Abstract
Inflammatory thermal hyperalgesia is principally mediated through transient receptor potential vanilloid 1 (TRPV1) channels, as demonstrated by prior studies using models of cutaneous inflammation. Muscle pain is significantly different from cutaneous pain, and the involvement of TRPV1 in hyperalgesia induced by muscle inflammation is unknown. We tested whether TRPV1 contributes to the development of mechanical and heat hypersensitivity of the paw in TRPV1−/− mice after muscle inflammation. Because TRPV1−/− mice lack TRPV1 at the site of inflammation (muscle) and at the testing site (paw), we do not know whether TRPV1 is important as a mediator of nociceptor sensitization in the muscle or as a heat sensor in the paw. Using recombinant herpesviruses, we reexpressed TRPV1 in TRPV1−/− mice in primary afferents innervating skin, muscle, or both to determine which sites were important for the behavioral deficits. Responses to repeated application of noxious mechanical stimuli to the hind paw were enhanced in TRPV1−/− mice; this was restored by reexpression of TRPV1 into skin. Withdrawal latencies to noxious heat were increased in TRPV1−/− mice; normal latencies were restored by reexpression of TRPV1 in both skin and muscle. Heat hypersensitivity induced by muscle inflammation did not develop in TRPV1−/− mice; mechanical hypersensitivity was similar between TRPV1−/− and TRPV1+/+ mice. Heat hypersensitivity induced by muscle inflammation was restored by reexpression of TRPV1 into both muscle and skin of TRPV1−/− mice. These results suggest that TRPV1 serves as both a mediator of nociceptor sensitization at the site of inflammation and as a heat sensor at the paw.
Keywords: Mechanical hypersensitivity, Muscle inflammation, Muscle pain, Thermal hyperalgesia
1. Introduction
The transient receptor potential vanilloid 1 (TRPV1) channel, a member of the TRP family of ion channels, is a polymodal receptor expressed on sensory neurons. TRPV1 channels are activated by a variety of noxious physicochemical stimuli that lead to inflammatory thermal hyperalgesia [6,14]. TRPV1 is predominantly expressed in a subset of sensory neurons that send sensory afferents to innervate skin, muscle, joint and viscera [10,28,30,35, 39,43]. TRPV1 is directly activated by temperatures greater than 43 °C, acidic pH less than 6.0, and a variety of endogenous lipid metabolic products. Further, inflammatory mediators such as prostaglandins and bradykinin potentiate TRPV1 mainly through phosphorylation-dependent upregulation of channel function [8,11,25, 44,52]. Such potentiation decreases the TRPV1 channel’s temperature-activation threshold, decreases channel desensitization, and increases cell surface expression of the channel protein [1,2,32, 34,44,52]. Further, after tissue injury and inflammation there is increased TRPV1 protein expression in sensory neurons [27,50]. Overall, TRPV1 serves as a key peripheral sensor of heat and acidic pH under normal physiological conditions.
The most compelling evidence in support of the role of TRPV1 in the development of inflammatory thermal hyperalgesia are deficits in inflammatory thermal hyperalgesia and heat sensitivity of TRPV1−/− mice [3,6,14]. However, these mice seem to have normal mechanical sensitivity and mechanical hypersensitivity induced by inflammation [3,6,14]. In contrast, systemic or intrathecal administration of TRPV1 antagonists in normal animals results in a reversal of both heat and mechanical hyperalgesia [12,50]. Prior studies in TRPV1−/− mice and studies using TRPV1 antagonists in wild-type animals have all used cutaneous paw inflammation and measured hyperalgesia at the site of inflammation, ie, primary hyperalgesia. Cutaneous pain and muscle pain utilize different mechanisms [15]. Furthermore, primary hyperalgesia and secondary hyperalgesia are thought to have distinct underlying mechanisms.
We hypothesized that TRPV1−/− mice would develop similar mechanical hyperalgesia, but not heat hyperalgesia, after muscle inflammation when compared to TRPV1+/+ mice. We further hypothesized that the loss of heat hyperalgesia was a result of the loss of TRPV1 in the afferent fibers innervating the skin where the testing occurred. In this study, we utilized a mouse muscle inflammation model to examine secondary hyperalgesia in TRPV1−/− mice by measuring mechanical and heat sensitivities of the paw. We reexpressed TRPV1 in TRPV1−/− mice in the skin, muscle, or both simultaneously, then examined the resultant effects on the hypersensitivity in uninjured animals and development of thermal hypersensitivity after muscle inflammation.
2. Methods
2.1. Mice
TRPV1+/+ and congenic TRPV1−/− mice were obtained from Jackson Laboratories and were bred at the University of Iowa. All the experiments involving mice were performed in accordance with the animal care and use protocol approved by the University of Iowa Institutional Animal Care and Use Committee.
2.2. Behavioral assessments
All behavior experiments were performed with the tester blinded to group, ie, genotype or virus injection. Importantly, TRPV1+/+ and TRPV1−/− mice were tested simultaneously over multiple days. Similarly, those injected with virus into a particular tissue type (ie, muscle) were always tested simultaneously with those injected with the control virus and several sets of animals were tested over multiple days. This ensured that we always tested control and experimental animals on the same days, and that control and experimental animals were tested in multiple litters.
