Abstract
Low pH in tissue can evoke pain in animals and humans, and is an important factor in hyperalgesia. Research has also implicated acidosis in psychiatric and neurological diseases. One emerging class of pH-detecting receptors is that of the acid-sensing ion channels (ASICs). Advances in ASIC research have improved the understanding of the role played by pH dynamics in physiological and pathophysiological processes. Increasing evidence suggests that targeting ASICs with pharmacological agents may offer an effective and novel approach for treating pain and diseases of the CNS. However, the development of pharmaceuticals that target ASICs and are suitable for clinical use remains an obstacle. This review provides an update on ASICs and their potential for therapeutic modification in pain and CNS diseases.
Keywords: Acid-sensing ion channel, anxiety, ASIC, depression, pain, pH
Introduction
Acid-sensing ion channels (ASICs) are an H+-gated subgroup of the degenerin/epithelial sodium channel (DEG/ENaC) family of proteins (for reviews of these channels, see references [1–3]). The crystal structure of chicken ASIC1a, minus the intracellular amino- and carboxy-termini, revealed that three subunits combine to form a channel [4], a mode of assembly that is probably shared by other members of the DEG/ENaC family [3, 5]. In addition to this trimeric subunit assembly, intracellular disulfide bonds can unite ASIC1a subunits into a larger complex [6]. Each subunit has two transmembrane domains, a prominent extracellular domain with 14 characteristically spaced cysteine residues, and several conserved amino acid motifs. ASICs conduct cations, including Na+ and Ca2+ [7–9], but their conductance of Na+ is probably the most physiologically relevant process [10–12]. At least five subunits (ASIC1a, ASIC1b, ASIC2a, ASIC2b and ASIC3) encoded by three genes (ACCN2, ACCN1 and ACCN3) can combine to form acid-activated channels. Additional subunits (eg, ASIC4 and BLINaC) have not been shown to produce or modify H+-evoked currents [13], suggesting that channels containing these subunits might constitute a distinct functional class.
The ASIC subunit composition of a channel determines its properties, including pH sensitivity, ion selectivity, activation kinetics and desensitization kinetics. In the case of ASICs that are expressed in heterologous cells, ASIC1a and ASIC3 homomeric channels are activated if the extracellular pH decreases to ≤ 7 [14, 15], whereas ASIC2a homomeric channels are not activated until a pH < 6 [14–16]. The combination of different subunits into heteromeric channels can produce channel properties that are different from those of homomeric channels. For example, the desensitization rate of ASIC1a/ASIC2a heteromeric channels is more rapid than that of homomeric channels composed of either ASIC1a or ASIC2a [16]. An increasing number of molecules that modulate ASIC function have been identified. For example, RFamide (Arg-Phe-NH2 C-terminal) neuropeptides, arachidonic acid, Zn2+, nitric oxide and lactate all alter channel properties such as pH sensitivity and desensitization [2, 17]. Both variability in subunit composition and modulation of activity by external factors therefore enable ASICs to detect a range of pH changes and to respond in diverse ways.
Tissue injury and inflammation cause acidosis (Figure 1), and acidic pH can trigger pain [18–22]. These observations led to the hypothesis that ASICs [23, 24], and possibly other acid-activated receptors, have a critical role in nociception. Despite some puzzling contrary results (reviewed in reference [2]), an increasing volume of literature supports the hypothesis that ASICs contribute to acid-evoked pain. Whether ASICs transduce or modulate painful stimuli has not been established. However, the data discussed in this review indicate that ASIC inhibitors might relieve pain in a variety of clinical conditions. In addition, because their mechanism of action is distinct, ASIC antagonists may provide new treatment options for patients who do not benefit from, or are unable to, tolerate the adverse side effects of current pain medications.
Figure 1. A model of tissue injury causing acidosis and activating ASICs on nociceptive fibers.
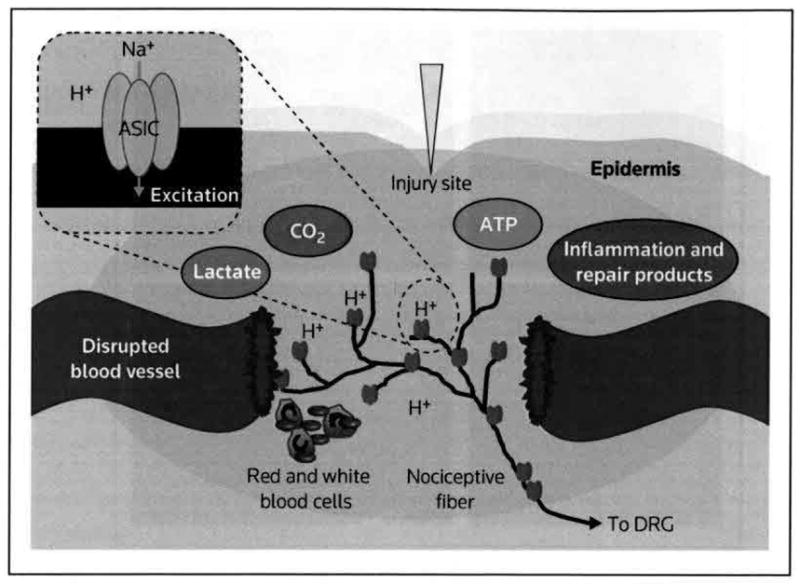
The disruption of blood vessels decreases tissue oxygenation and increases anaerobic metabolism, which increase levels of carbon dioxide and lactate, and thus lowers pH. Inflammation and tissue repair further increase metabolic activity and acidic products. ATP and lactate potentiate pH effects on ASIC-expressing peripheral sensory neurons [67]. The resulting Na+ influx and depolarization activate DRG fibers, leading to pain and hyperalgesia.
ASIC acid-sensing ion channel, DRG dorsal root ganglion
ASICs and acid-evoked pain
The pH sensitivity of ASICs is the property that most strongly links these channels to pain. Acidification of the skin or muscle produces pain [18, 25–27]. Even the earliest papers describing H+-activated channels recognized that the channels might function as nociceptors [28, 29]. Inflammation, infection, ischemia and exercise all cause local pH to decrease, frequently to a pH < 7 [21, 30], which is sufficient to activate ASIC1a and ASIC3 channels in vitro [14, 15]. Thus, ASICs are strong candidates for detecting the variations in pH that occur with a variety of painful conditions.
ASICs are suitably located to detect or modulate pain
Another fundamental property of ASICs suggesting their involvement in pain is that these channels are expressed at key pain-related sites in the peripheral nervous system (PNS), the CNS and in non-neuronal cells. In the PNS, ASIC subunits are expressed in primary afferent fibers. Many of these ASIC-expressing neurons are nociceptive, as defined by the expression of substance P and/or the calcitonin gene-related peptide [31–35]. Early immunohistochemical studies that identified ASIC1a in substance P-containing neurons of the dorsal root ganglion (DRG) supported the initial enthusiasm for the hypothesis that ASICs mediate nociception [31]. Shortly after ASIC1a was identified [36], ASIC3 was also discovered [37], and was determined to be localized to primary afferent nociceptive fibers innervating the skin, muscles, joints and viscera [23, 27, 32–35] (Figure 2). Interestingly, ASIC3 is expressed in a greater number of nociceptive neurons innervating muscle (~ 50%) than skin (~ 10%), suggesting that ASIC3 might be particularly important for detecting muscle acidosis [38].
