Abstract
IL-17–producing CD4 T cells play a key role in immune responses against extracellular bacteria and autoimmunity. Nuclear factor κB (NF-κB) is required for T-cell activation and selected effector functions, but its role in Th17 differentiation is controversial. Using genetic mouse models that impede T-cell–NF-κB signaling either downstream of the T-cell receptor (TCR) or of IκB kinase β (IKKβ), we demonstrate that NF-κB signaling controls not only survival and proliferation of activated T cells, but, if cell survival and cell-cycle progression are enabled, has an additional role in promoting completion of Th17 differentiation. CARD-containing MAGUK protein 1 (CARMA1), an adapter required for TCR/NF-κB signaling, was necessary for acquisition of IL-17A, IL-17F, IL-21, IL-22, IL-23R, and CCR6 expression in T cells cultured under Th17 conditions. In proliferating cells, lack of CARMA1 selectively prevented Th17, but not Th1 or Th2 differentiation, in a cell-intrinsic manner. Consistent with these data, CARMA1-KO mice were resistant to experimental autoimmune encephalomyelitis. Surprisingly, transcription factors essential for Th17 differentiation such as RORγt, AHR, and IRF4 were normally induced in CARMA1-KO T cells activated under Th17 conditions, suggesting that the Th17 differentiation program was initiated normally. Instead, chromatin loci of Th17 effector molecules failed to acquire an open conformation in CARMA1-KO T cells. Our results demonstrate that TCR/CARMA1/NF-κB controls completion of Th17 differentiation by enabling chromatin accessibility of Th17 effector molecule loci.
Keywords: IL-2, chromatin remodeling, histone 3 lysine 4 trimethylation, acetylated histone 3, 2D2
Th17-mediated immune responses are involved in immunity against extracellular bacteria, development of some autoimmune diseases (1), and acute rejection of certain solid organ transplants (2). Commitment of CD4 naïve T cells to the Th17 phenotype occurs when T cells are stimulated in the presence of the cytokines IL-6 and TGFβ and is dependent on activation of STAT3 and mothers against decapentaplegic homolog 2/3 (Smad2/3) (3). Th17 cells are characterized by the expression of the cytokines IL-17A, IL-17F, IL-21, and IL-22, and of the receptors IL-23R and CCR6. During the differentiation process, gene expression depends on the transcription factors RAR-related orphan receptor γt (RORγt), RORα, aryl hydrocarbon receptor (AHR), and runt-related transcription factor 1 (Runx1), among others, whereas the chromatin of the Th17 genetic loci acquires an open conformation.
The transcription factor NF-κB is a homo- or heterodimer composed of RelA, RelB, cRel, NF-κB1/p50, and NF-κB2/p52 subunits. NF-κB is important for T-cell activation, survival, and IL-2–mediated proliferation (4). In quiescent T cells, NF-κB dimers are sequestered in the cytoplasm by their inhibitor IκBα. Activation of the canonical NF-κB signaling pathway requires phosphorylation of the IκBα by the IκB kinase (IKK) complex (IKKα, IKKβ, and IKKγ), targeting it for degradation, thus releasing NF-κB dimers to translocate into the nucleus (5). T-cell receptor (TCR)-mediated canonical NF-κB activation requires PKCθ-dependent recruitment of the CARMA1 B-cell CLL/lymphoma 10 (BCL10)/mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1) adaptosome to the cell membrane for IKK complex activation and nuclear translocation of RelA/p50 and cRel/p50 heterodimers.
Initial studies had proposed that unique NF-κB family members may be required for the specification of activated T cells into the Th1 versus the Th2 phenotype, with RelB implicated in Th1 differentiation (6), and NF-κB1 and Bcl-3 contributing to Th2 cell commitment (6, 7). Intriguingly, the role of the NF-κB subunits RelA and cRel in Th17 differentiation is controversial as both NF-κB subunits have been shown to be either required or dispensable for Th17 differentiation (8–10). Deciphering the role of NF-κB in Th17 differentiation is essential to design proper immune therapies for Th17-mediated diseases. To better understand the role of NF-κB in Th17 differentiation, we used genetically modified mice deficient in the T-cell–NF-κB signaling pathway, either by deficiency of the TCR/NF-κB adapter molecule CARMA1, by expression of a nondegradable form of IκBα in T cells or by using an IKKβ pharmacological inhibitor. These approaches inhibit the canonical NF-κB signaling pathway independently of the NF-κB subunit composition. Our results identify the TCR/CARMA1/NF-κB axis as an essential T-cell–intrinsic pathway for functional activity of the Th17 transcriptional machinery.
Results
CARMA1 Is Required for Th17 Differentiation.
