Abstract
The anaerobic archaeon Pyrococcus furiosus grows by fermenting carbohydrates producing H2, CO2, and acetate. We show here that it is surprisingly tolerant to oxygen, growing well in the presence of 8% (vol/vol) O2. Although cell growth and acetate production were not significantly affected by O2, H2 production was reduced by 50% (using 8% O2). The amount of H2 produced decreased in a linear manner with increasing concentrations of O2 over the range 2–12% (vol/vol), and for each mole of O2 consumed, the amount of H2 produced decreased by approximately 2 mol. The recycling of H2 by the two cytoplasmic hydrogenases appeared not to play a role in O2 resistance because a mutant strain lacking both enzymes was not more sensitive to O2 than the parent strain. Decreased H2 production was also not due to inactivation of the H2-producing, ferredoxin-dependent membrane-bound hydrogenase because its activity was unaffected by O2 exposure. Electrons from carbohydrate oxidation must therefore be diverted to relieve O2 stress at the level of reduced ferredoxin before H2 production. Deletion strains lacking superoxide reductase (SOR) and putative flavodiiron protein A showed increased sensitivity to O2, indicating that these enzymes play primary roles in resisting O2. However, a mutant strain lacking the proposed electron donor to SOR, rubredoxin, was unaffected in response to O2. Hence, electrons from sugar oxidation normally used to produce H2 are diverted to O2 detoxification by SOR and putative flavodiiron protein A, but the electron flow pathway from ferredoxin does not necessarily involve rubredoxin.|
The anaerobic archaeon Pyrococcus furiosus grows optimally near 100 °C by fermenting various carbohydrates and peptides to form organic acids, CO2, and H2, or, in the presence of elemental sulfur (S0), H2S (1). In the metabolism of sugars to acetate via a modified Embden-Meyerhof pathway, the two oxidation steps are catalyzed by glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) and pyruvate ferredoxin oxidoreductase (POR), both of which use oxidized ferredoxin as an electron acceptor rather than nicotinamide–adenine dinucleotide or NADP (2, 3). In the absence of S0, the reduced ferredoxin is oxidized by a membrane-bound [NiFe]-hydrogenase (MBH) that conserves energy in the form of an ion gradient that can be used to generate ATP via a membrane-bound ATP synthase (4). It is estimated that for each glucose molecule oxidized, the formation of H2 by MBH is able to add 1.2 ATP to the energy pool. Substrate level phosphorylation from the conversion of phosphoenolpyruvate to pyruvate and the conversion of acetyl-CoA to acetate is responsible for 2.0 ATP per glucose molecule for a total of 3.2 ATP/glucose (4).
In addition to MBH, P. furiosus has two cytoplasmic [NiFe]-hydrogenases termed soluble hydrogenases I and II (SHI and SHII). Both enzymes are extremely active in vitro using H2 to reduce NADP to NADPH; these are assumed to be the physiological reactions (5). Support for this comes from the related hyperthermophile, Thermococcus kodakarensis, in which a mutant strain lacking the homolog of P. furiosus SHI produced higher levels of H2. It was proposed that T. kodakarensis recycles H2 via SHI to reduce NADP in the cell (6, 7).
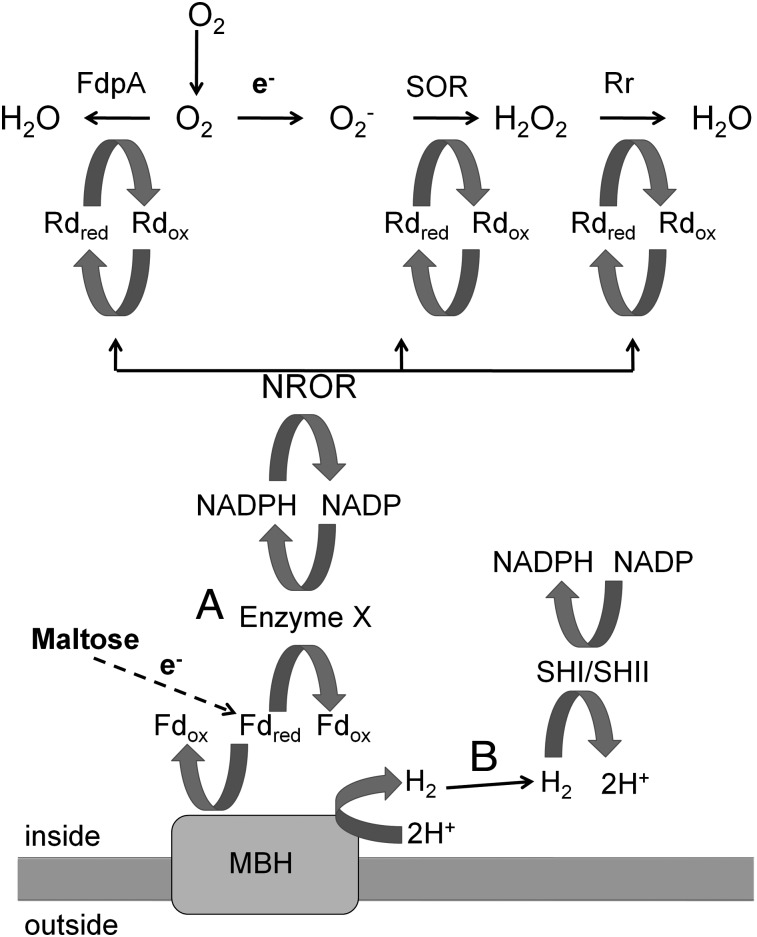
In general, anaerobic organisms lack the classical defense mechanism against reactive oxygen species (ROS) found in aerobic organisms, which includes superoxide dismutase and catalase, although there are some exceptions (8–10). This is the case for P. furiosus because its genome contains no homologs of either of these enzymes (11). In contrast, anaerobes have been proposed to contain a superoxide-reducing system based on superoxide reductase (SOR), an enzyme first identified in P. furiosus (12). An SOR homolog from Desulfoarculus baarsii was first implicated in dealing with superoxide (O2−) when it was able to suppress the phenotypes of an E. coli mutant lacking both superoxide dismutase enzymes (13). It was later shown that SOR reduces O2− rather than dismutating it (12). Other proteins in P. furiosus have been implicated in an oxidative stress response to ROS, including the peroxidase rubrerythrin (Rbr (14)), the electron carrier rubredoxin (Rd (15)), and NADP rubredoxin oxidoreductase (NROR (16), Fig. 1). The entire reduction pathway has been reconstituted in vitro using NADPH as the ultimate reductant for O2− reduction (17). The mononuclear iron site of SOR is reduced by Rd that is in turn reduced by the flavoprotein NROR, which uses NAD(P)H as the electron donor. The same pathway has been shown in vitro to reduce Rbr, which can reduce H2O2 rather than dismutating it like catalase (14). One particular advantage to reduction rather than dismutation of O2− is that O2 is not generated, which could contribute to additional toxicity.
