Abstract
Nitrite (NO2−) is a central intermediate in the nitrogen metabolism of microorganisms and plants, and is used as a cytotoxin by macrophages as part of the innate immune response. The bacterial membrane protein NirC acts as a specific channel to facilitate the transport of nitrite anions across lipid bilayers for cytoplasmic detoxification. Despite NirC’s importance in nitrogen metabolism and in the pathogenicity of enteric bacteria, available biochemical data are scarce. Here we present a functional and structural characterization of NirC from Salmonella typhimurium by lipid bilayer electrophysiology and X-ray crystallography. NirC is a pentameric member of the formate/nitrite transporter family of membrane proteins that operates as a channel with high conductance. Single-channel measurements reveal fast and slow gating events but, in contrast to the related FocA formate channel, no pH-dependent gating. A 2.4Å crystal structure of NirC at pH 5 shows similarity to FocA and aquaporins, but lacks the structural asymmetry observed in the formate channel at similarly low pH. Resolved water molecules in the protomers suggest a transport mechanism that also permits a facultative NO2−/H+ symport.
Keywords: ion channels, protein crystallography, planar lipid bilayer
The nitrite anion (NO2−) is a central branching point in nitrogen metabolism (1). It is generated through the two-electron reduction of nitrate (NO3−) or the four-electron oxidation of hydroxylamine (H2NOH) (2). Intracellular accumulation of nitrite is critical for most cells, because it can be reduced to the cytotoxic radical nitric oxide (NO) as a side reaction of nitrate reductase (NarGHI) (3). NO has been reported to cause damage to DNA (4) and iron-sulfur clusters (5), so that cytoplasmic nitrite is rapidly removed by a nitrite reductase to yield ammonium (NH4+) or dinitrogen (N2) (1, 6). This is achieved mainly through the NADH-dependent siroheme-containing nitrite reductase (NirBD), an assimilatory enzyme in bacteria, archaea, and eukaryotes, which consequently depends on an import system to transport its charged substrate across the cytoplasmic membrane. This is the task of the nitrite transport protein NirC, a member of the widespread formate/nitrite transporter (FNT) family (transporter class 2.A.44) (7) that also includes the formate channel FocA and the formate uptake permease FdhC from Methanobacterium thermoformicium (8), as well as the hydrosulfide channel HSC (9). In enteric bacteria, such as Escherichia coli and Salmonella typhimurium, NirC is encoded by the third gene of the nirBDCcysG operon (10). Its expression is induced by the transcription factors FNR (anoxicity), NarL (stimulated by nitrate), and NarP (stimulated by both nitrate and nitrite) (11, 12).
The functional mechanism of NirC remains under debate, with evidence presented to support both a passive transport of nitrite and a possibly active uptake of the anion through proton symport (13). A recent electrophysiological study of NirC on solid-supported membranes showed that the protein acts as a channel for both nitrate and nitrite anions, with a possible additional mode of action as an active NO2−/H+ antiporter (14). The human pathogen S. typhimurium imports nitrite for cytoplasmic reduction in response to the production of peroxynitrite by the inducible NO synthase of eukaryotic hosts (15–17). Das et al. (18) reported increased production of NO by a murine macrophage culture infected with a ΔnirC strain of Salmonella in parallel with a marked decrease in the intracellular proliferation rate of the pathogen in spleen, liver, and lymph tissues. NirC thus plays an important role in enterobacterial pathogenesis, and its absence in mammals marks it as a highly suitable target for the development of novel antimicrobial agents.
The recently reported high-resolution crystal structures of the FNT channel FocA from E. coli (19), Vibrio cholerae (20), and S. typhimurium (21) reveal an unprecedented, pentameric architecture with structural similarity to aquaporins and glyceroporins (22). The hydrosulfide channel HSC closely follows this architectural principle (9). Distinct channels are seen in each individual protomer, in contrast to ligand-gated ion channels in which all subunits form a common, central pore (23). Direct electrophysiological measurements of S. typhimurium FocA (StFocA) show that StFocA acts as a channel for a range of monovalent anions at high pH values (24) and is gated in a pH-dependent manner (21). Gating is mediated through conformational changes of the N termini of the protomers that are disordered when FocA acts as a channel, but attain three different distinct conformations at low pH. This provides a structural basis for the in vivo observation that under these conditions, the protein acts as an active H+/formate importer (25). No comparable functional switch has been proposed for NirC. To examine the functional properties of NirC, we recombinantly produced S. typhimurium NirC (StNirC) in E. coli and conducted structural and electrophysiological studies.
Results
NirC Is a Symmetric Pentamer with Structural Similarities to Other FNT Channels.
