Abstract
Purpose
Lexatumumab is an agonistic, fully human monoclonal antibody against tumor necrosis factor–related apoptosis-inducing ligand receptor 2 with preclinical evidence of activity in pediatric solid tumors.
Patients and Methods
This phase I dose-escalation study examined the safety, tolerability, pharmacokinetics, and immunogenicity of lexatumumab at doses up to, but not exceeding, the adult maximum-tolerated dose (3, 5, 8, and 10 mg/kg), administered once every 2 weeks to patients age ≤ 21 years with recurrent or progressive solid tumors.
Results
Twenty-four patients received a total of 56 cycles of lexatumumab over all four planned dose levels. One patient had grade 2 pericarditis consistent with radiation recall, and one patient developed grade 3 pneumonia with hypoxia during the second cycle. Five patients experienced stable disease for three to 24 cycles. No patients experienced complete or partial response, but several showed evidence of antitumor activity, including one patient with recurrent progressive osteosarcoma who experienced resolution of clinical symptoms and positron emission tomography activity, ongoing more than 1 year off therapy. One patient with hepatoblastoma showed a dramatic biomarker response.
Conclusion
Pediatric patients tolerate 10 mg/kg of lexatumumab administered once every 14 days, the maximum-tolerated dose identified in adults. The drug seems to mediate some clinical activity in pediatric solid tumors and may work with radiation to enhance antitumor effects.
INTRODUCTION
Tumor necrosis factor (TNF) and TNF-related ligands are cell-associated and -secreted molecules that can induce death in a wide variety of cancer cells. The clinical development of TNFα and Fas agonists, both members of this family, has been hampered by systemic toxicity. TNF-related apoptosis-inducing ligand (TRAIL, Apo2L) is produced by natural killer cells and has been shown to play a role in immunosurveillance.1 Binding of TRAIL to TRAIL receptor TRAIL-R1 or TRAIL-R2 activates the extrinsic apoptosis pathway. TRAIL-R1 and TRAIL-R2 have been identified in multiple adult and pediatric tumors but have limited expression in normal cells, making them attractive targets for anticancer therapy.2–8
Lexatumumab (HGS-ETR2) is a recombinant, fully human IgG1λ monoclonal antibody. Two previous phase I trials revealed that lexatumumab was well tolerated in adult patients when administered once every 14 or 21 days.9,10 Dose-limiting toxicities (DLTs) at 20 mg/kg once every 21 days included transaminitis and asymptomatic elevation of amylase or bilirubin. At the maximum-tolerated dose (MTD) of 10 mg/kg, lexatumumab was well tolerated, and several patients received prolonged therapy, with five patients receiving more than three cycles.9,10
Several pediatric solid tumors have been shown to undergo apoptosis after activation of the extrinsic death pathway by TRAIL receptor agonists.11–14 The extent of apoptosis observed in pediatric tumors such as Ewing sarcoma, rhabdomyosarcoma, osteosarcoma, and neuroblastoma is extensive after TRAIL-R2 agonist binding and, in many cases, results in 100% death of pediatric sarcoma cell lines in vitro.3,11,14,15 Preclinical testing has also revealed delays in tumor growth after TRAIL-R2 agonist treatment in xenograft models of Ewing sarcoma and osteosarcoma.11,16 Although carcinomas frequently display inhibition of the type II or mitochondrial-dependent pathway of death receptor apoptosis,17 pediatric tumors such as Ewing sarcoma, rhabdomyosarcoma, and osteosarcoma more frequently have intact type I and type II pathways, leading to efficient apoptosis after death ligand binding.4,13,18 Such intact apoptosis pathways suggest that TRAIL mimetics such as lexatumumab may be potent in the clinical treatment of pediatric tumors.
PATIENTS AND METHODS
Patients
The study population consisted of patients age 2 to 21 years with recurrent or progressive solid tumors after standard therapy. Patients must have completed their last dose of irradiation, chemotherapy, or investigational therapy at least 4 weeks before enrollment. Other inclusion criteria included Karnofsky or Lansky score > 50, hemoglobin concentration > 8 g/dL, absolute granulocyte count > 1,000/μL, platelet count > 75,000/μL, AST and ALT ≤ 2.5-fold the upper limit of normal, and bilirubin and serum creatinine within normal limits. Because of hepatotoxicity at > MTD in the first adult phase I study, patients with hepatic metastases were excluded. Patients with primary CNS malignancies or active brain metastases were excluded from the trial because of unknown penetration into the CNS.