2.2.1. Mechanical sensitivity
Mechanical sensitivity was tested by measuring the threshold to withdrawal to a series of von Frey filaments (0.07, 0.2, 0.4, 0.7, 1.6, 3.92, 5.88, 9.8 mN) applied to the paw. The lowest force that produced a withdrawal was recorded as the withdrawal threshold. We also tested the responsiveness of mice to repeated application of 3 different von Frey filaments (0.4, 0.7, 1.6 mN) [49]. Von Frey filaments were applied to the paw once every second, 10 times. For baseline sensitivity before inflammation, the number of withdrawals out of 10 trials was measured twice and averaged. The 0.4, 0.7, and 1.6 mN forces were chosen because they all produced a withdrawal response to the stimulus; lower forces did not routinely result in withdrawal responses. Thus, we interpret these forces as a mildly noxious stimulus.
For responses before and after inflammation, the sensitivity to mechanical stimulation was assessed using a single von Frey filament (0.4 mN) and was tested in separate groups of animals (TRPV1−/− n = 15, TRPV1+/+ n = 15) as previously described [40]. The 0.4 mN filament was applied 5 times, and 10 trials were averaged. We routinely use the 0.4 mN force to test mechanical hypersensitivity in mice after tissue insult because normal animals show a low number of responses (1 or less out of 5) and increases are clearly observable [40].
2.2.2. Heat sensitivity
Heat sensitivity was tested by measuring withdrawal latency of the paw to radiant heat with 3 different intensities of stimulation based on the voltage output of the stimulator (115 V, 125 V, 135 V). This resulted in baseline latencies in TRPV1+/+ mice of 15.2 ± 0.63 s, 10.3 ± 0.55 s, and 8.6 ± 0.31 s for the 115 V, 125 V, and 135 V of stimulation. Thus, the rate of temperature increase was different for each voltage so that higher voltages had a faster rate of increase and resulted in faster withdrawal thresholds. Three trials per intensity were tested and averaged. Prior work by Yeomans and colleagues showed longer thermal latencies activate C-fiber nociceptors and shorter latencies activate Aδ nociceptors [48].
For responses before and after inflammation, the sensitivity to heat stimuli was assessed with 125 V of stimulation in TRPV1−/− (n = 15) and TRPV1+/+ mice (n = 15). Three trials were tested and averaged. We routinely use this voltage to test responses after tissue injury [48].
2.3. Induction of inflammation
Inflammation was induced by injecting 20 μL of 3% carrageenan into the left gastrocnemius muscle of mice anesthetized with 4% isoflurane.
2.4. Virus construction
Recombinant herpesviruses (HSV-1) were constructed as previously described [51]. NPG is the control virus (HSV-GFP), and NPG-TRPV1 (HSV-GFP-TRPV1) is similar to NPG and contains the cDNA for rat TRPV1 inserted between the herpes UL36 and UL37 genes, downstream of the human cytomegalovirus immediate–early enhancer promoter. Expression of enhanced green fluorescent protein (eGFP) is also driven by the human cytomegalovirus promoter in both HSV-GFP and HSV-GFP-TRPV1 viruses. These vectors yield replication-conditional viruses, which do not replicate in nondividing cells because they do not express ICP434.5 or thymidine kinase. The viruses were used at a titer of 107 plaque-forming units/μL.
2.5. Injection of virus
HSV-GFP or HSV-GFP-TRPV1 viruses were injected into the skin of the hind paw, the gastrocnemius muscle, or both sites while the mice were anesthetized with 2–4% isoflurane. All injections were made with a Hamilton syringe with a 30-gauge needle attached at flow rate of 5 μL per minute. For skin, two 10 μL injections of recombinant viruses were injected intradermally, one into the rostral and the other into the caudal portion of the paw. For muscle, the skin overlying the gastrocnemius muscle was incised, and two 10 μL injections of viruses were injected 2 min apart. After injection, saline-soaked sterile gauze was placed over the muscle for 10 min to minimize leakage of the virus into the overlying skin. The skin was then sutured closed with 5-0 silk. Behavioral testing began 4 weeks after injection with HSV-1.
2.6. Real-time PCR
To confirm expression of TRPV1, we performed real-time PCR on L4–L6 dorsal root ganglion (DRG) from TRPV1−/− mice injected with virus into the skin, muscle, or both, as well as DRGs from TRPV1+/+ mice. RNA was purified from ipsilateral and contralateral L4–L6 DRGs with Trizol reagent (Invitrogen, Carlsbad, CA). RNA concentration and purity were assessed by spectrophotometric measurement at 260 and 280 nm. First strand cDNA was synthesized from 0.2–1 μg of each RNA sample using Superscript III or VILO reverse transcriptase (Invitrogen, Carlsbad, CA). Taqman PCR was carried out using an ABI prism 7900HT sequence detector (Applied Biosystems, Inc., Foster City, CA) on diluted cDNA samples (University of Iowa, DNA Facility, Iowa City, IA). Reactions were carried out for 40 cycles in triplicate. Rat TRPV1 (Rn01460299_m1) and the mouse control assay for glyceraldehyde-3-P-dehydrogenase (GAPDH) were obtained from Applied Biosystems (Foster City, CA). Quantitative RT-PCR data were normalized with GAPDH mRNA levels.
2.7. Electrophysiological recordings on cultured DRG neurons
To confirm the functional expression of TRPV1 in DRG neurons, we recorded capsaicin-induced currents in L4–L6 DRG from uninjected mice as well in as mice injected with HSV-GFP or HSV-GFP-TRPV1 virus into the hind paw skin or gastrocnemius muscle in both TRPV1−/− and TRPV1+/+ mice. Two weeks after virus injections, L4–L6 DRGs from each mouse were dissected, dissociated, and cultured (separately for each injection and genotype) on poly-L-ornithine/laminin-coated glass coverslips, as previously described [18]. Cultured neurons were used 48 h after plating for whole-cell voltage-clamp recordings.