Using a construct with a C-terminal deletion of 17 residues, crystals of StNirC diffracted to 2.4-Å resolution, permitting unambiguous modeling of residues 1–250 of the protein. StNirC crystallized in space group C2, with two disk-shaped pentamers per asymmetric unit, each with a diameter of 80 Å and a maximum thickness of 48 Å (Fig. 1A). The overall architecture is similar to that of StFocA, although the two membrane proteins share only 22% sequence identity. The NirC protomers interact tightly, with a total buried surface area of 11,700 Å2 that contains both hydrophobic and hydrophilic residues. Although the cytoplasmic face of StNirC shows the typical positive electrostatic surface potential distribution of cytoplasmic membrane proteins (26), the periplasmic face is subdivided into negatively charged patches on the periphery and strongly positively charged areas surrounding the entries to the transmembrane channels (Fig. 1B). This is in stark contrast to the surface potential distribution of StFocA, where the periplasmic face has a pronounced negative charge (21). Such differences directly reflect the functional properties of the two types of FNT channels.
Fig. 1.
The 3D structure of StNirC. (A) The NirC pentamer as seen from the cytoplasmic side (Left) and from within the membrane plane (Right). Each protomer contains an individual transport channel. (B) Electrostatic surface potential distribution of the cytoplasmic face (Left) and the periplasmic face (Right) of NirC, colored from blue at +10 kB⋅T to red at −10 kB⋅T. Both faces of the channel show an overall positive charge, in stark contrast to the formate exporter FocA, which hinders anion reimportation through a negatively charged periplasmic surface.
Depending on external pH, FocA functions either as a passive formate channel or as an active formic acid importer, which in either case does not require positive surface patches to facilitate the entry of cargo anions from the periplasmic side. In contrast, Jia et al. (13) found that during cytoplasmic nitrate reduction by NarGHI, the product nitrite was mostly first exported to the periplasm and then reimported for reduction by NirBD. NirC and the nitrate/nitrite antiporter NarK engaged in both export and import processes, but although NirC was a less active exporter than NarK, its import rate for nitrite was 10-fold higher (13). Thus, NirC is presumed to act in nitrite import to the cytoplasm as its main physiological role.
The NirC pentamer surrounds a central pore with a diameter of 12 Å that is lined by hydrophobic residues and measures 4.1 Å at the narrowest constriction close to the cytoplasmic side (Fig. 1B). In StNirC, a bundle of six parallel, elongated electron density features was observed within this pore facing the periplasmic side, likely representing the aliphatic chains of the detergent n-octyl-β-d-glucopyranoside. In vivo, these positions would be occupied by phospholipid molecules, so that transport through the center can be ruled out. The corresponding pore in the FocA pentamer is slightly narrower because of an extended linker region between TM1 and TM2, and in the case of StFocA, electron density was observed for only five detergent molecules (21).
NirC Protomer.
In contrast to the low-pH structure of StFocA (21), the five protomers of the StNirC pentamer are highly similar, with pairwise rmsd of 0.16–0.35 Å for all positions of nonhydrogen atoms. The polypeptide folds into eight α-helical segments, one hydrophilic helix comprising 21 residues at the N terminus, and six transmembrane helices, TM1–TM6. TM2 and TM5 are interrupted by loop regions L2 (residues A75-F80) and L5 (S193-S198) within the membrane, giving rise to two distinct subhelices each (TM2a/TM2b and TM5a/TM5b) (Fig. 2A). In addition, the loop connecting TM3 and TM4 contains a short helical stretch on the periplasmic side of NirC that forms part of the entrance funnel into the channel.
Fig. 2.
Architecture of the NirC protomer. (A) The polypeptide spans the membrane in six helical segments that form a barrel-like structure with a right-handed tilt (stereo image). Helices TM2 and TM5 are split into two separate segments and are involved in the formation of the substrate channel [dotted surface; calculated with HOLE (35)]. A short helical fragment (PH) on the periplasmic side, as well as an N-terminal helix, are oriented parallel to the membrane in close proximity to the respective entrances of the transport channel. (B) Superposition of Cα traces for StNirC (red) and for the intermediate conformation of StFocA (21). StNirC lacks an extended periplasmic loop found in StFocA and shows no structural flexibility in the N-terminal helix. (C) Structural profile of the transport channel of StNirC (blue) and StFocA (red), calculated with HOLE (35). Both proteins contain two constrictions along the channel that likely act as a hydrophobic barrier for water molecules. (D) Electrostatic surface potential properties of the periplasmic and cytoplasmic openings of the NirC channel facilitate anion import.
