Abstract
Infection with the hepatitis B virus (HBV) promotes the development of hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) and is a leading cause of morbidity and mortality worldwide. HBV X protein (HBx) is an important effector for HBV pathogenesis, but its cellular targets and acting mechanisms remain elusive. We show here that HBx interacts with the anti-apoptotic proteins Bcl-2 and Bcl-xL through a Bcl-2 homology 3 (BH3)-like motif in mammalian cells. Importantly, mutations in the BH3-like motif that prevent HBx binding to Bcl-2 and Bcl-xL abrogate cytosolic calcium elevation and cell death induced by HBx expression in hepatocytes and severely impair HBV viral replication, which can be substantially rescued by restoring cytosolic calcium. These results suggest that HBx binding to Bcl-2 family members and subsequent elevation of cytosolic calcium are important for HBV viral replication. Consistently, RNAi knockdown of Bcl-2 or Bcl-xL results in reduced calcium elevation by HBx and decreased viral replication in hepatocytes. Our results suggest that HBx targets Bcl-2 proteins through its BH3-like motif to promote cytosolic calcium elevation, cell death, and viral replication during HBV pathogenesis, which presents an excellent therapeutic intervention point in treating patients with chronic HBV.
Keywords: calcium signaling, apoptosis, necrosis
Hepatitis B virus (HBV) is a hepatocyte-specific DNA virus, which encodes several different viral proteins, including DNA polymerase, surface antigen, core antigen, and the X protein (HBx) (1, 2). Whereas the functions of the other viral genes in HBV DNA replication and virion assembly are better understood, the roles and mechanisms of HBx in HBV infection and pathogenesis remain enigmatic. HBx has been implicated in mediating multiple viral and cellular events in HBV-infected cells, including viral replication, transactivation of transcription factors, signal transduction, cell-cycle progression, and cell death (1, 2). Although HBx is found in both the cytoplasm and the nucleus, mitochondria appear to be an important site for HBx action, because expression of HBx has been shown to induce aggregation of mitochondria, loss of mitochondrial membrane potential, and cytochrome c release (3–6). How HBx interacts with mitochondria to cause these changes is not understood.
HBx is critical for viral pathogenesis and oncogenesis in HBV-infected livers (1, 2, 7). The HBx gene is the most frequently integrated viral sequence in hepatocellular carcinoma (HCC) and HBx protein is detected in most patients with HBV-related HCC, even in the absence of viral DNA replication (1, 8–10). In addition, HBV variants carrying mutations in HBx have been identified in HCC tissues and result in the loss of HBx-dependent activities, suggesting that evolving HBx functions may underlie HBV-related liver disease (11, 12). Moreover, HBx promotes liver tumorigenesis in transgenic mice lacking the other components of the HBV virion (13–17). Therefore, HBx plays an important role in the development of HBV-related HCC.
Calcium signaling is critical for multiple HBx activities (2, 18). HBx-induced elevation of cytosolic calcium has been shown to be important for HBV DNA replication, HBV core assembly, and activation of several transcriptional events and signaling cascades (19–24). Induction of apoptosis or necrosis by HBx also requires increased cytosolic calcium and mitochondria permeability transition (MPT), a process by which mitochondria regulate cellular calcium during homeostasis and cell death (5, 25). However, the cellular targets with which HBx interacts to induce MPT and cytosolic calcium increase have not been identified.
Many proteins were found to interact with HBx in various in vitro systems (1, 7, 18). However, most of these protein interactions have not been confirmed in conditions that recapitulate HBV infection in hepatocytes. Genetic redundancy of complex mammalian systems has been a major hurdle to definitive identification of HBx cellular targets. Using a simple, genetically tractable Caenorhabditis elegans animal model, we found that HBx interacts directly with the Bcl-2 homolog, CED-9, to induce cytosolic calcium increase and cell death, mimicking two important events downstream of HBx expression in hepatocytes (companion article, ref. 26). Here we demonstrate that HBx interacts with two Bcl-2 family members, Bcl-2 and Bcl-xL, in hepatocytes to induce cytosolic calcium elevation, cell death, and viral DNA replication. Therefore, Bcl-2 proteins are key cellular targets of HBx during HBV pathogenesis and may prove to be an effective point of pharmacologic intervention for the treatment of HBV-related liver disorders.