Study Design and Treatment
VEG10003 was a multicenter, open-label, nonrandomized phase I study to test the safety and pharmacokinetics of lexatumumab up to the MTD in the adult studies (NCT00428272). Lexatumumab (HGS-ETR2; Human Genome Sciences, Rockville, MD) was supplied as a sterile, single-use, lyophilized product stored in the dark at 2°C to 8°C. On reconstitution with 5.0 mL water for injection, lexatumumab was diluted in normal saline for intravenous administration over 60 minutes. Patients were premedicated with acetaminophen and diphenhydramine. Lexatumumab was administered once every 14 days. Cycles were 28 days long.
DLTs
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (version 3). Nonhematologic DLT was defined as any grade 3 or 4 toxicity occurring during cycle one or the failure of any toxicity to recover to grade ≤ 1 in 21 days after dosing of lexatumumab. Because it was anticipated that many patients enrolling onto this trial would have limited marrow reserve, hematologic DLT was defined as grade 4 neutropenia or thrombocytopenia persisting for at least 5 days.
Pharmacokinetics
This trial studied similar doses9,10 and schedule10 and used the same assay method as adult trials.9,10 Blood samples (5 mL) were collected from a site other than the infusion line for determination of serum lexatumumab concentrations on days 1 and 15. Day 1 samples were collected before dosing and at end of infusion, 5 minutes postinfusion, and 6, 24, 48, 96, and 168 hours postdose. On day 15, samples were collected before dosing, at end of infusion, 5 minutes postinfusion, and 6 hours postinfusion completion, and trough samples were collected before subsequent cycles. Blood was processed to obtain serum, and aliquots were frozen at −80°C until assayed. Samples were analyzed for lexatumumab concentration and immunogenicity by qualified enzyme-linked immunosorbent assays, as previously described.9 Lexatumumab concentration-time data were analyzed using noncompartmental methods. Peak lexatumumab concentration (Cmax) and time to peak concentration were determined from each patient's concentration-time plot. Area under the concentration-time curve (AUC) to last measured time point after dose one was calculated with the linear trapezoidal method and extrapolated to infinity (AUC0-∞) by adding the last serum concentration after dose one divided by the rate constant, which was derived from the slope of the natural log-transformed concentrations and times on the terminal elimination phase of the concentration-time curve. Half-life was calculated by dividing 0.693 by the terminal rate constant. Clearance (CL) was determined from dose/AUC0-∞. Volume of distribution at steady-state (Vdss) and mean resident time were calculated from the area under the moment curve. The relationship between CL and age was evaluated by linear regression. Accumulation of lexatumumab was assessed by dividing the trough concentrations after the second and subsequent doses by the trough concentration after the first dose.
Evaluation of Clinical Activity
Baseline imaging was assessed within 14 days of the first lexatumumab dose, after the first two doses (4 weeks), and then every 8 weeks until withdrawal from the study. Baseline imaging included computed tomography (CT) and/or magnetic resonance imaging of target lesions in addition to positron emission tomography (PET)/CT or bone scan when appropriate for the disease. Restaging was planned after the first cycle and then every other cycle after that. Response was evaluated according to the RECIST guidelines.