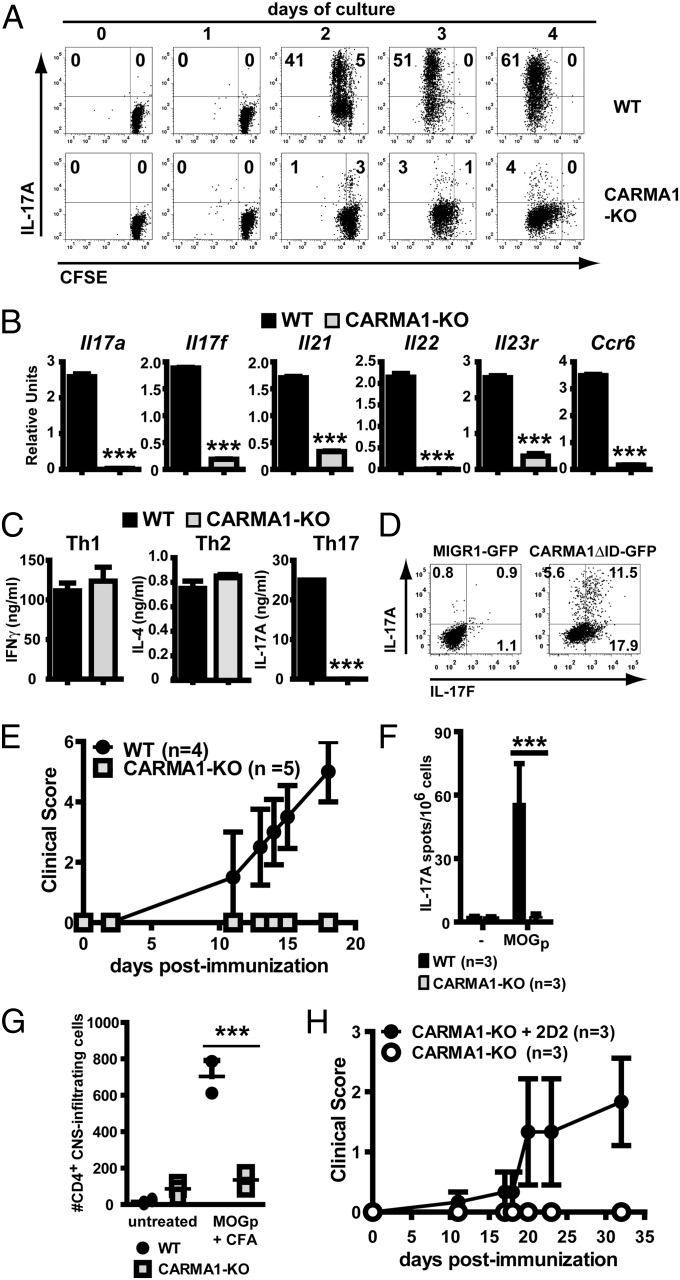
Stimulation through the TCR activates multiple signaling pathways that are important in T-cell function. CARMA1, a molecule restricted to lymphocytes, is an adapter that links the TCR to NF-κB activity, cell survival, and proliferation. To test the role of TCR/CARMA1/NF-κB in acquisition of IL-17 production, expression of IL-17A was analyzed in CFSE-labeled WT and CARMA1-KO CD4+CD44lo naïve T cells stimulated under Th17 polarizing conditions (anti-CD3, anti-CD28, TGFβ, and IL-6). To compensate for the defect of activated CARMA1-KO T cells in proliferation and survival in vitro (Fig. S1 A–C) and be able to investigate the role of CARMA1 in differentiation at checkpoints downstream of cell-cycle progression, a low dose of IL-2 (10 units/mL) was added to the in vitro cultures throughout the study. This restored survival and enhanced proliferation of CARMA1-KO–deficient cells without reducing the expression of IL-17A by Th17-stimulated WT cells (Fig. S1D). As assessed by flow cytometry, intracellular IL-17A expression was strongly reduced in CARMA1-KO compared with WT T cells, despite significant proliferation (Fig. 1A), suggesting that IL-17A expression in proliferating cells depends on the presence of CARMA1. The Th17 phenotype refers not only to expression of IL-17A, but also IL-17F, IL-21, IL-22, IL-23R, and CCR6. mRNA levels for these molecules were reduced in CARMA1-KO CD4 T cells stimulated in Th17 conditions compared with WT T cells (Fig. 1B), indicating that the presence of CARMA1 is required for Th17 differentiation, rather than only for IL-17A production.
Fig. 1.
Th17 differentiation depends on CARMA1. (A) Kinetics of proliferation and IL-17A expression were assessed in CFSE-labeled WT and CARMA1-KO naïve CD4 T cells stimulated in Th17 conditions and analyzed at the indicated time points by intracellular flow cytometry in Live Aqua− cells following 4-h restimulation with PMA and ionomycin in the presence of brefeldin A. (B) mRNA for Il7a, Il17f, Il21, Il22, Il23r, and Ccr6 was assessed by RT-qPCR in WT and CARMA1-KO naïve CD4 T cells stimulated for 72 h in Th17 conditions. Expression was normalized to levels of Actb (mean ± SD). (C) WT and CARMA1-KO naïve CD4 T cells cultured in Th1, Th2, or Th17 conditions for 72 h, washed extensively, and the same number of live cells were restimulated for 24 h with PMA and ionomycin (mean ± SD). (D) Expression of IL-17A and IL-17F was assessed in CARMA1-KO naïve CD4 T cells transduced with MIGR1-eGFP or MIGR1-CARMA1-ΔID-eGFP retroviruses and further cultured for 72 h in Th17 conditions before PMA/ionomycin stimulation. Graphs display CD4+GFP+-gated events (transduction rates: WT, 40–60% and CARMA1-KO, 20–35%). (E) WT and CARMA1-KO mice were immunized with MOG35–55/CFA plus pertussis toxin and followed for signs of EAE (mean clinical score ± SEM). (F) On day 10 after immunization, splenocytes were isolated and restimulated with MOG35–55, and IL-17 production was assessed by ELISpot (mean ± SD). (G) Count of CD4+ CNS infiltrating cells in mice subjected or not to MOG35–55 immunization for 20 d. (H) Clinical assessment of CARMA1-KO mice adoptively transferred with WT 2D2 cells and subjected to MOG35–55 immunization. Experiments were performed at least twice, with similar results. **P < 0.01, ***P < 0.001.
NF-κB induces the expression of the prosurvival factor Bcl-xL (Bcl2l1) in activated T cells (11) and CARMA1-KO cells displayed reduced levels of Bcl2l1 mRNA (Fig. S2A). Although addition of IL-2 effectively reduced death of activated CARMA1-KO T cells (Fig. S1B), an approach to exclude that reduced detection of Th17 cytokines was due to apoptosis, CARMA1-KO CD4 naïve T cells transgenic for BclxL (CARMA1-KO × BclxL-Tg) were stimulated in Th17 conditions (>85% viable cells). Forced expression of BclxL did not restore expression of IL-17A and IL-17F in CARMA1-KO × BclxL-Tg T cells (Fig. S2B), suggesting that the requirement of CARMA1 for Th17 differentiation is independent of BclxL-mediated cell survival.