Within this channel, a central vestibule is separated from the periplasmic and cytoplasmic entrances by two narrow constrictions. The side chains of L69, L79, V164, and I168 form the cytoplasmic (in) constriction, whereas the chain on the periplasmic side (out) comprises F65, F190, H197, and A200 (Fig. 2F). Loop regions L2 and L5 are directly involved in forming these constrictions, and the increased structural flexibility of a loop over a rigid helix may be required for the passage of anions through the controlled extension of the channel. The residues forming the two constriction sites are highly similar in FocA, NirC, and HSC sequences, but although the L5 loop is almost fully conserved [SG(F/Y)EHS], the L2 loop of StFocA (ILCVIC) is completely different from that of StNirC (AGSELF) and HSC (TGTELF). Notably, S77 in the L2 loop is in a fully stretched conformation in all protomers (φ = ψ = 180°), indicating structural strain. The two pores in StNirC are very narrow, with average diameters of 1.4 Å for the inner constrictions and 1.7 Å for the outer contrictions, similar to those seen in structures of FocA [1.2/1.9 Å in S. typhimurium (21) and 1.4/1.8 Å in E. coli (19, 20)] (Fig. 2 B and C). Such diameters are too small to allow passage of a nitrite or formate anion, and thus small rearrangements are necessary for the translocation of cargo. All available structures of FNT channels represent such a closed state, but the pore may have inherent flexibility that allows for key residues, such as H197, to quickly shift on the passage of permeating ions without necessarily forming a persistent open state. Note that in a form of VcFocA described as “open,” the two constrictions have conformations identical to those in all other structures (20).
With a pKa of 3.4, nitrous acid (HNO2) is a stronger Lewis acid than formic acid (pKa 3.8), and the nitrite anion also has a lone electron pair situated at the nitrogen atom. The differences between FocA and NirC are not sufficient to explain the selectivity of NirC for nitrite over formate, however. We suggest instead that the positive electrostatic potential field of the periplasmic funnel favors the importation of nitrite, in line with the physiological role of the protein. NirC transport responds to the directionality of the membrane potential (see below), and the charge distribution within the channel is asymmetric (Fig. 2D).
NirC Is a Monovalent Anion Channel but Prefers Nitrite to Formate.
Full-length StNirC was solubilized using the detergent n-octyl-β-d-glucopyranoside and reconstituted into proteoliposomes that were then fused with a planar lipid bilayer membrane, as described previously (21, 24). A nitrite gradient of 20 mM/100 mM of was applied, and currents were recorded at varying holding potentials (Fig. 3A). The current response demonstrated ohmic behavior, with the expected reversal of direction at a potential of −41 mV, compared with −18 mV in an analogous experiment with a nitrate gradient of 50 mM/100 mM (Fig. 3B). Both values correspond to the reversal potentials calculated using to Nernst’s equation. These findings indicate that NirC acts as a voltage-independent specific channel for nitrite anions, in agreement with observations from solid-supported membrane electrophysiology (14). With symmetric nitrite concentrations of 20 mM and a membrane potential of 150 mV, unambiguous single-channel currents of 3.6 pA were observed, corresponding to a conductance of 24 pS (Fig. 3C). No such currents were found in control experiments with protein-free liposomes.
Fig. 3.
Electrophysiological analysis of StNirC. (A) Voltage-clamp measurements with 20/100 mM nitrite showing specific conductance of nitrite. (B) Current-voltage diagram of macroscopic current measurements with different gradients of nitrite. The currents reverse at the potentials predicted by Nernst’s equation. (C) Single-channel currents from individual NirC protomers detected at a membrane potential of 150 mV that change direction on reversal of polarity. (D) Variations of single-channel currents with holding potential. (E) Current-voltage dependence based on single-channel currents as shown in D. The reversal potential corresponds to the calculated value, confirming channel specificity. (F) Dependence on anion concentration. At a potential of 150 mV, the currents increase linearly with nitrite concentration. At the same time, the opening probabilities decrease. All concentrations were measured on the same membrane. (G) Statistical analysis of open probabilities for single channels at two different holding potentials at nitrite concentrations of (20 mM/20 mM). (H) StNirC showing bursts of fast gating in a slower gating mode on a second time scale. All data were recorded at pH 7.9.