Figure 2. Immunofluorescent localization of mouse ASIC3 and ASIC1a protein at pain-related sites.
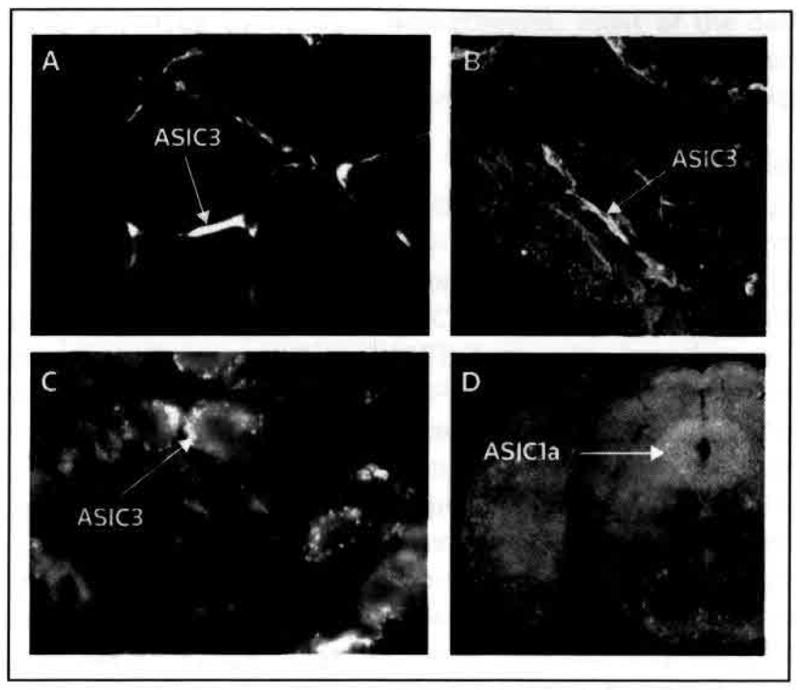
(A) ASIC3 in primary afferent fibers innervating muscle (cross-section); (B) ASIC3 in primary afferent fibers innervating the knee joint synyovium; and (C) ASIC3 in dorsal root ganglion neurons. (D) ASIC1a is abundantly expressed in brainstem periaqueductal gray matter (coronal section).
(B adapted with permission from The International Association for the Study of Pain and Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA: Role of ASIC3 in the primary and secondary hyperalgesia produced by joint Inflammation in mice. Pain (2008) 137(3):662–669 © 2008 The International Association for the Study of Pain; C adapted with permission from The International Association for the Study of Pain and Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ: Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain (2003) 106(3):229–239 © 2003 The International Association for the Study of Pain; and D adapted with permission from Elsevier Ltd and Coryell M, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA: Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry (2007) 62(10):1140–1148 © 2007 Elsevier Ltd)
ASIC acid-sensing ion channel
In the CNS, ASICs are also situated in regions that are critically involved in pain. ASIC1a and ASIC2 are located in the dorsal horn of the spinal cord [23, 39–42], where pain-related plasticity occurs and through which pain signals are transmitted to the brain. ASIC1a is also abundantly expressed in both the ventral and dorsal regions of the periaqueductal gray matter (Figure 2D) [43], both of which are important sites in pain modulation. Compelling evidence that ASIC1a in the CNS contributes to pain was provided by observations that the spinal or supraspinal inhibition of ASIC1a reduced pain-related behaviors in mice [40, 44]. ASIC2a and ASIC2b are also expressed in the CNS [45, 46], but their role in the brain has not been established thoroughly. Despite initial reports that ASIC3 was expressed primarily in the PNS [37], subsequent investigation identified this channel in the CNS [47], although its importance remains unknown.
ASIC expression has also been observed in non-neuronal cells, including monocytes (ASIC1, 2 and 3), arthritic chondrocytes (ASIC1, 2 and 3), osteoblasts (ASIC1, 2, 3 and 4), osteoclasts (ASIC1, 2 and 3) [48], carotid body glomus cells (ASIC1 and 3) [49], nucleus pulposus cells (ASIC3) [50], muscle cells (ASIC1 and 3) [51], and synovicytes of the joints (ASIC3) (Figure 2) [34]. ASICs in these non-neuronal cells may also be critical for detecting acidosis and modulating pain or inflammation.
Inflammation, nerve injury and bone cancer increase the expression of ASIC
In the process of hyperalgesia, tissue injury increases the nociceptive response to noxious stimuli. If ASICs contribute to hyperalgesia, the upregulation of ASIC expression would be expected occur as a result of inflammation or tissue injury. For example, inflammation of a rodent paw increased ASIC currents and the expression of ASIC1, 2 and 3 mRNA in DRG neurons [52]. Similarly, inflaming a mouse knee with carrageenan increased ASIC3 protein expression in nociceptive DRG neurons innervating the joint, and at the peripheral terminals of the neurons [34, 35]. Compression of the lumbar nerve root in a rat model of spinal disk herniation increased ASIC3 protein expression in DRG neurons, and the co-application of lidocaine at the same site prevented both this increase in ASIC3 and the development of mechanical hyperalgesia [50]. In an animal model of bone cancer, the expression of ASIC1 mRNA in DRG was increased, but no alteration in the expression of ASIC3 mRNA was observed [53]. Thus, tissue injury appears to increase the expression of ASICs at sites at which clinical hyperalgesia is commonly observed.
The interaction of ASIC with established anti-nociceptive pathways
Although acid receptors are novel targets for combating pain, several studies provide evidence of an overlap between ASIC function and two well-established pain treatments, NSAIDs and opioids. NSAIDs inhibit ASIC currents in cultured DRG neurons, hippocampal neurons and COS cells by directly binding to ASICs [52, 54]. NSAIDs also inhibit the ability of inflammation to induce ASIC expression in DRG neurons [52]. COX inhibition and reduced prostaglandin synthesis are thought to be the main mechanisms by which NSAIDs exert their analgesic/anti-inflammatory effects. However, the proportion of NSAID effects caused by ASIC blockade or reduced ASIC expression is unknown.
Another study proposed that ASICs in the CNS interact with the opioid system; the analgesic effects of centrally administered psalmotoxin (PcTx1), a peptidic ASIC1a inhibitor extracted from the South American tarantula Psalmopoeus cambridgei, were blocked by μ- and δ-opioid receptor antagonists [44]. In addition, PcTx1 increased levels of the endogenous opioid met-enkephalin in cerebrospinal fluid, and the analgesia produced by PcTx1 was absent in preproenkephalin knockout mice [44]. This activity of PcTx1 likely results from the inhibition of ASIC1a: antisense ASIC1a RNA had similar effects, and other PcTx1-mediated effects were observed to be ASIC1a-specific [43, 55]. These data suggest that blocking ASIC1a in the CNS results in opioid-mediated analgesia.
The relationship between ASIC1a and opioid-mediated analgesia raises several interesting questions, including which mechanism is used by PcTx1 and ASIC1a to influence opioid levels; whether centrally administered ASIC1a inhibitors provide any advantages compared with current opioid medications; and if ASIC1a inhibitors experience the same therapeutic limitations as opioids (for example, the development of tolerance to the analgesic effects of PcTx1 was observed to resemble the tolerance produced by repeated morphine injections [44]). Given that tolerance is an important factor in opioid addiction, this observation of developed tolerance suggests that ASICs might be used to reduce tolerance and addiction.