To assess whether CARMA1 was required for naïve T cells to enter any differentiation pathway or was selective for the Th17 phenotype, WT and CARMA1-KO T cells were stimulated under Th1, Th2, and Th17 conditions. Although T-cell–NF-κB has been reported to play a role in Th1 and Th2 differentiation (6, 7), IFNγ production was restored (Fig. S3D) if survival (Fig. S3 A and B) and proliferation (Fig. S3C) were corrected in the presence of IL-2, while T-cell–dependent IL-4 production was dependent on exogenous IL-2 and IL-4 (12) (Fig. S3 E and F) to prevent cell death (Fig. S3 A and B) and partially restore proliferation (Fig. S3C). Compared with WT CD4 T cells, production of IL-17A but not IFNγ and IL-4 was dramatically reduced in CARMA1-KO cells, as assessed by ELISA in supernatants of Th17-, Th1-, and Th2-stimulated cells (Fig. 1C) and intracellular cytokine staining (Fig. S3D), indicating that when cell-cycle progression is partially restored, there is an additional selective requirement of CARMA1 for Th17 but not Th1 or Th2 differentiation. To exclude the possibility of a thymic developmental defect in CARMA1-KO CD4 T cells that would render them incapable of Th17 differentiation, constitutively active CARMA1 (CARMA1-ΔID) was retrovirally introduced into CARMA1-KO CD4 T cells. Transduction with CARMA1-ΔID but not empty MIGR1 retrovirus restored production of IL-17A and IL-17F following culture under Th17 conditions (Fig. 1D), indicating that CARMA1-KO CD4 T cells have the intrinsic ability to produce IL-17 if CARMA1 expression is restored.
Experimental autoimmune encephalomyelitis (EAE) is dependent on Th17 differentiation (13). To determine whether Th17 differentiation could proceed in vivo in the absence of CARMA1, wild-type and CARMA1-KO mice were immunized with MOG35–55 peptide in complete Freund’s adjuvant (CFA) and injected with pertussis toxin. Whereas WT mice rapidly developed severe EAE (Fig. 1E) associated with antigen-specific production of IL-17A upon restimulation of splenocytes with MOG35–55 peptide (Fig. 1F) and with CD4 T-cell infiltration of the central nervous system (CNS; Fig. 1G), CARMA1-KO mice did not develop any clinical signs of disease (Fig. 1E), nor did they generate MOG35–55-reactive IL-17A–secreting cells (Fig. 1F). Few CARMA1-KO T cells infiltrated the CNS of MOG35–55 immunized mice (Fig. 1G), consistent with their lack of CCR6 up-regulation (Fig. S4A). Among the few CD4 cells present in the CNS, CARMA1-KO cells had a reduced proportion of IL-17A–, but not IFNγ-producing cells compared with WT counterparts (Fig. S4B). To determine whether lack of EAE development in CARMA1-KO mice was solely due to T-cell defects, CARMA1-KO mice were adoptively transferred with myelin-specific 2D2 TCR transgenic CD4 T cells before immunization with MOG35–55. Indeed, transfer of naïve CARMA1-expressing 2D2 cells restored EAE in CARMA1-KO mice (Fig. 1H), suggesting that antigen presenting cells (APC) function is normal in the absence of CARMA1. Consistent with this interpretation, CARMA1-KO dendritic cells (DCs) were competent as assessed by up-regulation of the costimulatory molecules CD80 and CD86, of MHC class II, and by antigen presentation to TCR-transgenic OT-II CD4 T cells using 24-h LPS-stimulated ovalbumin OVA323–339-loaded WT and CARMA1-KO splenic DCs (Fig. S4 C and D). Thus, CARMA1 is required in T cells to mount a Th17 response in vivo. In contrast, CARMA1 expression is not necessary for Th1 responses in vivo, as allogeneic skin transplants were successfully rejected in CARMA1-KO mice (Fig. S4E) and induced a similar IFNγ alloresponse to that in WT recipients (Fig. S4F).
Cell-Intrinsic Requirement of CARMA1 for Th17 Differentiation.
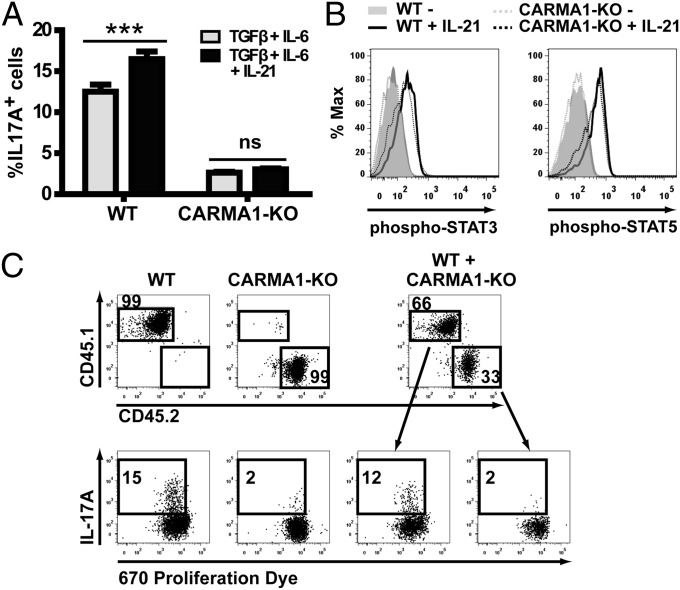
It has been previously demonstrated that IL-21, produced during Th17 differentiation, amplifies the Th17 commitment (14). As lack of CARMA1 prevented IL-21 expression (Fig. 1B), we tested whether addition of exogenous IL-21 could restore Th17 differentiation in CARMA1-KO CD4 T cells. However, addition of IL-21 was not sufficient to restore IL-17A expression in CARMA1-KO T cells (Fig. 2A), despite inducing equivalent phosphorylation of STAT3 and STAT5 as in WT T cells (Fig. 2B).
Fig. 2.
Cell-intrinsic requirement of CARMA1 for Th17 differentiation. (A) IL-17A expression was assessed in WT and CARMA1-KO naïve CD4 T cells stimulated with anti-CD3 and anti-CD28 mAb in the presence of IL-2 and the indicated cytokines (mean ± SD). (B) Phospho-STAT3 (Left) and phospho-STAT5 (Right) levels were determined in WT and CARMA1-KO CD4 naïve T cells stimulated for 30 min in the presence or absence of IL-21. (C) Proliferation, as assessed by 670 proliferation dye dilution and IL-17A expression were analyzed in CD45.1+ WT and CD45.2+ CARMA1-KO naïve CD4 T cells cultured under Th17 conditions alone or in combination. Data are representative of at least three independent experiments. ***P < 0.001.
