Abstract
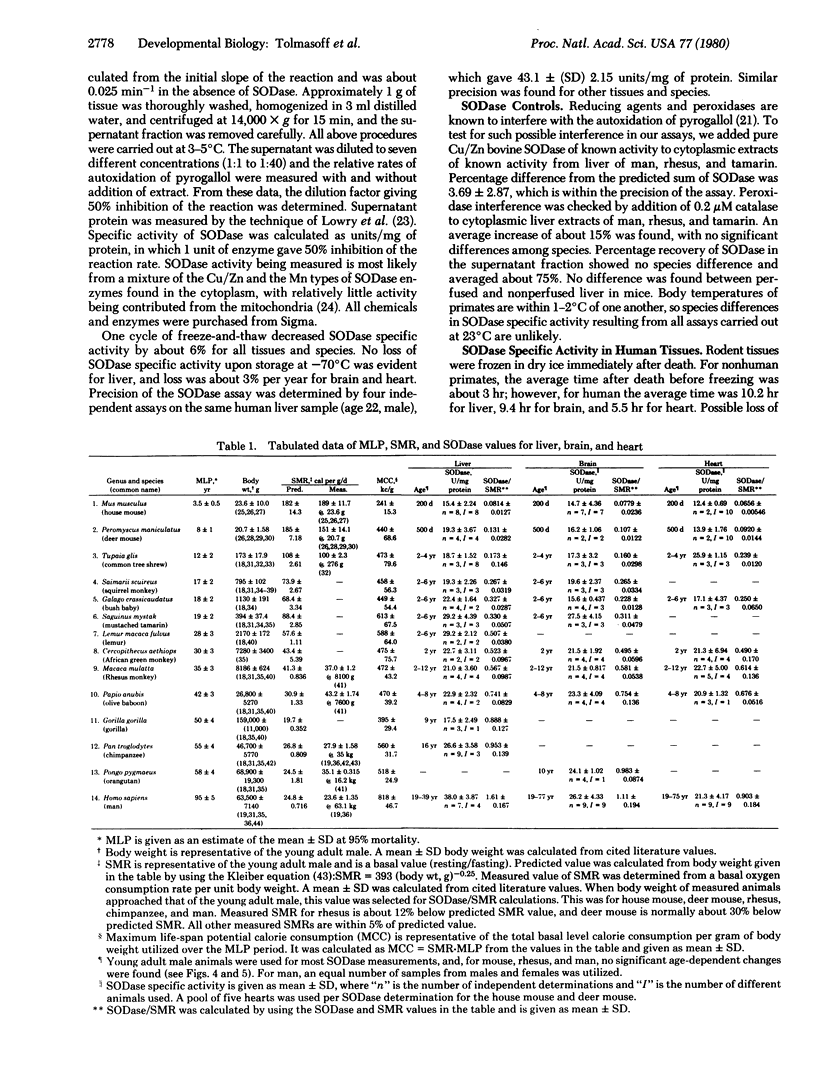
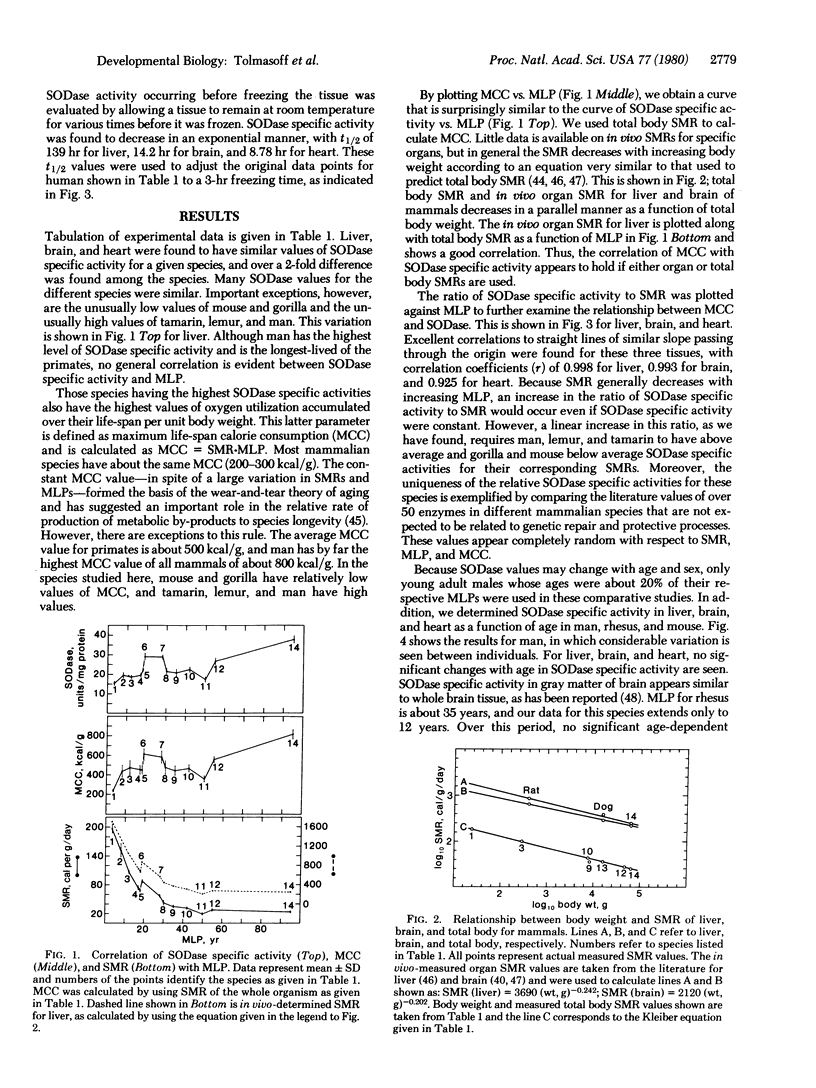
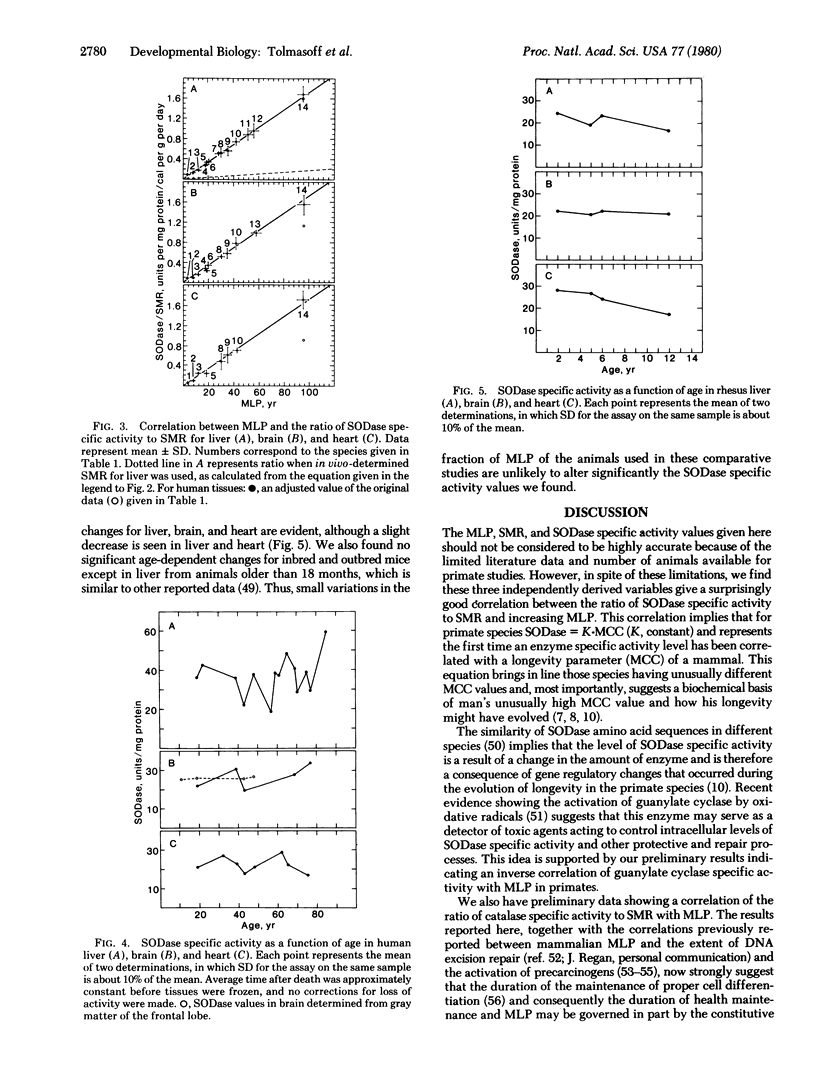
Much evidence now suggests that superoxide dismutase (superoxide:superoxide oxidoreductase, EC 1.15.1.1) may be a major intracellular protective enzyme against oxygen toxicity by catalyzing the removal of the superoxide radical. We examined the possible role this enzyme may have in determining the life-span of primate species. Superoxide dismutase specific activity levels were measured in cytoplasmic fractions of liver, brain, and heart of 2 rodent and 12 primate species. These species had maximum life-span potentials ranging from 3.5 to 95 years. Liver, brain, and heart had similar specific activity levels for a given species, but the levels for different species varied over 2-fold, with man having the highest level. No general correlation was found in the levels with life-span. However, the ratio of superoxide dismutase specific activity to specific metabolic rate of the tissue or of the whole adult organism was found to increase with increasing maximum lifespan potential for all the species. This correlation suggests that longer-lived species have a higher degree of protection against by-products of oxygen metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUER R. W. Liver circulation and function. Physiol Rev. 1963 Jan;43:115–213. doi: 10.1152/physrev.1963.43.1.115. [DOI] [PubMed] [Google Scholar]
- Cutler R. G. Evolution of human longevity and the genetic complexity governing aging rate. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4664–4668. doi: 10.1073/pnas.72.11.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. G. Evolution of human longevity: a critical overview. Mech Ageing Dev. 1979 Feb;9(3-4):337–354. doi: 10.1016/0047-6374(79)90110-6. [DOI] [PubMed] [Google Scholar]