Opportunities for reducing clinical pain with ASIC inhibitors
Preclinical data, mostly derived from pain models in mice with genetic and pharmacological manipulations of ASICs, suggest that ASIC inhibitors might be useful for treating some, but not all, types of pain. For example, these models have revealed that ASICs are not implicated in pain produced in the absence of tissue injury; ASIC1a−/− mice exhibit normal responses to nociceptive mechanical and thermal stimulation of the paw, and have normal responses to mechanical stimulation of the muscle and joint [23, 24, 34, 56, 57]. Similarly, ASIC3 gene disruption did not change behavioral responses to these stimuli [32, 57, 58], but may have increased primary behavioral responses to painful mechanical and thermal stimuli [32, 58–60]. Although the data do not indicate a role for ASICs in all types of pain, ASIC inhibitors might be particularly beneficial for the treatment of clinical pain in circumstances of low pH, given the activation of these channels under acidic conditions.
Inflammatory pain
Inflammation causes hyperalgesia, whereby noxious stimuli such as pressure are more painful than usual. Hyperalgesia can be primary (occurring at the inflamed site) or secondary (occurring away from the inflamed site). Painful inflammatory conditions include infection (eg, abscesses and cellulitus), rheumatological disorders (eg, arthritis and myositis) and trauma. Given that inflammation lowers pH, ASICs might play a critical role in inflammatory pain and hyperalgesia; this hypothesis has been investigated using different approaches involving various types of inflammation, tissue injury and hyperalgesia, and diverse methods of manipulating ASICs. Perhaps as a consequence of this diversity in experimental methodology, the data from these studies are often difficult to compare, and are contradictory in some cases. Nevertheless, most of the data confirm that ASICs play a role in inflammatory pain, and that disruption of ASICs reduces inflammatory hyperalgesia.
The local or systemic administration of non-selective ASIC antagonists reduced primary hyperalgesia of the paw in rats following inflammation induced with complete Freund’s adjuvant (CFA) [61]. The specific targeting of ASIC3 with either the selective antagonist peptide toxin APETx2 or with intrathecally delivered siRNA prevented CFA-induced heat hyperalgesia and the flinching behaviors caused by injecting acidified capsaicin, serotonin or formalin [62, 63]. The disruption of ASIC3 significantly reduced hyperalgesia 24 to 48 h after CFA-induced inflammation, but not 4 h after inflammation [64]. Similarly, non-selective ASIC antagonists reduced hyperalgesia induced by muscle inflammation evoked by carrageenan [57] or exercise [65]. Although the swelling after muscle inflammation was normal in a study in ASIC3−/− mice, the disruption of ASIC3 reduced histological evidence of inflammation [66]. Thus, disrupting ASIC3 appears to inhibit the inflammatory process itself [64], although the mechanism underlying this effect has not been established. Additional research suggested a role for other ASICs in inflammatory pain; for example, the disruption of ASIC1a in mice completely eliminated mechanical hyperalgesia of the muscle caused by carageenan-induced muscle inflammation [57].
Some of the results highlighted suggest that ASIC inhibitors have the ability to reduce primary hyperalgesia, which appears to involve the sensitization of peripheral neurons. Interestingly, research has also suggested that the disruption or inhibition of ASIC reduces secondary hyperalgesia, which is thought to involve the sensitization of central neurons. Disrupting ASIC3 in mice eliminated secondary mechanical hyperalgesia of the paw following joint and muscle inflammation [24, 34] (Figure 3). In ASIC3−/− mice, the re-expression of ASIC3 with a recombinant HSV-1 vector of the channel in primary afferent fibers innervating the gastrocnemius muscle restored secondary mechanical hyperalgesia [24], indicating that the muscle afferent fiber is a key site of ASIC3 action in secondary hyperalgesia. In contrast to these effects observed with ASIC3, the disruption of ASIC1a did not reduce secondary hyperalgesia after muscle inflammation (Figure 3) [57], suggesting that ASIC3 may play a unique role in central pain sensitization. Together, these data indicate that ASICs contribute to both peripheral and central pain sensitization.
Figure 3. ASIC1a- and ASIC3-mediated hyperalgesia.
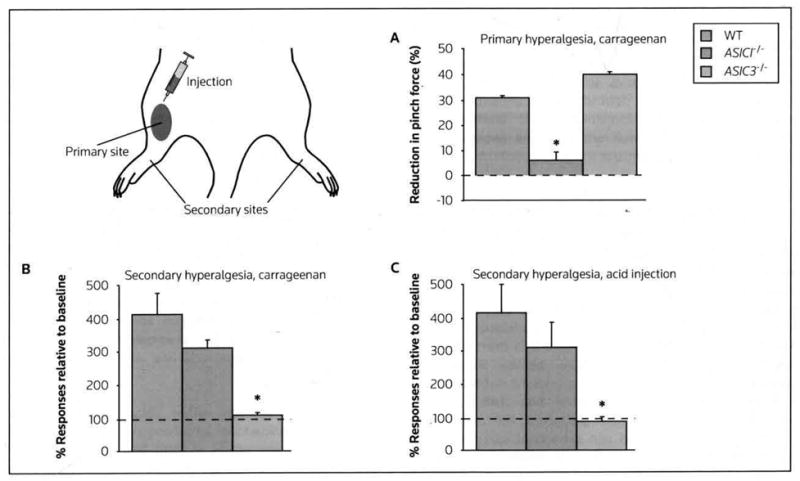
Hyperalgesia was tested 24 h after the administration of carrageenan (A and B) or acid (C) into the leg muscle of mice. (A) Primary hyperalgesia was assessed by pinching the previously injected muscle and measuring the force required to induce leg withdrawal (reduced force relative to baseline indicates hyperalgesia). ASIC1a−/− mice exhibited a significant reduction in primary hyperalgesia. (B and C) Secondary hyperalgesia was measured at the paw; a 0.4-mN von Frey filament-increased paw withdrawal response relative to baseline indicated hyperalgesia. ASIC3−/− mice demonstrated a significant deficit in secondary hyperalgesia following both carageenan and acid injection.
*p < 0.05 compared with the other two groups.
ASIC acid-sensing ion channel, WT wild-type
Although the evidence described previously is substantial, other research indicated that disruption of ASIC1 and ASIC3 in models of hyperalgesia had no effect, or increased hyperalgesia [23, 24, 32, 34, 58–60, 67]. The reasons for these apparently contradictory results are unclear, but may be a result of differences among the inflammatory models and research methodologies used. Alternatively, the role of ASICs in pain may be more complex than existing knowledge suggests. Additional crucial factors might be involved; for example, extracellular ATP and lactate may be important for increasing the neural response to low pH in some DRG sensory neurons [67]. The mechanisms underlying hyperalgesia may also differ among sites; for example, ASICs may exert a greater effect following deep tissue inflammation of the muscle and joint than at the skin [57]. Because of the apparent discrepancies in the published data, consistent results obtained across different laboratories would be encouraging. Nevertheless, the published data generally support the hypothesis that non-selective ASIC inhibitors can reduce primary and/or secondary hyperalgesia. However, until pharmacological agents suitable for clinical trials are available, the value of these inhibitors in the treatment of inflammatory pain will remain undetermined.