To test whether any factor produced by differentiating Th17 WT CD4 T cells and missing in CARMA1-KO cells was necessary for amplification or stabilization of the Th17 commitment, CD45.1+ WT and CD45.2+ CARMA1-KO CD4 T cells were cocultured under Th17 conditions. As depicted in Fig. 2C, IL-17A expression was not restored in CARMA1-KO cells cocultured with WT T cells, indicating that CARMA1 is required in a T-cell–intrinsic manner for Th17 specification.
NF-κB Activity Is Required for Priming of Th17 Differentiating Cells.
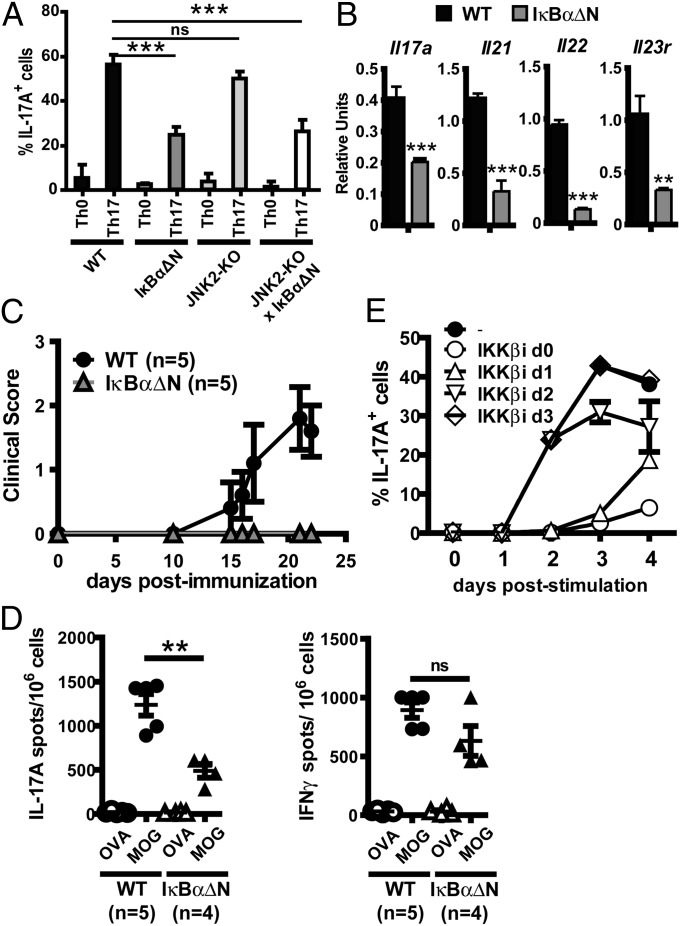
Formation of the CARMA1/Bcl10/MALT1 complex following TCR engagement results in activation of NF-κB and c-Jun kinase 2 (JNK2) signaling. To assess whether NF-κB and/or JNK2 were required for Th17 commitment, IL-17A expression was analyzed following Th17 differentiation of CD4 T cells from JNK2-KO mice, or mice expressing a transgenic superrepressor of NF-κB in T cells (IκBαΔN), or mice containing both genetic alterations (JNK2-KO × IκBαΔN). A reduction in IL-17A expression was observed in NF-κB–impaired T cells (Fig. 3A), although the defect was less pronounced than in CARMA1-KO CD4 cells, perhaps because IκBαΔN but not CARMA1-deficient CD4 cells have residual TCR-mediated NF-κB activity (Fig. S5). In contrast, no impairment in IL-17A induction was observed in JNK2-deficient CD4 T cells (Fig. 3A) and levels of IL-17 production in JNK2-KO × IκBαΔN T cells were similar to those in T cells with the IκBαΔN transgene alone. These data suggest that defective IL-17A expression in CARMA1-KO cells is due, at least in part, to impaired NF-κB but not JNK2 signaling.
Fig. 3.
NF-κB but not JNK2 is required for Th17 differentiation. (A) IL-17A expression was assessed by intracellular flow cytometry in WT, IκBαΔN, JNK2-KO, or IκBαΔN × JNK2-KO naïve CD4 T cells stimulated for 72 h in Th0 or Th17 conditions (mean ± SD of triplicates). (B) mRNA levels for Il17a, Il21, Il22, and Il23r in WT and IκBαΔN naïve CD4 T cells stimulated in Th17 conditions were assessed by RT-qPCR and normalized to levels of Actb gene expression. (C) WT and IκBαΔN mice were immunized with MOG35–55 peptide and disease progression was assessed over time. (D) IL-17– and IFNγ-producing splenocytes from WT and IκBαΔN mice immunized with MOG35–55 14 d before. (E) IL-17A expression was assessed in WT naïve CD4 T cells stimulated under Th17 conditions in the presence or absence of an IKKβ inhibitor added at day 0, 1, 2, or 3 poststimulation (mean ± SD of triplicates). **P < 0.01, ***P < 0.001. NS, not significant.
Similarly to CARMA1-KO cells, IκBαΔN CD4 T cells stimulated in Th17 culture conditions displayed reduced mRNA levels of Il17a, Il21, Il22, and Il23r, as assessed by RT-qPCR (Fig. 3B). IκBαΔN mice were also protected from EAE (Fig. 3C) and showed reduced antigen-specific IL-17 responses, whereas MOG-specific IFNγ responses were not significantly affected (Fig. 3D). Taken together, these results suggest that the TCR/CARMA1/NF-κB axis plays an important role in Th17 differentiation both in vitro and in vivo.
To assess the time frame during which NF-κB affects Th17 differentiation, IL-17A expression was analyzed in Th17-cultured WT T cells in the presence of an IKKβ-specific pharmacological inhibitor, which was added at different time points poststimulation. The IKKβ inhibitor only reduced IL-17A expression if added during the first 48 h of culture but not at later time points (Fig. 3E), indicating that NF-κB is required during the initiation of Th17 differentiation and not for production of IL-17 once T cells have committed to the Th17 phenotype.
CARMA1 and Transcription Factors Involved in Th17 Differentiation.