To further confirm the specificity of NirC, single-channel currents were determined at asymmetric nitrite concentrations of 20 mM/100 mM for different holding potentials (Fig. 3D), verifying that a current–voltage diagram derived from these data shows reversal at the theoretically expected value (Fig. 3E). Even in extended recordings (>50 min from 50 independent measurements) with variations in voltage and concentrations, it was not possible to obtain all six current levels for a single pentamer (i.e., the nonconducting L0 level plus five levels, L1–L5, for the opening of each protomer). Either three to five levels attributable to a single NirC pentamer and lacking L0 or L5 appeared, or a greater number of steps appeared when several pentamers were incorporated into the bilayer (Fig. 3F). These results are consistent with an independent noncooperative gating of the protomers in which the all-open and all-closed states, L0 and L5, are least likely and thus are sparsely populated. Although the currents increased linearly with nitrite concentrations, the opening probability and the lifetime of the conducting states decreased (Fig. 3F). Thus, NirC favors a state of lower conductance, with the open probability of the system decreasing even when the single-channel currents show linear increases. State histograms derived from such measurements over at least 20 s support the presence of defined states, with an apparent preference of a low-conducting form of the pentamer in which not all pores are open (Fig. 3G).
The gating of StNirC also exhibited two distinct modes, with fast gating events on a millisecond time scale with an apparent gating valence of 1, likely reflecting minor independent conformational changes within each NirC monomer resulting in intermittent opening of the transport pathway, and a second, slow mode of gating with significantly longer lifetimes of the open states of individual channels, possibly involving significant conformational rearrangements within the protein (Fig. 3H). Gated currents of this type have been reported for multimeric systems, such as the dimeric ClC chloride channel (27).
Given the chemical similarity of nitrite and formate, we determined the relative permeability of StNirC for HCOO− ions from the shift in reversal potential under bi-ionic conditions, that is, in the presence of both formate and nitrite. StNirC also transported formate, but with only 70% of the permeability of the physiological cargo nitrite. This reverses the situation seen in StFocA, where nitrite passes the channel with 70% of the permeability for formate (24). Thus, although NirC is able to transport both anions, it has the capability of distinguishing two molecules as similar as nitrite and formate.
Discussion
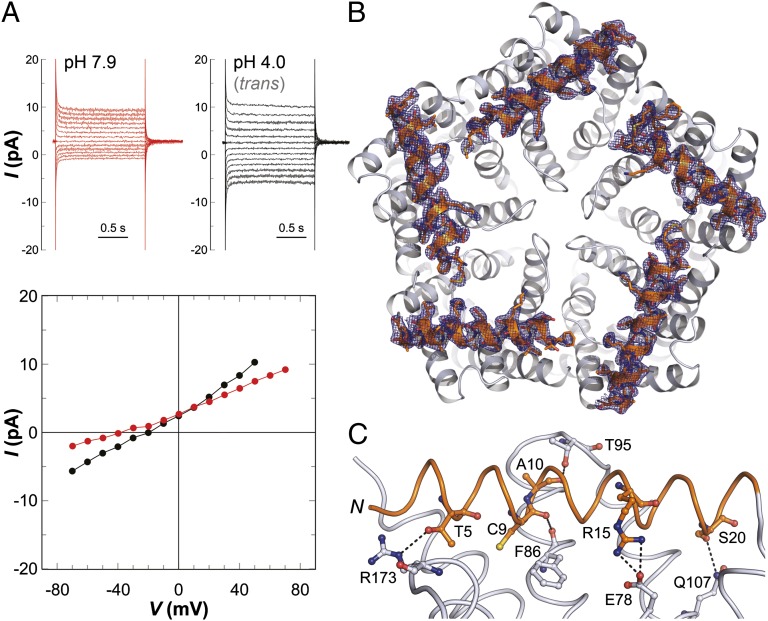
In StFocA, the pH-dependent gating and the resulting change in transport mechanism are crucial for the switch in formate utilization from the periplasmic formate dehydrogenase to the cytoplasmic formate:hydrogen lyase (25, 28). The structure of StFocA at pH 4.0 shows systematic conformational differences in the N termini of the five protomers (21). StNirC was crystallized at a similarly low pH value of 5.0, but here the conformations of all N termini in the two pentamers were identical and well-defined (Fig. 4B). This is also the case in the structurally similar HSC channel (9), a physiological HS− exporter. The position of the N-terminal helix most closely resembles the intermediate state of the StFocA protomer (Fig. 2B) (21). In contrast to StFocA, voltage-clamp experiments with StNirC at pH 7.9 and 4.0 revealed only minor variations in conductance and no distinct gating behavior (Fig. 4A). The observed reversal potential shifted with pH, however, indicating an effect of proton concentration on transport and providing mechanistic clues, as discussed below. In the nitrite channel, the N terminus is held in place by an array of hydrogen bonds that includes the interactions T5–R173, C9–F86(O; main chain oxygen atom), A10(O)–T95, R15–E78, and S20–Q107, and interactions at the base of the helix (Fig. 4C). Of these, T5, R173, C9, F86, R15, and E78 are highly conserved in NirC and HSC proteins, but not in FocA orthologs. The fixed N-terminal helix and the shortened periplasmic connection between TM3 and TM4 with respect to FocA result in a significantly altered shape of NirC. The periplasmic face of StNirC contains distinct entrance funnels to the five individual channels marked by positive surface potential and separated by negatively charged ridges (Fig. 1B). On the cytoplasmic side, all channels terminate in a common, deep groove surrounded by the ring of N-terminal helices (Figs. 1B and 4C),. In contrast, the different conformations of the StFocA protomers at pH 4.0 lead to an asymmetric surface structure (21).