Immunohistochemistry
Biopsies were not required for study entry, but an attempt was made to receive blocks or unstained slides from previous biopsies or surgeries. Slides were stained for TRAIL-R1, TRAIL-R2, and caspase 8. Priority was given to TR2 staining if fewer slides were available than needed for all stains. Immunohistochemistry (IHC) for TRAIL-R2 was performed as previously described.9 Of note, the TRAIL-R2 reagent was selected for greater sensitivity in an IHC assay than the human lexatumumab, but both bind to the extracellular region of TRAIL-R2. IHC for caspase 8 and survivin used mouse monoclonal anticaspase 8 (clone 1 to 1-37; Upstate; Millipore, Billerica, MA) and rabbit polyclonal antisurvivin (Alpha Diagnostic International, San Antonio, TX). Primary antibodies were applied for 1 hour at room temperature at a concentration of 1:100 for both antibodies, using antibody diluent with background-reducing components (Dako, Carpinteria, CA). Secondary antibodies were performed using EnVision+ System-HRP (DAB) antimouse and antirabbit (Dako) for 30 minutes at room temperature. The peroxidase reaction was developed with 3,3-diaminobenzidine chromogen solution (Dako) for 5 minutes. Negative controls excluded the primary antibody. Only cytoplasmic immunoreactivity was considered positive for caspase 8 and survivin expression.
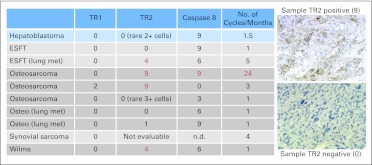
Stained specimens were reviewed by two observers and scored for each target antigen according to a standardized method in which both membrane and cytoplasmic staining were captured according to intensity (grades 0, 1, 2, 3) and percentage of tumor cells positive: grade 0 (0% to 1%), grade 1 (2% to 10%), grade 2 (11% to 50%), and grade 3 (51% to 100%). Final score is calculated as described in the legend of Figure 1.
Fig 1.
Immunohistochemical analysis of archived tissues. Scores were calculated based on intensity (0, 1, 2, or 3) multiplied by the grade corresponding to the percent of positive cells (grade 0 [0% to 1%], grade 1 [2% to 10%], grade 2 [11% to 50%], grade 3 [51% to 100%]). Maximum score of 9 when 3+ in > 50% of cells, as shown in top example. ESFT, Ewing sarcoma family of tumors; met, metastasis; n.d., not done; osteo, osteosarcoma; TR1, tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptor 1; TR2, TRAIL receptor 2.
RESULTS
Patient Characteristics
A total of 24 patients were enrolled onto this multicenter trial, and all patients were eligible and evaluable for toxicity. Patient ages ranged from 2 to 21 years, with a median age of 16 years (Table 1). The majority of patients had relapsed pediatric sarcomas, and three patients had a diagnosis of hepatoblastoma, nephroblastoma, and spindle epithelial tumor with thymus-like differentiation, respectively. Three patients were enrolled in each of the first two dose levels. Because of a DLT in a single patient at the 8-mg/kg dose level, six patients were treated at the third dose level, and an expanded cohort of 12 patients was enrolled at the highest dose level. The highest dose tested was the adult MTD of 10 mg/kg. No further escalation was planned based on toxicities identified at 20 mg/kg in adult studies. Patients in the study received a range of one to 24 cycles of lexatumumab treatment.
Table 1.
Demographics and Clinical Characteristics of Patients Enrolled Onto Trial (N = 24)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 16 | |
| Range | 2-21 | |
| Mean ± SD | 14.5 ± 5 | |
| Primary tumor type | ||
| Ewing sarcoma | 4 | 17 |
| Osteosarcoma | 9 | 28 |
| Rhabdomyosarcoma | 3 | 13 |
| Synovial sarcoma | 2 | 8 |
| Alveolar soft part sarcoma | 1 | 4 |
| Undifferentiated sarcoma | 2 | 8 |
| Hepatoblastoma | 1 | 4 |
| Nephroblastoma | 1 | 4 |
| SETTLE | 1 | 4 |
| Sex | ||
| Male | 15 | 62 |
| Female | 9 | 38 |
| Lansky/Karnofsky score | 60-90 | |
| No. of prior chemotherapy regimens | 2-6 | |
Abbreviations: SETTLE, spindle epithelial tumor with thymus-like differentiation; SD, standard deviation.
Toxicity
One patient with synovial sarcoma who received 8 mg/kg of lexatumumab experienced a DLT with hypoxia and pleural effusion associated with a change in pleural-based tumor, probably related to drug administration. No other DLT was observed in patients on this protocol. All 12 patients in the expanded final dose level tolerated 10 mg/kg of lexatumumab without any grade 3 or 4 toxicities. Common non–dose-limiting reactions were: grade 1 to 2 fatigue, grade 1 to 2 pain at tumor site, grade 1 hypokalemia, and grade 1 transaminitis. One patient had grade 2 radiation recall pericarditis, thought to be related to lexatumumab. Two patients had propagation of venous thrombi, judged possibly related to drug administration.