Capsaicin (100 nM, 5 s) induced inward currents were recorded from cultured DRG neurons (both small/medium-and large-diameter neurons) with whole-cell voltage-clamp technique, using an Axopatch-200B amplifier connected to a Digidata 1440A data acquisition system and controlled with the pClamp10 software (Molecular Devices, Sunnyvale, CA). Holding potential was −70 mV. Current recordings were sampled at 2 kHz and filtered at 1 kHz with a low-pass Bessel filter. Patch pipettes were pulled from borosilicate glass tubes (World Precision Instruments, Sarasota, FL) and heat polished at the tip to give a resistance of 3–6 MΩ when filled with the intracellular solution. Currents were recorded with an extracellular solution containing (in mM) 140 NaCl, 5 KCl, 0.1 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, and adjusted to pH 7.3 with NaOH; pipettes were filled with intracellular solution containing (in mM) 5 NaCl, 140 KCl, 1 MgCl2, 10 EGTA, 3 MgATP, 0.3 NaGTP, and 10 HEPES with the pH adjusted to 7.3 with KOH. Capsaicin was dissolved in DMSO, and the final working capsaicin solution was made by dilution with extracellular buffer. Electrophysiological data were analyzed and figures were prepared by Origin 7.0 software. Current density was calculated by dividing the peak capsaicin (100 nM, 5 s) current (pA) to the whole-cell capacitance (pF) of that particular neuron, as reported earlier [32]. The experimenter performing the voltage-clamp experiments was blinded for the mouse genotypes and the type and the site of virus injections.
3. Results
3.1. Baseline pain behaviors
Mechanical paw withdrawal thresholds were similar between TRPV1−/− and TRPV1+/+ mice (Fig. 1A, inset), as previously described [6,14]. However, responses to repeated stimulation with multiple von Frey filaments (0.4, 0.7, 1.6 mN) were significantly increased in TRPV1−/− mice when compared to TRPV1+/+ mice (Fig. 1A), suggesting an increased responsiveness to mechanical stimuli. Paw withdrawal latency to heat was significantly increased with higher intensities of stimulation but not with lower intensities of stimulation in TRPV1−/− mice when compared to TRPV1+/+ mice (Fig. 2A), suggesting a decreased responsiveness to heat stimuli at higher intensity.
Fig. 1.
Mechanical sensitivity of the hind paw in TRPV1+/+ and TRPV1−/− mice. Mechanical sensitivity of the paw was tested by measuring the number of responses to repeated stimulation with 0.4, 0.7 and 1.6 mN von Frey filaments and by measuring the paw withdrawal thresholds (insets). No changes in paw withdrawal thresholds were observed in any groups (insets). (A) The number of responses in TRPV1−/− (open symbols) and TRPV1+/+ (closed symbols) mice are shown. TRPV1−/− mice show a significantly enhanced responsiveness to all 3 forces *P < .05. (B) Injection of HSV-GFP-TRPV1 virus into the skin of TRPV1−/− mice (closed symbols) significantly restores the normal mechanical sensitivity to repeated mechanical stimulation. The responses in the TRPV1−/− injected with HSV-GFP-TRPV1 virus (closed symbols) is similar to that observed in TRPV1+/+ mice in A (closed symbols) and the responses observed in TRPV1−/− mice injected with HSV-GFP virus (control) (open symbols) is similar to TRPV1−/− mice in A (open symbols). *P < .05 significantly less than TRPV1−/− injected with HSV-GFP virus (open symbols). (C) Injection of HSV-GFP-TRPV1 virus into the gastrocnemius muscle had no effect on the mechanical sensitivity of TRPV1−/− mice and was similar to TRPV1−/− mice injected with control HSV-GFP virus. (D) Injection of HSV-GFP-TRPV1 into the hind paw skin and gastrocnemius muscle had no effect on the mechanical sensitivity of TRPV1−/− mice and was similar to control TRPV1−/− mice injected with control HSV-GFP virus. Data are presented as mean ± SEM.
Fig. 2.
Heat sensitivity of the hind paw in TRPV1+/+ and TRPV1−/− mice. Heat sensitivity of the paw was tested by measuring the withdrawal latency to radiant heat from 3 intensities of stimulation (115, 125, and 135 V). (A) Paw withdrawal latencies to heat were significantly increased in TRPV1−/− mice with higher intensities of stimulation (125 and 135 V) but not the lower intensity of stimulation (115 V). *P < .05 compared to TRPV1+/+ mice. (B) Injection of HSV-GFP-TRPV1 into the skin of TRPV1−/− mice had no effect on the paw withdrawal latency to heat when compared to control TRPV1−/− mice injected with HSV-GFP. (C) Injection of HSV-GFP-TRPV1 into the gastrocnemius muscle of TRPV1−/− mice had no effect on the paw withdrawal latency to heat when compared to control TRPV1−/− mice injected with HSV-GFP. (D) Injection of HSV-GFP-TRPV1 into the hind paw skin and gastrocnemius muscle in TRPV1−/− mice restored the normal heat sensitivity at 135 V. *P < .05 significant differences between TRPV1−/− mice injected with HSV-GFP-TRPV1 and HSV-GFP. Data are presented as mean ± SEM.