Results
HBx Binds Bcl-2 and Bcl-xL in Human Hepatocytes Through Its Bcl-2 Homology 3-Like Motif.
Our analyses of HBx activities in C. elegans have suggested that HBx interacts with CED-9 in C. elegans to induce cytosolic calcium increase and cell death. Moreover, HBx can interact with human Bcl-2 and Bcl-xL in vitro through its Bcl-2 homology 3 (BH3)-like motif and this interaction is disrupted by two substitutions in conserved residues of the BH3-like motif (G124L and I127A) (26). We therefore investigated whether HBx interacts with Bcl-2 and Bcl-xL in human hepatic HepG2 cells by coimmunoprecipitation assay. In HepG2 cells transfected with pcDNA3.1-Flag-HBx, endogenous Bcl-2 and Bcl-xL, but not the anti-apoptotic Bcl-2 family member Mcl-1, were coprecipitated with Flag-HBx, using an antibody to the Flag epitope (Fig. 1A, lane 3). In contrast, no Bcl-xL and only a trace amount of Bcl-2 were coprecipitated with Flag-HBx(G124L, I127A) (Fig. 1A, lane 4), which was expressed in HepG2 cells at a comparable level to Flag-HBx (Fig. 1A, lanes 1 and 2). These results indicate that HBx associates with Bcl-2 and Bcl-xL in human cells through its BH3-like motif. Importantly, in HepG2 cells transfected with a 140% head-to-tail DNA copy of the HBV genome (pHBV) with or without HBx(G124L, I127A) mutations, endogenous Bcl-2 and Bcl-xL, but not Mcl-1, were coprecipitated with HBx, but not with HBx(G124L, I127A), using an antibody to HBx (Fig. 1B, lanes 3 and 4). Therefore, HBx expressed from its native promoter in a replicating HBV genome associates with endogenous Bcl-2 and Bcl-xL in human hepatic cells through its BH3-like motif.
Fig. 1.
HBx binds Bcl-2 and Bcl-xL and induces elevation of cytosolic Ca2+ in human hepatocytes through its BH3-like motif. (A) HBx associates with Bcl-2 and Bcl-xL in HepG2 cells. A coimmunoprecipitation (co-IP) experiment was performed in HepG2 cells transfected with pcDNA3.1-Flag-HBx or pcDNA3.1-Flag-HBx(G124L, I127A) (Materials and Methods). The cell lysate was precipitated with an anti-Flag antibody and analyzed by immunoblotting (IB), using anti-Flag, anti-Bcl-2, anti-Bcl-xL, and anti-Mcl-1 antibodies, respectively (lanes 3 and 4). One portion of the cell lysate was used in immunoblotting analysis to examine the expression levels of the HBx protein and Bcl-2 family proteins (lanes 1 and 2). (B) HBx expressed from its native promoter in a replicating HBV genome binds Bcl-2 and Bcl-xL in HepG2 cells. The co-IP experiment was performed as in A. The cell lysate was precipitated with an anti-HBx monoclonal antibody (16F9) and analyzed by IB analysis as in A. (C) Expression of HBx in HepG2 cells increases cytosolic Ca2+ through the BH3-like motif. HepG2 cells cotransfected with pcDNA3-mCherry and pcDNA3.1-Flag-HBx or pcDNA3.1-Flag-HBx(G124L, I127A) were treated with 4 μM Fura-2-AM 48 h posttransfection. Ca2+ concentrations were calculated from the measured Fura-2 ratios as previously described (27). Error bars indicate SEM (n > 12). **P < 0.01.
HBx Induces Cell Killing and Cytosolic Calcium Increase in Hepatocytes Through Its BH3-Like Motif.
We analyzed the cell-killing activity of HBx in human cells by staining HBx-transfected HepG2 cells with Annexin-V Pacific Blue and propidium iodide (PI) to distinguish living cells from apoptotic and necrotic cells. Flow cytometry analysis of cells transfected with pcDNA3.1-Flag-HBx showed 10.6% apoptotic cells (Annexin-V positive and PI negative) and 7.9% necrotic cells (Annexin-V positive and PI positive) (Fig. 2B). Significantly less cell death was observed in HepG2 cells transfected with the same amount of pcDNA3.1-Flag-HBx(G124L, I127A) (4.87% apoptotic cells and 1.14% necrotic cells; Fig. 2C) or empty pcDNA3.1 vector (1.14% apoptotic cells and 0.32% necrotic cells; Fig. 2A). Therefore, as in C. elegans, HBx uses its BH3-like motif to bind anti-apoptotic Bcl-2 and Bcl-xL proteins and induces both apoptosis and necrosis in human hepatocytes.
