Abstract
Despite a general trend for larger mammals to have larger brains, humans are the primates with the largest brain and number of neurons, but not the largest body mass. Why are great apes, the largest primates, not also those endowed with the largest brains? Recently, we showed that the energetic cost of the brain is a linear function of its numbers of neurons. Here we show that metabolic limitations that result from the number of hours available for feeding and the low caloric yield of raw foods impose a tradeoff between body size and number of brain neurons, which explains the small brain size of great apes compared with their large body size. This limitation was probably overcome in Homo erectus with the shift to a cooked diet. Absent the requirement to spend most available hours of the day feeding, the combination of newly freed time and a large number of brain neurons affordable on a cooked diet may thus have been a major positive driving force to the rapid increased in brain size in human evolution.
Keywords: encephalization, expensive tissue hypothesis, brain metabolism
The human brain is a linearly scaled-up primate brain in its relationship between brain size and number of neurons (1), having evolved while being subjected to the same cellular scaling rules that apply to primates as a whole (2, 3), including great apes (4). With the largest brain among primates, humans thus have the largest number of neurons among these and possibly all mammals (5), a number that we estimate to be close to three times larger than in gorillas and orangutans, owners of the next largest brains among extant primates (4). We are not outstanding primates, on the contrary, in body size: gorillas can grow to be three times larger than humans. This discrepancy between body and brain size led to the predominant view that encephalization (that is, a larger brain size than expected for body size) is the main characteristic that sets humans apart from other primates and mammals as a whole (6). Although a relatively large brain compared with body size is expected to bring cognitive advantages (6), and despite the well-documented evidence for an increase in brain size during human evolution (7), there is still no consensus on what mechanisms or reasons led to brain enlargement in the Homo lineage.
Why are the largest primates not those endowed with the largest brains as well? Rather than evidence that humans are an exception among primates, we consider this disparity to be a clue that, in primate evolution, developing a very large body and a very large brain have been mutually excluding strategies, probably because of metabolic reasons (4, 8). The brain is the third most energy-expensive organ in the human body, ranking in total organ metabolic cost below only skeletal muscle and liver (9). Accordingly, several studies have suggested that the main constraints to increasing primate brain size in evolution are metabolic in nature (10–18). The human brain, in particular, has come to cost ∼20% of the total body resting metabolic rate, even though it represents only 2% of total body mass (MBD), whereas, in other primates, the brain consumes a lower percentage of the body resting metabolic rate of approximately 9% (17). Even though a greater relative brain size in mammals has recently been found not to correlate with a smaller relative size of the digestive tract or other expensive organs (19), as predicted by the expensive-tissue hypothesis (10), increases in absolute brain size are expected to have direct metabolic consequences. The trend toward a larger brain size in primate evolution (20), particularly in the hominin lineage, has thus presumably occurred in the face of limitations imposed by the increasing metabolic cost of larger brains. Such limitations, however, have not been evaluated quantitatively.
Larger bodies, another related trend in primate evolution that is often [but not always (20)] related to larger brains, also require more energy for their maintenance. The daily energetic cost (measured from the basal metabolic rate) of mammalian bodies scales across species as a power function of MBD with an average exponent of 0.75 (21–23). The greater the caloric need of a species, the greater the time that must be spent on feeding, which is modulated by factors such as food availability (24), time required for ingestion [which varies depending on the food composition and the structure and capacity of the oral cavity (25)], the digestive rate of the gastrointestinal system (26), and the caloric income of the diet (27, 28).
Despite the obvious metabolic costs entailed by increasing brain mass (MBR) and MBD, it remains to be determined whether brain and body size are indeed metabolically limiting in a way that would be physiologically relevant and constraining for primate evolution. The energetic viability of a nonhibernating primate species depends on the balance between the energy requirements associated to its MBD and its brain size, and its daily caloric intake (EIN) during the hours available for eating. It has previously been considered that the energetic cost of the brain scales more slowly than MBR, varying with MBR0.85 (29, 30). Given that the metabolic cost of the body also scales less than linearly, with MBD0.75, and that MBR scales linearly at most, if not less than linearly, with MBD (3), it would suffice for the daily EIN to scale with MBD raised to an exponent of at least 0.85 for metabolism not to be a limiting factor to brain and body expansion in primate evolution—as long as there were enough hours in the day available for feeding.
However, we have recently shown the total daily metabolic cost of primate and rodent brains is a linear function of their numbers of neurons, regardless of how MBR changes depending on its number of neurons, varying at an average cost of 6 × 10−9 kcal per neuron, or 6 kCal per billion neurons (8). Given that primate MBR scales linearly to its number of neurons (2, 3), a relationship that also applies to humans, great apes, and thus supposedly to extinct hominins (1, 4), the finding that brain metabolism scales linearly with its number of neurons implies that, in primates, brain metabolism also scales linearly with brain size (8). This is in contrast to earlier reports that brain metabolism scaled more slowly than MBR, the results of which were skewed by combining primates and other mammals in the analysis under the assumption, now known to be wrong, that MBR scaled hypermetrically with number of neurons across all species (17, 30). Because this is a much faster rate of scaling of brain metabolism than thus far acknowledged, it raises the possibility that metabolism may indeed have been a much more limiting factor than previously suspected to increasing numbers of brain neurons in evolution, particularly given that, across non–great-ape primates, MBR can scale linearly with MBD (2, 3).