Fig. 4.
pH dependence and gating in NirC-mediated ion translocation. (A) Voltage-clamp studies of NirC at pH 7.9 (red) and after acidification of the trans chamber to pH 4.0 (black) show that StNirC is not gated in a pH-dependent manner, but shows a shift in reversal potential. (B) The N termini that mediate gating in FocA are fully ordered in NirC, as shown in a Fo − Fc omit electron density map contoured at the 2.5 σ level. (C) Multiple interactions stabilize the conformation of the N-terminal helix of NirC.
The structural model of StNirC suggests a translocation mechanism centered on H197 as a pivotal component. This residue is strictly conserved in FNT family proteins. It is part of the L5 loop at the base of helix TM5b (Fig. 5A), and in all structures its Nδ1 atom forms a hydrogen bond to the backbone nitrogen of an alanine at the N-terminal end of helix TM5b (A200 in StNirC). Because the A200 amide nitrogen will not deprotonate, H197 can act exclusively as an acceptor in this H bond. Moreover, the location of H197 at the positive end of the TM5b helix dipole further stabilizes a deprotonated state of the histidine (Fig. 5C). Based on the data presented here, we can hypothesize a transport mechanism in which the increased acidity of H197 is a necessary prerequisite for the protonation of the weak base nitrite. In the uncharged state of HNO2, the cargo will be able to cross the two hydrophobic constrictions in the channel. H197 and a water molecule coordinated to T81 and N161 are the sole protonable residues in the proximity of the outer constriction and inner constriction, respectively (Fig. 5C). T81 and N161 polarize the water and make it a suitable proton acceptor from HNO2 after passing the second constriction. T81 also forms a 2.9-Å hydrogen bond to Nε2 of H197, creating a closed H-bonding network through which the proton can return to its origin (Fig. 5B). The hydrogen-bonding partners of H197, A200 and T81, are not in plane with the imidazole moiety, so that the strengthening of one H bond leads to destabilization of the other. Singly protonated histidines undergo rapid tautomeric rearrangements between the Nδ1-protonated and Nε2-protonated states (29), allowing efficient regeneration of the imidazolium form of H197. In this model, NirC attains specific transport through internal proton cycling. Note that this mechanism can be readily transformed into the active H+-symport mechanism proposed by Cole and co-workers (11). if after deprotonation of the cargo, the proton does not return to the histidine, but rather exits to the cytoplasm and is replenished at H197 from the periplasmic side. This idea is supported by the observed influence of pH on the reversal potential of NirC (Fig. 4A). Consequently, the channel requires a surplus proton to be in a conducting state. Deprotonation and the time required for reprotonation of H197 from the environment may be the basis for the observed slow gating, whereas the fast gating events likely reflect more subtle conformational rearrangements around the two constrictions.
Fig. 5.
Transport channel and amino acid residues involved in proton cycling. (A) The StNirC protomer in cartoon representation. The segment TM2a/L2/TM2b is shown in green; the TM5a/L5/TM5b region, in red. The course of the channel was calculated using HOLE (35). (B) Proposed mechanism for NirC. In its imidazolium form, H197 can donate a proton to NO2−, driven by the formation of an H bond to the backbone nitrogen of A200 that stabilizes the deprotonated histidine. The remaining proton at Nε2 of H197 can undergo a tautomeric shift to Nδ1, after which the H-bonding network among H197, T81, and a water molecule rearranges. After passing the constrictions, nitrous acid readily deprotonates and creates a hydronium ion at the water site. The positive charge can then relocate to the imidazole moiety, rearranging the H-bonding network again to reach the initial state. (C) Stereo representation of the region of the two constrictions. The residues involved in the H-bonding network required for the proton cycling mechanism are shown in a ball-and-stick representation.
