remained open to accrual, a larger number of patients contributed data to earlier study time points than later. To determine the impact of having a lower number of evaluations at 4 years postdiagnosis and beyond, the models were examined using only observations up to and including 3 years postdiagnosis. For social problems and social competence, the results remained identical to the models using all time points. A similar pattern of results was found for withdrawn/depressed behaviors. Though the sex-by-time and PFS-by-time interactions were not retained in the best-fitting 3-year model, single covariate models including the interactions of sex and PFS with time since diagnosis were significant at 3 and 5 years. Therefore, it was concluded that including observations at later time points, although fewer in number, did not significantly alter the interpretation of study results.
DISCUSSION
To our knowledge, this is the largest longitudinal study of parent-reported social outcomes for pediatric brain tumor survivors. Importantly, our sample was relatively homogeneous with respect to diagnosis and treatment, factors that have been difficult to disentangle in previous research on social outcomes. We found that few patients were reported to have clinically elevated scores on measures of social competence, social problems, or withdrawn/depressed behavior; however, the proportion of survivors with clinically elevated scores often exceeded the expected proportion based on population data. Despite the fact that observed scores differed from population norms, survivors, in general, scored within the average range on measures of social functioning. Although these findings are promising and largely suggest positive social adjustment several years after diagnosis and treatment, a number of factors associated with unfavorable patterns of change in social functioning emerged and may be important precursors of late social outcomes.
Patients with high-risk treatment status had a significantly greater increase in parent-reported social problems and withdrawn/depressed behaviors over time compared with average-risk patients. The major difference in treatment approach for high- versus average-risk patients involves CSI dose. Although cranial radiation therapy has been implicated in poor social adjustment15 and peer relationships8 in previous studies involving small heterogeneous samples, our results support and extend these findings by revealing that higher CSI dose may be a risk factor for decline in social functioning over time. Importantly, we were unable to examine the impact of treatment for progressive disease as a contributor to change in social functioning given the small number of patients with outcome data after documented disease progression.
Posterior fossa syndrome was associated with reduced social competence and greater withdrawn/depressed behaviors at baseline, as well as with increasing social problems over time. These findings are consistent with a past report of persistent psychosocial problems for children who develop PFS after surgical resection for medulloblastoma.19 Of interest, parents of patients with PFS reported fewer withdrawn/depressed behaviors over time. This may reflect a shift in parental expectations and/or perceptions of child behavior after resolution of acute symptoms of emotional lability often associated with PFS.24
General intellectual ability emerged as a significant contributor to parent-reported social competence and social problems. Specifically, patients with higher intelligence scores at diagnosis were reported to have fewer social problems at diagnosis. Moreover, patients with higher intelligence scores at diagnosis demonstrated greater gains in social competence over time. Previous reports have documented an association between parent-reported social difficulties and low intelligence quotient among brain tumor survivors.11,25 Though similar, our results also seem to suggest a potential protective role of baseline cognitive ability on later social outcomes. Comparable findings have been reported in the traumatic brain injury literature, as estimates of premorbid functioning seem to predict postinjury behavioral outcomes.26 It is important to note that general intellectual ability is a global construct that is dependent on specific cognitive processes, such as attention and memory,27,28 which may have more direct effects on social functioning.
Parents reported that female brain tumor patients demonstrated more withdrawn/depressed behaviors over time compared with male patients. This is consistent with reports from the general pediatric population, indicating that female patients with special health care needs are more likely to exhibit internalizing symptoms than male patients.29 Further, our results parallel a report from Barrera et al,30 indicating that female brain tumor survivors are at greater risk for depression than their male counterparts, and that limited social skills and low self-confidence increase risk for depression in females. Taken together, these data suggest that female brain tumor survivors may be especially vulnerable to the impact of disease and treatment on internalizing behavior.
We also found an association between older age at diagnosis and greater parent-reported social competence and fewer social problems at diagnosis. Given that social competence and problems scores are adjusted for age, this finding may reflect differences in parent perceptions of child behavior rather than true differences in child competence. A study of coping in parents of pediatric patients with brain tumors revealed that parents of children who were older at the time of diagnosis scored significantly higher on positive reappraisal than parents of younger children.31 Our findings suggest that parental efforts to conceptualize their child's diagnosis and treatment in a positive manner may have a broader impact on parental perception and reporting of child behavior.