Here we examine whether, given the existent raw diet of nonhuman primates, brain and body size can indeed pose metabolic constraints to brain and MBD expansion in primate and human evolution. By estimating how EIN scales with MBD and using this estimate to calculate how many hours of feeding on raw foods would be required to afford increasing MBD and number of brain neurons, we provide evidence that brain and MBD in large primates are indeed limiting, such that, on a raw foods diet, a tradeoff must exist between MBD and number of brain neurons, with direct implications for human brain evolution.
Results
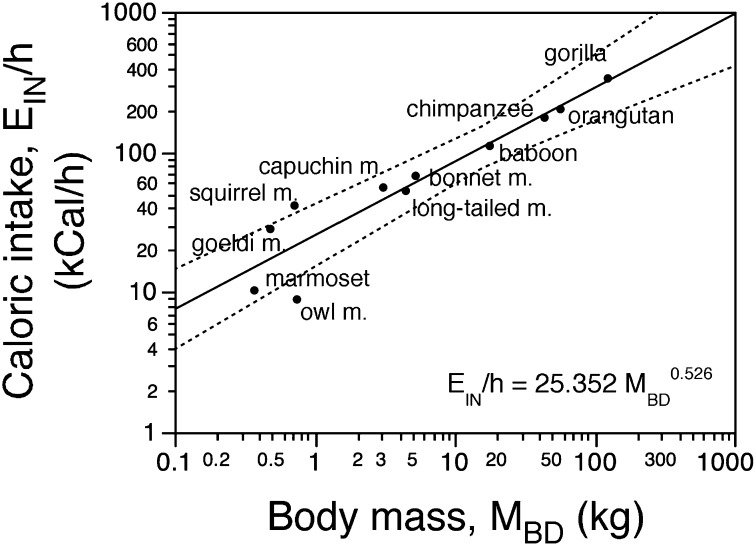
For each of 11 nonhuman primate species varying by a factor of 335 in MBD, from Callithrix jacchus to Gorilla gorilla, we calculated the average EIN per hour spent feeding by dividing the average daily caloric need per species [estimated from the law of Kleiber (21, 22) using body masses previously published (25) and not excluding MBR, given that it is relatively very small, of the order of 2% of MBD] by the average number of hours per day spent feeding (24, 31–40) (Table S1). We find that the average EIN per hour increases together with MBD such that EIN/h is equal to 25.352 × MBD0.526 (P < 0.0001 for both constant and exponent; 95% CI for the exponent, 0.392–0.660) and varies from 8.9 kCal/h in the owl monkey and 10.3 kCal/h in the marmoset to 334.7 kCal/h in the gorilla (Fig. 1).
Fig. 1.
Estimated EIN per hour of feeding for each species, EIN/h, scales with MBD0.526. Values of EIN/h were calculated from the predicted daily caloric need (from Kleiber law applied to the MBD values in Table S1) divided by the number of hours spent feeding per day (Table S1). Individual species are indicated.
The total daily EIN for a species is dependent on the number of hours it spends feeding per day, such that EIN is equal to the number of hours × 25.352 × MBD0.526. Two limitations become evident from this relationship. First, EIN scales much more slowly than MBD, with approximately its square root, and thus also more slowly than the caloric requirement of the body. This finding by itself indicates that there is an upper limit to the body size that a primate can afford. Second, considering a practical limitation of 10 h available for feeding in a day, which is the most that gorillas are know to spend feeding (24), the maximal total EIN per day for a primate species is limited to 253.52 × MBD0.526.
Given that the maximal total EIN per day and the daily metabolic cost of the body scale with MBD, it is possible to estimate the maximal MBD for a primate that spent a putative limit of 10 h/d eating as the value of MBD for which EIN equals the metabolic cost of the body. This calculation, which does not consider the cost that would be accrued by the number of neurons in the brain, yields an estimate of 312.7 kg. For an animal that feeds for 8 h/d, such as the gorilla, the maximal MBD estimated would decrease to 115.5 kg.
Considering that MBR is a very small fraction of MBD, and that the metabolic cost of the brain depends directly on its number of neurons (8), we next calculate separately the metabolic cost of body and brain. The first, MBD, is estimated by Kleiber law to amount to 70 × MBD0.75 kCal/d. The second, EBR, is estimated to equal 6 × 10−9 × N kCal/d, where N is the number of neurons in the brain (8). By equating the total estimated daily energy expenditure MBD+EBR to the daily EIN, we can calculate the number of neurons that a primate of a certain MBD can afford upon feeding a certain number of hours per day.