3.2. Pain responses after muscle inflammation
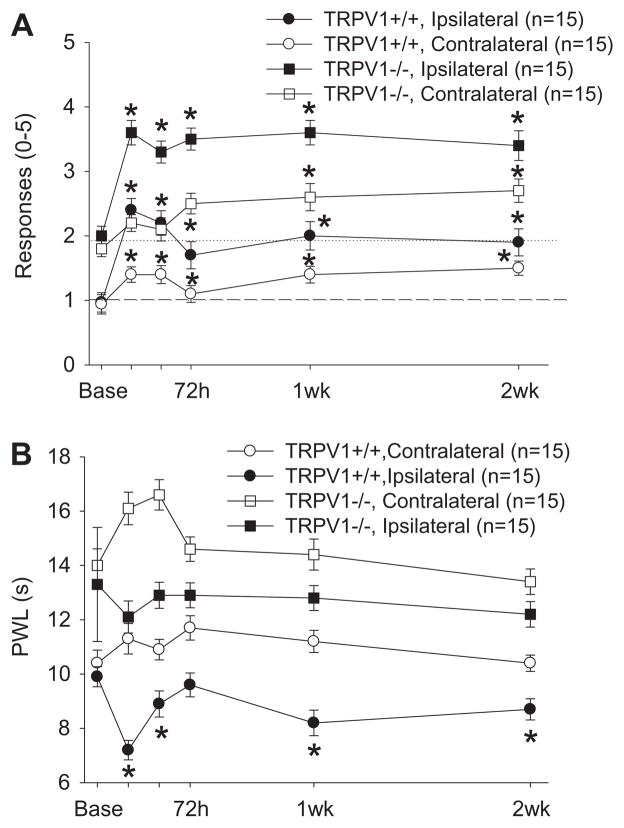
Mechanical sensitivity to repeated application of a 0.4 mN von Frey filament increased significantly in TRPV1+/+ mice both ipsilaterally and contralaterally after inflammation of the gastrocnemius muscle with carrageenan. There was also an increase in the mechanical sensitivity in TRPV1−/− mice bilaterally after carrageenan-induced muscle inflammation, and the magnitude of increase was similar to that observed in TRPV1+/+ mice (Fig. 3A). There was no difference between mechanical paw withdrawal thresholds in TRPV1−/− and TRPV1+/+ mice after inflammation when baseline withdrawal thresholds are used as a covariate or when analyzed as a magnitude of change relative to the baseline values (P = .143). Paw withdrawal latency to heat in TRPV1+/+ mice decreased ipsilaterally at 24 h to 2 weeks after carrageenan-induced muscle inflammation. This decrease in paw withdrawal latency to heat was absent in TRPV1−/− mice. Further, there were significant differences between TRPV1+/+ mice and TRPV1−/− mice when baseline latency was used as a covariate (Fig. 3B, P = .0001).
Fig. 3.
Mechanical (A) and heat (B) sensitivity of the hind paw after induction of muscle inflammation with 3% carrageenan in TRPV1+/+ mice and TRPV1−/− mice. (A) An increased sensitivity to repeated applications of a 0.4 mN von Frey filament was observed 24 h to 2 weeks after induction of muscle inflammation in both TRPV1−/− and TRPV1+/+ mice (*P < .05). No significant differences between TRPV1−/− and TRPV1+/+ mice were noted when baseline was used as a covariate (P > .05). (B) A decreased paw withdrawal latency to heat, when compared to baseline, occurred in the TRPV1+/+ mice but not the TRPV1−/− mice (*P < .05). Significant differences between groups were observed when baseline was used as a covariate (*P < .05). Data are presented as mean ± SEM.
3.3. Reexpression of TRPV1 into skin, muscle, or muscle and skin
Injection of HSV-GFP-TRPV1 into the skin, muscle or both skin and muscle of TRPV1−/− mice increased the mRNA levels for TRPV1 in the L4–L6 DRGs, ipsilaterally 4 weeks after injection. Relative expression of TRPV1 to GAPDH (2−ΔΔCT) in skin was 5.6 ± 1.8, muscle was 59.2 ± 28.9 and skin and muscle combined was 195.7 ± 84.7. The lowest levels of reexpression were in the skin innervating the paw which has the smallest volume of tissue injected. The highest volume of tissue injected, skin and muscle combined, showed the highest expression of TRPV1 mRNA. As expected, TRPV1 expression levels after reexpression in DRG innervating selective peripheral tissues was less than that observed in DRGs from TRPV1+/+ mice where TRPV1 is expressed in sensory neurons that send sensory afferents to other regions in the body (2−ΔΔCT: 8437.6 ± 459.1 4).