Fig. 1.
Model of electron flow in the oxidative stress defense system of P. furiosus. The oxidation of maltose results in the reduction of ferredoxin, which is used to produce H2 by the MBH. Two different routes are proposed to connect the reduced ferredoxin (Fd) pool to the NADPH pool, which is the proposed electron source for the oxidative stress defense enzymes FdpA, SOR, and Rbr. Route A: An unknown enzyme (X) directly transfers electrons from reduced Fd to NADP. Route B: The H2 that is produced by MBH is recycled by oxidation by the soluble hydrogenases SHI and SHII forming NADPH. NROR and Rd are proposed to transfer electrons from NADPH to SOR, Rbr, and FdpA.
Another family of proteins important in the protection from ROS in anaerobes is flavodiiron proteins (FDPs). Two activities have been reported for these proteins: the reduction of O2 and the reduction of NO (18). The FDP of Desulfovibrio gigas is termed rubredoxin oxygen oxidoreductase (ROO) and it reduces O2 in vitro using Rd as the electron donor (19). In vivo evidence for a role of FDP in O2 detoxification was found in Desulfovibrio vulgaris. A strain lacking ROO was shown to be more sensitive than its parent when exposed to air (20). P. furiosus contains two FDPs, putative FdpA encoded by PF0751 and FdpB encoded by PF0694, but their physiological roles are not known. In this study, we investigated the O2 sensitivity of P. furiosus, the possible mechanisms that it uses for protection, and the relationship between O2 resistance and H2 metabolism. The organism was reported to be a strict anaerobe unable to grow in the presence of O2 (1).
Results
Growing P. furiosus Cells Are O2-Resistant.
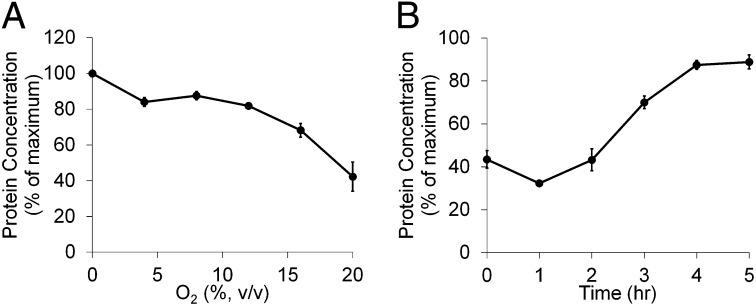
The sensitivity of P. furiosus to O2 exposure was investigated in sealed 100-mL culture bottles containing 40-mL cultures by injecting increasing amounts of pure O2 to the headspace at different times during the growth cycle (Fig. 2). The organism was surprisingly resilient to O2 when O2 was added in midlog phase, with concentrations close to 20% (vol/vol in headspace) required to show a 50% decrease in cell yield (after 8 h; Fig. 2A). However, if O2 was present before inoculation or was added during the lag phase of growth (before 2 h), 8% O2 caused a decrease in cell yield by almost 70% (Fig. 2B). To study the effects of O2 exposure in P. furiosus, the experimental model used an 8% (vol/vol) concentration of O2 added ∼4 h after inoculation when the cell density reached 1 × 107 cells/mL. As shown in Fig. 3, this partially but not completely inhibited the growth of the parent strain, designated COM1c2, making it possible to compare strains harboring various gene deletions.
Fig. 2.
Exposure of P. furiosus cultures to different concentrations of O2 at different points of growth. Cultures (40 mL) in 100-mL bottles were grown at 95 °C in medium containing 5 g/L maltose, 0.5 g/L yeast extract, and no sulfide on a shaking platform (150 rpm). Growth was monitored near the end of log phase (8 h) using total cell protein. (A) Cultures were exposed to 8% O2 at various times in the growth cycle. (B) Cultures were exposed to increasing amounts of O2 after 4 h of growth (about 20% grown). Values are % protein produced compared with a culture not challenged with O2.
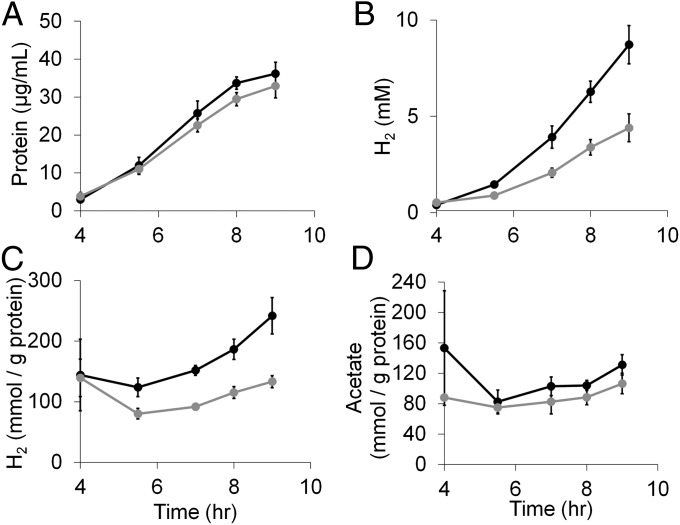
Fig. 3.