Neuropathic pain
Neuropathic pain, defined as pain initiated or caused by a primary lesion or dysfunction in the nervous system, occurs in a variety of conditions, including nerve compression and diabetic neuropathy. The pain resulting from such nerve injuries could be a result of local inflammation and both peripheral and central sensitization of the nervous system. Several potential mechanisms for neuropathic pain have been identified, including the involvement of ASICs. Spinal nerve root compression causes hyperalgesia, and nerve compression induces ASIC3 expression [50]. In addition, the spinal delivery of PcTx1 reduced hyperalgesia in mice with a chronic constriction injury of the sciatic nerve [44]. Thus, ASIC antagonists may be useful for targeting neuropathic pain. Although these observations are promising, further research is needed to define specific neuropathic pain types that might be alleviated by ASIC blockade.
Post-operative and visceral pain
Treatments for post-operative pain are recognized to be inadequate. The mechanisms by which surgical incisions lower pH may resemble those underlying the acidosis associated with trauma and inflammation (Figure 1); disrupted blood flow impedes oxygen delivery, slows the removal of carbon dioxide and favors anaerobic metabolism (which promotes the accumulation of lactate); concurrently, the healing process accelerates metabolism. Consistent with these observations, the incision of a rat hind paw lowers local pH, reduces weight bearing and increases paw mechanosensitivity, all of which are measures of hyperalgesia [68]. Interestingly, the non-specific ASIC inhibitors amiloride and A-317567 improved weight bearing on incised rat paws [61], suggesting that ASIC inhibitors may relieve postoperative pain.
ASICs may also contribute to pain in visceral organs. ASIC3-like currents are robustly expressed in the nociceptive DRG neurons innervating the heart [69, 70], and the acidosis that occurs during myocardial ischemia may activate these channels, causing anginal pain. ASICs also contribute to mechanosensitivity in the intestine [56, 71, 72], suggesting a role for ASICs in the pain accompanying gastritis, inflammatory bowel disease and other gastrointestinal pain disorders.
Fibromyalgia and chronic non-specific low back pain
Fibromyalgia and chronic low back pain are pain syndromes that involve little or no tissue damage or inflammation, and are likely to be mediated by CNS sensitization. In susceptible individuals, acidosis occurring with unaccustomed exercise or mild injury might trigger exaggerated CNS responses, causing CNS sensitization and widespread pain. However, the mechanisms by which hyperalgesia develops in fibromyalgia and chronic low back pain have not been determined.
Fibromyalgia results in widespread hyperalgesia, with increased sensitivity of the skin, muscle and viscera, whereas low back pain involves focal hyperalgesia. In rodent models developed for these centrally maintained conditions, two injections of acidic saline into muscle tissue produced long-lasting, widespread hyperalgesia of the skin, muscle and viscera [73–75]. Histological analysis did not identify any inflammation or tissue damage after these acidic saline injections [73], a result that concurs with the absence of tissue damage and inflammation observed in fibromyalgia and chronic non-specific low back pain. Remarkably, secondary mechanical hyperalgesia of the paw failed to develop in ASIC3−/− mice after these acidic injections (Figure 3) [66]. In addition, the central sensitization of dorsal horn neurons that normally occurs after a second acid injection failed to develop in ASIC3−/− mice [66]. In contrast, the ASIC1a−/− mice developed normal secondary mechanical hyperalgesia [66]. Together, these findings suggest an important role for ASIC3 in the development of central sensitization, secondary hyperalgesia, and also possibly in fibromyalgia-like pain syndromes.
In humans, infusing acidic buffer into the anterior tibialis muscle in the lower leg produced mechanical hyperalgesia at the infusion site and distally at the ankle [18]. These results suggest that similar pain sensitization and processing mechanisms may operate in both humans and rodents.
ASICs in psychiatric and neurological diseases
As described previously, ASICs are expressed in the CNS, and may contribute to central pain processing. However, the abundance of pH receptors in the CNS raises additional questions, such as what activates these channels under normal conditions and the consequences of this activation. The published data suggest the possibility that ASICs in the CNS might be targeted to treat several disorders, including anxiety, depression, stroke, neurodegeneration and seizures.
The dynamics of pH in the brain
Although pH levels in the brain are generally controlled within a narrow range, dynamic pH fluctuations occur with neural activity and in neurological disease. Neurotransmitter-containing vesicles release protons into the synaptic cleft during neurotransmission, resulting in rapid reductions in pH; however, this effect is likely to be of short duration because of buffering and diffusion [76]. This brief acidosis is sufficient to inhibit pH-sensitive Ca2+ channels in the presynaptic membrane and to reduce vesicle release at ribbon synapses [77–79]. Intense neural activity also produces slower and more sustained acidosis, which depends on the degree of activity. For example, intense neural activity during a seizure can lower pH to ≤ 6.5. Metabolism is also thought to play an important role in neural acidosis. Mitochondria absorb Ca2+ and extrude H+, which can subsequently be transported out of the cell [80]. Neural activity also increases levels of lactate [81], which is an important determinant of extracellular pH [82]. Although the source of lactate is not well established, increased lactate levels may result from glycolytic activity in astrocytes [83]. More recent research in invertebrates suggests that tight baseline pH control provides an opportunity for even small pH changes to exert a signaling effect through acid receptors [84, 85]. To understand the physiological importance of these pH fluctuations and how the fluctuations are generated, improved strategies for measuring pH changes in vivo are needed.
The location of ASIC1a and mediating acidosis effects on synaptic and neural activity
Most of the established understanding of ASICs in the CNS is derived from knowledge of ASIC1a. ASIC1a, located in cell bodies and dendrites, is enriched in post-synaptic dendritic spines [2]. Experimental observations suggest that an association with ASIC2a assists the movement of ASIC1a from cell bodies to the dendritic spines [6, 86]. At dendritic spines, ASIC1a confers acid sensitivity, increases intracellular Ca2+ concentration, regulates spine number [6, 86], and facilitates synaptic plasticity [87]. In addition, an intriguing ASIC1a-dependent inhibition of synaptic vesicle release has been observed in cultured hippocampal neurons [88]. The mechanism for this effect of ASIC1a on vesicle release is unknown, but appears to be presynaptic and pH-independent [88]. ASIC-1, an ASIC homolog in Caenorhabditis elegans, was determined to be located presynaptically and facilitated associated learning by C elegans by increasing dopamine signaling [85]. Together, these observations suggest important roles for ASICs at synapses. Given that synaptic transmission is critical for brain function and behavior, ASIC dysfunction might contribute to psychiatric and neurological diseases. Emerging evidence from animal models supports this possibility.