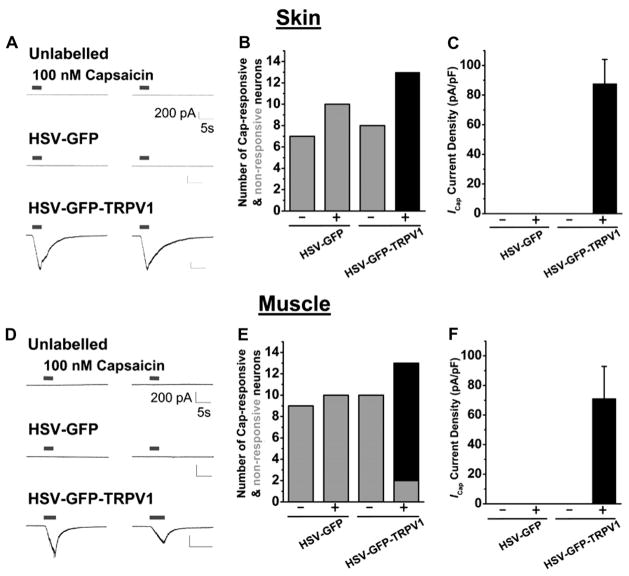
Injection of HSV-GFP-TRPV1 into skin or muscle in TRPV1−/− mice resulted in the reappearance of capsaicin-induced inward currents in DRG neurons infected with the virus (GFP labeled but not in uninfected DRG neurons (unlabeled; Fig. 4A,B,D,E). Specifically, in small-and medium-diameter neurons from the L4–L6 DRGs of TRPV1−/− mice injected with HSV-GFP-TRPV1, the capsaicin current densities were 87.76 ± 16.28 pA/pF (n = 13) and 71.15 ± 21.7 pA/pF (n = 11) for skin-and muscle-injection groups, respectively (Fig. 4C,F). In contrast, there were no responses to capsaicin in DRG neurons that were not labeled with GFP or in both GFP labeled and unlabeled DRG neurons from mice injected with HSV-GFP (Fig. 4). In addition, capsaicin currents were also found in a number of large-diameter L4–L6 DRG neurons from TRPV1−/− mice after injection of HSV-GFP-TRPV1 into the skin or muscle. These results from our qRT-PCR and electrophysiological analysis suggest that the TRPV1 channel can be functionally reexpressed in the DRG neurons innervating the skin and muscles of TRPV1−/− mice.
Fig. 4.
Reexpression of TRPV1 in TRPV1−/− mice. Injection of HSV-GFP-TRPV1 into either the hind paw skin (A–C) or the gastrocnemius muscle (D–F) in TRPV1−/− mice led to the functional reexpression of TRPV1 in L4–L6 DRG neurons. (A, D) Representative current traces obtained from unlabeled, HSV-GFP-labeled and HSV-GFP-TRPV1-labeled small/medium-diameter cultured DRG neurons recorded under whole-cell voltage-clamp with 2 successive capsaicin applications with an interval of 30 s. (B, E) Bar graphs showing the total number of neurons recorded that responded (black bars) and did not respond (gray bars) to 100 nM capsaicin in whole-cell voltage-clamp experiments. A – indicates they were from animals injected with virus but did not express GFP, and + from animals injected with virus that also expressed GFP. The group injected with HSV-GFP-TRPV1 and expressed GFP were the only neurons that responded to capsaicin. (C, F) Current densities (pA/pF) of unlabeled and labeled cultured small/medium-diameter neurons from L4–L6 DRGs in response to 100 nM capsaicin in whole-cell voltage-clamp. – were from animals injected with virus but did not express GFP; + were from animals injected with virus that also expressed GFP. Data are presented as mean ± SEM.
To test whether TRPV1 at the site of behavioral testing is important in the baseline responses, as well as for the development of heat hyperalgesia, we tested whether reexpression of TRPV1 into the hind paw skin of TRPV1−/− mice restored these behavioral responses.
Surprisingly, reexpression of TRPV1 into the skin of TRPV1−/− mice restored the mechanical sensitivity of the hind paw to the levels observed in TRPV1+/+ mice (P = .01, Fig. 1B). However, reexpression of TRPV1 into the skin had no effect on the heat sensitivity, or the development of heat hyperalgesia after muscle inflammation (Fig. 2B).
Next, we tested whether TRPV1 reexpression at the site of inflammation was important in the development of secondary heat hyperalgesia of the hind paw. Reexpression of TRPV1 into the muscle alone had no effect on baseline mechanical (Fig. 1C) or heat sensitivities (Fig. 2C). Further reexpression of TRPV1 into the muscle had no effect on heat responsiveness after inflammation (Fig. 4), ie, TRPV1−/− mice did not develop heat hyperalgesia with reexpression of TRPV1 in the muscle.
Because reexpression of TRPV1 in skin alone or muscle alone did not restore the heat sensitivity in uninjured animals or the heat hypersensitivity after muscle inflammation, we reexpressed TRPV1 in both skin and muscle. Reexpression of TRPV1 into skin and muscle had no effect on the mechanical sensitivity in TRPV1−/− mice (Fig. 1D). Interestingly, reexpression of TRPV1 into both the muscle and skin in TRPV1−/− mice significantly decreased the paw withdrawal latency to high intensity heat stimuli (P = .04, Fig. 2D). Further, reexpression of TRPV1 into both the muscle and skin in TRPV1−/− mice restored the heat hypersensitivity induced by carrageenan-induced muscle inflammation (P = .01, Fig. 5).
Fig. 5.
Percentage changes in withdrawal latencies to radiant heat after carrageenan-induced inflammation. The percent change in withdrawal latencies 24 h after carrageenan-induced inflammation in TRPV1−/− mice when HSV-GFP-TRPV1 was injected into the skin, muscle, or both skin and muscle (black bars) as compared to control TRPV1−/− mice injected with HSV-GFP (open bars). Heat hypersensitivity was restored in the group injected with HSV-GFP-TRPV1 into both skin and muscle (*P < .05), but not in the groups injected with HSV-GFP-TRPV1 into the skin or muscle alone. For comparison, the change in withdrawal latencies of TRPV1+/+ mice with inflammation are shown (gray bar). The heat hypersensitivity in TRPV1+/+ mice was similar to the group injected with HSV-GFP-TRPV1 into both skin and muscle.