- DAVIES M. On body size and tissue respiration. J Cell Comp Physiol. 1961 Jun;57:135–147. doi: 10.1002/jcp.1030570302. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Hart R. W., Setlow R. B. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2169–2173. doi: 10.1073/pnas.71.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday M. A., Potter D., Jarrah A., Bearg S. The relation of metabolic rate to body weight and organ size. Pediatr Res. 1967 May;1(3):185–195. doi: 10.1203/00006450-196705000-00005. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide dismutase in the rat and mouse as a function of age and longevity. J Gerontol. 1976 Jul;31(4):405–408. doi: 10.1093/geronj/31.4.405. [DOI] [PubMed] [Google Scholar]
- King M. C., Wilson A. C. Evolution at two levels in humans and chimpanzees. Science. 1975 Apr 11;188(4184):107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loomis T. C., Yee G., Stahl W. L. Regional and subcellular distribution of superoxide dismutase in brain. Experientia. 1976 Nov 15;32(11):1374–1376. doi: 10.1007/BF01937382. [DOI] [PubMed] [Google Scholar]
- Malinow M. R., Wagner R. Oxygen uptake in squirrel monkeys (Saimiri sciurea). Lab Anim Care. 1966 Apr;16(2):105–108. [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Middleton C. C., Rosal J. Weights and measurements of normal squirrel monkeys (Saimiri sciureus). Lab Anim Sci. 1972 Aug;22(4):583–586. [PubMed] [Google Scholar]
- Milton K., May M. L. Body weight, diet and home range area in primates. Nature. 1976 Feb 12;259(5543):459–462. doi: 10.1038/259459a0. [DOI] [PubMed] [Google Scholar]
- Mittal C. K., Murad F. Properties and oxidative regulation of guanylate cyclase. J Cyclic Nucleotide Res. 1977 Dec;3(6):381–391. [PubMed] [Google Scholar]