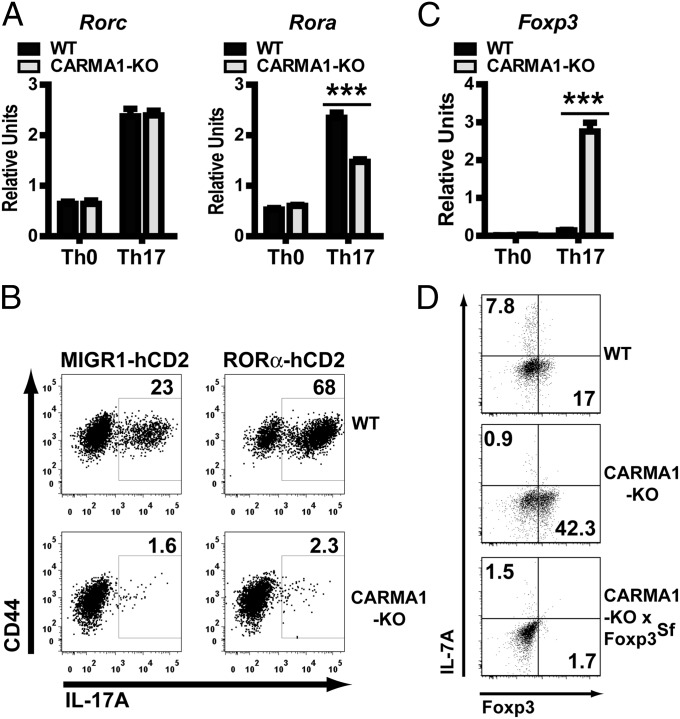
Several transcription factors have been reported to promote Th17 differentiation, including RORγt, RORα, AHR, and IRF4, among others (1). To investigate whether the Th17 differentiation defect in CARMA1-KO CD4 T cells was due to impaired induction of Th17-promoting transcription factors, RT-qPCR analysis was performed in WT and CARMA1-KO T cells stimulated for 72 h in Th0 or Th17 conditions. Surprisingly, expression of Rorc (the gene encoding RORγt) (Fig. 4A), Ahr, Irf4, Runx1, and Stat3 (Fig. S6A) was similar in WT and CARMA1-KO CD4 T cells. Similarly, IκBαΔN CD4 cells did not have any defects in Rorc expression (Fig. S6B). Protein expression levels of RORγt as determined by intracellular flow cytometry (Fig. S6C), and DNA-binding activity of RORγt as assessed by electrophoretic mobility shift assay (EMSA) (Fig. S6D), were also similarly induced in CARMA1-KO and WT T cells stimulated in Th17 conditions. In contrast, mRNA expression of Rora (the gene for RORα) was slightly, but reproducibly, diminished (Fig. 4A). However, transduction of CARMA1-KO cells with a retrovirus encoding RORα did not restore IL-17A production following Th17 differentiation, although it did augment the percentage of WT T cells that expressed IL-17 (Fig. 4B). Nuclear factor of kappa light chain gene enhancer in B cells inhibitor zeta (NFKBiz) has been shown to cooperate with RORγt and RORα to drive transcription of IL-17 (15). Nevertheless, levels of Nfkbiz mRNA were similar in WT and CARMA1-KO CD4 cells activated under Th17 differentiating conditions (Fig. S6E).
Fig. 4.
CARMA1 and transcription factors controlling Th17 differentiation. (A) mRNA for Rorc and Rora was assessed by RT-qPCR in WT and CARMA1-KO naïve CD4 T cells stimulated for 72 h in Th0 or Th17 conditions. Results (mean ± SD) were normalized to levels of Actb mRNA. (B) IL-17A expression of WT and CARMA1-KO naïve CD4 T cells transduced with MIGR1-hCD2 or MIGR1-hCD2-RORα retroviral particles, and subsequently incubated under Th17 conditions for 72 h. (C) Foxp3 mRNA levels were assessed from cells stimulated and processed as in A. (D) IL-17A and Foxp3 expression was assessed in WT, CARMA1-KO, and CARMA1-KO × Foxp3Scurfy naïve CD4 T cells stimulated for 72 h under Th17 conditions. All experiments were performed at least three times. ***P < 0.001.
Transcription factors inhibiting Th17 commitment have also been identified, raising the possibility that CARMA1-dependent signaling may prevent the expression of some of these molecules. Levels of Atf4, Gfi1, Nr2f6, and Stat5a mRNA, as well as phosphorylation of STAT5 upon IL-2 stimulation, were similar in WT and CARMA1-KO CD4 T cells activated under Th17 conditions (Fig. S6 F and G). Interestingly, Foxp3 expression was strongly induced in CARMA1-KO but not in WT T cells undergoing Th17 differentiation (Fig. 4C). Foxp3 has been shown to interact with RORγt and prevent it from driving Th17 differentiation (16). To investigate whether the increment in Foxp3 was causing the failure of Th17 commitment, IL-17A expression was analyzed in CARMA1/Foxp3-double KO CD4 naïve T cells stimulated for 72 h in Th17 conditions. Surprisingly, genetic ablation of Foxp3 was not sufficient to restore expression of IL-17A or IL-17F (Fig. 4D and Fig. S7) in CARMA1-KO cells. Taken together, these data suggest that requirement of CARMA1 for Th17 differentiation is independent of expression levels of the known Th17-promoting or -antagonizing transcription factors. Thus, the Th17 differentiation program is initiated normally in the absence of CARMA1 or NF-κB but fails to complete.
CARMA1 Is Not Required for STAT3/STAT5 Control of Th17 Differentiation.
The transcription factor STAT3 is critical for Th17 differentiation, and is used by CD4 T cells for transcription of both RORγt and Th17 loci. NF-κB is required for STAT3-dependent cell transformation in oncogenic cell lines (17), suggesting that the TCR/CARMA1/NF-κB axis may modulate STAT3-dependent Th17 differentiation. Thus, we analyzed the effect of CARMA1 deficiency in STAT3 acetylation and phosphorylation. As assessed by Western blot and flow cytometry, presence of CARMA1 was not required for either STAT3 acetylation or phosphorylation in tyrosine 705 (Fig. S8 A and B). Recently, Laurence and colleagues showed that IL-2–activated STAT5 inhibits Th17 differentiation by displacing STAT3 from the DNA-binding sites in Th17 loci (18). However, by chromatin immunoprecipitation, STAT3 and STAT5 recruitment to the Il17a locus was similar in CARMA1-KO and WT Th17-polarized cells (Fig. S8C), suggesting that the STAT3 and STAT5 chromatin-binding ratio is independent of TCR/CARMA1/NF-κB signaling.