Oxygen challenge of P. furiosus. Cultures (40 mL) in 100-mL bottles were grown at 95 °C in medium containing 5 g/L maltose, 0.5 g/L yeast extract, and no sulfide on a shaking platform (150 rpm). Cultures were grown anoxically (filled circles) or were exposed to 8% O2 (vol/vol in headspace) after 4 h of growth (gray circles). (A) Growth was monitored using total cell protein. (B) Total H2 production. (C) Specific H2 production where the amount of H2 produced was divided by the protein concentration of the culture. (D) Specific acetate production where the amount of acetate produced was divided by the protein concentration of the culture.
Exposure to O2 Decreases H2 Production by P. furiosus.
The effects of 8% O2 on the growth of COM1c2 at 95 °C were investigated using maltose as the carbon source. Throughout growth, samples were taken to monitor growth (measured by protein production), acetate, and H2. As shown in Fig. 3, the O2 challenge had a greater effect on H2 production than it did on cell growth. After 9 h, the O2 challenge caused a 9% decrease in growth but a 50% decrease in H2 yield. The specific H2 production curve (Fig. 3C) demonstrates the decrease in specific H2 production throughout the course of the O2 challenge experiment. Unlike H2 production, the production of acetate, the main organic acid by-product of P. furiosus growth, was not specifically decreased compared with protein production (Fig. 3D).
Quantitation of O2 Effect on H2 Production.
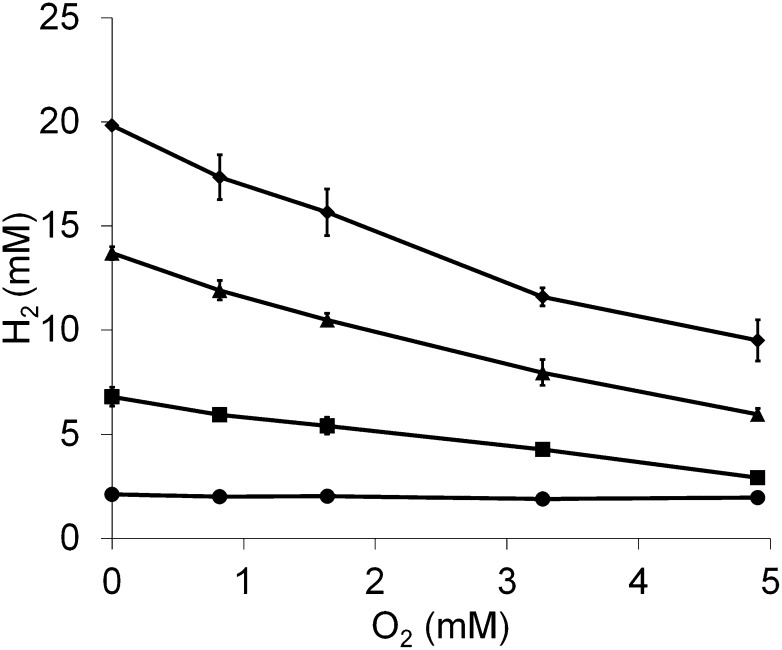
To quantify the effect of O2 on H2 production, various concentrations of O2 (2–12%, or 0.8–4.9 mM in the gas phase) were added to actively growing P. furiosus cultures, and protein, H2 production, and O2 consumption was measured at various intervals (Fig. S1A). As shown in Fig. 4, the amount of H2 produced decreased in a linear manner with increasing concentrations of O2. At the highest O2 concentration used (4.9 mM in the gas phase), the amount of O2 remaining after 6 h exposure was almost zero (Fig. S1B). Moreover, the slope of a plot of H2 concentrations versus O2 concentrations measured 6 h after O2 addition was −2.1 (Fig. 4), indicating that for each mole of O2 consumed, approximately 2 mol of H2 were used. This agrees with the expected stoichiometry, in which electrons that would otherwise be used to reduce protons to H2 (a two-electron process) are used for the reduction of O2 to H2O (a four-electron process). At the 2- and 4-h time points (Fig. 4) when the O2 was not completely removed from the cultures, the slopes of the O2 versus H2 concentration plots were −0.8 and −1.6, respectively. Hence, H2 production continues in the presence of O2, but at a diminished rate in proportion to concentration of the O2 present.
Fig. 4.
Exposure of P. furiosus cultures to O2 decreases H2 production in a linear fashion. Cultures (40 mL) in 100-mL bottles were grown at 95 °C in medium containing 5 g/L maltose, 0.5 g/L yeast extract but no added sulfide on a shaking platform (150 rpm). After 6 h of growth, cultures were exposed to a range of O2 concentrations (0.8–4.9 mM in the gas phase) and the amount of H2 produced was measured at 0 (circles), 2 (squares), 4 (triangles), and 6 h (diamonds) after O2 exposure.
Working Models of O2 Detoxification in P. furiosus.
There are several mechanisms by which O2 stress could specifically affect H2 production by growing P. furiosus cells. The H2-generating hydrogenase MBH could be inactivated as a result of the O2 stress, thereby impairing H2 production. Conversely, the flow of electrons from carbohydrate oxidation via ferredoxin could be diverted from MBH and used to reduce and detoxify O2, which would result in less H2 produced. In other words, O2 detoxification and H2 production would be competing processes in P. furiosus. A third possibility is that the H2 already produced by P. furiosus is recycled by one or both of the cytoplasmic hydrogenases, SH1 and SHII, to form NADPH, which in turn provides electrons for O2 reduction. The working model shown in Fig. 1 illustrates the proposed roles of the enzymes involved in how P. furiosus resists oxidative stress and how they are interconnected. In pathway A, the reduced ferredoxin and NADPH pools are connected by an unknown enzyme (X) thereby connecting H2 production and O2 detoxification. Pathway B shows how H2 could be recycled to generate NADPH for O2 detoxification. Next we describe experiments designed to differentiate between these possibilities and determine how H2 production and O2 detoxification are linked.
H2 Recycling Does Not Play a Major Role in O2 Detoxification in P. furiosus.