The role of ASICs in anxiety and depression-related behaviors
In the mouse brain, ASIC1a is abundant in regions that are critical for anxiety and depression. Immunohistochemistry identified robust ASIC1a expression in the basolateral amygdala, bed nucleus of the stria terminalis, cingulate cortex, hypothalamus and periaqueductal gray matter [43, 89]. Consistent with this distribution of the protein, disrupting ASIC1a in mice reduced auditory cue and contextual fear conditioning [89], whereas the overexpression of ASIC1a increased contextual fear conditioning [90]. Similarly, disrupting ASIC1a in these animals reduced the unconditioned fear response to trimethylthiazoline (TMT; a predator odor from foxes), as well as to acoustic startle and fear of open spaces [43]. Disrupting ASIC1a in mice also attenuated depression-related behavior in the forced swim, tail suspension and chronic mild stress tests [55]. In addition, the loss of ASIC1a attenuated an important biomarker of depression: the ability of stress to reduce brain-derived neurotrophic factor (BDNF) levels in the hippocampus. The basolateral amygdala was identified as a key site of ASIC1a action; restoring ASIC1a expression to the basolateral amygdala of ASIC1a−/− mice (with an adeno-associated virus vector) reversed the deficit in contextual fear memory and normalized forced swim behavior [55, 91]. However, the amygdala is not the only important site of ASIC1a activity; restoring ASIC1a expression to the basolateral amygdala of ASIC1a−/− mice did not rescue the freezing deficit observed immediately following foot-shock [91]. In addition, restoring ASIC1a expression to the basolateral amygdala did not improve the deficit in the unconditioned fear response to TMT in ASIC1a−/− mice, a behavior that might rely on the presence of ASIC1a in the bed nucleus of the stria terminalis or the medial amygdala [91].
Anxiety- and depression-related effects of pharmacologically inhibiting ASICs
The observation that ASIC1a−/− mice exhibit behavior similar to that induced by antidepressant or anxiolytic medications [55] suggested that pharmacological blockade of ASICs might provide a novel approach to treating anxiety and depression. In support of this hypothesis, three ASIC antagonists were observed to reduce anxiety, fear and depression-related behaviors in mice. PcTx1 attenuated TMT-evoked fear and reduced immobility in the forced swim test [43, 55] (Figure 4). Similarly, PcTx1 reduced punished crossings in the four-plate test, and decreased stress-induced elevations in core body temperature [92]. No effect from PcTx1 was observed in ASIC1a−/− mice, suggesting that PcTx1 exhibits an ASIC1a-specific action [43, 55]. The non-specific ASIC inhibitors A-317567 [61] and amiloride [92] exhibited similar fear-reducing effects, which were comparable to those produced by the GABAA-receptor agonist alprazolam. A-317567 also mimicked the effects of antidepressants in the forced swim test [55] (Figure 4). The depletion of serotonin did not lessen the antidepressant-like effect of ASIC1a inhibition [55], implying that ASIC1a blockers might operate through a novel mechanism. For example, the effects of ASIC1a were additive in combination with several common antidepressants [55]. Therefore, ASIC1a inhibitors might improve antidepressant efficacy if used in conjunction with current therapies. Because of their novel mode of action, ASIC1a inhibitors might also benefit patients who do not respond to current antidepressants.
Figure 4. Attenuated depression-related behavior with the ASIC1a inhibitor A-317567.
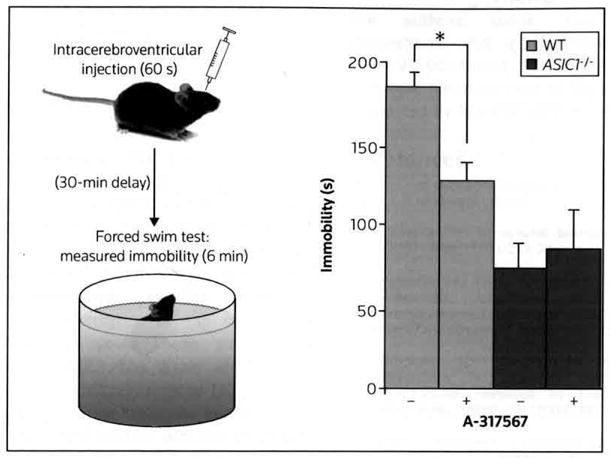
In the forced swim test, mice were placed in a beaker of tepid water, and the time spent immobile was quantified; antidepressants are known to reduce immobility in this test. Wild-type mice injected with A-317567 exhibited significantly less immobility than those injected with vehicle. ASIC1a−/− mice were less immobile than wild-type mice, and the administration of A-317567 produced no effect in the knockout mice. *p < 0.01
(Adapted with permission from The Society for Neuroscience and Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z et al: Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci (2009) 29(17):5381–5388 © 2009 The Society for Neuroscience)
ASIC acid-sensing ion channel, WT wild-type
Ischemic stroke: A recipe for ASIC activation and cell death
Ischemia dramatically reduces brain pH levels, which aggravate cell damage and death [93–95]. Synergistic factors in the ischemic brain may promote ASIC-mediated cell death. Energy consumption by ATP hydrolysis generates protons, which, coupled with lactate production by anaerobic glycolysis, cause brain pH levels to decrease significantly below the normal physiological range (to ≤ pH 6.5) [96, 97]. Lactate, arachidonic acid and neuropeptide release, cellular swelling and glutamate receptor activation may all occur during ischemia, and may facilitate ASIC activation and cell death [9, 88, 95, 98, 99]; the resulting toxicity is likely to be Ca2+-mediated, Ischemic conditions may be ideal for activating ASICs, and therefore there may be potential benefits of blocking ASICs during a stroke. Several studies support the concept that blocking ASIC1a may be a useful approach to stroke therapy.
In cells heterologously expressing ASICs and in hippocampal neurons, the removal or blockade of ASIC1a reduced acidosis-related cell death [7]. In addition, genetically and pharmacologically disrupting ASIC1a diminished infarct volume in mice and rats following middle cerebral artery occlusion (MCAO) [7,100]. Interestingly, PcTx1 reduced infarct volume even when administered 5 h post MCAO [100]; therefore, the timeframe for administering ASIC inhibitors might be larger than the 3-h window recognized for current anticoagulant therapy. ASIC inhibition might also explain the benefits of other potential interventions. σ1 receptor agonists have been reported to reduce infarct volume when administered up to 24 h post MCAO [101]; the same σ receptor agonists have been demonstrated to reduce ASIC1a-evoked Ca2+ influx [12]. As well as reducing Ca2+-mediated toxicity, ASIC inhibition might provide protection from brain injury via other mechanisms. For example, neurotrophic factors could play an important role in stroke recovery; therefore, the recent observation that ASIC1a−/− mice are resistant to stress-induced BDNF reduction [55] implies that ASIC1a blockade might help to maintain levels of BDNF following an ischemic insult.
Following the failure of glutamate receptor antagonists to be developed as successful stroke therapies, the need for the development of improved therapies for this significant cause of mortality remains. Novel targets such as ASICs and combination therapies targeting multiple pathological pathways might provide opportunities for the development of more effective stroke treatments. These drug development efforts might also lead to new therapies for other neurological diseases.
The potential for targeting ASICs in neurodegenerative diseases
Inflammation and accompanying acidosis are symptoms shared by several neurodegenerative diseases, suggesting that ASIC inhibitors might minimize the damaging effects of inflammatory acidosis in these conditions. Emerging research suggests that ASIC inhibitors may reduce damage in animal models of neurodegenerative diseases, including multiple sclerosis [102], Parkinson’s disease [103] and Huntington’s disease [104]. Traumatic brain injury (TBI), which can lead to neurogenerative diseases, is also known to lower brain pH [105]; thus, ASIC inhibitors could potentially reduce TBI-associated damage. These data indicate that ASIC blockade may be a potential neuroprotective strategy.