CARMA1 Is Required for Th17 Loci Accessibility.
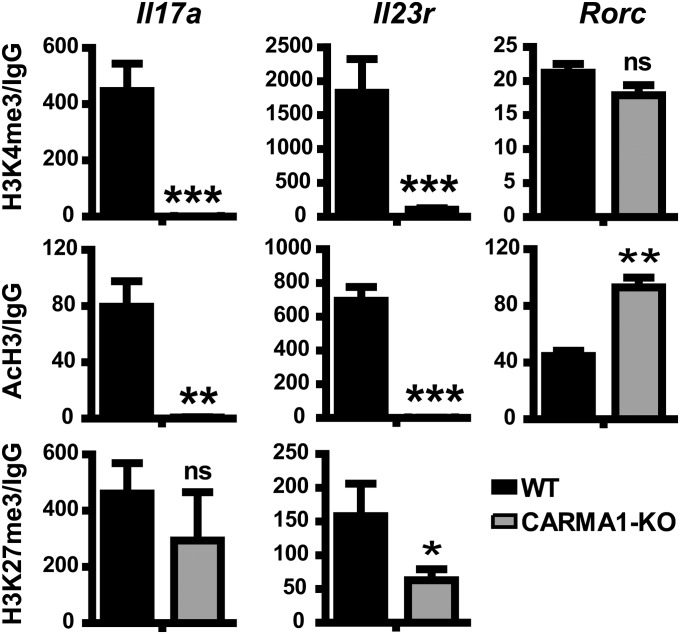
In silico analysis of potential NF-κB–binding sites in the Il17a locus revealed only one nonfunctional sequence (8) making it unlikely that NF-κB directly regulates expression of the Th17 loci. NF-κB has the potential to modulate the chromatin remodeling machinery (19), suggesting that TCR/CARMA1/NF-κB may control chromatin accessibility to the loci of the Th17 effector molecules. Transcriptionally active genes are associated with multiple “active” histone modifications, such as histone 3 lysine 4 trimethylation (H3K4me3) and acetylated histone 3 (AcH3), whereas “repressive” histone modifications, such as histone 3 lysine 27 trimethylation (H3K27me3) are associated with silent genes (20). To assess whether CARMA1 is necessary for opening the Th17 loci or for preventing their repression, the presence of AcH3 and H3K4me3 and H3K27me3 in Il17a, Il23r, and Rorc loci was assessed by chromatin immunoprecipitation. Indeed, CARMA1-KO CD4 T cells cultured in Th17 conditions for 3 d lacked both H3K4me3 and AcH3 marks in Il17a and Il23r but not Rorc loci, without increased enrichment in H3K27me3 (Fig. 5), suggesting that CARMA1 is required for chromatin accessibility to the loci of Th17 effector molecules, but not to prevent gene silencing. Taken together, our results suggest that the TCR/CARMA1/NF-κB axis controls the Th17 differentiation program by making chromatin of Th17 effector molecules loci accessible for gene transcription.
Fig. 5.
CARMA1 is required for chromatin accessibility of Th17 loci. Chromatin immunoprecipitation for H3K4me3, AcH3, and H3K27me3 in promoter regions for Il17a, Il23r, and Rorc genes was performed on the same numbers of WT and CARMA1-KO naïve CD4 T cells stimulated in Th17 conditions for 72 h. Data represent fold enrichment to isotype control IgG and normalized to input DNA (mean ± SD of triplicates). Experiments were performed at least three times. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In the present report, we show that in addition to controlling the survival and proliferation of activated T cells, the TCR/CARMA1/NF-κB axis is critical downstream of cell-cycle progression for completion of Th17 differentiation. Our data point to a cell-intrinsic role of CARMA1 and an early (first 48 h) requirement of NF-κB after TCR engagement for Th17 differentiation, whereas JNK2 is dispensable. Rather than governing the level of expression of Th17-promoting transcription factors, CARMA1/NF-κB activity facilitates chromatin accessibility to the loci of Th17 effector molecules, thus revealing a unique mechanism of control of Th17 differentiation.
The role of NF-κB in Th17 differentiation is controversial. Whereas the subunit RelB, which plays a role in Th1 differentiation (6), is unanimously dispensable for the Th17 program (8, 9), RelA was reported to be either dispensable or necessary for Th17 differentiation (8, 9). Similarly, two recent reports have shown that cRel mediates Th17 differentiation through transcriptional control of RORγt (8, 21), whereas an earlier study suggested that cRel was dispensable for Th17 differentiation (10). Interestingly, both the Chen et al. (21) and Visekruna et al. (10) reports agreed that cRel was dispensable for Th17 differentiation once CD28 costimulation was present. Discrepancies in the published articles may be due to compensatory expression by other NF-κB subunits. By using CARMA1-KO and IκBαΔN mice (genetic models that inhibit NF-κB upstream of activation of the individual NF-κB subunits), as well as a pharmacological IKKβ inhibitor, our studies should avoid potential caveats of subunit compensation. In contrast to the reports showing that cRel controls Th17 differentiation via up-regulation of RORγt and RORα expression, our data demonstrate that CARMA1-KO and IκBαΔN T cells differentiated under Th17 conditions display normal levels of RORγt transcripts and protein, which in turn exhibits normal DNA-binding capacity. Taken together, these data suggest that the mechanism by which the TCR/CARMA1/NF-κB axis regulates Th17 differentiation is different from the mechanism reported to be used by the cRel subunit.