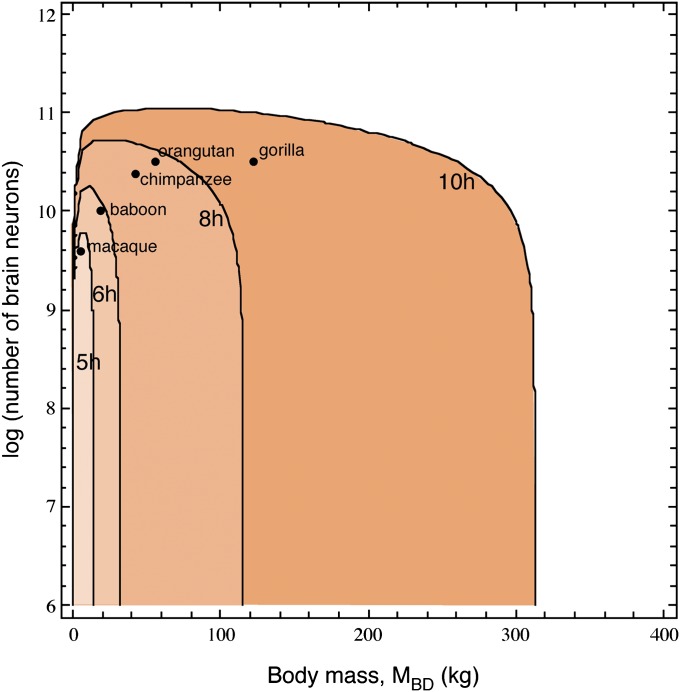
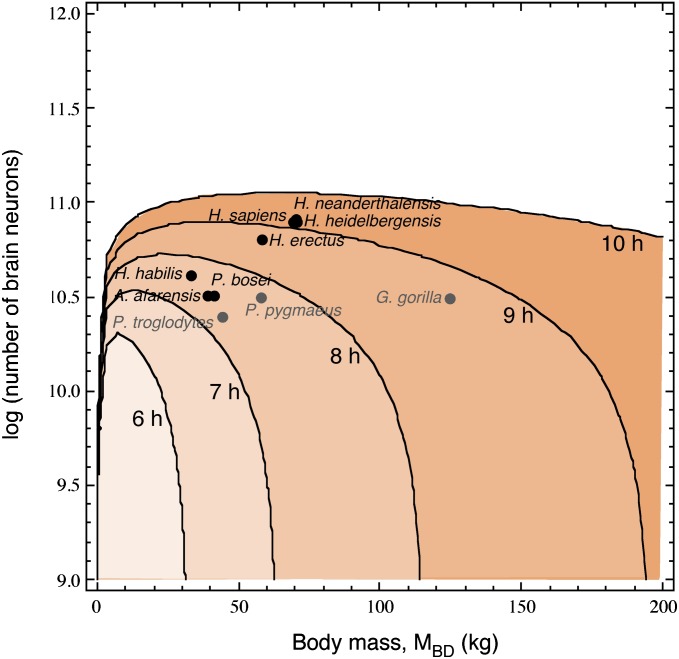
The result of this three-variable analysis is illustrated in Fig. 2, in which the area beneath each curve depicts the metabolically viable combinations of number of brain neurons and MBD that a primate could afford by feeding a certain number of hours per day. The inverted U-shaped curves show that there are tradeoffs between MBD and number of neurons affordable by a given number of daily hours of feeding (maximal values given in Table S2). The rising left side of the curves indicates that a minimal body size is required for a primate to afford a certain number of brain neurons, and there is an optimal combination of MBD and number of neurons (and thus MBR) that maximizes both for each feeding regimen. Moreover, increasing the number of daily feeding hours allows for larger maximal combinations of MBD and number of brain neurons. However, the declining right side of each curve indicates that, when past the maximal number of brain neurons afforded by a certain number of daily hours of feeding, any further increases in MBD come at a cost of a smaller number of brain neurons. For instance, a primate that fed the putative maximum of 10 h/d could afford a brain of, at most, 113 billion neurons, in which case it could weigh no more than 64 kg; if it fed 8 h/d, it could afford a brain of no more than 53 billion neurons, but a body no larger than 24 kg; and if it fed 6 h/d, it could afford up to 23 billion neurons in the brain, but, in that case, its body could weigh only 8 kg. The maximal viable number of brain neurons depending on MBD and the number of daily hours of feeding are given in Table S3.
Fig. 2.
Viable combinations of total numbers of brain neurons and MBD among primate species on a diet of raw foods. Each curve represents the combination of values for which the energy requirements for body and brain is equal to the energy intake calculated for that MBD, given a certain number of daily hours of feeding (indicated); the shaded area beneath each curve indicates the viable combinations of MBD and number of brain neurons for a primate species that fed for that number of hours per day.
Fig. 2 also depicts the five largest nonhuman species in our sample, plotted according to their actual combinations of MBD and number of brain neurons (1–4). Remarkably, these data points fall within the viability curves that match reported daily feeding times for these species: 5.5 h for the baboon (31), 6.8 h for the chimpanzee (32–34), and 7.2 h for the orangutan (35, 36). This match suggests that the actual combinations of MBD and number of brain neurons in large nonhuman primates indeed impose a certain number of daily feeding hours. Although the estimated 8.8 daily hours of feeding for the gorilla to afford a combination of 124.7 kg (25) and an estimated 33.4 billion neurons (4) is higher than the reported 7.8 h used in our estimates (37–40), in some extreme conditions, gorillas are known to spend as much as 10 h/d feeding (24). This difference suggests that the gorilla lives on the limit of viability, where increases in MBD are constrained by metabolic requirements.