Pharmacokinetics and Immunogenicity
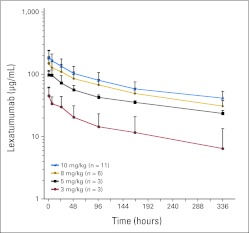
Pharmacokinetic parameters are summarized in Table 2. There was substantial variability in the pharmacokinetic parameters at the first dose level and a disproportionate increase in drug exposure from the 3- (n = 3) to 5-mg/kg (n = 3) dose level. Less pharmacokinetic variability was observed at the higher dose levels, and a dose-proportionate increase in Cmax and drug exposure from the 5- to 8- and 10-mg/kg dose levels. The concentration-time profile captured drug exposure incompletely (Appendix Fig A1, online only); approximately 30% of the AUC0-∞ was extrapolated, and the average terminal half-life was 8.3 days. No relationship between age and CL normalized to body weight or body surface area was observed. Drug exposure (AUC0-∞, 30,907 μg × h/mL) of the only patient who experienced a DLT at 8 mg/kg was similar to that of other patients treated at the same dose level. The mean trough concentrations for eight patients at the 10-mg/kg dose after the first and second doses were 31.2 and 41.5 μg/mL, respectively, exceeding levels required for apoptosis in Ewing sarcoma cells in vitro after 1× dosing and incubation for 72 hours.2 The mean accumulation index in patients who had samples obtained for this analysis after the first dose was 1.8 at 3 (n = 2), 1.5 at 5 (n = 2), 1.6 at 8 (n = 3), and 1.5 at 10 mg/kg (n = 8). The mean accumulation index after the second dose for eight patients was 2.0 (range, 1.9 to 2.3).
Table 2.
Pharmacokinetic Parameters for Lexatumumab by Dose Level
| Dose Level (mg/kg) | No. | Cmax (μg/mL) |
AUC0-last (μg × h/mL) |
AUC0-∞ (μg × h/mL) |
Percent Extrapolated |
t1/2 (days) |
CL (mL/d/kg) |
Vdss (mL/kg) |
Cmin (μg/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | Mean | Coefficient of Variation (%) | ||
| 3 | 3 | 46 | 37 | 4,695 | 64 | 7,063 | 87 | 21 | 99 | 6.8 | 70 | 20 | 92 | 93.7 | 3.3 | 6 | 108 |
| 5 | 3 | 99 | 21 | 13,757 | 8 | 21,835 | 7 | 37 | 15 | 9.9 | 13 | 17 | 116 | 79.7 | 12 | 24 | 12 |
| 8 | 6 | 155 | 26 | 19,872 | 24 | 28,969 | 22 | 32 | 20 | 8.5 | 16 | 6.9 | 20 | 74.4 | 48 | 31 | 23 |
| 10 | 9 | 193 | 29 | 26,499 | 27 | 37,886 | 27 | 30 | 31 | 7.9 | 37 | 6.8 | 29 | 72.5 | 34 | 42 | 19 |
Abbreviations: AUC, area under the serum concentration-time curve; Cmax, peak serum concentration; Cmin, trough serum concentration before second dose; CL, clearance; t1/2, half-life; Vdss, volume of distribution at steady state.
IHC
Fourteen patients had sufficient tissue available to test for TRAIL-R1 and TRAIL-R2, whereas only nine tumors were available to test for caspase 8 expression. Biopsy material was obtained from initial diagnosis in 12 patients and from lung metastases in three patients. TRAIL-R2 was highly expressed in seven samples, whereas five had rare positive cells or < 10% positive, and two tumors had no staining at all (Fig 1). Of the nine tissues stained for caspase 8, seven tissues had high expression, one had low expression, and one had no expression. There was no correlation found between amount of TRAIL-R2 expression and cycles of therapy. However, lack of TRAIL-R2 was associated with shorter time to progression in the clinical trial (Appendix Table A1, online only). TRAIL-R1 was not expressed on any of the malignant tissues except for rare positive TRAIL-R1 cells in two osteosarcomas.