StNirC is a nitrite channel with high conductance that does not undergo the pH-dependent functional switch characteristic of FocA formate channels/transporters. Similar to the two formate-oxidizing systems described above, enteric bacteria such as E. coli and S. typhimurium also contain both periplasmic (NrfA) and cytoplasmic (NirBD) nitrite reductases. Nevertheless, a switch in the transport mechanism is obviously not a physiological requirement for NirC. This may be related largely to the presence of a second membrane transporter for nitrite anions. NarK and NarU, members of the major facilitator superfamily of secondary active transport proteins, have been reported to function as nitrate/nitrite exchangers to provide the cytoplasmic nitrate reductases NarGHI and NarZYW with substrate and expel their toxic product, nitrite (11). These systems are induced by the same regulatory factors that also initiate transcription of nirC, so that the nitrite channel is not required for the export, allowing its evolutionary optimization to be directed toward an import function that can be passive or active, depending on whether a proton is cotransported. Reconciling the recent observation that StNirC reconstituted in a solid-supported membrane system seems to act as an NO2−/H+ antiporter (14) with this physiological role of NirC is not straightforward, however. This would imply that the transporter coexpressed from an operon with cytoplasmic nitrite reductase NirBD would actively remove the substrate of this enzyme from the cell. We note that in the solid-supported membrane electrophysiology study, everted membrane vesicles were energized by the addition of lactate (14). The monovalent lactate anion is a permeating species for StFocA (24) and likely for NirC as well, and may substantially influence the measurements in this case. Further studies are needed to clarify this apparent contradiction.
Its role in infection and its absence in higher eukaryotes make NirC a promising target for the development of novel types of antibacterial agents. Peroxynitrite and nitrite generated by the inducible NO synthase of macrophages as part of the innate immune response are detoxified by enteric pathogens through NirC-dependent uptake and cytoplasmic NirBD-dependent reduction to uncritical ammonia (16–18). Given the defined and rigid structure of the nitrite channel and the particularity of a strongly positive electrostatic surface potential around the entrance sites to the channels of the pentamer, it appears feasible to identify and optimize specific nitrite uptake inhibitors that may give the host a decisive edge over a pathogen.
Materials and Methods
Protein Production and Purification.
A variety of NirC orthologs were produced recombinantly and isolated as full-length proteins following previously described protocols (21). N-octyl-β-d-glucopyranoside (Anatrace) was used as a detergent for membrane solubilization, and each construct was purified by two chromatographic steps, affinity chromatography followed by size-exclusion chromatography. Several of the channels obtained were stable and could be crystallized by the sitting-drop vapor diffusion method, but all crystals obtained diffracted X-rays to only low resolution and with significant anisotropy. Thus, we generated a large number of N-terminal and C-terminal truncation variants with the aim of improving crystal packing and diffraction quality. The optimal construct obtained in this process was StNirC, with 17 amino acid residues deleted at the C terminus of the 269-aa polypeptide.
Electrophysiological Studies on NirC.
Full-length StNirC was reconstituted into liposomes generated from E. coli polar lipid extract (Avanti) at a protein:lipid ratio of 10 μg·mg–1 as described previously (30). The proteoliposomes were prepared in 20 mM of Tris/HCl buffer (pH 8.0) with 450 mM NaCl. For electrophysiological measurements using a Planar Lipid Bilayer Workstation (Warner Instruments), proteoliposomes were fused with a 200-μm-diameter lipid membrane created with E. coli polar lipid extract. Initially, both the cis and trans chambers contained 10 mM histidine and 20 mM sodium nitrite at a final pH of 7.9. A nitrite gradient between the two compartments was created by adding 30.4 μL of 8 M sodium nitrite to the cis side, yielding 100 mM of nitrite in cis and 20 mM in trans. Measurements with holding potentials varying from −70 mV to +70 mV in 10-mV steps were carried out in triplicate. Changes in pH were achieved by the stepwise addition of 0.7 N HCl to the trans chamber only, and the resulting pH was derived from a predetermined calibration curve. Single-channel recordings were made in the same buffer with a holding potential of ±100 mV or ±150 mV. Variations in nitrite concentrations were symmetric; that is, equivalent amounts of sodium nitrite were added to both the cis and trans chambers.
Crystallization of StNirC.
StNirC was crystallized by sitting-drop vapor diffusion. First, 2 µL of an 8 mg·mL−1 protein solution was mixed with 2 µL of a reservoir solution containing 27% (wt/vol) of polyethylene glycol 1000 and 0.1 M sodium citrate buffer at pH 4.5. As an additive, 0.4 µL of a 350 mM solution of the detergent cyclohexylbutanoyl-N-hydroxyethylglucamide was added to the crystallization drop. The final pH of the mixture was 5.0. Crystals appeared within 2–3 d at 4 °C. Before the crystals were mounted on cryo loops and flash-frozen in liquid nitrogen, the crystallization drop was left on air for 5–10 min, to allow some water evaporation. The resulting concentrated buffer was suitable as a cryoprotectant.