Altogether these results indicate that TRPV1 can be functionally reexpressed in DRG neurons of mice lacking TRPV1, and that reexpression of TRPV1 in the L4–L6 DRG neurons innervating both muscle and skin of TRPV1−/− mice leads to the restoration of mechanical and thermal sensitivities in uninjured animals, as well as of heat hypersensitivity after muscle inflammation.
4. Discussion
4.1. TRPV1 mediates cutaneous heat sensitivity in uninjured animals
Our present study demonstrates a small effect on the paw withdrawal latency to radiant heat in TRPV1−/− mice without tissue injury at higher heat intensities (Fig. 2A). Previous reports demonstrated a similar pattern in TRPV1−/− mice with an increase in paw withdrawal latencies to heat at higher intensities but not to lower heat intensities [6,14,47]. We further demonstrate that paw withdrawal latencies to heat were decreased by reexpressing TRPV1 in both muscle and skin simultaneously in TRPV1−/− mice, but not in the skin alone or muscle alone. These data suggest that peripheral receptors at the site of radiant heat application alone, ie, skin, are not sufficient to mediate the decreased heat sensitivity observed in TRPV1−/− mice. It is possible that localization of TRPV1 on central terminals of afferent fibers from different tissues at the spinal cord dorsal horn is necessary for TRPV1-mediated heat sensitivity. Recent reports that demonstrate abolished noxious heat sensitivity in mice upon ablation of the central terminals of nociceptors expressing the TRPV1 channel [7,26] supports this possibility.
It is highly likely that distinct nociceptive afferents that express TRPV1 are critical for normal heat sensation. However, pharmacological blockade of TRPV1 channels spinally has no effect on baseline withdrawal latencies to heat. It is likely that TRPV1 in the peripheral site innervating the test area, as well as a sufficient amount of centrally located TRPV1 channels are necessary to produce normal heat sensitivity.
4.2. TRPV1 mediates cutaneous mechanical sensitivity in uninjured animals
The current study indicates that mechanical sensitivity of the hind paw to repeated application of von Frey filaments is significantly increased in TRPV1−/− mice, as compared to that in TRPV1+/+ mice. This effect, while significant, is relatively small representing a difference of around 2/5 withdrawals. In animal models of hypersensitivity we routinely see differences of 3–4/5 withdrawals to the 0.4 mN von Frey filament [5,40,41]. Further, reexpression of TRPV1 into the skin of TRPV1−/− mice, the site of behavioral testing, normalizes mechanical sensitivity similar to that observed in TRPV1+/+ mice. In direct contrast, the current study, in combination with previous reports, demonstrates that TRPV1−/− mice display no visible difference in mechanical sensitivity when measuring paw withdrawal thresholds [3,6,47], and removal of central terminals of TRPV1 expressing nociceptors has no effect on mechanical sensitivity in normal animals [7,26]. These data suggest that responses to repeated application of mechanical stimuli of the hind paw are a uniquely different measure of mechanical sensitivity. It is highly likely that the repeated application test is more like a temporal summation effect because the noxious mechanical stimulation is applied approximately once per second. Temporal summation is thought to be the behavioral correlate to wind-up, the enhanced dorsal horn neuron response to repeated stimulation of nociceptors. However, recordings from central neurons in TRPV1−/− mice are inconclusive with respect to mechanical responsiveness of the hind paw.
Specifically, responses of dorsal horn neurons to a single 15 s application of a von Frey filament was reduced by systemic or spinal application of a TRPV1 antagonist [31], while there were no differences in responses to innocuous brushing, noxious pressure, or noxious pinching of the skin in TRPV1−/− mice, as compared to those in TRPV1+/+ mice [16]. Further, systemically administered TRPV1 antagonists reduced the responses to mechanical stimulation in the hind paw of CFA-inflamed animals, as compared to animals without inflammation [31]. Therefore, while responses to a single mechanical stimuli are unaffected in TRPV1−/− mice or by the administration of TRPV1 antagonists, repeated application of a mechanical stimuli results in enhanced sensitivity as a result of the precise location of TRPV1 on cutaneous afferents.
4.3. TRPV1 mediates heat hyperalgesia in animals with muscle inflammation
Our present study demonstrates the loss of secondary heat hyperalgesia in TRPV1−/− mice after muscle inflammation, and to our knowledge, ours is the first report regarding the involvement of TRPV1 channel in inflammatory muscle hyperalgesia. The loss of heat hyperalgesia in TRPV1−/− mice is consistent with several prior studies utilizing animal models of tissue inflammation: CFA-paw, carrageenan-paw and mustard-oil-paw [6,14]. In further agreement, systemic administration of TRPV1 antagonists reduces heat hyperalgesia induced by CFA-paw inflammation, carrageenan-paw inflammation, or nerve injury [9,12,13,19,24,37,46]. We further demonstrate that heat hyperalgesia requires TRPV1 channels to be located both in the skin, where the behavioral testing occurs, as well as in the muscle, where inflammation occurs, and leads to the development of heat hyperalgesia. This suggests that TRPV1 in muscle afferents is important in sending information centrally regarding the inflammatory environment, and that TRPV1 in the skin afferents is necessary for full heat sensitivity. A loss of TRPV1 in either place eliminates the development of heat hyperalgesia. Although the loss of heat hyperalgesia is in agreement with many prior studies [12,13,19,24,37,46], our study extends these by showing that TRPV1 plays a role in sensing the inflammatory environment, as well as in sensing the elevated temperature. Sensory neurons expressing TRPV1 are critically important for the development of heat hyperalgesia because elimination of the central terminals of nociceptors expressing TRPV1 prevents the development of heat hyperalgesia in mouse models of tissue injury/inflammation and nerve injury [7,26].