- Moore C. J., Schwartz A. G. Inverse correlation between species lifespan and capacity of cultured fibroblasts to convert benzo(a)pyrene to water-soluble metabolites. Exp Cell Res. 1978 Oct 15;116(2):359–364. doi: 10.1016/0014-4827(78)90459-7. [DOI] [PubMed] [Google Scholar]
- NELSON L. E., ASLING C. W. Metabolic rate of tree-shrews (Urogale everetti). Proc Soc Exp Biol Med. 1962 Mar;109:602–604. doi: 10.3181/00379727-109-27281. [DOI] [PubMed] [Google Scholar]
- Ono T., Cutler R. G. Age-dependent relaxation of gene repression: increase of endogenous murine leukemia virus-related and globin-related RNA in brain and liver of mice. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4431–4435. doi: 10.1073/pnas.75.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. G. Correlation between species life span and capacity to activate 7,12-dimethylbenz(a)anthracene to a form mutagenic to a mammalian cell. Exp Cell Res. 1975 Sep;94(2):445–447. doi: 10.1016/0014-4827(75)90514-5. [DOI] [PubMed] [Google Scholar]
- Schwartz A. G., Moore C. J. Inverse correlation between species life span and capacity of cultured fibroblasts to bind 7,12-dimethylbenz(a)anthracene to DNA. Exp Cell Res. 1977 Oct 15;109(2):448–450. doi: 10.1016/0014-4827(77)90026-x. [DOI] [PubMed] [Google Scholar]
- Spikes J. D., Swartz H. M. International conference on singlet oxygen and related species in chemistry and biology: review and general discussion. Photochem Photobiol. 1978 Oct-Nov;28(4-5):921–933. doi: 10.1111/j.1751-1097.1978.tb07041.x. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Yousef M. K., Chaffee R. R., Johnson H. D. Oxygen consumption of tree shrews: effects of ambient temperatures. Comp Biochem Physiol A Comp Physiol. 1971 Mar 1;38(3):709–712. doi: 10.1016/0300-9629(71)90138-1. [DOI] [PubMed] [Google Scholar]