Clinical Antitumor Activity
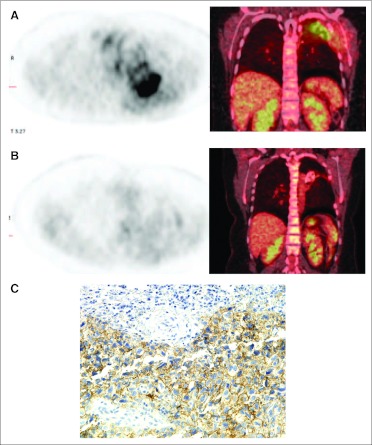
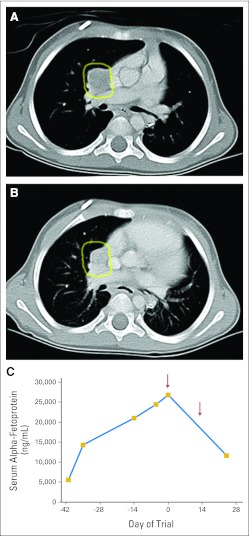
There were no complete or partial responses by standard RECIST criteria. However, one patient with osteosarcoma enrolled at the 10-mg/kg dose level derived clinical benefit, with resolution of chest wall pain, cough, and shortness of breath interfering with activities of daily living at the time of enrollment. This patient was enrolled onto the study 4 weeks after irradiation, when baseline scanning revealed progressive, metabolically active chest wall and pulmonary disease. She received a total of 24 months of lexatumumab therapy and continues to be asymptomatic, with stable PET-negative imaging more than 1 year off therapy. CT scans showed ossification of the mass in her left chest, and PET scan activity returned to background levels during treatment (Fig 2). One patient age 2 years with hepatoblastoma had dramatic biomarker decrease after a single dose of lexatumumab (Fig 3) but developed seizures revealing CNS metastases within 2 months of starting therapy and was removed from study for CNS radiotherapy. A total of four patients had confirmed stable disease during the trial, and one patient had an unconfirmed stable disease evaluation.
Fig 2.
Prolonged clinical benefit after administration of lexatumumab 10 mg/kg in a 16-year-old patient with osteosarcoma of the chest wall. (A) Baseline positron emission tomography (PET)/computed tomography (CT) in patient with cough, shortness of breath, dyspnea on exertion, and chest wall pain. (B) PET/CT 6 months off therapy after 24 months of therapy, with no respiratory complaints or pain. (C) TR2 staining on archived osteosarcoma tissue. TR2, tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptor 2.
Fig 3.
Clinical activity with biomarker response in first cycle in a 2-year-old patient with hepatoblastoma. (A) Computed tomography with metastasis outlined in yellow. (B) Follow-up imaging after two doses, with 24% reduction in longest diameter and 54% reduction in volume by three-dimensional volumetric assessment. (C) Alpha fetoprotein decline over first cycle (arrows indicate lexatumumab administration).
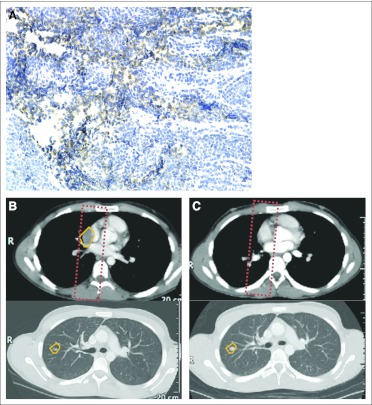
One patient with Ewing sarcoma received radiation therapy 1 month before lexatumumab treatment without clearance of his pericardiac mass. He developed radiation recall pericarditis after lexatumumab administration, which was controlled with a short course of steroids. The bulky disease within the radiation field showed complete regression (Fig 4). Over the course of the next 5 months while receiving lexatumumab, he experienced progression of target lesions outside of the radiation field but no recurrence within the field.
Fig 4.