A central aim of this longitudinal study was to gain insight into when social difficulties begin to emerge for these patients. We found a decline in parent-reported social competence during the first year after diagnosis. This finding is not surprising in the context of the clinical protocol on which these patients are treated. During the first year of therapy, patients receive 6 weeks of CSI followed by several cycles of chemotherapy. Owing to clinic visits, hospitalizations, and possible immunosuppression, these patients miss numerous opportunities for social engagement (ie, school, extracurricular activities). The social competence scale on the CBCL largely reflects participation in social organizations and frequency of contact with peers. A reduction in these activities would not be uncommon during this intense phase of cancer treatment. However, what raises concern is the lack of reported recovery in social competence after this first year of treatment, especially for high-risk patients. This may be related to continued medical problems prohibiting opportunity or ability to re-enter social groups or activities. This suggests that intervention efforts directed at promoting social re-engagement for patients during or after the first year of treatment could be particularly beneficial. It will be important to consider patient cognitive status and tailor interventions accordingly.
Importantly, the majority of survivors were reported to be doing well with respect to social functioning, especially during the first several years after diagnosis and treatment. Despite the longitudinal nature of our study, questions regarding the continued trajectory of social outcomes for this particular cohort of survivors remain unanswered. Consistent with our findings, Mabbott et al15 reported progressively worsening social adjustment over a median follow-up period of 4 years from diagnosis (maximum, 15 years); however, mean scores were only beginning to approach the clinically significant range. Long-term follow-up studies of childhood brain tumor survivors unequivocally report social difficulties in adulthood including reduced rates of dating, marriage, and independent living.32,33 Taken together, these data highlight a gap in our knowledge of social functioning for this patient population. Although the trajectory of social adjustment is beginning to be elucidated, information pertaining to social functioning during emerging adulthood is still needed.
Future work is necessary to identify when clinically significant social difficulties emerge, as well as the optimal time for intervention delivery. Our study provides important insights toward understanding factors associated with social functioning after diagnosis and treatment for a pediatric embryonal tumor; however, the broad behavioral constructs from the CBCL lack the specificity necessary to inform targeted intervention development. Future studies should assess specific social skills that provide the foundation for successful navigation of the social environment and development of interpersonal relationships. Bonner et al18 have provided a model for such work with their research on facial expression recognition, a specific skill deficit observed in brain tumor survivors that may be amenable to intervention. Other factors that may contribute to social outcomes include family environment, parent coping, and child language and motor skills. In fact, a recent conceptual model highlights the interdependence of social problem-solving skills, affective social competence, and executive functions, as well as the potential contribution of family variables on social adjustment following childhood brain injury.34 There remains a need for improved conceptual development of social competence in survivors of brain tumors.
We must acknowledge the limitation of relying exclusively on parent reports of child social functioning. Given that the majority of social opportunities for children occur in the context of peer groups and in school settings, parents may not have the opportunity to observe their children in their most natural social context, especially during active treatment. In addition, discrepancies between parent, teacher, and child reports have been documented and these are especially salient in the areas of social and internalizing behaviors.35–37 The validity of the CBCL as a sole measure of social functioning as well as its application to the pediatric psycho-oncology patient population have been questioned.38 Future research should employ multimethod and multi-informant approaches toward the assessment of social functioning.
Appendix
Participant Attrition
As noted in the article, because the clinical treatment protocol remains open to accrual, fewer patients contributed data to later time points in the study. We examined this along with other reasons for participant attrition. A total of 40 patients contributed Child Behavior Checklist (CBCL) data to at least one study time point but were not yet far enough from diagnosis to contribute data at later time points (eg, 12 patients contributed data at Year 4 but were not yet 5 years from diagnosis at the time of data analysis). In addition, our study did not require patients treated at institutions other than St Jude Children's Research Hospital to provide neurocognitive data at Year 2 (n = 42) and Year 4 (n = 23). Forty-nine patients died or experienced disease progression during the study. Patients were also missing data owing to parent refusal (n = 14), medical issues restricting assessment (n = 4), missed appointments or scheduling conflicts (n = 6), current age older than 18 years (n = 4), and other related reasons (n = 2). An additional 16 patients had undergone partial neurocognitive testing that did not include the CBCL. Finally, study sites had not yet submitted the CBCL data for four patients in time for inclusion in the current study.
Table A1.