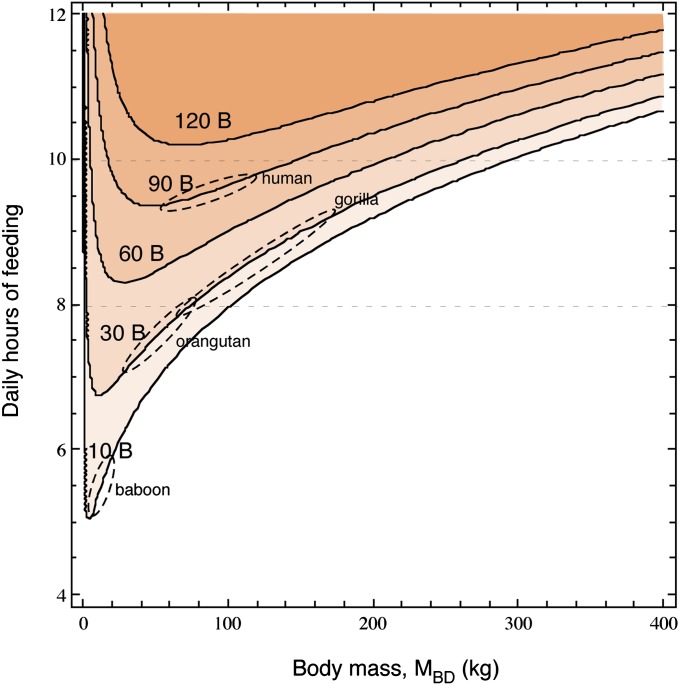
Interestingly, these species do not have optimal combinations of MBD and number of brain neurons. Fig. 3 shows that, for a given number of brain neurons, there is an optimal MBD that minimizes the required number of hours of feeding by decreasing the total energetic cost of body and brain while still allowing a large enough EIN per hour. Beyond that MBD, the lines show that, for a given number of brain neurons, a larger body requires longer feeding hours until it becomes prohibitively large (that is, requiring longer than 10 h/d of feeding; Fig. 3, upper dashed line). This is indicated in Fig. 3 for baboons, great apes, and humans by the shapes spanning the range of observed MBDs for each species, given their measured (i.e., baboon) or estimated numbers of brain neurons (i.e., orangutan and gorilla). The reverse is also true: for a given MBD, increasing the number of neurons in the brain (i.e., shifting curves upward) increases steeply the number of daily hours of feeding required to maintain viability. According to this analysis, for the gorilla to afford a larger number of brain neurons (and hence a proportionately larger brain) while still feeding at most for 8 h/d, a steep decrease in MBD would be required.
Fig. 3.
Hours of feeding required per day to sustain a certain number of neurons and MBD. The horizontal lines illustrate that increasing MBD comes at a cost of a reduced number of brain neurons if the number of daily hours of feeding is maintained. The line at 8 h/d indicates the average maximal number of daily hours of feeding practiced by extant great apes, and the line at 10 h/d indicates the putative maximal number of hours per day that could be spent feeding.
Having a larger relative MBR may thus be metabolically unviable for the largest nonhuman primates. To address this possibility, we next determined the energy requirement for generic primates of different MBDs to afford a brain of 2% relative size, as observed in several primate species, including humans. Relative MBR, calculated as a percentage of total MBD, varies among primates from 0.4% to more than 4% in a way that is inversely correlated with MBD (Spearman correlation, −0.532, P < 0.0001, excluding Homo spp. and hominins), with the smallest relative brain sizes, of 0.4% to 0.6% of MBD, found among the great apes (41, 42).
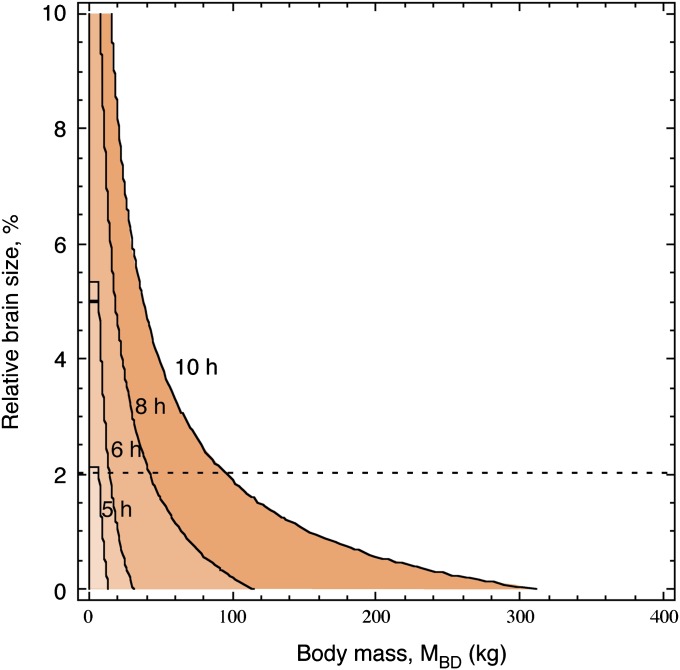
Because the number of brain neurons in primates can be expressed as a linear function of MBR such that the number is equal to 700,332,022.773 + 56,605,881,094 × MBR [3], we can express EBR as a function of MBR, fix MBR at 2% of MBD, and then calculate the limits of viable MBD depending on the number of feeding hours. We find that the maximal MBD of a primate is markedly dependent on feeding hours and severely limited if MBR is to be maintained at 2% of MBD (Fig. 4). For example, a primate that fed for 8 h/d [approximately like a gorilla (37–40)] would be limited to a body of 42.4 kg and 49 billion neurons; if it fed for 7 h/d [close to the values for a chimpanzee or orangutan (32–36)], it would be limited to a body of 25.8 kg and 32 billion neurons; or if it fed for 6 h/d [close to the values for a baboon (31)], it would be limited to a body of 14.3 kg and 17 billion brain neurons. If a primate could consistently feed at the estimated limit of 10 h/d, it would still be limited to a body of 95.2 kg to afford a relative brain size of 2% (in which case, its brain would have 108 billion neurons). This shows that, to maintain a relative MBR of 2%, a primate must keep its MBD below a certain limit, or else spend more time feeding if it increases MBD (but only to a limit of 95 kg if it fed 10 h/d). Any increase in MBD to greater than this limit must occur at the expense of the number of neurons in the brain, that is, of relative brain size.