It has been proposed that the homolog of SHI in the related organism, T. kodakarensis, can function as an uptake hydrogenase oxidizing H2 and reducing NADP (6, 7). If H2 can be recycled in P. furiosus by either of the cytosolic hydrogenases SHI or SHII, this could explain the decreased H2 production seen after O2 exposure. If H2 recycling plays a role in O2 defense, a strain lacking both SHI and SHII would be unable to oxidize the H2 that is produced and therefore might be more sensitive to O2. In addition, the exogenous addition of H2 to the headspace of the growing cultures might protect P. furiosus from O2 stress by providing additional reductant for O2 reduction. However, as shown in Fig. S2, when exposed to 8% O2, the double-mutant SHI–SHIIc strain behaved in a similar fashion to the COM1c2 strain, and both had similar rates of H2 production during growth (Fig. S2A). Hence, SHI and SHII do not appear to play a role in O2 resistance. This was confirmed by exposing the COM1c2 strain to 12.5% O2 with varying amounts of H2 (up to 15%, vol/vol) present in the headspace. The increased concentration of O2 (to 12.5%) was used to accentuate the growth phenotype because H2 production could not be accurately measured when adding high concentrations of H2 to the headspace. Growth of the COM1c2 strain was inhibited by O2, but increasing the concentrations of H2 up to 15% (vol/vol) in the presence of O2 had no significant effect on growth (Fig. S2B). If H2 metabolism and O2 metabolism are connected by electron flow, pathway B shown in Fig. 1 appears to represent the pathway of electron flow, at least from sugar oxidation to NADPH. This raises the questions of whether the lower yield of H2 in the presence of O2 is due to the partial inactivation of MBH by O2.
Decrease in H2 Production on O2 Exposure Is not Caused by Direct Damage of MBH.
To determine if the O2 growth challenge resulted in direct damage to the MBH, the H2 production activity of MBH was measured in cell-free extracts using ferredoxin reduced by POR as the electron donor. Extracts were prepared from cells that were and were not challenged with 8% O2 for 30 min before harvesting. The activities of the two types of extracts were similar: 0.22 ± 0.01 units/mg for unexposed cells and 0.21 ± 0.01 units/mg protein for O2-challenged cells. The lack of difference in MBH activity indicates that the 50% decrease in the yield of H2 seen with the O2 challenge is not due to inactivation of MBH (or of POR, which generates the reduced ferredoxin). Therefore, we propose that, as shown in Fig. 1, reductant from sugar oxidation is diverted away from MBH at the level of reduced ferredoxin, which is used instead to generate NADPH. This then raises the issue of how the apparent increased amount of NADPH generated in cells in the presence of O2 is used to confer resistance to O2.
Response of Mutants Lacking SOR, FdpA, and Rd to O2 Challenge.
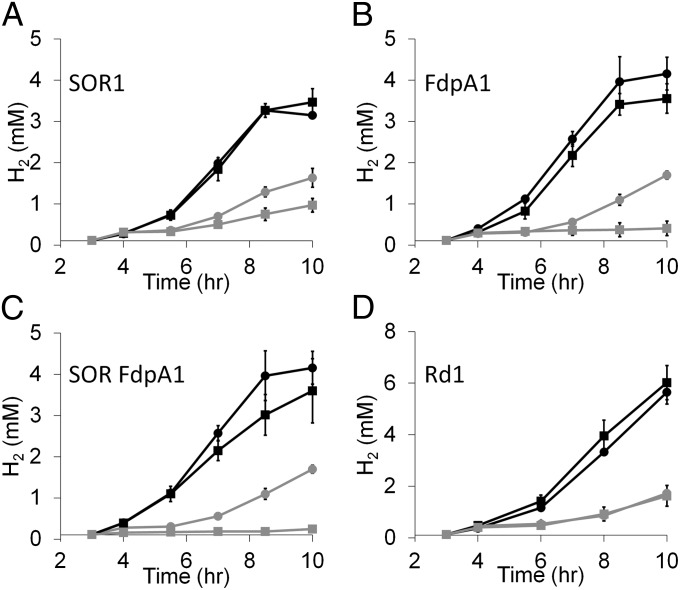
Strains were constructed that lacked one or more of the genes that encode the enzymes that are proposed to be involved in the oxidative stress response of P. furiosus (Table 1). These included SOR, FdpA, and Rd. The strains were tested for O2 sensitivity by exposure to 8% O2 after 4 h of growth at 95 °C. H2 production was measured over time and the results are shown in Fig. 5. As with the COM1c2 parental strain, all of the mutant strains when exposed to O2 exhibited a lag and decrease in H2 production. However, although the mutant lacking Rd was similarly sensitive to O2, the mutants lacking SOR1 or FdpA1, and the double-mutant SOR FdpA1, were much more sensitive to O2 than their respective parent strains. In fact, the FdpA1 and double mutant SOR FdpA1 strains were much more sensitive to O2 than the SOR1 strain and displayed no H2 production after the O2 challenge. Both strains lacking FdpA did not grow after O2 exposure, explaining their lack of H2 production (Fig. S3).
Table 1.
P. furiosus strains constructed and/or used in this study
| Strain | Genotype | Deleted ORF(s) | Source |
| COM1 (MW0002)* | ΔpyrF | PF1114 | (41) |
| COM1c2 (MW0004) | ΔpyrF::PgdhpyrF | None, restored | This work |
| SOR1 (MW0017) | ΔpyrF Δsor | PF1114, PF1281 | This work |
| Rd1 (MW0019) | ΔpyrF Δrd::PgdhpyrF | PF1282 | This work |
| FdpA1 (MW0020) | ΔpyrF ΔfprA::PgdhpyrF | PF0751 | This work |
| SOR FdpA1 (MW0021) | ΔpyrF Δsor ΔfprA::PgdhpyrF | PF1281, PF0751 | This work |
| ΔshI ΔshII (MW0015) | ΔpyrF ΔshI ΔshII | PF1114, PF0891–PF0894, PF1329–PF1332 | (41) |
| SHI SHIIc (MW0016) | ΔpyrF::PgdhpyrF ΔshI ΔshII | PF0891–PF0894, PF1329–PF1332 | This work |
*MW strain codes in parentheses are laboratory strain designations.