Our results reveal a selective role for CARMA1 in Th17 but not Th1 or Th2 differentiation, when survival and proliferative defects secondary to reduced production of IL-2 are partially corrected (22). Th1 and especially Th2 differentiation are cell-cycle dependent (23), but partial restoration of proliferation was sufficient to restore acquisition of IL-4 and IFNγ production by CARMA1-KO T cells, suggesting that defects in Th1 and Th2 differentiation previously reported with CARMA1-KO T cells may be mostly secondary to defects in cell-cycle progression (22). A recent report showed lack of IL-4 expression in CARMA1-KO CD4 T cells stimulated in nonpolarizing conditions, due to an inability to up-regulate the transcription factor GATA3 (12). We reproduced these results and showed that when CARMA1-KO CD4 T cells were stimulated in the presence of IL-4/anti-IFNγ/IL-2 (Th2 polarizing conditions), expression of both GATA3 and IL-4 was restored, suggesting that exogenous IL-4 can bypass the requirement for CARMA1 in Th2 differentiation. In contrast, the defect in acquisition of IL-17 production persisted with significantly corrected proliferation and cell survival or after addition of differentiating WT Th17 cells as a source of other potential factors that may help amplify Th17 differentiation and was obvious even in cells having undergone several rounds of proliferation as assessed by CFSE dilution. Similarly to Th1 and Th2 differentiation, we and others have previously shown that induced regulatory T cell (iTreg) differentiation can proceed independently of CARMA1 when exogenous IL-2 was provided (24, 25). Although the mechanisms behind the differential dependence on CARMA1 for Th1/Th2/iTreg versus Th17 differentiation remain to be investigated, our data suggest that CARMA1/NF-κB controls T-cell activation at two distinct levels: driving TCR-dependent survival/cell-cycle progression, which is required for differentiation of all effector phenotypes, and selectively enabling completion of Th17 differentiation via a mechanism downstream to cell-cycle progression.
Th17 differentiation requires the orchestrated cooperation of several transcription factors. Some of these transcription factors control Th17 differentiation by inducing Rorc transcription (Runx1, IRF4, and cRel) (8, 26–28), whereas others repress Rorc gene expression (T-bet, Gfi1) (29, 30). Other transcription factors promote (NF-κBiz) or prevent the function of the RORγt protein (Foxp3) (15, 16), and yet others inhibit nuclear factor of activated T cells (NFAT)- and AP-1–mediated Th17 differentiation (Nr2F6) (31), indicating that RORγt is necessary but not sufficient for Th17 differentiation. None of these known factors was differentially expressed in WT and CARMA1-KO T cells in Th17 conditions, indicating that deficiency in CARMA1 does not prevent initiation of the Th17 differentiation program. Our results show a unique mechanism of regulation of Th17 differentiation, where CARMA1/NF-κB rather governs chromatin accessibility to Th17 effector molecules loci and completion of the program.
Chromatin exists in open and closed conformations (32). The open conformation allows recruitment of the transcriptional machinery to enhancers and promoters. The transcription factor NF-κB has been suggested to enable chromatin remodeling and accessibility in T cells. For instance, Brg1, a protein of the SWI/SNF nucleosome remodeling complex, requires RelA/p65 for its recruitment to the GM-CSF enhancer (19). Furthermore, it has been reported in macrophages that RelA/p65 mediates the LPS-dependent transcription of metastatic tumor antigen 1 (MTA1), a component of the nucleosome remodeling and deacetylase (NuRD) complex (33). Our results suggest that CARMA1/NF-κB generates a permissive landscape for subsequent action by pro-Th17 transcription factors, perhaps by regulating the chromatin remodeling machinery necessary to open the Th17 effector molecules’ loci.
The restricted pattern of CARMA1 expression to lymphocytes makes it an appealing therapeutic target for immunological diseases with a strong Th17 component, such as multiple sclerosis and inflammatory bowel disease. However, our results also suggest that for such drugs to be effective, inhibition of CARMA1/NF-κB activation would have to occur early in the disease, before all Th17 differentiation has taken place. In summary, our study identifies a unique pathway of regulation of Th17 differentiation by CARMA1/NF-κB that may be able to be modulated for therapeutic purposes.
Materials and Methods
Mice.
C57BL/6 mice were purchased from Harlan. IκBαΔN mice (34), expressing a superrepressor form of IκBα directed by the Lck promoter and the CD2 enhancer were bred in house. CARMA1−/− mice (23) were originally generated in the 129 background but were backcrossed for at least six generations to C57BL/6 animals. BclxL-Tg mice, expressing BclxL specifically in T cells, were provided by Jeffrey Rathmell (Duke University, Durham, NC). JNK2−/− and Foxp3Scurfy mice on a C57BL/6 background were purchased from Jackson Laboratories. BclxL-Tg and Foxp3Scurfy mice were crossed to CARMA1−/− mice to generate CARMA1−/−/BclxL-Tg and CARMA1−/−/Foxp3Scurfy mice, respectively. The 2D2 TCR-transgenic mice were generously provided by Stephen Miller (Northwestern University, Evanston, IL), whereas OT-II mice were bred in house. All experiments were performed in agreement with University of Chicago Institutional Animal Care and Use Committee and according to the National Institutes of Health guidelines for animal use (https://researchadmin.uchicago.edu/iacuc/).
Experimental Autoimmune Encephalomyelitis.
Eight- to 10-wk old C57BL/6, CARMA1-KO, or IκBαΔN female mice were injected s.c. with an emulsion containing 100 ng of MOG35–55 peptide (MEVGWYRSPFSROVHLYRNGK, CSBio) and CFA (Sigma), and i.v. with 200 ng of pertussis toxin (Sigma) in saline solution. Pertussis toxin injection was repeated on d2 poststimulation. In some experiments CARMA1-KO mice were injected i.v. with 1.5 × 106 2D2 encephalitogenic CD4 T cells at the time of immunization. Mice were monitored daily for signs of disease. Disease score was as follows: 0, no clinical signs; 1, loss of tail tonicity; 2, flaccid tail and abnormal gait; 3, hind limb paresis; 4, hind leg paralysis; 5, hind and fore leg paralysis; and 6, death.
Skin Grafting.
Skin allograft rejection was performed as previously described (35) (SI Materials and Methods).
T-Cell Differentiation.
Naïve CD4 T cells were first enriched from spleen and peripheral lymph nodes with CD4+ T-cell enrichment kit (Stemcell Technologies) and CD4+CD25–CD44lo cells were then sorted with a BD FACSAria. Naïve CD4+ T cells were stimulated with plate-bound anti-CD3 (1–5 μg/mL) and anti-CD28 (1 μg/mL) mAbs for 3 d in either Th0 (10 units/mL of rhIL-2), Th17 (10 units/mL of rhIL-2, 50 ng/mL of mIL-6, 2.5 ng/mL of rhTGFβ1, 5 μg/mL of anti-IFNγ, and 5 μg/mL of anti–IL-4), Th1 (10 units/mL of rhIL-2, 5 ng/mL of rmIL-12, and 5 μg/mL of anti–IL-4), and Th2 (10 units/mL of rhIL-2, 10 ng/mL of IL-4, and 5 μg/mL of anti-IFNγ) culture conditions. Alternatively, cells were cultured with 50 ng/mL of mIL-21. In some experiments WT naïve CD4+ cells were treated with a pharmacological IKKβ inhibitor [10 nM of NF-κB activation inhibitor, 6-amino-4-(4-phenoxyphenylethylamino)quinazoline; Calbiochem] at the indicated times.