Comparison of Patients With and Without Year 5 Data
| Characteristic | Only Baseline |
Baseline and Year 5 |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Sex* | .66 | ||||
| Male | 21 | 51.2 | 24 | 57.1 | |
| Female | 20 | 48.8 | 18 | 42.9 | |
| PFS* | .76 | ||||
| Yes | 6 | 14.6 | 5 | 11.9 | |
| No | 35 | 85.4 | 37 | 88.1 | |
| Risk* | .005 | ||||
| High | 19 | 46.3 | 7 | 16.7 | |
| Average | 22 | 53.7 | 35 | 83.3 | |
| Age at baseline† | .69 | ||||
| Mean | 11.1 | 10.7 | |||
| SD | 3.7 | 3.8 | |||
| Age at diagnosis† | .70 | ||||
| Mean | 11.0 | 10.6 | |||
| SD | 3.7 | 3.8 | |||
| Social competence† | .22 | ||||
| Mean | 50.2 | 52.8 | |||
| SD | 8.6 | 8.2 | |||
| Withdrawn/depressed† | .02 | ||||
| Mean | 54.0 | 58.0 | |||
| SD | 5.6 | 8.3 | |||
| Social problems† | .23 | ||||
| Mean | 52.7 | 54.1 | |||
| SD | 3.8 | 5.4 | |||
Abbreviations: PFS, posterior fossa syndrome; SD, standard deviation.
Fisher's exact test for equality of proportions.
t test for equality of means.
Table A2.
Parent-Reported Social Outcomes by Patients' Risk Status
| Year | Social Competence |
Social Problems |
Withdrawn/Depressed |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Risk |
High Risk |
Average Risk |
High Risk |
Average Risk |
High Risk |
|||||||||||||
| No. of Patients | Mean | SD | No. of Patients | Mean | SD | No. of Patients | Mean | SD | No. of Patients | Mean | SD | No. of Patients | Mean | SD | No. of Patients | Mean | SD | |
| Baseline | 120 | 49.8 | 9.5 | 48 | 50.2 | 7.9 | 121 | 53.7 | 5.0 | 48 | 52.9 | 3.8 | 121 | 55.8 | 7.3 | 48 | 56.5 | 7.3 |
| 1 | 100 | 45.7 | 9.3 | 35 | 42.1 | 7.6 | 103 | 54.9 | 5.9 | 37 | 54.7 | 5.1 | 103 | 57.3 | 8.6 | 37 | 56.8 | 6.9 |
| 2 | 46 | 46.6 | 9.8 | 17 | 46.3 | 6.9 | 45 | 55.3 | 5.6 | 17 | 56.2 | 8.3 | 45 | 56.6 | 6.9 | 17 | 56.5 | 7.0 |
| 3 | 59 | 45.3 | 9.8 | 16 | 45.9 | 7.0 | 60 | 56.9 | 7.5 | 16 | 54.4 | 5.5 | 60 | 57.2 | 7.2 | 16 | 56.7 | 9.9 |
| 4 | 32 | 47.5 | 9.7 | 9 | 46.9 | 7.0 | 32 | 56.0 | 6.7 | 9 | 56.0 | 6.5 | 32 | 55.4 | 7.5 | 9 | 55.4 | 6.9 |
| 5 | 26 | 46.7 | 10.6 | 7 | 42.7 | 9.2 | 26 | 57.8 | 8.1 | 7 | 55.9 | 7.6 | 26 | 56.7 | 6.2 | 7 | 57.9 | 10.6 |
NOTE. T scores: mean, 50; SD, 10. Higher social competence scores reflect better functioning. Lower social problems and withdrawn/depressed scores reflect better functioning.
Abbreviation: SD, standard deviation.
Table A3.