Fig. 5.
Exposure of P. furiosus mutants lacking SOR, FdpA, and Rd to O2 challenge. Cultures (40 mL) in 100-mL bottles were grown at 95 °C in medium containing 5 g/L maltose, 0.5 g/L yeast extract, and no sulfide on a shaking platform (150 rpm). Cultures parent (circles) or mutant (squares) were grown anoxically (black) or were exposed to 8% O2 (vol/vol in headspace) after 4 h of growth (gray). Total H2 production was measured throughout growth. (A) SOR1, (B) FdpA1, (C) SOR FdpA1, and (D) Rd1.
Discussion
P. furiosus is regarded as a fastidious anaerobe (1) and so one of the most surprising results of the current study was its tolerance to exposure to O2. It can withstand atmospheric levels of O2 (21%) when exposed during growth. A critical aspect is that cells must be actively growing, however, because the final cell yield decreased dramatically (30% of the control culture) when a culture was inoculated into the standard medium with O2 at 8% (vol/vol) present. Other organisms that were once considered to be strictly anaerobic have also been shown to be relatively aerotolerant and able to survive exposure to air (21% O2 vol/vol), including some of the sulfate-reducing Desulfovibrio species (20, 21) and methanogens from the genus Methanosarcina (22). On the other hand, the hyperthermophilic bacterium Thermotoga maritima could grow in the presence of only 0.5% (vol/vol) O2 in the gas phase when exposed at the beginning of growth (23). It has been proposed that some Desulfovibrio species can even respire O2, resulting in proton translocation (21, 24). Whole-genome transcriptional analysis has shown that in the response of P. furiosus to peroxide stress, the genes encoding the proposed oxidative defense system of P. furiosus shown in Fig. 1 are not up-regulated. Rather, these genes are constitutively expressed, indicating that P. furiosus is always prepared for exposure to ROS (25). This notion is consistent with the idea that in their natural environments, hyperthermophilic organisms such as P. furiosus are exposed to O2 suddenly when cool, oxygenated seawater mixes with hydrothermal vent fluid (26, 27).
There was a dramatic reduction (50%) in the amount of H2 produced by growing cells when O2 was present (Fig. 3). Theoretically, 1.2 ATP of the total 3.2 ATP gained from converting one glucose molecule to acetate is derived from the production of H2 by MBH, so the amount of ATP lost from the decreased H2 production can be estimated to be around 19%. The presence of O2 resulted in a decrease in cell protein yield of about 9%, indicating that ATP production is not limiting growth under these conditions. Therefore, although the effect on H2 production is dramatic, the cells are able to compensate and continue to grow despite the loss of ATP. This also suggests that O2 is not directly damaging cells to a large extent and that metabolism persists despite O2 exposure. This is consistent with the fact that the H2-producing hydrogenase of P. furiosus, MBH, was unaffected in terms of its activity in cells exposed to O2. We also show here that the 50% reduction in H2 production as a result of O2 exposure is not due to an increase in the amount of H2 recycled by the cytoplasmic hydrogenases SHI and SHII. In fact, there appeared to be no connection between these enzymes and O2 metabolism.
Quantitation of the effect O2 exposure had on H2 production revealed that each mole of O2 added to the culture resulted in 2 mol less H2 produced. This ratio is consistent with the proposed model in which electrons from sugar fermentation that are normally used to generate H2 via MBH are diverted to reduce O2 when the cells are exposed to O2 stress. This reduction in H2 yield also means that less energy (ATP) is conserved, but this also does not affect cell growth. Hence, there must be a mechanism to transfer electrons from reduced ferredoxin to NADP (Fig. 1). One possibility is that P. furiosus can reduce NADP directly during glycolysis using the enzyme nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPN), which generates 3-phosphoglycerate and reduces NADP, bypassing the ferredoxin-reducing enzyme GAPOR (28, 29). Interestingly, strains of the related organism T. kodakarensis lacking either GAPN or GAPOR were unable to grow by glucose fermentation, suggesting that GAPN does play an essential role. In addition, the two reductant pools of reduced ferredoxin and NADPH could be connected by cytoplasmic oxidoreductase I (CORI) (PF1327 and PF1328). The true physiological function of CORI is not known because in vitro it catalyzes several reactions, including the reduction of NADP with reduced P. furiosus ferredoxin (30). CORI can also use NADPH to both reduce Rd and reduce polysulfide to H2S (31). Interestingly, the genome of P. furiosus also contains a homolog of CORI termed CORII (PF1910 and PF1911) and they are reciprocally regulated during growth on sugars and peptides (32). Determining the pathways of electron flow between the two major electron pools in P. furiosus is a focus of future research.
Surprisingly the P. furiosus mutant lacking the intermediate electron transfer protein Rd did not have an obvious growth phenotype in the presence of O2. However, O2-sensitive growth phenotypes were seen for the deletion strains lacking FdpA and SOR. These data support the activities shown in Fig. 1, in which these two enzymes serve as the terminal reductases and reduce O2 and O2−, respectively. Such a role for FdpA is in agreement with what has been proposed in Desulfovibrio sp. in which FdpA is termed ROO (19, 20), although the biochemical activity of FdpA from P. furiosus was not investigated here. Interestingly, the fdpA mutant in P. furiosus was more sensitive to O2 than the sor mutant. Because O2− is derived in vivo from adventitious electron transfer to O2 (33, 34), the presence of FdpA in the sor mutant could limit the amount of O2− produced, but in the fdpA strain, the first line of O2 stress defense is missing. Nevertheless, these data demonstrate that both FdpA and SOR play roles in the oxidative stress response of P. furiosus.