Fig. 4.
Viable relative brain size (as percentage of MBD) depending on MBD and daily hours of feeding. Viable combinations of relative brain size and MBD are within the colored areas for the respective indicated daily hours of feeding. The horizontal line indicates the required increase in daily hours of feeding to maintain a relative brain size of 2% with increasing MBD.
Based on the estimated MBDs of extinct hominins (7) and their predicted numbers of brain neurons (4), we next used the raw diet of extant primates to calculate the feeding hours that would have been required for different extinct species in the human lineage. Fig. 5 shows that the putative first hominin species in the human lineage, Australopithecus afarensis, Paranthropus boisei, and Homo habilis, with a predicted 30 to 40 billion neurons (4), would have had similar feeding requirements of more than 7 h/d if feeding on a raw diet equivalent to that of extant great apes. Homo erectus, with a predicted 62 billion neurons, on the contrary, would have had to spend more than 8 h/d feeding on raw foods; and Homo heidelbergensis, Homo neanderthalensis, and Homo sapiens would have had to spend consistently more than 9 h/d feeding to afford their 76 to 86 billion neurons, longer than extant great apes spend feeding (Table S1). Given the difficulties that the largest great apes have to feed for more than 8 h/d (as detailed later), it is unlikely, therefore, that Homo species beginning with H. erectus could have afforded their combinations of MBD and number of brain neurons on a raw diet.
Fig. 5.
Required daily feeding time for hominin and great ape species to afford combinations of MBD and total number of brain neurons. Notice that H. heidelbergensis, H. neanderthalensis, and H. sapiens fall well over the viability curve for 8 h/d of feeding if they had a raw foods diet similar to extant nonhuman primates.
Discussion
Although several authors have pointed out that increasing MBR has energetic consequences (10–17, 23), it remained to be determined whether these would indeed be physiologically relevant to the extent of actually making metabolic cost a limiting factor in brain evolution. By using a simple model of scaling of energetic intake and expenditure with increasing MBD and number of brain neurons, here we demonstrate that, for primates feeding exclusively on a raw diet, metabolism is indeed a physiologically relevant limiting factor in evolution such that a tradeoff between MBD and number of brain neurons is imposed. This tradeoff is particularly clear in animals the size of great apes, as sustaining an MBD of ∼50 kg already requires feeding appoximately 8 h/d, beyond which increasing both MBD and the number of brain neurons becomes rapidly dangerous. Such a tradeoff provides a simple explanation for why great apes have the smallest relative brain sizes among primates: on their diet based on raw foods, and given that, per gram of tissue, larger brains came at a higher metabolic cost than larger bodies, the largest great apes cannot afford both a large body and a larger number of neurons.
Adding neurons to the primate brain apparently comes at a fixed cost of approximately 6 kCal per billion neurons (8). Because of the scaling of EIN with MBD, adding 1 billion neurons to the brain can cost as much as one extra hour of feeding per day for a small primate, but only extra minutes to a large primate. However, adding large numbers of neurons can be prohibitively expensive, even to a large primate: the additional number of neurons necessary (122 billion) for a gorilla to have a brain corresponding to 2% of its MBD would cost the animal an extra 733 kCal, which we estimate would require another 2 h 12 min of feeding—when a gorilla already spends as much as 80% of 12 h of day in feeding (24, 37–40).
The feeding behavior of gorillas indicates that feeding time is indeed limiting, and possibly already maximal in this species, at an average of almost 8 h/d and peaking at close to 10 h/d (24, 37–40). During intense or prolonged rainfall, gorillas usually stop feeding, but resume feeding if rainfall continues for more than 2 h, and compensate for the lost feeding time by shortening the periods of rest (24). Similarly, orangutans, feeding for approximately 8 h/d, lose MBD during the periods of low food availability (43). Likewise, the cranial capacity of orangutans in the forests of Borneo, where food quality is low, is smaller than that of orangutans in Sumatra (44), where food quality is higher, despite the increased capacity and resistance to chewing of the jaw of the Borneo orangutans, as expected if brain metabolism were indeed limiting to survival. Interestingly, and in agreement with our observation that very small body sizes are similarly constraining, energetic availability may also be limiting for survival of the smallest primates, as suggested by the recent report of hibernation in lemurs that is related not to low ambient temperatures, but to food scarcity (45). Taken together, the susceptibility of gorillas and orangutans to fluctuations in weather and food availability suggests that feeding times longer than approximately 8 h/d are not viable for long periods of time, particularly considering that other daily activities, such as nesting and socializing, also require time.
