Abstract
Background
Retrocyclins are cyclic antimicrobial peptides that have been shown to be both broadly active and safe in animal models. RC-101, a synthetic retrocyclin, targets important human pathogens and is a candidate vaginal microbicide. Its activity against microbes associated with bacterial vaginosis is unknown.
Methods
We investigated the effect of RC-101 on toxin activity, bacterial growth and biofilm formation of Gardnerella vaginalis in vitro.
Results
RC-101 potently inhibits the cytolytic activity of vaginolysin, the Gardnerella vaginalis toxin, on both erythrocytes and nucleated cells. RC-101 lacks inhibitory activity against planktonic G. vaginalis but markedly decreases biofilm formation.
Conclusions
These dual properties, toxin inhibition and biofilm retardation, justify further exploration of RC-101 as a candidate agent for bacterial vaginosis prevention.
Keywords: defensin, vaginolysin, bacterial vaginosis, biofilm
Introduction
Bacterial vaginosis (BV) is a highly prevalent vaginal dysbiosis that has been linked to adverse pregnancy outcomes and enhanced transmission of sexually transmitted infections (STIs). The precise pathogenesis of BV remains unclear. However, loss of vaginal Lactobacillus and overgrowth of Gardnerella vaginalis are thought to be key elements of the disease process. G. vaginalis forms biofilms at the vaginal mucosal surface in vivo, and these appear to contribute to persistence and the frequent failure of antimicrobial therapy for BV.1,2 G. vaginalis biofilms may also be found in male partners of women with BV and may represent a transmissible state for this organism.3 In addition, G. vaginalis produces vaginolysin (VLY), a human-specific cholesterol-dependent cytolysin that lyses epithelial cells and erythrocytes and is thought to play an important role in BV pathogenesis.4 VLY is inactive at normal vaginal pH (<4.5) but fully active at the higher pH levels noted in the BV milieu.5
Defensins are antimicrobial peptides that have important roles in immune defence and in shaping microbial ecology at mucosal surfaces.6 Θ-Defensins, also known as retrocyclins, are 18-residue circular peptides related to the α- and β-defensins. Retrocyclins have broad antibacterial and antiviral activity, but the human retrocyclin gene does not produce a functional product owing to a premature stop codon.7 Based on several studies showing that retrocyclins prevent HIV and herpes simplex virus (HSV) infection, efforts are now under way to develop a topical intravaginal preparation of synthetic retrocyclin RC-101 for STI prophylaxis.8 RC-101 has been shown to lack significant effects on lactobacilli that normally colonize the vaginal tract and to be non-toxic to human cells. We hypothesized that RC-101 might have antibacterial and antitoxin effects against G. vaginalis and VLY that could make it useful for BV prophylaxis.
Materials and methods
Bacterial strains and growth conditions
Gardnerella vaginalis strain 49145 was purchased from ATCC and was grown on solid human blood-bilayer Tween agar (BD Biosciences) prior to growth in broth medium. For planktonic growth, G. vaginalis was grown in brain–heart infusion broth (BHI) supplemented with 10% fetal bovine serum, 5% Fildes enrichment and 1 mg/L amphotericin B in 96-well plates for the indicated times at 37°C with 5% CO2. For biofilm assays, bacteria were grown in BHI supplemented with 0.3% starch, 0.3% glucose and 1 mg/L amphotericin B at 37°C with 5% CO2.
Reagents
RC-101 (a generous gift from Robert I. Lehrer University of California Los Angeles, CA, USA) was produced using solid-phase synthesis as described and was resuspended in sterile, deionized water.9 We used purified, recombinant VLY, produced as described.5
Cytotoxicity assays
Both haemolysis and HeLa cell lysis experiments were performed as described.5 RC-101 or vehicle control was added at the concentrations indicated. The use of human erythrocytes was approved by the Columbia University Institutional Review Board.
Biofilm assays
Biofilms were allowed to form in 24-well plates with RC-101 or vehicle control added either at the beginning of the experiment or after 24 h of growth, as indicated, with incubation for an additional 24 h. At the end of the experiment, biofilms were washed, air dried, stained with safranin and quantified as described.1 This strategy allowed us to test the effect of RC-101 on either newly forming or pre-formed G. vaginalis biofilms. Each experiment was performed in triplicate and repeated at least three times; representative results from one experiment are shown. Statistical analysis was by analysis of variance (ANOVA) with Tukey post-test as appropriate (GraphPad Prism).