Inflammation is associated with a decrease in tissue pH, and a pH below 6.0 can directly activate the TRPV1 channel [4,6]. However, pH levels below 6.0 are rare in inflammatory conditions, particularly in muscle. However, the TRPV1 channel activity to other agonists such as temperature and lipid metabolites can be upregulated at mild/moderated acidic pH [20–23,25]. Further, release of inflammatory mediators, such as prostaglandins, bradykinin, nerve growth factor, and ATP, can sensitize TRPV1 channels through activation of a variety of G-coupled protein receptors, and downstream activation of a number of protein kinases [8,11,25,44,52]. Specifically, phosphorylation of TRPV1 by protein kinases C and A (PKC and PKA) leads to channel activation at temperatures below the body temperature (<37 °C) [38]. Additionally, phosphorylation of TRPV1 protein by PKC and PKA increases the channel probability and decreases channel desensitization, respectively, and phosphorylation of TRPV1 by the tyrosine kinase src enhances TRPV1 channel trafficking to the cell surface [1,2,32–34,44,52]. Thus, sensitization of TRPV1 by inflammatory mediators at the site of inflammation would result in sensitization of TRPV1-expressing nociceptors, subsequently increasing the nociceptive input to the spinal cord. Increased central sensitization could be manifested as secondary heat hyperalgesia and removal of TRPV1 presumably eliminates the increased central sensitization and thus the manifestation of secondary heat hyperalgesia.
Although sensitization of TRPV1 is one potential mechanism for the enhanced nociception, there could also be an increased expression of TRPV1 after inflammation. After muscle inflammation TRPV1 expression, measured by qPCR is unchanged 12 h after inflammation [17] and is similar to that observed after paw inflammation [27,42,45]. However, after CFA-induced paw inflammation there is an increased expression of TRPV1 protein in DRG neurons measured either by Western blot or immunohistochemistry [27,50].
4.4. TRPV1 has no effect on mechanical hyperalgesia after muscle inflammation
The current study demonstrates that genetic elimination of TRPV1 has no effect on secondary mechanical hyperalgesia that develops after muscle inflammation. This finding is in agreement with prior studies in TRPV1−/− mice revealing no effects on inflammation-induced or nerve injury-induced mechanical hyperalgesia [3,6]. However, cutaneous mechanical hyperalgesia induced by cystitis was reduced in TRPV1−/− mice [47]. This difference may be specific to visceral nociception as TRPV1−/− mice demonstrate reduced sensitivity to colorectal distention, and reduced afferent sensitization to stretching [28]. Similarly, elimination of TRPV1 in the central terminals of primary afferents still results in the development of mechanical hyperalgesia after inflammation [7,26].
Interestingly and in direct contrast, TRPV1 antagonists can reverse mechanical hyperalgesia induced by paw inflammation, nerve injury, capsaicin, and cystitis [12,13,19,24,29,31,37,46, 47,50]. These effects occur if provided systemically or intrathecally, and studies therefore suggest the importance of TRPV1 on central terminals and/or supraspinal sites. In support, endogenous or exogenous TRPV1 ligands injected intrathecally evoke mechanical hyperalgesia that are blocked by intrathecal blockade of TRPV1 [36]. Further, antibodies to endogenous TRPV1 ligands have been demonstrated to attenuate inflammation-induced mechanical hyperalgesia, which suggest that inflammation results in release of endogenous TRPV1 ligands spinally to produce hyperalgesia [36]. However, it should be noted that mechanical hyperalgesia induced by carrageenan-induced muscle inflammation is unaffected by the blockade of TRPV1 with capsazepine either systemically or intramuscularly [17]. This may be related to species specificity of capsazepine [46], or to differences between muscle and paw inflammation [15].
4.5. Conclusion
Our data demonstrate that TRPV1 mediates mechanical sensitivity to repeated stimulation, heat sensitivity to high intensity stimulation, and the development of heat hypersensitivity after muscle inflammation. In uninjured animals, the enhanced sensitivity to repeated mechanical stimulation can be normalized by reexpression of TRPV1 in the sensory neurons innervating the skin, and heat sensitivity is restored by reexpression in neurons innervating both the skin and muscle. Because TRPV1 is not directly involved in the detection of noxious mechanical stimulation, we conclude that lack of TRPV1 on the central terminals of cutaneous nociceptors mediates the enhanced mechanical sensitivity. We further suggest that TRPV1 serves as both a mediator of nociceptor sensitization at the site of inflammation and a heat sensor at the site of testing because reexpression of TRPV1 in both the muscle and skin is necessary to restore normal heat sensitivity and heat hypersensitivity induced by inflammation.
Acknowledgments
The authors thank Ann C. Lawler for editorial assistance. This work was supported by the National Institutes of Health grant (R01-AR053609, AR052316 to KAS; and R01-NS069898 to DPM).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Conflict of interest statement
The authors report no conflict of interest.