The importance of ASICs in seizures
In addition to the possible benefits of inhibiting ASICs as described previously, the activation of ASICs might also prove to be therapeutically relevant in some cases. For example, although some research suggests that ASIC activation contributes to seizures and associated cellular damage [7], other studies propose evidence that acidosis inhibits seizures, and that ASICs may be involved in the termination of seizures [106]. These apparently contradictory observations lead to questions such as whether ASIC blockade reduces or worsens seizures, what are the underlying causes of these conflicting results, and if ASIC activation and central acidosis could be used to control seizures. The different seizure models used in these studies may explain some of variable results observed. ASIC blockade might produce some unwanted side effects, but because ASIC1a−/− mice did not exhibit spontaneous seizures [106], ASIC1a inhibitors will likely not cause seizures to develop de novo. However, ASIC1a inhibitors might worsen seizures in seizure-prone individuals. Conversely, ASIC1a activators might be used to halt the unabated seizures of status epilepticus.
The role of ASICs in retinal function
ASIC1, ASIC2 and ASIC3 are present in the retina, and exhibit multifaceted effects, including the probable mediation of pH responses [107–111]. Alterations in retinal pH can dramatically shift light sensitivity, and ASICs appear to have an important role in this response. However, different ASICs have different effects; for example, blocking ASIC1a decreased light sensitivity [109], whereas blocking ASIC2 increased light sensitivity and the risk of light-induced retinal degeneration [108]. ASIC3 may also play an important role in protecting against late-onset photoreceptor death [112]. Thus, ASIC inhibitors could improve some aspects of vision, but might also increase the risk of retinal degeneration. These differential effects of disrupting ASIC suggest that it might be necessary to target ASICs in specific tissues or to target specific subunits to avoid unwanted side effects.
The roles of other ASICs and ASIC modulators
Advances in the understanding of ASICs are encouraging, but many questions remain unanswered. These questions include what normally activates ASICs in the brain; what is the physiological role of H+-evoked currents in diverse brain regions such as the striatum [113] and suprachiasmatic nucleus [114]; if ASICs at these sites influence motivation, movement, circadian rhythm or sleep; and what is the importance of the other ASIC subunits in the brain, and could these subunits be therapeutically modified. ASIC3 has been identified in the rat hypothalamus and other brain regions [47], and the functional effects of ASIC3 in the brain will be interesting to elucidate. ASIC2a, 2b, 4 and BLINaC are also expressed in the brain, but little information is known regarding their function. Thus, whether other ASICs and ASIC modulators could be targeted therapeutically remains to be determined.
Conclusion
Although potential therapeutic indications for targeting ASICs have been outlined in this review, several challenges must be overcome before the ASICs can be exploited in the clinic. For example, the current understanding of ASICs in pain and in psychiatric and neurological diseases is derived mostly from animal models, and thus requires human validation. An important first step in this process was demonstrating that the non-specific ASIC inhibitor amiloride could reduce cutaneous acid-evoked pain in humans [115]. The validation of ASICs as targets for the treatment of human disease might be derived from research in genetics, and might also result from an improved understanding of the role of pH in human physiology. Establishing the nature of the pH changes that activate ASICs in vivo will lead to greater knowledge of the physiological purposes of these intriguing channels. However, the most important challenge that remains is the development of more specific and effective pharmaceuticals targeting ASICs. Amiloride has been used safely to treat hypertension in the clinic, but the concentration of amiloride required to block ASICs is higher than doses used for hypertension. Thus, whether the administration of ASIC-blocking doses of amiloride might produce dangerous side effects such as hyperkalemia, resulting from the inhibition of DEG/ENaC channels in the kidney, remains unknown. Research has identified more potent and specific ASIC antagonists [61, 116], but has not yet produced compounds that are suitable for clinical use. Fundamentally, ASICs (and pH more generally) are promising therapeutic targets, but more knowledge and tools are needed to translate this promise into reality.
Acknowledgments
The authors thank Jennifer Brown for helpful suggestions. JAW is supported by the VA Merit Review, NIH R21 00230884, NIH RO100468381, NARSAD and the McKnight Neuroscience of Brain Disorders Award. KAS is supported by NIH RO1 AR053509.
Abbreviations
- ASIC
acid-sensing ion channel
- BDNF
brain-derived neurotrophic factor
- DEG/ENaC
degenerin/epithelial sodium channel
- DRG
dorsal root ganglion
- PcTx1
psalmotoxin
- TMT
trimethylthiazoline
References
•• of outstanding interest
• of special interest
- 1.Canessa CM. Structural biology: Unexpected opening. Nature. 2007;449(7160):293–294. doi: 10.1038/449293a. [DOI] [PubMed] [Google Scholar]
- 2•.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: Advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29(10):578–586. doi: 10.1016/j.tins.2006.06.014. Review of the structure, function and physiological roles of ASICs. [DOI] [PubMed] [Google Scholar]
- 3.Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life. 2008;60(9):620–628. doi: 10.1002/iub.89. [DOI] [PubMed] [Google Scholar]
- 4••.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 2007;449(7160):316–323. doi: 10.1038/nature06163. Discloses the first crystal structures of ASICs and DEG/ENaCs. [DOI] [PubMed] [Google Scholar]
- 5.Qadri YJ, Berdiev BK, Song Y, Lippton HL, Fuller CM, Benos DJ. Psalmotoxin-1 docking to human acid sensing ion channel-1. J Biol Chem. 2009;284(26):17625–17633. doi: 10.1074/jbc.M109.003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zha XM, Wang R, Collier DM, Snyder PM, Wemmie JA, Welsh MJ. Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc Natl Acad Sci USA. 2009;106(9):3573–3578. doi: 10.1073/pnas.0813402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118(6):687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA. 2004;101(17):6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48(4):635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci USA. 2006;103(44):16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samways DS, Harkins AB, Egan TM. Native and recombinant ASIC1a receptors conduct negligible Ca2+ entry. Cell Calcium. 2009;45(4):319–325. doi: 10.1016/j.ceca.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrera Y, Katnik C, Rodriguez JD, Hall AA, Willing A, Pennypacker KR, Cuevas J. σ1, receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther. 2008;327(2):491–502. doi: 10.1124/jpet.108.143974. [DOI] [PubMed] [Google Scholar]
- 13.Gründer S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport. 2000;11(8):1607–1611. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- 14.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimerics of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA. 2002;99(4):2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesselager M, Timmermann DB, Ahring PK. pH-dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279(12):11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- 16.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2003;279(18):18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- 17.Cadiou H, Studer M, Jones NG, Smith ES, Ballard A, McMahon SB, McNaughton PA. Modulation of acid-sensing ion channel activity by nitric oxide. J Neurosci. 2007;27(48):13251–13260. doi: 10.1523/JNEUROSCI.2135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140(2):254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993;154(1–2):113–116. doi: 10.1016/0304-3940(93)90184-m. [DOI] [PubMed] [Google Scholar]
- 20.Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res. 1996;113:143–151. doi: 10.1016/s0079-6123(08)61085-7. [DOI] [PubMed] [Google Scholar]
- 21.Goldie I, Nachemson A. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop Scand. 1969;40(5):634–641. doi: 10.3109/17453676908989529. [DOI] [PubMed] [Google Scholar]