Results
Retrocyclin inhibits of VLY-mediated cytolysis
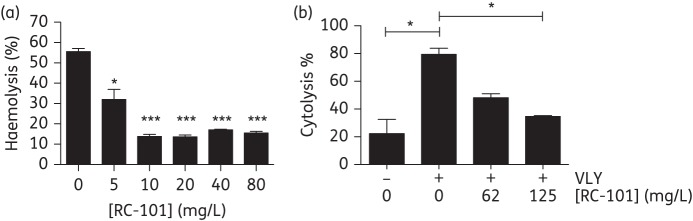
Using VLY at a concentration (20 ng/mL) approximating the 50% haemolytic dose (HD50) combined with either vehicle control or a range of concentrations of purified RC-101, we demonstrated potent inhibition of VLY-mediated lysis of primary human erythrocytes (Figure 1a). The 50% inhibitory concentration (IC50) of RC-101 in the haemolytic assay was between 5 and 10 mg/L. VLY also causes cytolysis of epithelial cells.4 Using HeLa cells as a model system and lactate dehydrogenase release as an assay of disrupted membrane integrity, we showed that RC-101 inhibited epithelial cell destruction caused by VLY (1 μg/mL) with an IC50 between 62 and 125 mg/L (Figure 1b). Of note, at low concentrations of VLY and high concentrations of RC-101 (250 ng/mL and 125 mg/L, respectively), there was no detectable cytotoxic effect of RC-101 on HeLa cells (data not shown). Together, these two experiments suggest an antitoxin effect of RC-101 against VLY, similar to the reported interaction between α-defensins and cholesterol-dependent cytolysins.10
Figure 1.
RC-101 inhibits VLY-mediated cytolysis. (a) VLY (20 ng/mL) and indicated concentrations of RC-101 or vehicle control were added to a 1% solution of human erythrocytes, and haemolysis was measured by haemoglobin release assay. (b) VLY (1 μg/mL) and indicated concentrations of RC-101 or vehicle control were added to HeLa cells and lysis assessed by lactate dehydrogenase release. Percentage lysis is reported relative to cells exposed to 0.05% Triton X-100 (representing 100% lysis). Bars represent standard errors. *P < 0.05; ***P < 0.001.
Retrocyclin inhibits G. vaginalis biofilm formation but not planktonic growth
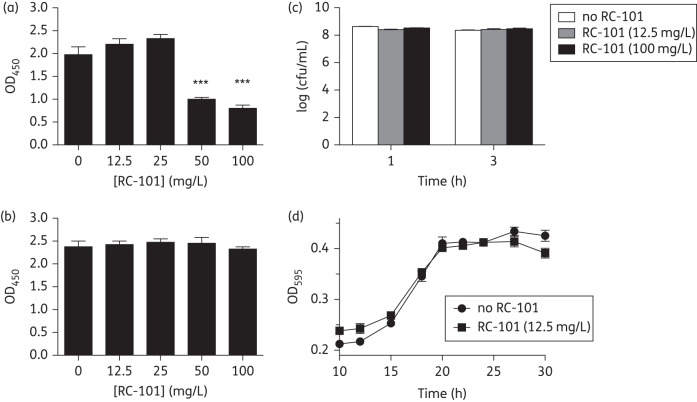
Because of the importance of biofilms to BV pathogenesis, we investigated potential anti-biofilm effects of RC-101. RC-101 inhibited the formation of new Gardnerella biofilms in a dose-dependent manner (Figure 2a). However, the addition of RC-101 after 24 h of bacterial growth did not lead to disruption of pre-existing biofilms (Figure 2b). It was not clear from these results whether this differential effect of RC-101 was due to direct inhibition of G. vaginalis growth. Addition of RC-101 or vehicle control to planktonic G. vaginalis (∼108 cfu/mL) did not decrease recoverable colony-forming units at 1 or 3 h post addition (Figure 2c). No significant effects were observed of RC-101 on G. vaginalis growth over a >18 h period, as assessed in a 96-well plate assay (Figure 2d).
Figure 2.