Fig. 2.
HBx induces cell killing in human hepatic cells through its BH3-like motif. HepG2 cells were transfected with 1 μg of empty pcDNA3.1 vector (A), pcDNA3.1-Flag-HBx (B), or pcDNA3.1-Flag-HBx(G124L, I127A) (C), using pEGFP-C1 (1 μg) as a cotransfection marker. Only the HBx-transfected cells (GFP positive) are shown (Materials and Methods). Lower Left square indicates the percentage of living cells, Lower Right square indicates the percentage of apoptotic cells, and Upper Right square indicates the percentage of necrotic cells.
Because calcium signaling is an important event downstream of HBx, we examined whether the BH3-like motif of HBx is necessary for HBx-induced elevation of cytosolic calcium. Cytosolic calcium in HepG2 cells transfected with pcDNA3.1-Flag-HBx or pcDNA3.1-Flag-HBx(G124L, I127A) was determined using the ratiometric fluorescent calcium indicator Fura-2 (27). We found that the resting calcium concentration was significantly increased upon expression of HBx compared with the vector-only control (Fig. 1C), whereas expression of HBx(G124L, I127A) failed to do so. The HBx-induced calcium elevation is not due to increased cell death, because a similar level of calcium increase was observed in the presence of Z-VAD, a pan-caspase inhibitor that blocks cell death (Fig. S1A). These results indicate that HBx can induce an increase in cytosolic calcium that is dependent on its BH3-like motif and association with Bcl-2 family proteins.
BH3-Like Motif of HBx Is Critical for HBV Viral Replication.
Given the critical role of HBx in HBV DNA replication (20), we examined whether interactions between HBx and Bcl-2 proteins are important for HBV replication. Cytoplasmic viral core particles, where HBV DNA replication occurs, were isolated from HepG2 cells transfected with the pHBV replicon with or without the HBx(G124L, I127A) mutations. The level of HBV DNA replication was examined by Southern blot analysis. Compared with cells transfected with wild-type pHBV, HBV DNA replication was significantly reduced in cells transfected with the mutant pHBV (Fig. 3A). The level of the HBV core protein (HBcAg), which correlates with the level of HBV DNA (28), was also greatly reduced in cells transfected with the mutant HBV genome (Fig. 3B). Northern blot analysis showed no reduction in HBV pregenomic (pg)/precore (pc) RNA, preS/S mRNA, or HBx mRNA in cells transfected with the mutant pHBV replicon (Fig. 3C). Quantitative real-time PCR (Q-PCR) analysis of isolated viral particles revealed an eight- to ninefold reduction in HBV DNA replication in cells transfected with the mutant HBV genome compared with cells transfected with the wild-type HBV genome (Fig. 3D). These results indicate that the BH3-like motif of HBx is critical for HBV DNA replication but dispensable for HBV transcription. Importantly, HBV DNA replication in cells transfected with the mutant HBV genome was largely rescued by treatment with 5 μM ionomycin (Fig. 3D), an ionophore that increases cytosolic calcium (29). This result suggests that increased cytosolic calcium is an important signaling event downstream of HBx interaction with Bcl-2 proteins that stimulates HBV DNA replication.
Fig. 3.