- 22.Geborek P, Saxne T, Pettersson H, Wollheim FA. Synovial fluid acidosis correlates with radiological joint destruction in rheumatoid arthritis knee joints. J Rheumatol. 1989;16(4):468–472. [PubMed] [Google Scholar]
- 23•.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106(3):229–239. doi: 10.1016/S0304-3959(03)00269-0. Identifies a role for ASIC3 in mechanical hyperalgesia after repeated intramuscular injections of acid. [DOI] [PubMed] [Google Scholar]
- 24.Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129(1–2):102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: A mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208(3):191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- 26.Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993;154(1–2):113–116. doi: 10.1016/0304-3940(93)90184-m. [DOI] [PubMed] [Google Scholar]
- 27.Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24(48):10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishtal OA, Pidoplichko VI. A ‘receptor’ for protons in small neurons of trigeminal ganglia: Possible role in nociception. Neurosci Lett. 1981;24(3):243–246. doi: 10.1016/0304-3940(81)90164-6. [DOI] [PubMed] [Google Scholar]
- 29.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272(34):20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 30.Rehncrona S. Brain acidosis. Ann Emerg Med. 1985;14(8):770–776. doi: 10.1016/s0196-0644(85)80055-x. [DOI] [PubMed] [Google Scholar]
- 31.Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuron. 1998;9(6):1109–1113. doi: 10.1097/00001756-199804200-00028. [DOI] [PubMed] [Google Scholar]
- 32.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32(6):1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 33.Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137(3):662–669. doi: 10.1016/j.pain.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain. 2009;10(3):336–342. doi: 10.1016/j.jpain.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386(6621):173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 37.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997;272(34):20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 38.Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279(42):43716–43724. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- 40.Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu L, Chen J, Xu TL. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci. 2007;27(41):11139–11148. doi: 10.1523/JNEUROSCI.3364-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008;28(6):1498–1508. doi: 10.1523/JNEUROSCI.4975-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira J, Santos AR, Calixto JB. Antinociception produced by systemic, spinal and supraspinal administration of amiloride in mice. Life Sci. 1999;65(10):1059–1066. doi: 10.1016/s0024-3205(99)00336-7. [DOI] [PubMed] [Google Scholar]
- 43.Coryell M, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62(10):1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 44••.Mazzuca M, Heurteaux C, Alloui A, Diochot S, Baron A, Voilley N, Blondeau N, Escoubas P, Gélot A, Cupo A, Zimmer A, et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nat Neurosci. 2007;10(8):943–945. doi: 10.1038/nn1940. Describes fascinating interactions between ASICs and opioid signaling. [DOI] [PubMed] [Google Scholar]
- 45.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271(14):7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Añoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA. 1997;94(4):1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng QY, Wang W, Chen XN, Xu TL, Zhou JN. Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience. 2009;159(3):1126–1134. doi: 10.1016/j.neuroscience.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 48.Jahr H, van Driel M, van Osth GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun. 2005;337(1):349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 49.Tan ZY, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ Res. 2007;101(10):1009–1019. doi: 10.1161/CIRCRESAHA.107.154377. [DOI] [PubMed] [Google Scholar]
- 50.Ohtori S, Inoue G, Koshi T, Ito T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of acid-sensing ion channel 3 in dorsal root ganglion neurons following application of nucleus pulposus on nerve root in rats. Spine. 2006;31(18):2048–2052. doi: 10.1097/01.brs.0000231756.56230.13. [DOI] [PubMed] [Google Scholar]
- 51.Gitterman DP, Wilson J, Randall AD. Functional properties and pharmacological inhibition of ASIC channels in the human SJ-RH30 skeletal muscle cell line. J Physiol. 2005;562(Pt 3):759–769. doi: 10.1113/jphysiol.2004.075069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21(20):8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39(5):1107–1115. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 54.Dorofeeva NA, Barygin OI, Staruschenko A, Bolshakov KV, Magazanik LG. Mechanisms of non-steroid anti-inflammatory drugs action on ASICs expressed in hippocampal interneurons. J Neurochem. 2008;106(1):429–441. doi: 10.1111/j.1471-4159.2008.05412.x. [DOI] [PubMed] [Google Scholar]
- 55•.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, et al. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29(17):5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. First paper to identify a role for ASICs in depression-related behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54(10):1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia following inflammatory muscle injury. J Pain. 2009 doi: 10.1016/j.jpain.2009.07.004. in press. Reveals the role of ASI3 in inflammation-evoked and acid-evoked secondary hyperalgesia and the role of ASIC1 in primary hyperalgesia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Séguéla P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25(43):9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA. 2002;99(13):8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staniland AA, McMahon SB. Mice lacking acid-sensing ion channels (ASIC) 1 or 2, but not ASIC3, show increased pain behaviour in the formalin test. Eur J Pain. 2009;13(6):554–563. doi: 10.1016/j.ejpain.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honoré P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117(1–2):88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 62•.Deval E, Noël J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27(22):3047–3055. doi: 10.1038/emboj.2008.213. Suggests that, with inflammatory pain, ASIC3 mediates the response to a combination of factors, including hypertonicity and arachidonic acid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Rocha-Gonzalez HI, Herrejon-Abreu EB, López-Santillán FJ, García-López BE, Murbartián J, Granados-Soto V. Acid increases inflammatory pain in rats: Effect of local peripheral ASICs inhibitors. Eur J Pharmacol. 2009;603(1–3):56–61. doi: 10.1016/j.ejphar.2008.12.017. Suggests that ASICs contribute to pain-related behavioral responses to serotonin, capsaicin and low pH, and to formalin when pH levels are low. [DOI] [PubMed] [Google Scholar]
- 64.Yen YT, Tu PH, Chen CJ, Lin YW, Hsieh ST, Chen CC. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol Pain. 2009;5:1. doi: 10.1186/1744-8069-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140(2):292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 66.Sluka KA, Price MP, Wemmie JA, Welsh MJ. ASIC3, but not ASIC1, channels are involved in the development of chronic muscle pain. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain, Progress in Pain Research and Management. Vol. 24. IASP Press; Seattle, USA: 2003. pp. 71–79. [Google Scholar]
- 67.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100(3):1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101(2):468–475. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 69.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84(8):921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 70.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA. 2001;98(2):711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, Blackshaw LA. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127(6):1739–1747. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 72.Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25(47):10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24(1):37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 74.Yokoyama T, Maeda Y, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8(5):422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 75.Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Wemmie JA, Zha X-M, Welsh MJ. Acid-sensing ion channels (ASICs) and pH in synapse physiology. In: Hell JW, Ehlers MD, editors. Structural and functional organization of the synapse. Springer Science+Business Media LLC; New York, NY, USA: 2008. pp. 661–682. [Google Scholar]