Regression of nontarget lesion in a recently irradiated field, with progressive disease outside the radiation field. (A) TR2 staining on archival tissue from patient age 16 years metastatic recurrent Ewing sarcoma; (B) lung metastasis (outlined in yellow) and prior field of radiation (red dashed line); (C) disappearance of lung metastasis within prior radiation field after three cycles of lexatumumab but concurrent growth of tumor outside radiation field (lower panel).
DISCUSSION
Lexatumumab was well tolerated at all dose levels tested, including the adult MTD of 10 mg/kg. This is despite the fact that pediatric patients were more heavily pretreated than patients enrolled onto previous adult studies with this agent. Only one DLT possibly related to drug effect was observed. There were no grade 4 toxicities, and drug-related toxicities ≤ grade 3 were readily managed. No cumulative toxicities were observed in the patient who received 2 years of lexatumumab.
The pharmacokinetic profile of lexatumumab in children is similar to that of other monoclonal antibodies, with a small volume of distribution (mean Vdss at MTD, 72.5 mL/kg) and slow elimination (mean CL at MTD, 6.8 mL/d/kg). Variability in the pharmacokinetic parameters and small sample size (n = 3) limit conclusions for the lowest dose level (3 mg/kg). A dose-proportionate increase in drug exposure was observed for the 5-, 8-, and 10-mg/kg dose levels, as described for adult phase I trials of lexatumumab. Average trough concentrations at the 10-mg/kg dose level after the first and second dose exceeded the 10- to 100-ng/mL concentrations required in vitro for apoptosis of pediatric sarcoma cell lines as well as adult carcinoma lines.2,15 A comparison of the pharmocokinetics from the six patients age < 12 years with those of older adolescents in the trial revealed no age dependence in CL. The accumulation indexes of 1.5 after the first dose and 2.0 after the second dose of lexatumumab at the MTD justifies the dosing schedule of once every 14 days and are consistent with the adult phase I trial using a schedule of once every 14 days. CL, Vdss, and Cmax in children in this study were similar to those of adults treated with lexatumumab on schedules of once every 21 days and once every 14 days.9,10 Mean AUC0-∞ at 10 mg/kg was lower in children (37,886 ± 10,179 μg × h/mL standard deviation [SD]) compared with adults treated with the same dose on schedules of once every 14 days (56,448 ± 24,816 μg × h/mL SD) and once every 21 days (50,400 ± 25,488 μg × h/mL SD), respectively, but within one SD of the adult drug exposure. The calculation of the terminal half-life in our study was limited by the duration of the sampling interval to 168 hours after the first dose. This may have contributed to the shorter mean half-life observed in children in this trial, which was shorter (7.3 days) compared with those in adult studies (13.710 and 16.4 days9).
Although it was not the primary aim of this phase I trial, clinical activity was observed in our study. The most striking activity was in a teenager with progressive, unresectable chest wall/lung osteosarcoma who received 2 years of lexatumumab with resolution of symptoms, ossification of her lesion, and loss of PET activity and has remained symptom free with stable imaging for more than 1 year after cessation of therapy. Furthermore, we saw clear evidence for a biomarker response in hepatoblastoma, but this was associated with progressive disease in the CNS. Previous confirmed partial responses in chondrosarcomas and prolonged stable disease in several sarcomas in adult trials suggest that solid tumors of mesenchymal origin may be responsive to TRAIL-targeting agents and should be targeted in future clinical trials of such agents.9,20,21
With the caveat that few biopsies in this study were from relapsed disease, and receptor expression may evolve during the evolution of cancer in an individual patient, TRAIL-R2 expression seemed to correlate with prolonged stable disease during therapy. Only one patient with rare TR2-positive staining in the tumor received more than three cycles, and all patients with tumors lacking TRAIL-R2 had rapid progression. TRAIL-mediated apoptosis in Ewing sarcoma and rhabdomyosarcoma cells has been shown to be dependent on caspase 8.2,22 In our study, caspase 8 also seemed to correlate with clinical activity. Agents that increase TRAIL-R2 and/or caspase 8 expression may improve the efficacy of TRAIL mimetics.