Data Collection, Structure Determination, and Refinement.
Diffraction data were collected on beamline X-06-SA of the Swiss Light Source (Villigen, Switzerland) on a Pilatus 6M pixel detector (Dectris). All data were indexed, integrated, and scaled using XDS (31). The structure was solved by molecular replacement using MOLREP (32), with the StFocA structure (PDB ID code 3Q7K) as the initial search model. Model building was done in Coot (33), and the structure was refined with REFMAC (34) to 2.4-Å resolution, using automatically generated TLS parameters and NCS restraints. Data collection and refinement statistics are presented in Table 1.
Table 1.
Data collection and refinement statistics
| Dataset | Native |
| Data collection | |
| Space group | C2 |
| Cell dimensions | |
| a, b, c, Å | 176.3, 101.8, 205.3 |
| α, β, γ, degrees | 90.0, 101.2, 90.0 |
| Resolution, Å | 87.75–2.40 (2.46–2.40)* |
| Rmerge | 0.065 (0.518) |
| I/σI | 12.3 (2.7) |
| Completeness, % | 98.3 (98.0) |
| Redundancy | 3.9 (4.0) |
| Refinement | |
| Resolution, Å | 87.0–2.4 (2.46–2.40) |
| No. of reflections | 136,898 (19,855) |
| Rwork/ Rfree | 0.192/0.236 (0.297/0.344) |
| No. of atoms | |
| Protein | 18,780 |
| Ligand/ion | 0 |
| Water | 196 |
| B-factors | |
| Protein | 49.1 |
| Ligand/ion | 0 |
| Water | 44.8 |
| rmsd | |
| Bond length, Å | 0.018 |
| Bond angle, degrees | 1.845 |
*The highest-resolution shell is shown in parentheses.
Atomic coordinates and structure factor data for NirC were deposited in the Protein Data Bank (PDB ID code 4FC4).
Acknowledgments
We thank the staff at beamline X-06-SA at the Swiss Light Source for their excellent assistance with data collection. This work was supported by Deutsche Forschungsgemeinschaft Grants Ei 520/3 (to O.E.), Ei 520/6 (to O.E.), An 676/1 (to S.L.A.A.), and International Research Training Grant 1478.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4FC4).
References
- 1.Einsle O, Kroneck PMH. Structural basis of denitrification. Biol Chem. 2004;385(10):875–883. doi: 10.1515/BC.2004.115. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-Espinosa RM, Cole JA, Richardson DJ, Watmough NJ. Enzymology and ecology of the nitrogen cycle. Biochem Soc Trans. 2011;39(1):175–178. doi: 10.1042/BST0390175. [DOI] [PubMed] [Google Scholar]
- 3.Gilberthorpe NJ, Poole RK. Nitric oxide homeostasis in Salmonella typhimurium: Roles of respiratory nitrate reductase and flavohemoglobin. J Biol Chem. 2008;283(17):11146–11154. doi: 10.1074/jbc.M708019200. [DOI] [PubMed] [Google Scholar]
- 4.Wink DA, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254(5034):1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 5.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 6.Canfield DE, Glazer AN, Falkowski PG. The evolution and future of Earth’s nitrogen cycle. Science. 2010;330(6001):192–196. doi: 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- 7.Saier MH, Jr, et al. Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim Biophys Acta. 1999;1422(1):1–56. doi: 10.1016/s0304-4157(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 8.White WB, Ferry JG. Identification of formate dehydrogenase-specific mRNA species and nucleotide sequence of the fdhC gene of Methanobacterium formicicum. J Bacteriol. 1992;174(15):4997–5004. doi: 10.1128/jb.174.15.4997-5004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czyzewski BK, Wang DN. Identification and characterization of a bacterial hydrosulphide ion channel. Nature. 2012;483(7390):494–497. doi: 10.1038/nature10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peakman T, et al. Nucleotide sequence, organisation and structural analysis of the products of genes in the nirB-cysG region of the Escherichia coli K-12 chromosome. Eur J Biochem. 1990;191(2):315–323. doi: 10.1111/j.1432-1033.1990.tb19125.x. [DOI] [PubMed] [Google Scholar]
- 11.Clegg S, Yu F, Griffiths L, Cole JA. The roles of the polytopic membrane proteins NarK, NarU and NirC in Escherichia coli K-12: Two nitrate and three nitrite transporters. Mol Microbiol. 2002;44(1):143–155. doi: 10.1046/j.1365-2958.2002.02858.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu HC, Tyson KL, Cole JA, Busby SJW. Regulation of transcription initiation at the Escherichia coli nir operon promoter: A new mechanism to account for co-dependence on two transcription factors. Mol Microbiol. 1998;27(2):493–505. doi: 10.1046/j.1365-2958.1998.00699.x. [DOI] [PubMed] [Google Scholar]