The results presented here therefore led us to conclude that FdpA is the primary enzyme in removing O2, whereas SOR removes O2− when P. furiosus is exposed. The working model for how these enzymes obtain reductant is shown in Fig. 1. We have established that the response to O2 is independent of H2 and the hydrogenases MBH, SHI, and SHII, and that reductant is diverted from MBH to the enzymes involved in the oxidative stress response. How electrons are provided for removal of ROS still remains to be established, especially given that a mutant lacking Rd was not more sensitive to O2 than the parent strain. A potential explanation is that ferredoxin or another redox protein in P. furiosus can directly reduce both FdpA and SOR in the absence of Rd. The role of Rd in vivo is still not clear. For example, Escherichia coli contains an FdpA homolog termed flavorubredoxin that contains an Rd domain, in addition to the flavo and di-iron domains, and it catalyzes NO reduction (35). A mutant strain lacking flavorubredoxin had increased sensitivity to NO (36). However, the mutant was successfully complemented by FprA from Moorella thermoacetica and ROO from D. gigas and D. vulgaris, even though these proteins do not contain an Rd domain and no other Rd-like protein is present in E. coli (37–39).
The surprising discovery that FdpA, SOR, and Rbr are enzymes that deal with oxidative stress in anaerobes has been followed by much debate as to why anaerobes use the strategy of reduction to detoxify ROS rather than using dismutation like most aerobic organisms (40). Another side to the story, however, is the effects that using reduction, rather than dismutation, to detoxify ROS have on the metabolism of anaerobic organisms. The in vivo evidence presented here indicates that under certain conditions, the metabolic strain of using reduction to detoxify O2 can result in detectable phenotypes, such as decreased H2 production. This observation can be used to analyze both the oxidative stress defense system and the flow of electrons in anaerobic organisms such as P. furiosus; such studies are under way.
Materials and Methods
Strains and Strain Construction.
P. furiosus strains used in this study are listed in Table 1. All strains were constructed using the methodology previously described (41). The marker-replaced deletions of fdpA and rd were constructed by transforming linear DNA products made by overlap PCR in which 500 bp immediately upstream and downstream of the target gene were overlapped flanking the PgdhpyrF cassette with terminator (41) into the COM1 strain and/or the SOR1 strain. Transformants were selected on solid defined media for uracil prototrophy. To construct the markerless deletion of sor (SOR1), 1-kb flanking regions of the target gene were cloned into the pGLW015 plasmid (41), which contains the PgdhpyrF cassette for prototrophic selection. The plasmid was transformed into P. furiosus COM1 (ΔpyrF) selecting uracil prototrophy on solid defined medium and counterselected for plasmid loss using 5-fluoroorotic acid resistance as previously described (41). All deletion strains constructed were screened for deletion by PCR amplification of the locus using primers outside the homologous flanking regions. Isolates containing the deletions were colony-purified by serial passage on solid defined medium. Further strain conformation was obtained by PCR sequencing of the PCR amplifications and by quantitative PCR analysis using primers internal to the deleted gene in question.
The COM1c2 and SHI SHIIC strains in which the pyrF gene was reintroduced were constructed by transforming their parent strains with a construct containing the PgdhpyrF cassette flanked by a 1-kb sequence surrounding the original pyrF locus (Table 1). A markerless deletion of sor (SOR1) and marker-replaced deletions of fdpA (FdpA1) and rd (Rd1) were constructed from the COM1 background strain (ΔpyrF). In addition, a marker-replaced deletion of fdpA was made in the SOR1 background stain forming the Δsor ΔfdpA (SOR FdpA1) double-mutant strain. All strains were confirmed using PCR and sequence analysis. In addition, the quantitative PCR products of deleted genes could not be detected in any of the newly constructed strains.
Growth Conditions.
All strains were grown as previously reported (42) in complex growth medium using maltose as the carbon source and containing yeast extract (0.5 g/L), but no sulfide was added to prevent chemical interference in the O2 challenge experiments. Unless otherwise stated, growth experiments were carried out in triplicate in 40-mL cultures at 95 °C on a shaking incubator (150 rpm). To add O2 or H2 to the cultures, separate sealed bottles at room temperature were filled with 100% of the indicated gas and a syringe was used to transfer known amounts to growth temperature cultures. Percentages of O2 and H2 reported are calculated vol/vol in the headspace of the culture bottle. The solubility of O2 at 95 °C in a brine solution with similar salt concentration to the growth medium used was measured at 25 μM (43).
Analyses for Protein, H2, O2, and Acetate.
Protein assays were used to monitor growth throughout the course of this study. The Bradford method (44) was used to quantitate cell protein using BSA as the standard. To monitor H2 production and O2, 1-mL samples from the headspace of cultures at the growth temperature were transferred to argon filled sealed 5-mL vials at room temperature. From the secondary vials, 1 mL was injected into a Shimadzu GC-8A gas chromatograph for H2 and O2 measurements. Acetate production was measured from 1-mL culture samples as previously described (45). Error bars were calculated as the SD of measurements taken from independent cultures grown in triplicate, and slopes were calculated using linear regression trend lines.
Isolation of RNA and Quantitative PCR Analyses.
Acid-phenol was used to extract total RNA from P. furiosus cells (46). A second acid-phenol isolation followed by Turbo DNase (Ambion) treatment (30 min, 37 °C) and Absolutely RNA cleanup kit (Agilent Technologies) was performed to further purify the RNA. cDNA was made with the AffinityScrpt QPCR cDNA Synthesis Kit (Agilent). Quantitative PCR experiments were carried out in technical duplicate using an Mx300P instrument (Agilent) and the Brilliant SyBR green QPCR master mix (Agilent) out to 40 cycles. The absence of signal in the deletion mutant strains compared with the positive control of the parent or COM1 strain was used to confirm the deletion mutants.
Hydrogenase Assays.