It is in this context that one must consider our finding that, on a raw diet similar to that of extant nonhuman primates, Homo species would be required to feed consistently more than 9 h/d to afford their estimated MBD and number of neurons. These values are similar to the recent estimate by another group that humans would be required to feed 48% of the day, calculated by regressing feeding time on MBD for wild populations of nonhuman primates (46). Intriguingly, the species H. habilis, A. afarensis, and P. boisei have similar estimated required daily feeding times of 7.3 to 7.5 h (Table S1), close to the feeding times of extant great apes, despite their different presumptive MBDs of 33 kg, 38 kg, and 41 kg (7), given their estimated numbers of neurons of 40 billion, 34.7 billion, and 32.8 billion, respectively (4). These possibly compensatory changes between body size and number of brain neurons suggest that daily feeding time was already maximal then, and thus limiting for MBD and number of brain neurons to increase jointly without a change in diet.
We do not discard the influence of other modifications in human or nonhuman primate evolution, such as changes in the distribution of glucose between brain and skeletal muscle (47, 48) or an overall decrease in basal metabolic rate (49), which might themselves be adaptations to limited energetic availability. Our findings are also compatible with the general essence of the “maternal energy hypothesis,” according to which the maternal supply of energy to the fetus may be limiting to brain expansion (23), although the specific link proposed by that hypothesis is between the mother’s basal metabolic rate (rather than simply energy availability, as proposed here) and the brain size of her offspring. However, by showing that metabolism is indeed limiting at physiologically relevant combinations of body and MBR, our data provide evidence that metabolic cost is limiting enough to impose tradeoffs in brain evolution, and thus offer direct support for the proposition of Wrangham (50, 51) that such a metabolic limitation was overcome in the human lineage by the advent of cooking food, which greatly increases the caloric yield of the diet, as a result of the greater ease of chewing, digestion, and absorption of foods (27, 28, 52). In line with this proposition, a cooked diet is preferred by extant nonhuman great apes (53). Although the earlier addition of raw meat to the diet of earlier hominins may also have contributed to increase its caloric content (54), raw meat is difficult to chew and ingest, whereas cooked meat is easier to chew and has a higher caloric yield (51, 52). Besides increasing the caloric yield and making previous metabolic limitations irrelevant, cooking would also have increased the time available for social and more cognitively demanding activities, which in turn would impose a positive pressure for increased numbers of neurons, now affordable by the new diet. We propose that the combination of a newly affordable larger number of neurons with the accompanying time now available to use these neurons in cognitively demanding tasks that improved species fitness drove the rapid increase in numbers of brain neurons encountered in human evolution from H. erectus onward (4).
Materials and Methods
Data on average body and MBR of each species were obtained from the literature (7, 25, 55). We limited our study to primates species for which we had determined experimentally the total number of brain neurons (1–3), and to the living great apes and extinct hominins for which we have recently estimated the total number of brain neurons (4). The total of 17 species analyzed range in MBD from the common marmoset (C. jacchus) to the gorilla (G. gorilla) and include our own species, H. sapiens, the primate with the largest number of brain neurons (1, 4, 5).
We calculated the body daily metabolic cost by applying the law of Kleiber (21, 22) to the MBD (minus the MBR) of each species. The body energy expenditure is estimated as 70 × MBD0.75 kcal/d. This calculation, in which we subtracted MBR from total MBD, overestimates the energy needs of the body unprovided with the brain. On the contrary, given that the law of Kleiber applies to animals during fasting and rest, two conditions known to reduce the body’s metabolism (56), it is therefore more likely that our calculation actually underestimates the real energy needs of the body without the brain. The metabolic cost of the brain (EBR) was estimated separately as a linear function of its number of neurons, assuming an average energy cost per individual neuron of 6 × 10−9 kcal/d (8), such that EBR is equal to the total number of brain neurons × 6 × 10−9. The total energy requirement of a species is therefore the sum of EBR and the metabolic cost of the body.
The number of daily hours devoted by each primate to feeding were obtained from previous publications (25, 31-40). These data sources were selected because they clearly provide numbers of feeding hours per day, and not just percentages of the day, daylight time, or active time that might complicate a direct correspondence across species and studies. Although our dataset may thus not represent the entire range of variation recorded in a species, it is compatible with datasets used in other recent studies (46). Average values used in our equations are listed in Table S1. By dividing the required daily EIN (estimated by Kleiber law for a given body weight) by the number of daily feeding hours, we estimated the actual EIN per hour of each species. The average hourly EIN was then plotted as a function of MBD of each species to establish how it scales with MBD.
All mathematical models were programmed in Mathematica software (Wolfram), and all mathematical analysis were also performed on that platform.
Supplementary Material
Acknowledgments
We thank Jon Kaas for collaborating on all previous primate studies that made this study possible and Bruno Mota for help with mathematical models. This work was financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico - Edital Universal and Produtividade em Pesquisa), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro - Cientista do Nosso Estado), INNT/MCT (Instituto Nacional de Neurociência Translacional/Ministério de Ciência e Tecnologia), and the James S. McDonnell Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206390109/-/DCSupplemental.