RC-101 inhibits G. vaginalis de novo biofilm formation but does not break down established biofilms or inhibit planktonic growth. (a) Bacteria were grown for 24 h in the presence of vehicle control or RC-101 at the indicated concentrations and biofilm formation determined by safranin staining, solubilization and measurement of OD450. (b) G. vaginalis biofilms were grown for 24 h without treatment and then exposed to vehicle control or RC-101 at the indicated concentrations for an additional 24 h prior to staining. (c) G. vaginalis was exposed to RC-101 or vehicle control for 1 or 3 h and bacterial concentration determined by quantitative culture. (d) G. vaginalis growth was measured by monitoring OD590 in the presence of RC-101 (final concentration 12.5 mg/L) or vehicle control. *P < 0.05; ***P < 0.001.
Discussion
We have demonstrated that RC-101 inhibits both the activity of VLY, the protein toxin produced by G. vaginalis, and the de novo formation of biofilms by this organism. While the exact role of G. vaginalis and its toxin in BV pathogenesis is uncertain, the organism is a major component of the altered microbiota, and VLY may contribute to local immunodysregulation. One limitation of our approach is that we examined only a single strain of G. vaginalis. There is genomic variability among strains, and there could be differences with respect to toxin production and biofilm formation. VLY inhibition has been proposed as a potential tool for BV prevention and/or treatment. We believe that RC-101, already under development as topical STI prophylaxis, may be useful in this respect.
Our studies did not reveal a direct bactericidal or bacteriostatic effect of RC-101 against G. vaginalis. However, RC-101 did inhibit formation of G. vaginalis biofilms. The mechanism of this inhibition remains unclear. It may result from adherence of the cationic RC-101 peptide to the bacterial surface, preventing bacterial self-association necessary for biofilm formation. Alternatively, G. vaginalis biofilm formation may depend on VLY or another secreted substance whose function is impeded by RC-101. The main weakness of our current study is the absence of in vivo data. Currently, there is no reliable animal model of BV. In prior studies, exposure of RC-101 to human vaginal fluid samples taken from women with BV caused a marked reduction in activity through an unknown mechanism.8 This finding represents a potential challenge to development of RC-101 for use in the setting of BV and requires both replication and a more detailed mechanistic understanding. However, our findings provide justification for further exploration of the potential utility of RC-101, or perhaps related molecules with enhanced stability in the vaginal milieu, for BV prevention.
Funding
This work was supported by the National Institutes of Health [R01 AI092743, R21 AI098654 to A. J. R.; T32 AI007531 to Lisa Saiman provided support for S. R. H.; K23 HD065844 to T. M. R.]. T. A. H. received support from an American Academy of Pediatrics Resident Research Grant.
Transparency declarations
All authors: none to declare.
Acknowledgements
We are grateful to Robert I. Lehrer for the gift of RC-101 and for productive discussions.
References
- 1.Patterson JL, Girerd PH, Karjane NW, et al. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am J Obstet Gynecol. 2007;197:170 e1–7. doi: 10.1016/j.ajog.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198:97 e1–6. doi: 10.1016/j.ajog.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 3.Swidsinski A, Doerffel Y, Loening-Baucke V, et al. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol Obstet Invest. 2010;70:256–63. doi: 10.1159/000314015. [DOI] [PubMed] [Google Scholar]
- 4.Gelber SE, Aguilar JL, Lewis KL, et al. Functional and phylogenetic characterization of vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol. 2008;190:3896–903. doi: 10.1128/JB.01965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rampersaud R, Planet PJ, Randis TM, et al. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J Bacteriol. 2011;193:1034–41. doi: 10.1128/JB.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehrer RI, Lu W. α-Defensins in human innate immunity. Immun Rev. 2012;245:84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 7.Venkataraman N, Cole AL, Ruchala P, et al. Reawakening retrocyclins: ancestral human defensins active against HIV-1. PLoS Biol. 2009;7:e95. doi: 10.1371/journal.pbio.1000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sassi AB, Bunge KE, Hood BL, et al. Preformulation and stability in biological fluids of the retrocyclin RC-101, a potential anti-HIV topical microbicide. AIDS Res Ther. 2011;8:27. doi: 10.1186/1742-6405-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole AM, Hong T, Boo LM, et al. Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc Natl Acad Sci USA. 2002;99:1813–8. doi: 10.1073/pnas.052706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehrer RI, Jung G, Ruchala P, et al. Human α-defensins inhibit hemolysis mediated by cholesterol-dependent cytolysins. Infect Immun. 2009;77:4028–40. doi: 10.1128/IAI.00232-09. [DOI] [PMC free article] [PubMed] [Google Scholar]