Cultures (300 mL) were grown in triplicate at 95 °C on a shaking incubator (150 rpm) to midlog phase (5 h) before cultures were challenged or not with 8% O2 for 30 min. Cells were harvested and whole cell extracts were prepared anaerobically and were measured for protein content. Assays were performed anaerobically in 5-mL sealed vials at 85 °C and were started by addition of whole cell extract. Assay mixtures contained 50 mM potassium phosphate buffer (pH 7.0), 1 mM MgCl2, 10 mM pyruvate, 0.5 mM coenzyme A, and 7 µM ferredoxin. The H2 produced was measured using a Shimadzu GC-8A gas chromatograph. Hydrogenase activity is reported in units where 1 unit is equal to 1 µmol of H2 produced per minute.
Supplementary Material
Acknowledgments
We acknowledge the Office of Biological and Environmental Research of the Office of Basic Energy Sciences, Office of Science of the Department of Energy for funding strain construction and analyses through Grant FG02-08ER64690 (to R.A.S.) and the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the US Department of Energy for funding in part strain characterization through Grant DE-FG05-95ER20175 (to M.W.W.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208605109/-/DCSupplemental.
References
- 1.Fiala G, Stetter KO. Pyrococcus furiosus sp. nov., represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch Microbiol. 1986;145(1):56–61. [Google Scholar]
- 2.Blamey JM, Adams MWW. Purification and characterization of pyruvate ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. Biochim Biophys Acta. 1993;1161(1):19–27. doi: 10.1016/0167-4838(93)90190-3. [DOI] [PubMed] [Google Scholar]
- 3.Mukund S, Adams MWW. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase, a novel tungsten-containing enzyme with a potential glycolytic role in the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem. 1995;270(15):8389–8392. doi: 10.1074/jbc.270.15.8389. [DOI] [PubMed] [Google Scholar]
- 4.Sapra R, Bagramyan K, Adams MW. A simple energy-conserving system: Proton reduction coupled to proton translocation. Proc Natl Acad Sci USA. 2003;100(13):7545–7550. doi: 10.1073/pnas.1331436100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma K, Adams MW. Hydrogenases I and II from Pyrococcus furiosus. Methods Enzymol. 2001;331:208–216. doi: 10.1016/s0076-6879(01)31059-5. [DOI] [PubMed] [Google Scholar]
- 6.Kanai T, et al. Distinct physiological roles of the three [NiFe]-hydrogenase orthologs in the hyperthermophilic archaeon Thermococcus kodakarensis. J Bacteriol. 2011;193(12):3109–3116. doi: 10.1128/JB.01072-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santangelo TJ, Cuboňová L, Reeve JN. Deletion of alternative pathways for reductant recycling in Thermococcus kodakarensis increases hydrogen production. Mol Microbiol. 2011;81(4):897–911. doi: 10.1111/j.1365-2958.2011.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCord JM, Keele BB, Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: The physiological function of superoxide dismutase. Proc Natl Acad Sci USA. 1971;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 10.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robb FT, et al. Genomic sequence of hyperthermophile, Pyrococcus furiosus: Implications for physiology and enzymology. Methods Enzymol. 2001;330:134–157. doi: 10.1016/s0076-6879(01)30372-5. [DOI] [PubMed] [Google Scholar]
- 12.Jenney FE, Jr, Verhagen MFJM, Cui XY, Adams MWW. Anaerobic microbes: Oxygen detoxification without superoxide dismutase. Science. 1999;286(5438):306–309. doi: 10.1126/science.286.5438.306. [DOI] [PubMed] [Google Scholar]
- 13.Liochev SI, Fridovich I. A mechanism for complementation of the sodA sodB defect in Escherichia coli by overproduction of the rbo gene product (desulfoferrodoxin) from Desulfoarculus baarsii. J Biol Chem. 1997;272(41):25573–25575. doi: 10.1074/jbc.272.41.25573. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg MV, Jenney FE, Jr, Cui XY, Adams MWW. Rubrerythrin from the hyperthermophilic archaeon Pyrococcus furiosus is a rubredoxin-dependent, iron-containing peroxidase. J Bacteriol. 2004;186(23):7888–7895. doi: 10.1128/JB.186.23.7888-7895.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blake PR, et al. Solution-state structure by NMR of zinc-substituted rubredoxin from the marine hyperthermophilic archaebacterium Pyrococcus furiosus. Protein Sci. 1992;1(11):1508–1521. doi: 10.1002/pro.5560011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma K, Adams MWW. A hyperactive NAD(P)H:Rubredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 1999;181(17):5530–5533. doi: 10.1128/jb.181.17.5530-5533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grunden AM, et al. In vitro reconstitution of an NADPH-dependent superoxide reduction pathway from Pyrococcus furiosus. Appl Environ Microbiol. 2005;71(3):1522–1530. doi: 10.1128/AEM.71.3.1522-1530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicente JB, Justino MC, Gonçalves VL, Saraiva LM, Teixeira M. Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Methods Enzymol. 2008;437:21–45. doi: 10.1016/S0076-6879(07)37002-X. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, et al. Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the “strict anaerobe” Desulfovibrio gigas. Biochem Biophys Res Commun. 1993;193(1):100–105. doi: 10.1006/bbrc.1993.1595. [DOI] [PubMed] [Google Scholar]
- 20.Wildschut JD, Lang RM, Voordouw JK, Voordouw G. Rubredoxin:oxygen oxidoreductase enhances survival of Desulfovibrio vulgaris Hildenborough under microaerophilic conditions. J Bacteriol. 2006;188(17):6253–6260. doi: 10.1128/JB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cypionka H. Oxygen respiration by desulfovibrio species. Annu Rev Microbiol. 2000;54:827–848. doi: 10.1146/annurev.micro.54.1.827. [DOI] [PubMed] [Google Scholar]