References
- 1.Azevedo FAC, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513(5):532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 2.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104(9):3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabi M, et al. Cellular scaling rules for the brains of an extended number of primate species. Brain Behav Evol. 2010;76(1):32–44. doi: 10.1159/000319872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herculano-Houzel S, Kaas JH. Gorilla and orangutan brains conform to the primate cellular scaling rules: Implications for human evolution. Brain Behav Evol. 2011;77(1):33–44. doi: 10.1159/000322729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herculano-Houzel S. The human brain in numbers: A linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerison HJ. Animal intelligence as encephalization. Philos Trans R Soc Lond B Biol Sci. 1985;308(1135):21–35. doi: 10.1098/rstb.1985.0007. [DOI] [PubMed] [Google Scholar]
- 7.Sousa A, Wood B. The hominin fossil record and the emergence of the modern human central nervous system. In: Kaas JH, editor. Evolution of Nervous Systems: A Comprehensive Reference: The Evolution of Primate Nervous Systems. Vol 4. Oxford: Elsevier; 2007. pp. 291–336. [Google Scholar]
- 8.Herculano-Houzel S. Scaling of brain metabolism with a fixed energy budget per neuron: Implications for neuronal activity, plasticity and evolution. PLoS ONE. 2011;6(3):e17514. doi: 10.1371/journal.pone.0017514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aschoff J, Günther B, Kramer K. Energiehaushalt und Temperaturregulation. Munich: Urban and Schwarzenberg; 1971. [Google Scholar]
- 10.Aiello L, Wheeler P. The expensive-tissue hypothesis. Curr Anthropol. 1995;36:199–121. [Google Scholar]
- 11.Leonard W, Robertson M. Nutritional requirements and human evolution: A bioenergetics model. Am J Hum Biol. 1992;4:179–195. doi: 10.1002/ajhb.1310040204. [DOI] [PubMed] [Google Scholar]
- 12.Leonard W, Robertson M. Evolutionary perspectives on human nutrition: the influence of brain and body size on diet and metabolism. Am J Hum Biol. 1994;6:77–88. doi: 10.1002/ajhb.1310060111. [DOI] [PubMed] [Google Scholar]
- 13.Leonard WR, Robertson ML. Comparative primate energetics and hominid evolution. Am J Phys Anthropol. 1997;102(2):265–281. doi: 10.1002/(SICI)1096-8644(199702)102:2<265::AID-AJPA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Fish JL, Lockwood CA. Dietary constraints on encephalization in primates. Am J Phys Anthropol. 2003;120(2):171–181. doi: 10.1002/ajpa.10136. [DOI] [PubMed] [Google Scholar]
- 15.Isler K, van Schaik CP. Metabolic costs of brain size evolution. Biol Lett. 2006;2(4):557–560. doi: 10.1098/rsbl.2006.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin RD. Relative brain size and basal metabolic rate in terrestrial vertebrates. Nature. 1981;293(5827):57–60. doi: 10.1038/293057a0. [DOI] [PubMed] [Google Scholar]
- 17.Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: Its constancy and functional basis. Am J Physiol. 1981;241(3):R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong E. Relative brain size and metabolism in mammals. Science. 1983;220(4603):1302–1304. doi: 10.1126/science.6407108. [DOI] [PubMed] [Google Scholar]
- 19.Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480(7375):91–93. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery SH, Capellini I, Barton RA, Mundy NI. Reconstructing the ups and downs of primate brain evolution: Implications for adaptive hypotheses and Homo floresiensis. BMC Biol. 2010;8:9. doi: 10.1186/1741-7007-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–353. [Google Scholar]
- 22.Kleiber M. Body size and metabolic rate. Physiol Rev. 1947;27(4):511–541. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- 23.Martin RD. Scaling of the mammalian brain: The maternal energy hypothesis. News Physiol Sci. 1996;11:149–156. [Google Scholar]
- 24.Watts DP. Environmental influences on mountain gorilla time budgets. Am J Primatol. 1988;15:195–211. doi: 10.1002/ajp.1350150303. [DOI] [PubMed] [Google Scholar]
- 25.Ross CF, et al. Ecological consequences of scaling of chew cycle duration and daily feeding time in primates. J Hum Evol. 2009;56(6):570–585. doi: 10.1016/j.jhevol.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Parra R. Comparison of foregut and hindgut fermentation in herbivores. In: Montgomery GG, editor. The Ecology of Arboreal Folivores. Washington, DC: Smithsonian Inst Press; 1978. pp. 205–230. [Google Scholar]
- 27.Urquiza-Haas T, Serio-Silva JC, Hernández-Salazar LT. Traditional nutritional analyses of figs overestimates intake of most nutrient fractions: A study of ficus perforata consumed by howler monkeys (Alouatta palliata mexicana) Am J Primatol. 2008;70(5):432–438. doi: 10.1002/ajp.20510. [DOI] [PubMed] [Google Scholar]
- 28.Carmody RN, Wrangham RW. The energetic significance of cooking. J Hum Evol. 2009;57(4):379–391. doi: 10.1016/j.jhevol.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Hofman MA. Energy metabolism, brain size and longevity in mammals. Q Rev Biol. 1983;58(4):495–512. doi: 10.1086/413544. [DOI] [PubMed] [Google Scholar]