Interaction between HBx and Bcl-2 proteins is critical for HBV DNA replication in human hepatic cells. (A) Southern blot analysis of HBV DNA replication in HepG2 cells transfected with the wild-type or mutant (G124L, I127A) pHBV replicon 3 d posttransfection. rc/ds DNA represents relaxed circular DNA and double-stranded DNA. ssDNA represents single-stranded DNA. (B) Measurement of the levels of the HBV core protein (HBcAg) in transfected HepG2 cells. The difference between cells transfected with the wild-type and mutant (G124L, I127A) pHBV is shown as fold change to wild type. The data represent mean ± SD from three independent experiments. ***P < 0.0001. (C) Northern blot analysis of HBV transcription in HepG2 cells transfected with the wild-type or mutant pHBV replicon 3 d posttransfection. Different HBV mRNAs are indicated. GFP mRNA from a cotransfection marker and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were used as controls. (D) Quantitative PCR analysis of HBV DNA replication in HepG2 cells transfected with the wild-type or mutant pHBV replicon with or without 5 μM ionomycin treatment. Three days posttransfection, cytoplasmic HBV viral particles were isolated and the viral DNA replication intermediates were quantified by real-time PCR (Materials and Methods). The results represent the fold change of the replicative intermediates from the mutant pHBV replicon compared with those from the wild-type pHBV replicon in HepG2 cells, using two different primer sets (one specific to the HBS ORF and one specific to the polymerase ORF). Data are presented as mean ± SEM. At least three independent experiments were performed for each dataset. ***P < 0.0001.
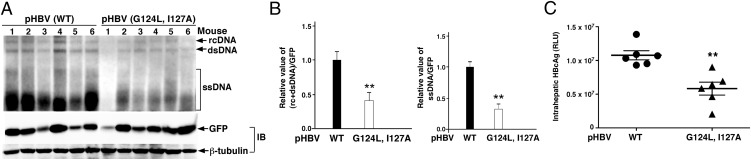
We next examined the importance of HBx interaction with Bcl-2 proteins for HBV replication in an established mouse model of chronic HBV infection (30). The pHBV replicon with wild-type HBx or HBx(G124L, I127A) was introduced into BALB/C mice (n = 6) through hydrodynamic tail vein injection, along with a pcDNA3-GFP reporter as a control for injection efficiency. Cytoplasmic viral core particles were isolated from the liver 2 d after injection and subjected to Southern blot analysis (Fig. 4A). The average level of replicative DNA intermediates in livers of mice receiving the mutant pHBV replicon was reduced by two- to threefold compared with that of mice receiving the wild-type pHBV replicon (Fig. 4B). Expression of intrahepatic HBV core antigen (HBcAg) was similarly reduced in mice receiving the mutant pHBV replicon (Fig. 4C). These results confirm the importance of the BH3-like motif of HBx, and thus the association of HBx with Bcl-2 family proteins, for HBV DNA replication in HBV-infected liver.
Fig. 4.
Interaction between HBx and Bcl-2 proteins is critical for HBV DNA replication in mouse hepatocytes. (A) Southern blot analysis of HBV DNA replication in mouse livers after hydrodynamical injection of the wild-type or the mutant (G124L, I127A) pHBV replicon 2 d postinjection. pcDNA3-GFP was coinjected as an injection marker. Equivalent amounts of liver tissues from six BALB/C mice in each injection group were collected and subjected to HBV DNA and HBcAg analyses (Materials and Methods). The levels of GFP and β-tubulin were determined by immunoblotting (IB) and used as controls to normalize the transfection efficiency. (B) Quantification of viral DNA intermediates from the Southern blot analysis in A. The results represent the relative value of different HBV DNA intermediates to the GFP control. The amounts of DNA and GFP were quantified by Quality One software (Bio-Rad). Data are presented as mean ± SEM. **P < 0.01. (C) A plot showing the levels of the HBV core protein (HBcAg) in mouse livers. All mice (n = 6) from each injection group were subjected to HBcAg analysis (Materials and Methods). RLU represents relative luminescence units (mean ± SEM). **P < 0.01.
Bcl-2 and Bcl-xL Are Important for HBx-Induced Cytosolic Calcium Elevation and HBV Viral Replication in Hepatocytes.