- 22.Kiener A, Leisinger T. Oxygen sensitivity of methanogenic bacteria. Syst Appl Microbiol. 1983;4:305–312. doi: 10.1016/S0723-2020(83)80017-4. [DOI] [PubMed] [Google Scholar]
- 23.Le Fourn C, Fardeau ML, Ollivier B, Lojou E, Dolla A. The hyperthermophilic anaerobe Thermotoga maritima is able to cope with limited amount of oxygen: Insights into its defence strategies. Environ Microbiol. 2008;10(7):1877–1887. doi: 10.1111/j.1462-2920.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- 24.Fitz RM, Cypionka H. A study on electron transport-driven proton translocation in Desulfovibrio desulfuricans. Arch Microbiol. 1989;152(4):369. doi: 10.1007/BF00409657. [DOI] [PubMed] [Google Scholar]
- 25.Strand KR, et al. Oxidative stress protection and the repair response to hydrogen peroxide in the hyperthermophilic archaeon Pyrococcus furiosus and in related species. Arch Microbiol. 2010;192(6):447–459. doi: 10.1007/s00203-010-0570-z. [DOI] [PubMed] [Google Scholar]
- 26.Fouquet Y, et al. Tectonic setting and mineralogical and geochemical zonation in the Snake Pit Sulfide Deposit (Mid-Atlantic Ridge at 23-degrees-N) Econ Geol. 1993;88:2018–2036. [Google Scholar]
- 27.Huber R, Dyba D, Huber H, Burggraf S, Rachel R. Sulfur-inhibited Thermosphaera aggregans sp. nov., a new genus of hyperthermophilic archaea isolated after its prediction from environmentally derived 16S rRNA sequences. Int J Syst Bacteriol. 1998;48(Pt 1):31–38. doi: 10.1099/00207713-48-1-31. [DOI] [PubMed] [Google Scholar]
- 28.Ettema TJ, Ahmed H, Geerling AC, van der Oost J, Siebers B. The non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPN) of Sulfolobus solfataricus: A key-enzyme of the semi-phosphorylative branch of the Entner-Doudoroff pathway. Extremophiles. 2008;12(1):75–88. doi: 10.1007/s00792-007-0082-1. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara K, Yokooji Y, Atomi H, Imanaka T. Biochemical and genetic characterization of the three metabolic routes in Thermococcus kodakarensis linking glyceraldehyde 3-phosphate and 3-phosphoglycerate. Mol Microbiol. 2011;81(5):1300–1312. doi: 10.1111/j.1365-2958.2011.07762.x. [DOI] [PubMed] [Google Scholar]
- 30.Ma K, Adams MW. Ferredoxin:NADP oxidoreductase from Pyrococcus furiosus. Methods Enzymol. 2001;334:40–45. doi: 10.1016/s0076-6879(01)34456-7. [DOI] [PubMed] [Google Scholar]
- 31.Ma K, Adams MW. Sulfide dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus: A new multifunctional enzyme involved in the reduction of elemental sulfur. J Bacteriol. 1994;176(21):6509–6517. doi: 10.1128/jb.176.21.6509-6517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schut GJ, Brehm SD, Datta S, Adams MWW. Whole-genome DNA microarray analysis of a hyperthermophile and an archaeon: Pyrococcus furiosus grown on carbohydrates or peptides. J Bacteriol. 2003;185(13):3935–3947. doi: 10.1128/JB.185.13.3935-3947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massey V, et al. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun. 1969;36(6):891–897. doi: 10.1016/0006-291x(69)90287-3. [DOI] [PubMed] [Google Scholar]
- 34.Messner KR, Imlay JA. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem. 1999;274(15):10119–10128. doi: 10.1074/jbc.274.15.10119. [DOI] [PubMed] [Google Scholar]
- 35.Gomes CM, Vicente JB, Wasserfallen A, Teixeira M. Spectroscopic studies and characterization of a novel electron-transfer chain from Escherichia coli involving a flavorubredoxin and its flavoprotein reductase partner. Biochemistry. 2000;39(51):16230–16237. doi: 10.1021/bi001844y. [DOI] [PubMed] [Google Scholar]
- 36.Gardner AM, Helmick RA, Gardner PR. Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J Biol Chem. 2002;277(10):8172–8177. doi: 10.1074/jbc.M110471200. [DOI] [PubMed] [Google Scholar]
- 37.Silaghi-Dumitrescu R, et al. A flavodiiron protein and high molecular weight rubredoxin from Moorella thermoacetica with nitric oxide reductase activity. Biochemistry. 2003;42(10):2806–2815. doi: 10.1021/bi027253k. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues R, et al. Desulfovibrio gigas flavodiiron protein affords protection against nitrosative stress in vivo. J Bacteriol. 2006;188(8):2745–2751. doi: 10.1128/JB.188.8.2745-2751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silaghi-Dumitrescu R, Ng KY, Viswanathan R, Kurtz DM., Jr A flavo-diiron protein from Desulfovibrio vulgaris with oxidase and nitric oxide reductase activities. Evidence for an in vivo nitric oxide scavenging function. Biochemistry. 2005;44(9):3572–3579. doi: 10.1021/bi0477337. [DOI] [PubMed] [Google Scholar]
- 40.Imlay JA. What biological purpose is served by superoxide reductase? J Biol Inorg Chem. 2002;7(6):659–663. doi: 10.1007/s00775-002-0361-3. [DOI] [PubMed] [Google Scholar]
- 41.Lipscomb GL, et al. Natural competence in the hyperthermophilic archaeon Pyrococcus furiosus facilitates genetic manipulation: construction of markerless deletions of genes encoding the two cytoplasmic hydrogenases. Appl Environ Microbiol. 2011;77(7):2232–2238. doi: 10.1128/AEM.02624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams MWW, et al. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 2001;183(2):716–724. doi: 10.1128/JB.183.2.716-724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cramer SD. The solubility of oxygen in brines from 0 to 300 C. Ind Eng Chem Proc DD. 1980;19(2):300–305. [Google Scholar]
- 44.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 45.Bridger SL, et al. Deletion strains reveal metabolic roles for key elemental sulfur-responsive proteins in Pyrococcus furiosus. J Bacteriol. 2011;193(23):6498–6504. doi: 10.1128/JB.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schut GJ, Bridger SL, Adams MW. Insights into the metabolism of elemental sulfur by the hyperthermophilic archaeon Pyrococcus furiosus: Characterization of a coenzyme A-dependent NAD(P)H sulfur oxidoreductase. J Bacteriol. 2007;189(12):4431–4441. doi: 10.1128/JB.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.