- 30.Karbowski J. Global and regional brain metabolic scaling and its functional consequences. BMC Biol. 2007;5:18. doi: 10.1186/1741-7007-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill RA, et al. Day length, latitude and behavioural (in)flexibility in baboons (Papio cynocephalus ursinus) Behav Ecol Sociobiol. 2003;53:278–286. [Google Scholar]
- 32.Ghiglieri MP. The Chimpanzees of Kibale Forest. A Field Study of Ecology and Social Structure. New York: Columbia Univ Press; 1984. [Google Scholar]
- 33.Wrangham RW. Feeding behavior of chimpanzees in Gombe National Park Tanzania. In: Clutton-Brock TH, editor. Primate Ecology. London: Academic Press; 1977. pp. 504–538. [Google Scholar]
- 34.Fawcett KA. 2000. Female relationships and food availability in a forest community of chimpanzees. PhD thesis (Univ Edinburgh, Edinburgh)
- 35.Galdikas BMF. Orangutan diet, range, and activity at Tanjung Puting, Central Borneo. Int J Primatol. 1988;9:1–35. [Google Scholar]
- 36.Fox EA, van Schaik CP, Sitompul A, Wright DN. Intra-and interpopulational differences in orangutan (Pongo pygmaeus) activity and diet: implications for the invention of tool use. Am J Phys Anthropol. 2004;125(2):162–174. doi: 10.1002/ajpa.10386. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann J, Korstjens AH, Dunbar RIM. Time management in Great Apes: Implications for gorilla biogeography. Evol Ecol Res. 2008;10:517–536. [Google Scholar]
- 38.Doran DM, McNeilage A. Gorilla ecology and behavior. Evol Anthropol. 1998;6:120–131. [Google Scholar]
- 39.Ilambu O. The Apes: Challenges for the 21st Century. Conference Proceedings. Brookfield, IL: Brookfield Zoo; 2001. Ecology of eastern lowland gorilla: is there enough scientific knowledge to mitigate conservation threats associated with extreme disturbances in its distribution range? pp. 307–312. [Google Scholar]
- 40.Stokes EJ, Parnell RJ, Olejniczak C. Female dispersal and reproductive success in wild western lowland gorillas (Gorilla gorilla gorilla) Behav Ecol Sociobiol. 2003;54:329–339. [Google Scholar]
- 41.Frahm HD, Stephan H, Stephan M. Comparison of brain structure volumes in Insectivora and Primates. I. Neocortex. J Hirnforsch. 1982;23(4):375–389. [PubMed] [Google Scholar]
- 42.Marino L. A comparison of encephalization between odontocete cetaceans and anthropoid primates. Brain Behav Evol. 1998;51(4):230–238. doi: 10.1159/000006540. [DOI] [PubMed] [Google Scholar]
- 43.Knott C. Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Intern J Prim. 1998;19:1061–1079. [Google Scholar]
- 44.Taylor AB, van Schaik CP. Variation in brain size and ecology in Pongo. J Human Evol. 2007;52:59–71. doi: 10.1016/j.jhevol.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. Physiology: Hibernation in a tropical primate. Nature. 2004;429(6994):825–826. doi: 10.1038/429825a. [DOI] [PubMed] [Google Scholar]
- 46.Organ C, Nunn CL, Machanda Z, Wrangham RW. Phylogenetic rate shifts in feeding time during the evolution of Homo. Proc Natl Acad Sci USA. 2011;108(35):14555–14559. doi: 10.1073/pnas.1107806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fedrigo O, et al. A potential role for glucose transporters in the evolution of human brain size. Brain Behav Evol. 2011;78(4):315–326. doi: 10.1159/000329852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfefferle AD, et al. Comparative expression analysis of the phosphocreatine circuit in extant primates: Implications for human brain evolution. J Hum Evol. 2011;60(2):205–212. doi: 10.1016/j.jhevol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pontzer H, Raichlen DA, Shumaker RW, Ocobock C, Wich SA. Metabolic adaptation for low energy throughput in orangutans. Proc Natl Acad Sci USA. 2010;107(32):14048–14052. doi: 10.1073/pnas.1001031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wrangham RW, Jones JH, Laden G, Pilbeam D, Conklin-Brittain NL. The Raw and the Stolen. Cooking and the Ecology of Human Origins. Curr Anthropol. 1999;40(5):567–594. [PubMed] [Google Scholar]
- 51.Wrangham RW. Catching Fire: How Cooking Made Us Human. New York: Basis Books; 2009. [Google Scholar]
- 52.Carmody RN, Weintraub GS, Wrangham RW. Energetic consequences of thermal and nonthermal food processing. Proc Natl Acad Sci USA. 2011;108(48):19199–19203. doi: 10.1073/pnas.1112128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wobber V, Hare B, Wrangham R. Great apes prefer cooked food. J Hum Evol. 2008;55(2):340–348. doi: 10.1016/j.jhevol.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Milton K. A hypothesis to explain the role of meat-eating in human evolution. Evol Anthropol. 1999;8:11–21. [Google Scholar]
- 55.Semendeferi K, Damasio H. The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. J Hum Evol. 2000;38(2):317–332. doi: 10.1006/jhev.1999.0381. [DOI] [PubMed] [Google Scholar]
- 56.Nagy KA. Field metabolic rate and body size. J Exp Biol. 2005;208(Pt 9):1621–1625. doi: 10.1242/jeb.01553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.