Preclinical studies have revealed that irradiation upregulates TRAIL-R2 on tumor cells without increasing the sensitivity of normal cells to TRAIL receptor agonists.6,23–25 There was some suggestion in our clinical trial that irradiation before or after TRAIL receptor agonist therapy may increase antitumor activity. Our findings support the future evaluation of combination trials with TRAIL agonists plus radiation therapy.
In conclusion, this study demonstrates that lexatumumab is well tolerated in heavily pretreated pediatric patients with solid tumors. TRAIL-R2 and caspase 8 are often expressed in pediatric solid tumors, whereas TRAIL-R1 is rarely expressed. Induction of apoptosis by agonists to TRAIL-R2 represents a novel targeting of tumors with intact death receptor pathways. A therapeutic window seems to be attainable, without significant off-target effects. On the basis of preclinical data and activity identified in this trial, the development of lexatumumab or other TRAIL-R2 agonists in combination with irradiation should be considered. Further study at both the bench and the bedside will be required to determine if concurrent treatment with irradiation and TRAIL-R2 is tolerable or if sequential treatment is best.
Acknowledgment
We thank the medical teams at the National Cancer Institute, Memorial Sloan-Kettering Cancer Center, and Cincinnati Children's Hospital Medical Center for their excellent clinical care. We also wish to acknowledge the work of the dedicated data managers and the rest of the clinical research team.
Appendix
Fig A1.
Pharmacokinetics of lexatumumab in children and adolescents.
Table A1.
Association of TR2 and Caspase 8 Expression With Duration of Therapy
| No. of Cycles | TR2 Positive, Caspase 8 Positive | TR2 Positive, Caspase 8 Negative | TR2 Negative, Caspase 8 Negative |
|---|---|---|---|
| ≤ Three | 2 | 0 | 4 |
| > Four | 2 | 1 | 0 |
| Total | 4 | 1 | 4 |
Abbreviation: TR2, tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptor 2.
Footnotes
See accompanying editorial on page 4059
Supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, and by the Clinical Research Training Program, National Institutes of Health (M.A., E.H.R.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00428272.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Melinda S. Merchant, Eunice H. Rhee, Crystal L. Mackall
Provision of study materials or patients: Melinda S. Merchant, James I. Geller, Kristin Baird, Leonard H. Wexler, Paul A. Meyers, Brigitte C. Widemann, Crystal L. Mackall
Collection and assembly of data: Melinda S. Merchant, James I. Geller, Kristin Baird, Alexander J. Chou, Ava Charles, Anita Price, Leonard H. Wexler, Paul A. Meyers, Brigitte C. Widemann, Maria Tsokos, Crystal L. Mackall
Data analysis and interpretation: Melinda S. Merchant, James I. Geller, Kristin Baird, Susana Galli, Martha Amaoko, Leonard H. Wexler, Paul A. Meyers, Brigitte C. Widemann, Maria Tsokos, Crystal L. Mackall
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Cretney E, Takeda K, Yagita H, et al. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 2.Kontny HU, Hämmerle K, Klein R, et al. Sensitivity of Ewing's sarcoma to TRAIL-induced apoptosis. Cell Death Differ. 2001;8:506–514. doi: 10.1038/sj.cdd.4400836. [DOI] [PubMed] [Google Scholar]
- 3.Petak I, Douglas L, Tillman DM, et al. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. 2000;6:4119–4127. [PubMed] [Google Scholar]
- 4.Evdokiou A, Bouralexis S, Atkins GJ, et al. Chemotherapeutic agents sensitize osteogenic sarcoma cells, but not normal human bone cells, to Apo2L/TRAIL-induced apoptosis. Int J Cancer. 2002;99:491–504. doi: 10.1002/ijc.10376. [DOI] [PubMed] [Google Scholar]
- 5.Zinonos I, Labrinidis A, Lee M, et al. Apomab, a fully human agonistic antibody to DR5, exhibits potent antitumor activity against primary and metastatic breast cancer. Mol Cancer Ther. 2009;8:2969–2980. doi: 10.1158/1535-7163.MCT-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belka C, Schmid B, Marini P, et al. Sensitization of resistant lymphoma cells to irradiation-induced apoptosis by the death ligand TRAIL. Oncogene. 2001;20:2190–2196. doi: 10.1038/sj.onc.1204318. [DOI] [PubMed] [Google Scholar]