Measurement of Cytokines.
Cytokines were measured by intracellular cytokine staining, ELISA and EliSpot (SI Materials and Methods).
Measurement of Total, Acetylated, and Phosphorylated STAT3 and STAT5.
These molecules were measured by Western blot and intracellular flow cytometry (SI Materials and Methods).
DNA Binding of Transcription Factors and Modified Histones.
DNA and chromatin binding were measured using EMSA and chromatin immunoprecipitation assays (ChIP) (SI Materials and Methods).
Retroviral Transduction.
Generation of CARMA1-ΔID retroviral vectors, retroviral particles production and transduction were performed as described in SI Materials and Methods.
RT-qPCR.
Generation of cDNA and quantitative PCR were performed as described in SI Materials and Methods, utilizing the described pairs of oligonucleotides (Table S1).
Statistical Analysis.
Statistical significance was evaluated using the two-tailed unpaired t test and one way ANOVA for multiple groups (Bonferroni for pairwise comparisons). Values of P <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Aki Ueda, Liang Zhou, Yonglian Sun, Marei Dose, Malay Mandal, and Michelle Miller, along with the rest of the M.-L.A. Laboratory members for technical assistance. This work was supported by National Institutes of Health Grant R01 AI1052352 (to M.-L.A.) and an American Heart Association postdoctoral fellowship (to L.L.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204557109/-/DCSupplemental.
References
- 1.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Chadha R, Heidt S, Jones ND, Wood KJ. Th17: Contributors to allograft rejection and a barrier to the induction of transplantation tolerance? Transplantation. 2011;91(9):939–945. doi: 10.1097/TP.0b013e3182126eeb. [DOI] [PubMed] [Google Scholar]
- 3.Hirahara K, et al. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21(6):425–434. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molinero LL, Alegre ML. Role of T cell-nuclear factor kappaB in transplantation. Transplant Rev (Orlando) 2012;26(3):189–200. doi: 10.1016/j.trre.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanarek N, London N, Schueler-Furman O, Ben-Neriah Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2(2):a000166. doi: 10.1101/cshperspect.a000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corn RA, Hunter C, Liou HC, Siebenlist U, Boothby MR. Opposing roles for RelB and Bcl-3 in regulation of T-box expressed in T cells, GATA-3, and Th effector differentiation. J Immunol. 2005;175(4):2102–2110. doi: 10.4049/jimmunol.175.4.2102. [DOI] [PubMed] [Google Scholar]
- 7.Das J, et al. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat Immunol. 2001;2(1):45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 8.Ruan Q, et al. The Th17 immune response is controlled by the Rel-RORγ-RORγ T transcriptional axis. J Exp Med. 2011;208(11):2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powolny-Budnicka I, et al. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin-dependent interleukin-17 production in γδ T cells. Immunity. 2011;34(3):364–374. doi: 10.1016/j.immuni.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Visekruna A, et al. c-Rel is crucial for the induction of Foxp3(+) regulatory CD4(+) T cells but not T(H)17 cells. Eur J Immunol. 2010;40(3):671–676. doi: 10.1002/eji.200940260. [DOI] [PubMed] [Google Scholar]
- 11.Khoshnan A, et al. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol. 2000;165(4):1743–1754. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 12.Blonska M, Joo D, Zweidler-McKay PA, Zhao Q, Lin X. CARMA1 controls Th2 cell-specific cytokine expression through regulating JunB and GATA3 transcription factors. J Immunol. 2012;188(7):3160–3168. doi: 10.4049/jimmunol.1102943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto K, et al. IkappaBzeta regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010;464(7293):1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15(4):283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holloway AF, Rao S, Chen X, Shannon MF. Changes in chromatin accessibility across the GM-CSF promoter upon T cell activation are dependent on nuclear factor kappaB proteins. J Exp Med. 2003;197(4):413–423. doi: 10.1084/jem.20021039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northrup DL, Zhao K. Application of ChIP-Seq and related techniques to the study of immune function. Immunity. 2011;34(6):830–842. doi: 10.1016/j.immuni.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G, et al. The NF-κB transcription factor c-Rel is required for Th17 effector cell development in experimental autoimmune encephalomyelitis. J Immunol. 2011;187(9):4483–4491. doi: 10.4049/jimmunol.1101757. [DOI] [PubMed] [Google Scholar]
- 22.Egawa T, et al. Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr Biol. 2003;13(14):1252–1258. doi: 10.1016/s0960-9822(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 23.Bird JJ, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9(2):229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 24.Barnes MJ, et al. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 2009;7(3):e51. doi: 10.1371/journal.pbio.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB-dependent manner. J Immunol. 2011;186(8):4609–4617. doi: 10.4049/jimmunol.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9(11):1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q, et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29(6):899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brüstle A, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8(9):958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 29.Lazarevic V, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat Immunol. 2011;12(1):96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, et al. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J Exp Med. 2009;206(2):329–341. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermann-Kleiter N, et al. The nuclear orphan receptor NR2F6 suppresses lymphocyte activation and T helper 17-dependent autoimmunity. Immunity. 2008;29(2):205–216. doi: 10.1016/j.immuni.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory PD, Wagner K, Hörz W. Histone acetylation and chromatin remodeling. Exp Cell Res. 2001;265(2):195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- 33.Pakala SB, et al. Regulation of NF-kappaB circuitry by a component of the nucleosome remodeling and deacetylase complex controls inflammatory response homeostasis. J Biol Chem. 2010;285(31):23590–23597. doi: 10.1074/jbc.M110.139469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185(11):1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molinero LL, et al. Epidermal Langerhans cells promote skin allograft rejection in mice with NF-kappa B-impaired T cells. Am J Transplant. 2008;8(1):21–31. doi: 10.1111/j.1600-6143.2007.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.