- 13.Jia WJ, Tovell N, Clegg S, Trimmer M, Cole J. A single channel for nitrate uptake, nitrite export and nitrite uptake by Escherichia coli NarU and a role for NirC in nitrite export and uptake. Biochem J. 2009;417(1):297–304. doi: 10.1042/BJ20080746. [DOI] [PubMed] [Google Scholar]
- 14.Rycovska A, Hatahet L, Fendler K, Michel H. The nitrite transport protein NirC from Salmonella typhimurium is a nitrite/proton antiporter. Biochim Biophys Acta. 2012;1818(5):1342–1350. doi: 10.1016/j.bbamem.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Xie Q, Nathan C. The high-output nitric oxide pathway: Role and regulation. J Leukoc Biol. 1994;56(5):576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 16.Brett PJ, Burtnick MN, Su H, Nair V, Gherardini FC. iNOS activity is critical for the clearance of Burkholderia mallei from infected RAW 264.7 murine macrophages. Cell Microbiol. 2008;10(2):487–498. doi: 10.1111/j.1462-5822.2007.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravortty D, Hensel M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003;5(7):621–627. doi: 10.1016/s1286-4579(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 18.Das P, Lahiri A, Lahiri A, Chakravortty D. Novel role of the nitrite transporter NirC in Salmonella pathogenesis: SPI2-dependent suppression of inducible nitric oxide synthase in activated macrophages. Microbiology. 2009;155(Pt 8):2476–2489. doi: 10.1099/mic.0.029611-0. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Structure of the formate transporter FocA reveals a pentameric aquaporin-like channel. Nature. 2009;462(7272):467–472. doi: 10.1038/nature08610. [DOI] [PubMed] [Google Scholar]
- 20.Waight AB, Love J, Wang DN. Structure and mechanism of a pentameric formate channel. Nat Struct Mol Biol. 2010;17(1):31–37. doi: 10.1038/nsmb.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lü W, et al. pH-dependent gating in a FocA formate channel. Science. 2011;332(6027):352–354. doi: 10.1126/science.1199098. [DOI] [PubMed] [Google Scholar]
- 22.Agre P, et al. Aquaporin water channels—from atomic structure to clinical medicine. J Physiol. 2002;542(Pt 1):3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilf RJC, Dutzler R. A prokaryotic perspective on pentameric ligand-gated ion channel structure. Curr Opin Struct Biol. 2009;19(4):418–424. doi: 10.1016/j.sbi.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Lü W, et al. The formate channel FocA exports the products of mixed-acid fermentation. Proc Natl Acad Sci USA. 2012;109(33):13254–13259. doi: 10.1073/pnas.1204201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawers RG. Formate and its role in hydrogen production in Escherichia coli. Biochem Soc Trans. 2005;33(Pt 1):42–46. doi: 10.1042/BST0330042. [DOI] [PubMed] [Google Scholar]
- 26.von Heijne G, Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440(7083):484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 28.Suppmann B, Sawers G. Isolation and characterization of hypophosphite-resistant mutants of Escherichia coli: Identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol. 1994;11(5):965–982. doi: 10.1111/j.1365-2958.1994.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 29.Wolff N, et al. Histidine pK(a) shifts and changes of tautomeric states induced by the binding of gallium-protoporphyrin IX in the hemophore HasA(SM) Protein Sci. 2002;11(4):757–765. doi: 10.1110/ps.3630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knol J, Sjollema K, Poolman B. Detergent-mediated reconstitution of membrane proteins. Biochemistry. 1998;37(46):16410–16415. doi: 10.1021/bi981596u. [DOI] [PubMed] [Google Scholar]
- 31.Kabsch W. XDS. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vagin A, Teplyakov A. Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 1):22–25. doi: 10.1107/S0907444909042589. [DOI] [PubMed] [Google Scholar]
- 33.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 35.Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MS. HOLE: A program for the analysis of the pore dimensions of ion channel structural models. J Mol Graph. 1996;14(6):354–360, 376. doi: 10.1016/s0263-7855(97)00009-x. [DOI] [PubMed] [Google Scholar]