- 7.Clodi K, Wimmer D, Li Y, et al. Expression of tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptors and sensitivity to TRAIL-induced apoptosis in primary B-cell acute lymphoblastic leukaemia cells. Br J Haematol. 2000;111:580–586. doi: 10.1046/j.1365-2141.2000.02404.x. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence D, Shahrokh Z, Marsters S, et al. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 9.Plummer R, Attard G, Pacey S, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 10.Wakelee HA, Patnaik A, Sikic BI, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchant MS, Yang X, Melchionda F, et al. Interferon gamma enhances the effectiveness of tumor necrosis factor-related apoptosis-inducing ligand receptor agonists in a xenograft model of Ewing's sarcoma. Cancer Res. 2004;64:8349–8356. doi: 10.1158/0008-5472.CAN-04-1705. [DOI] [PubMed] [Google Scholar]
- 12.Fulda S. Exploiting apoptosis pathways for the treatment of pediatric cancers. Pediatr Blood Cancer. 2009;53:533–536. doi: 10.1002/pbc.21922. [DOI] [PubMed] [Google Scholar]
- 13.Van Valen F, Fulda S, Truckenbrod B, et al. Apoptotic responsiveness of the Ewing's sarcoma family of tumours to tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) Int J Cancer. 2000;88:252–259. doi: 10.1002/1097-0215(20001015)88:2<252::aid-ijc17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiades N, Poulaki V, Mitsiades C, et al. Ewing's sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res. 2001;61:2704–2712. [PubMed] [Google Scholar]
- 15.Kang Z, Chen JJ, Yu Y, et al. Drozitumab, a human antibody to death receptor 5, has potent antitumor activity against rhabdomyosarcoma with the expression of caspase-8 predictive of response. Clin Cancer Res. 2011;17:3181–3192. doi: 10.1158/1078-0432.CCR-10-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picarda G, Trichet V, Téletchéa S, et al. TRAIL receptor signaling and therapeutic option in bone tumors: The trap of the bone microenvironment. Am J Cancer Res. 2012;2:45–64. [PMC free article] [PubMed] [Google Scholar]
- 17.Kurita S, Mott JL, Cazanave SC, et al. Hedgehog inhibition promotes a switch from type II to type I cell death receptor signaling in cancer cells. PLoS One. 2011;6:e18330. doi: 10.1371/journal.pone.0018330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Valen F, Fulda S, Schäfer KL, et al. Selective and nonselective toxicity of TRAIL/Apo2L combined with chemotherapy in human bone tumour cells vs. normal human cells. Int J Cancer. 2003;107:929–940. doi: 10.1002/ijc.11503. [DOI] [PubMed] [Google Scholar]
- 19. Reference deleted.
- 20.Camidge DR, Herbst RS, Gordon MS, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res. 2010;16:1256–1263. doi: 10.1158/1078-0432.CCR-09-1267. [DOI] [PubMed] [Google Scholar]
- 21.Herbst RS, Eckhardt SG, Kurzrock R, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28:2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Merchant MS, Romero ME, et al. Induction of caspase 8 by interferon gamma renders some neuroblastoma (NB) cells sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but reveals that a lack of membrane TR1/TR2 also contributes to TRAIL resistance in NB. Cancer Res. 2003;63:1122–1129. [PubMed] [Google Scholar]
- 23.Marini P. Drug evaluation: Lexatumumab, an intravenous human agonistic mAb targeting TRAIL receptor 2. Curr Opin Mol Ther. 2006;8:539–546. [PubMed] [Google Scholar]
- 24.Marini P, Denzinger S, Schiller D, et al. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: Enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene. 2006;25:5145–5154. doi: 10.1038/sj.onc.1209516. [DOI] [PubMed] [Google Scholar]
- 25.Marini P, Junginger D, Stickl S, et al. Combined treatment with lexatumumab and irradiation leads to strongly increased long term tumour control under normoxic and hypoxic conditions. Radiat Oncol. 2009;4:49. doi: 10.1186/1748-717X-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]