We examined the importance of Bcl-2 proteins for HBV replication by knocking down the expression of Bcl-2 or Bcl-xL in HepG2 cells through RNA interference (RNAi). Compared with control short hairpin RNA (shRNA), Bcl-2 and Bcl-xL shRNA significantly reduced the expression of Bcl-2 and Bcl-xL in HepG2 cells, respectively (Fig. 5 A and B). Importantly, Q-PCR analysis of isolated viral particles revealed a 21–41% reduction in HBV DNA replication in cells infected by lentivirus expressing Bcl-2 or Bcl-xL shRNA, compared with cells with control shRNA (Fig. 5 A and B). Overexpression of the anti-apoptotic Mcl-1 protein, which does not interact with HBx (Fig. 1 A and B), in cells treated with Bcl-2 or Bcl-xL shRNA did not prevent reduction of HBV DNA replication (Fig. S2), indicating that decreased HBV DNA replication caused by loss of Bcl-2 or Bcl-xL is unlikely due to impaired survival of the host cells. Moreover, RNAi knockdown of Bcl-2 or Bcl-xL dampened but did not obliterate intracellular calcium increase induced by HBx (Fig. S1 B and C), which is consistent with the finding that Bcl-2 or Bcl-xL knockdown reduced but did not block HBV DNA replication and indicates that Bcl-2 and Bcl-xL are partially redundant in mediating HBx functions. Bcl-2 and Bcl-xL double-knockdown cells were not viable for analysis of HBV viral replication and intracellular calcium changes. These results indicate that Bcl-2 and Bcl-xL are important for HBV viral replication and, together with the findings described above, provide strong evidence that HBx targets both Bcl-2 and Bcl-xL to increase intracellular calcium and to promote HBV DNA replication (Fig. 5C).
Fig. 5.
Bcl-2 and Bcl-xL are important for HBV DNA replication. (A and B) (Right) HepG2 cells infected by lentivirus expressing control, Bcl-2, or Bcl-xL shRNA were transfected with the pHBV replicon and subjected to Q-PCR analysis as described in Fig. 3D. (Left) One portion of the cells was analyzed by immunoblotting to examine the expression levels of Bcl-2 and Bcl-xL, using α-tubulin as a loading control. Data are presented as mean ± SEM. ***P < 0.0001. (C) A working model of HBx-dependent viral pathogenesis. HBx directly interacts with Bcl-2 and Bcl-xL to increase cytosolic Ca2+. Increased cytosolic Ca2+ then promotes HBV replication and cell death. This signaling pathway is conserved in C. elegans.
Discussion
Despite the critical role of HBx in HBV pathogenesis and oncogenesis, identification of HBx host targets has remained a major challenge in the last three decades (1, 2, 7). The intricacy of HBx activities, the lack of a powerful animal model to study HBV infection, variability among cell culture assays, and the complexity of the mammalian genome, which encodes at least six Bcl-2 family proteins, have all contributed to the longstanding questions regarding the functions of HBx, its interactions with host targets, and its mechanisms of action. We have engineered a C. elegans animal model to identify HBx targets and downstream signaling pathways (26). Mimicking the initial cellular events that unfold following liver infection by HBV (17, 31, 32), HBx induces both apoptosis and necrosis in C. elegans through canonical cell death pathways. Interestingly, a unique gain-of-function mutation (G169E) in the Bcl-2 homolog CED-9, which inhibits cell death in C. elegans by blocking the binding of the endogenous BH3-only cell death inducer EGL-1 to CED-9 (33), also completely blocks the interaction between CED-9 and the BH3-like motif of HBx and HBx-induced cell death in C. elegans. Remarkably, Bcl-2 can fully substitute for CED-9 in C. elegans to mediate HBx-induced cell killing, indicating that Bcl-2 likely interacts with HBx in mammals. Indeed, we demonstrate here that HBx associates with Bcl-2 and Bcl-xL in human hepatocytes through its BH3-like motif and that this protein interaction is crucial for HBx-induced cytosolic calcium elevation, cell death, and viral DNA replication. These findings suggest that molecular mimicry of endogenous BH3-only proteins by HBx enables its interactions with conserved host targets and hijacking of cell signaling pathways to benefit viral infection.