Parent-Reported Social Outcomes by Posterior Fossa Syndrome
| Year | Social Competence |
Social Problems |
Withdrawn/Depressed |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS |
No PFS |
PFS |
No PFS |
PFS |
No PFS |
|||||||||||||
| No. of Patients | Mean | SD | No. of Patients | Mean | SD | No. of Patients | Mean | SD | No. of Patients | Mean | SD | No. of Patients | Mean | SD | No. of Patients | Mean | SD | |
| Baseline | 25 | 44.8 | 7.6 | 143 | 50.8 | 9.0 | 25 | 53.6 | 3.2 | 144 | 53.5 | 4.9 | 25 | 56.9 | 7.0 | 144 | 55.8 | 7.4 |
| 1 | 26 | 45.2 | 8.7 | 109 | 44.7 | 9.1 | 27 | 58.3 | 6.1 | 113 | 54.0 | 5.3 | 27 | 60.2 | 9.6 | 113 | 56.5 | 7.7 |
| 2 | 11 | 44.1 | 4.8 | 52 | 47.0 | 9.6 | 11 | 57.5 | 4.6 | 51 | 55.1 | 6.7 | 11 | 59.8 | 8.2 | 51 | 55.8 | 6.4 |
| 3 | 17 | 43.4 | 7.3 | 58 | 46.1 | 9.7 | 17 | 60.6 | 7.9 | 59 | 55.2 | 6.6 | 17 | 59.7 | 8.2 | 59 | 56.3 | 7.5 |
| 4 | 8 | 44.9 | 8.3 | 33 | 47.9 | 9.3 | 8 | 57.9 | 3.9 | 33 | 55.6 | 7.0 | 8 | 57.5 | 6.8 | 33 | 54.9 | 7.4 |
| 5 | 7 | 45.4 | 7.5 | 26 | 46.0 | 11.1 | 7 | 59.6 | 8.0 | 26 | 56.8 | 8.0 | 7 | 55.6 | 6.8 | 26 | 57.3 | 7.3 |
NOTE. T scores: mean, 50; SD, 10. Higher social competence scores reflect better functioning. Lower social problems and withdrawn/depressed scores reflect better functioning.
Abbreviations: PFS, posterior fossa syndrome; SD, standard deviation.
Footnotes
Supported in part by Cancer Center Support Grant No. P30-CA21765 from the National Cancer Institute, the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer, and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Shawna L. Palmer, Amar Gajjar
Provision of study materials or patients: Melanie J. Bonner, Carol L. Armstrong, Amar Gajjar
Collection and assembly of data: Shawna L. Palmer, Karen Evankovich, Michelle A. Swain, Melanie J. Bonner, Laura Janzen, Sarah Knight, Carol L. Armstrong, Robyn Boyle, Amar Gajjar
Data analysis and interpretation: Tara M. Brinkman, Shawna L. Palmer, Si Chen, Hui Zhang, Carol L. Armstrong, Amar Gajjar
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: Childhood cancer survivor study. J Clin Oncol. 2003;21:3255–3261. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:946–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong GT. Long-term survivors of childhood central nervous system malignancies: The experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14:298–303. doi: 10.1016/j.ejpn.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: A report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12:1173–1186. doi: 10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulte F, Barrera M. Social competence in childhood brain tumor survivors: A comprehensive review. Support Care Cancer. 2010;18:1499–1513. doi: 10.1007/s00520-010-0963-1. [DOI] [PubMed] [Google Scholar]
- 8.Barrera M, Shaw AK, Speechley KN, et al. Educational and social late effects of childhood cancer and related clinical, personal, and familial characteristics. Cancer. 2005;104:1751–1760. doi: 10.1002/cncr.21390. [DOI] [PubMed] [Google Scholar]
- 9.Vannatta K, Gartstein MA, Short A, et al. A controlled study of peer relationships of children surviving brain tumors: Teacher, peer, and self ratings. J Pediatr Psychol. 1998;23:279–287. doi: 10.1093/jpepsy/23.5.279. [DOI] [PubMed] [Google Scholar]
- 10.Carey ME, Barakat LP, Foley B, et al. Neuropsychological functioning and social functioning of survivors of pediatric brain tumors: Evidence of nonverbal learning disability. Child Neuropsychol. 2001;7:265–272. doi: 10.1076/chin.7.4.265.8730. [DOI] [PubMed] [Google Scholar]
- 11.Poggi G, Liscio M, Galbiati S, et al. Brain tumors in children and adolescents: Cognitive and psychological disorders at different ages. Psychooncology. 2005;14:386–395. doi: 10.1002/pon.855. [DOI] [PubMed] [Google Scholar]
- 12.Kullgren KA, Morris RD, Morris MK, et al. Risk factors associated with long-term social and behavioral problems among children with brain tumors. J Psychosoc Oncol. 2003;21:73–87. [Google Scholar]
- 13.Aarsen FK, Paquier PF, Reddingius RE, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106:396–402. doi: 10.1002/cncr.21612. [DOI] [PubMed] [Google Scholar]
- 14.Schultz KA, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2007;25:3649–3656. doi: 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- 15.Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 16.Bhat SR, Goodwin TL, Burwinkle TM, et al. Profile of daily life in children with brain tumors: An assessment of health-related quality of life. J Clin Oncol. 2005;23:5493–5500. doi: 10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]