- 77.DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32(6):1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- 78.Vessey JP, Stratis AK, Daniels BA, Da Silva N, Jonz MG, Lalonde MR, Baldridge WH, Barnes S. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25(16):4108–4117. doi: 10.1523/JNEUROSCI.5253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmer MJ, Hull C, Vigh J, von Gersdorff H. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23(36):11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demaurex N, Poburko D, Frieden M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbabio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Maddock RJ, Buonocore MH, Copeland LE, Richards AL. Elevated brain lactate responses to neural activation in panic disorder: A dynamic 1H-MRS study. Mol Psychiatry. 2008;14(5):537–545. doi: 10.1038/sj.mp.4002137. [DOI] [PubMed] [Google Scholar]
- 82.Stewart PA. Independent and dependent variables of acid-base control. Respir Physiol. 1978;33(1):9–26. doi: 10.1016/0034-5687(78)90079-8. [DOI] [PubMed] [Google Scholar]
- 83.Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20(4–5):291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 84.Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM. Protons act as a transmitter for muscle contraction in C elegans. Cell. 2008;132(1):149–160. doi: 10.1016/j.cell.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Voglis G, Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C elegans by facilitating dopamine signalling. EMBO J. 2008;27(24):3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29(26):8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34(3):463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 88•.Cho JH, Askwith CC. Presynaptic release probability is increased in hippocampal neurons from ASIC1 knockout mice. J Neurophysiol. 2008;99(2):426–441. doi: 10.1152/jn.00940.2007. Identifies the effect of ASICs on presynaptic release probability. [DOI] [PubMed] [Google Scholar]
- 89.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23(13):5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wemmie J, Coryell M, Askwith C, Lamani E, Leonard S, Sigmund C, Welsh M. Overexpression of acid-sensing ion channel 1a in transgenic mice increases fear-related behavior. Proc Natl Acad Sci USA. 2004;101(10):3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, McBride JL, Davidson BL, Wemmie JA. Restoring acid-sensing ion channel-1a in the amygdala of knock-out mice rescues fear memory but not unconditioned fear responses. J Neurosci. 2008;28(51):13738–13741. doi: 10.1523/JNEUROSCI.3907-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dwyer JM, Rizzo SJ, Neal SJ, Lin Q, Jow F, Arias RL, Rosenzweig-Lipson S, Dunlop J, Beyer CE. Acid sensing ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in preclinical pharmacological models. Psychopharmacology (Berl) 2009;203(1):41–52. doi: 10.1007/s00213-008-1373-7. [DOI] [PubMed] [Google Scholar]
- 93.Obrenovitch TP, Scheller D, Matsumoto T, Tegtmeier F, Höller M, Symon L. A rapid redistribution of hydrogen ions is associated with depolarization and repolarization subsequent to cerebral ischemia reperfusion. J Neurophysiol. 1990;64(4):1125–1133. doi: 10.1152/jn.1990.64.4.1125. [DOI] [PubMed] [Google Scholar]
- 94.Diarra A, Sheldon C, Brett CL, Baimbridge KG, Church J. Anoxia-evoked intracellular pH and Ca2+ concentration changes in cultured postnatal rat hippocampal neurons. Neuroscience. 1999;93(3):1003–1016. doi: 10.1016/s0306-4522(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 95.Allen NJ, Attwell D. Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischaemia-related signals. J Physiol. 2002;543(Pt 2):521–529. doi: 10.1113/jphysiol.2002.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katsura K, Siesjŏ BK. Acid-base metabolism in ischemia. In: Kaila K, Ransom BR, editors. pH and brain function. Wiley-Liss; New York, NY, USA: 1998. pp. 563–582. [Google Scholar]
- 97.Siesjo BK, Katsura K, Kristián T. Acidosis-related damage. Adv Neurol. 1996;71:209–233. discussion 234–236. [PubMed] [Google Scholar]
- 98.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4(9):869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 99.Smith ES, Cadiou H, McNaughton PA. Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. Neuroscience. 2007;145(2):686–698. doi: 10.1016/j.neuroscience.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 100.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130(Pt 1):151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 101.Ajmo CT, Jr, Vernon DO, Collier L, Pennypacker KR, Cuevas J. σ receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3(2):89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- 102•.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13(12):1483–1489. doi: 10.1038/nm1668. Describes the role of acidosis and ASICs in a mouse model of multiple sclerosis. [DOI] [PubMed] [Google Scholar]
- 103.Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, Kagan N, Beyer C, Lin Q, Dwyer JM, Zaleska MM, et al. Amiloride is neuroprotective in an MPTP model of Parkinson’s disease. Neurobiol Dis. 2008;31(3):334–341. doi: 10.1016/j.nbd.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 104.Wong HK, Bauer PO, Kurosawa M, Goswami A, Washizu C, Machida Y, Tosaki A, Yamada M, Knöpfel T, Nakamura T, Nukina N. Blocking acid-sensing ion channel 1 alleviates Huntington’s disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet. 2008;17(20):3223–3235. doi: 10.1093/hmg/ddn218. [DOI] [PubMed] [Google Scholar]
- 105.Zhao X, Gorin FA, Berman RF, Lyeth BG. Differential hippocampal protection when blocking intracellular sodium and calcium entry during traumatic brain injury in rats. J Neurotrauma. 2008;25(10):1195–1205. doi: 10.1089/neu.2008.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106•.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, 3rd, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11(7):816–822. doi: 10.1038/nn.2132. Identifies role for ASIC1a in acidosis-induced seizure termination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol Cell Physiol. 2002;283(1):C126–C134. doi: 10.1152/ajpcell.00457.2001. [DOI] [PubMed] [Google Scholar]
- 108•.Ettaiche M, Guy N, Hofman P, Lazdunski M, Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci. 2004;24(5):1005–1012. doi: 10.1523/JNEUROSCI.4698-03.2004. The first paper to identify a role for ASICs in retinal function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ettaiche M, Deval E, Cougnon M, Lazdunski M, Voilley N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J Neurosci. 2006;26(21):5800–5809. doi: 10.1523/JNEUROSCI.0344-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brockway LM, Benos DJ, Keyser KT, Kraft TW. Blockade of amiloride-sensitive sodium channels alters multiple components of the mammalian electroretinogram. Vis Neurosci. 2005;22(2):143–151. doi: 10.1017/S0952523805222034. [DOI] [PubMed] [Google Scholar]
- 111.Lilley S, LeTissier P, Robbins J. The discovery and characterization of a proton-gated sodium current in rat retinal ganglion cells. J Neurosci. 2004;24(5):1013–1022. doi: 10.1523/JNEUROSCI.3191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ettaiche M, Deval E, Pagnotta S, Lazdunski M, Lingueglia E. Acid-sensing ion channel 3 in retinal function and survival. Invest Ophthalmol Vis Sci. 2009;50(5):2417–2426. doi: 10.1167/iovs.08-3028. [DOI] [PubMed] [Google Scholar]
- 113.Chu Y, Su X, Huang Q, Zhang X. Patterns of DNA sequence variation at candidate gene loci in black poplar (Populus nigra L) as revealed by single nucleotide polymorphisms. Genetica. 2009 doi: 10.1007/s10709-009-9371-1. [DOI] [PubMed] [Google Scholar]
- 114.Scott G, Rusak B. Activation of hamster suprachiasmatic neurons in vitro via metabotropic glutamate receptors. Neuroscience. 1996;71(2):533–541. doi: 10.1016/0306-4522(95)00438-6. [DOI] [PubMed] [Google Scholar]
- 115.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110(8):1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuduk SD, Di Marco CN, Chang RK, Dipardo RM, Cook SP, Cato MJ, Jovanovska A, Urban MO, Leitl M, Spencer RH, Kane SA, et al. Amiloride derived inhibitors of acid-sensing ion channel-3 (ASIC3) Bioorg Med Chem Lett. 2009;19(9):2514–2518. doi: 10.1016/j.bmcl.2009.03.029. [DOI] [PubMed] [Google Scholar]