Calcium signaling is a critical event downstream of HBx expression that promotes HBV replication, core assembly, cell death, and other HBx functions (2, 20, 21, 25). HBx has been proposed to effect MPT (4–6, 20), which is important for intracellular calcium homeostasis and cell death (34, 35). Importantly, both Bcl-2 and Bcl-xL are mitochondrial proteins and have been implicated in regulating MPT (34, 36). HBx binding to Bcl-2 and Bcl-xL is critical for calcium regulation by HBx, because expression of HBx, but not HBx(G124L, I127A), which fails to bind Bcl-2 and Bcl-xL, triggers elevation of cytosolic calcium in hepatocytes. The finding that G124L/I127A mutations in the BH3-like motif of HBx greatly reduce HBV DNA replication in human and mouse hepatocytes, which can be substantially rescued by restoring cytosolic calcium with ionomycin, and the observation that RNAi knockdown of either Bcl-2 or Bcl-xL significantly compromises HBx-induced intracellular calcium increase and HBV replication provide further confirmation that HBx targets Bcl-2 proteins to trigger cytosolic calcium elevation required for HBV replication and other events such as cell death (Fig. 5C).
Hepatocarcinogenesis is a complex and poorly understood process. Chronic hepatocyte cell death induced by HBV infection or carcinogens may trigger cycles of inflammation, immune response, and compensatory tissue regeneration and the acquisition of oncogenic mutations that lead to development of HCC (17, 31, 37). On the other hand, hepatocyte expression of prosurvival factors, such as Bcl-2 and p38α kinase, has been shown to be effective in preventing HCC development (37–39). Moreover, HBV viral replication plays an important contributing role in hepatocarcinogenesis. The development and progression of HCC in patients with chronic HBV strongly correlate with the viral DNA level in a dose-dependent manner (40, 41). Therefore, blocking HBV viral replication and HBV-induced cell death represents an effective strategy to treat patients with chronic HBV and to prevent the development of HCC. Our study suggests that the BH3-like motif of HBx is necessary for HBx binding to Bcl-2 family proteins, which results in elevated cytosolic calcium, efficient viral replication, and HBV-induced cell death. Therefore, therapeutically targeting the BH3-like motif of HBx could be a unique and effective strategy to treat patients with chronic HBV and to prevent development of HCC.
Materials and Methods
Immunoprecipitation Assays.
HepG2 cells transfected with the pcDNA3.1-Flag-HBx constructs or the pHBV replicons (wild-type and G124L/I127A mutations) were lysed and precipitated using an anti-Flag antibody or an anti-HBx antibody. The proteins pulled down with HBx were detected by immunoblotting analysis.
Calcium Imaging and Analysis.
HepG2 cells cotransfected with pcDNA3-mCherry and pcDNA3.1-Flag-HBx constructs (wild-type or G124L/I127A mutations) were incubated for 35 min with 4 μM Fura-2-AM and 0.04% Pluronic solution 48 h posttransfection, washed three times with buffer, and incubated for an additional 15 min to allow for cleavage of the acetoxymethyl (AM) ester, which trapped Fura-2 in the cells. Data were collected using the Metafluor software and analyzed by Excel (27). Statistical analysis was performed using a t test in the KaleidaGraph program. The error bars indicate SEM.
Quantification of HBV DNA Replication and HBcAg.
Southern hybridization analysis and quantitative real-time PCR were used to quantify the amount of HBV replication DNA intermediates isolated from HepG2 cells or from mouse livers. The level of cytoplasmic HBcAg was measured by chemiluminescence, using a commercial assay kit.
Hydrodynamic Injection.
Thirty micrograms of the pHBV replicon and 3 µg of pcDNA3-GFP were injected into the tail veins of BALB/c mice within 5 s in a volume of PBS equivalent to 10% of the mouse body weight. Livers of the injected mice were assayed for HBcAg and viral DNA 2 d after injection (30).
Detailed methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank T. Blumenthal and R. Garcea for comments and suggestions. This work was supported by National Institutes of Health Grants F30 NS070596 (to B.L.H.); R01 GM059083, GM079097, and GM088241 (to D.X.); and GM084027 (to A.E.P.); grants from China National Science Foundation (30925030) and the National Scientific and Technological Major Project (2013ZX10002-002) (to N.-S.X.); and a Burroughs Wellcome Fund Award (to D.X.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204668109/-/DCSupplemental.