References
- 1.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–5. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. CAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–31. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 3.Bolcskei K, Helyes Z, Szabo A, Sandor K, Elekes K, Nemeth J, Almasi R, Pinter E, Petho G, Szolcsanyi J. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–76. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Burnes LA, Kolker SJ, Danielson JF, Walder RY, Sluka KA. Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1347–55. doi: 10.1152/ajpregu.00687.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 7.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA. 2009;106:9075–80. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435–9. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Willcockson HH, Valtschanoff JG. Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Exp Neurol. 2009;220:383–90. doi: 10.1016/j.expneurol.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho WG, Valtschanoff JG. Vanilloid receptor TRPV1-positive sensory afferents in the mouse ankle and knee joints. Brain Res. 2008;1219:59–65. doi: 10.1016/j.brainres.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crandall M, Kwash J, Yu W, White G. Activation of protein kinase C sensitizes human VR1 to capsaicin and to moderate decreases in pH at physiological temperatures in Xenopus oocytes. Pain. 2002;98:109–17. doi: 10.1016/s0304-3959(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 12.Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–93. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culshaw AJ, Bevan S, Christiansen M, Copp P, Davis A, Davis C, Dyson A, Dziadulewicz EK, Edwards L, Eggelte H, Fox A, Gentry C, Groarke A, Hallett A, Hart TW, Hughes GA, Knights S, Kotsonis P, Lee W, Lyothier I, McBryde A, McIntyre P, Paloumbis G, Panesar M, Patel S, Seiler MP, Yaqoob M, Zimmermann K. Identification and biological characterization of 6-aryl-7-isopropylquinazolinones as novel TRPV1 antagonists that are effective in models of chronic pain. J Med Chem. 2006;49:471–4. doi: 10.1021/jm051058x. [DOI] [PubMed] [Google Scholar]
- 14.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–7. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 15.DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep. 2008;12:338–43. doi: 10.1007/s11916-008-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert WA, III, Julius D, Basbaum AI. Differential contribution of TRPV1 to thermal responses and tissue injury–induced sensitization of dorsal horn neurons in laminae I and V in the mouse. Pain. 2006;126:184–97. doi: 10.1016/j.pain.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140:292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Gautam M, Benson CJ, Sluka KA. Increased response of muscle sensory neurons to decreases in pH after muscle inflammation. Neuroscience. 2010;170:893–900. doi: 10.1016/j.neuroscience.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, Wang J, Zhu D, Vanderah TW, Porreca F, Doherty EM, Norman MH, Wild KD, Bannon AW, Louis JC, Treanor JJ. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2005;313:474–84. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- 20.Goldie I, Nachemson A. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop Scand. 1969;40:634–41. doi: 10.3109/17453676908989529. [DOI] [PubMed] [Google Scholar]
- 21.Goldie I, Nachemson A. Synovial pH in rheumatoid knee joints. II. The effect of local corticosteroid treatment. Acta Orthop Scand. 1970;41:354–62. doi: 10.3109/17453677008991521. [DOI] [PubMed] [Google Scholar]
- 22.Habler C. Uber den K+ und Ca2+-gehalt von eiter und exsudaten und seine beziehungen zum entuzundungschmerz. Klin Wochenschr. 1929;8:1569–72. [Google Scholar]
- 23.Habler C. Untersuchungen zur molekularpathologie der gelenkexsudate und ihre klinischen ergebnisse. Arch Klin Chirurgie. 1929;156:20–42. [Google Scholar]
- 24.Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, El KR, Lee CH, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314:410–21. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Zhang X, McNaughton PA. Inflammatory pain: the cellular basis of heat hyperalgesia. Curr Neuropharmacol. 2006;4:197–206. doi: 10.2174/157015906778019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009;4:e7021. doi: 10.1371/journal.pone.0007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. P38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 28.Jones RCW, Xu LJ, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–9. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanai Y, Nakazato E, Fujiuchi A, Hara T, Imai A. Involvement of an increased spinal TRPV1 sensitization through its up-regulation in mechanical allodynia of CCI rats. Neuropharmacology. 2005;49:977–84. doi: 10.1016/j.neuropharm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGaraughty S, Chu KL, Brown BS, Zhu CZ, Zhong C, Joshi SK, Honore P, Faltynek CR, Jarvis MF. Contributions of central and peripheral TRPV1 receptors to mechanically evoked and spontaneous firing of spinal neurons in inflamed rats. J Neurophysiol. 2008;100:3158–66. doi: 10.1152/jn.90768.2008. [DOI] [PubMed] [Google Scholar]
- 32.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–90. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 33.Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–32. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 34.Morenilla-Palao C, Planells-Cases R, Garcia-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–72. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 35.Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–87. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- 36.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci USA. 2009;106:18820–4. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. In vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:387–93. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- 38.Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: a common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–5. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rau KK, Jiang N, Johnson RD, Cooper BY. Heat sensitization in skin and muscle nociceptors expressing distinct combinations of TRPV1 and TRPV2 protein. J Neurophysiol. 2007;97:2651–62. doi: 10.1152/jn.00840.2006. [DOI] [PubMed] [Google Scholar]
- 40.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–39. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 41.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–12. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tohda C, Sasaki M, Konemura T, Sasamura T, Itoh M, Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J Neurochem. 2001;76:1628–35. doi: 10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- 43.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 44.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–25. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–33. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–67. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 48.Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–40. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain. 2007;8:692–9. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, Wan Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain. 2008;4:61. doi: 10.1186/1744-8069-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang G, Mohammad H, Peper BD, Raja S, Wilson SP, Sweitzer SM. Enhanced peripheral analgesia using virally mediated gene transfer of the mu-opioid receptor in mice. Anesthesiology. 2008;108:305–13. doi: 10.1097/01.anes.0000299836.61785.79. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–23. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]