- 17.Willard VW, Hardy KK, Bonner MJ. Gender differences in facial expression recognition in survivors of pediatric brain tumors. Psychooncology. 2009;18:893–897. doi: 10.1002/pon.1502. [DOI] [PubMed] [Google Scholar]
- 18.Bonner MJ, Hardy KK, Willard VW, et al. Social functioning and facial expression recognition in survivors of pediatric brain tumors. J Pediatr Psychol. 2008;33:1142–1152. doi: 10.1093/jpepsy/jsn035. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe-Christensen C, Mullins LL, Scott JG, et al. Persistent psychosocial problems in children who develop posterior fossa syndrome after medulloblastoma resection. Pediatr Blood Cancer. 2007;49:723–726. doi: 10.1002/pbc.21084. [DOI] [PubMed] [Google Scholar]
- 20.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 21.Gleissner U, Fritz NE, Von Lehe M, et al. The validity of the Child Behavior Checklist for children with epilepsy. Epilepsy Behav. 2008;12:276–280. doi: 10.1016/j.yebeh.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Woodcock RJ, McGraw K, Mather N. Woodcock-Johnson Tests of Cognitive Abilities(ed 3) Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 23.Spiegler BJ, Bouffet E, Greenberg ML, et al. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 24.Catsman-Berrevoets CE, Aarsen FK. The spectrum of neurobehavioural deficits in the posterior fossa syndrome in children after cerebellar tumour surgery. Cortex. 2010;46:933–946. doi: 10.1016/j.cortex.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Schulte F, Bartels U, Bouffet E, et al. Body weight, social competence, and cognitive functioning in survivors of childhood brain tumors. Pediatr Blood Cancer. 2010;55:532–539. doi: 10.1002/pbc.22543. [DOI] [PubMed] [Google Scholar]
- 26.Fay TB, Yeates KO, Wade SL, et al. Predicting longitudinal patterns of functional deficits in children with traumatic brain injury. Neuropsychology. 2009;23:271–282. doi: 10.1037/a0014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagel BJ, Delis DC, Palmer SL, et al. Early patterns of verbal memory impairment in children treated for medulloblastoma. Neuropsychology. 2006;20:105–112. doi: 10.1037/0894-4105.20.1.105. [DOI] [PubMed] [Google Scholar]
- 28.Reeves CB, Palmer SL, Reddick WE, et al. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006;31:272–280. doi: 10.1093/jpepsy/jsj019. [DOI] [PubMed] [Google Scholar]
- 29.Ghandour RM, Kogan MD, Blumberg SJ, et al. Prevalence and correlates of internalizing mental health symptoms among CSHCN. Pediatrics. 2010;125:e269–e277. doi: 10.1542/peds.2009-0622. [DOI] [PubMed] [Google Scholar]
- 30.Barrera M, Schulte F, Spiegler B. Factors influencing depressive symptoms of children treated for a brain tumor. J Psychosoc Oncol. 2008;26:1–16. doi: 10.1300/j077v26n01_01. [DOI] [PubMed] [Google Scholar]
- 31.Palmer SL, Lesh S, Wallace D, et al. How parents cope with their child's diagnosis and treatment of an embryonal tumor: Results of a prospective and longitudinal study. J Neurooncol. 2011;105:253–259. doi: 10.1007/s11060-011-0574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddrey AM, Bergeron JA, Lombardo ER, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72:245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- 33.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeates KO, Bigler ED, Dennis M, et al. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychol Bull. 2007;133:535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radcliffe J, Bennett D, Kazak AE, et al. Adjustment in childhood brain tumor survival: Child, mother, and teacher report. J Pediatr Psychol. 1996;21:529–539. doi: 10.1093/jpepsy/21.4.529. [DOI] [PubMed] [Google Scholar]
- 36.Eapen V, Revesz T, Mpofu C, et al. Self-perception profile in children with cancer: Self vs parent report. Psychol Rep. 1999;84:427–432. doi: 10.2466/pr0.1999.84.2.427. [DOI] [PubMed] [Google Scholar]
- 37.Hardy KK, Willard VW, Watral MA, et al. Perceived social competency in children with brain tumors: Comparison between children on and off therapy. J Pediatr Oncol Nurs. 2010;27:156–163. doi: 10.1177/1043454209357918. [DOI] [PubMed] [Google Scholar]
- 38.Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. J Pediatr Psychol. 2005;30:9–27. doi: 10.1093/jpepsy/jsi012. [DOI] [PubMed] [Google Scholar]