References
- 1.Kremsdorf D, Soussan P, Paterlini-Brechot P, Brechot C. Hepatitis B virus-related hepatocellular carcinoma: Paradigms for viral-related human carcinogenesis. Oncogene. 2006;25:3823–3833. doi: 10.1038/sj.onc.1209559. [DOI] [PubMed] [Google Scholar]
- 2.Ganem D, Schneider RJ. The molecular biology of the hepatitis B viruses. In: Knipe DM, et al., editors. Fields Virology. 4th Ed. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2923–2970. [Google Scholar]
- 3.Terradillos O, et al. The hepatitis B virus X protein abrogates Bcl-2-mediated protection against Fas apoptosis in the liver. Oncogene. 2002;21:377–386. doi: 10.1038/sj.onc.1205110. [DOI] [PubMed] [Google Scholar]
- 4.Shirakata Y, Koike K. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J Biol Chem. 2003;278:22071–22078. doi: 10.1074/jbc.M301606200. [DOI] [PubMed] [Google Scholar]
- 5.Clippinger AJ, Gearhart TL, Bouchard MJ. Hepatitis B virus X protein modulates apoptosis in primary rat hepatocytes by regulating both NF-kappaB and the mitochondrial permeability transition pore. J Virol. 2009;83:4718–4731. doi: 10.1128/JVI.02590-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahmani Z, Huh KW, Lasher R, Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J Virol. 2000;74:2840–2846. doi: 10.1128/jvi.74.6.2840-2846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Y, Neuveut C, Tiollais P, Buendia MA. Molecular biology of the hepatitis B virus and role of the X gene. Pathol Biol (Paris) 2010;58:267–272. doi: 10.1016/j.patbio.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Feitelson MA, Duan LX. Hepatitis B virus X antigen in the pathogenesis of chronic infections and the development of hepatocellular carcinoma. Am J Pathol. 1997;150:1141–1157. [PMC free article] [PubMed] [Google Scholar]
- 9.Su Q, et al. Expression of hepatitis B virus X protein in HBV-infected human livers and hepatocellular carcinomas. Hepatology. 1998;27:1109–1120. doi: 10.1002/hep.510270428. [DOI] [PubMed] [Google Scholar]
- 10.Peng Z, et al. Integration of the hepatitis B virus X fragment in hepatocellular carcinoma and its effects on the expression of multiple molecules: A key to the cell cycle and apoptosis. Int J Oncol. 2005;26:467–473. [PubMed] [Google Scholar]
- 11.Sirma H, et al. Hepatitis B virus X mutants, present in hepatocellular carcinoma tissue abrogate both the antiproliferative and transactivation effects of HBx. Oncogene. 1999;18:4848–4859. doi: 10.1038/sj.onc.1202867. [DOI] [PubMed] [Google Scholar]
- 12.Tu H, et al. Biological impact of natural COOH-terminal deletions of hepatitis B virus X protein in hepatocellular carcinoma tissues. Cancer Res. 2001;61:7803–7810. [PubMed] [Google Scholar]
- 13.Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991;351:317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- 14.Terradillos O, et al. The hepatitis B virus X gene potentiates c-myc-induced liver oncogenesis in transgenic mice. Oncogene. 1997;14:395–404. doi: 10.1038/sj.onc.1200850. [DOI] [PubMed] [Google Scholar]
- 15.Yu DY, et al. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999;31:123–132. doi: 10.1016/s0168-8278(99)80172-x. [DOI] [PubMed] [Google Scholar]
- 16.Madden CR, Finegold MJ, Slagle BL. Hepatitis B virus X protein acts as a tumor promoter in development of diethylnitrosamine-induced preneoplastic lesions. J Virol. 2001;75:3851–3858. doi: 10.1128/JVI.75.8.3851-3858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu BK, et al. Blocking of G1/S transition and cell death in the regenerating liver of Hepatitis B virus X protein transgenic mice. Biochem Biophys Res Commun. 2006;340:916–928. doi: 10.1016/j.bbrc.2005.12.089. [DOI] [PubMed] [Google Scholar]
- 18.Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725–12734. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Y, Gyoo Park S, Yoo JH, Jung G. Calcium ions affect the hepatitis B virus core assembly. Virology. 2005;332:454–463. doi: 10.1016/j.virol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001;294:2376–2378. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- 21.Bouchard MJ, Puro RJ, Wang L, Schneider RJ. Activation and inhibition of cellular calcium and tyrosine kinase signaling pathways identify targets of the HBx protein involved in hepatitis B virus replication. J Virol. 2003;77:7713–7719. doi: 10.1128/JVI.77.14.7713-7719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarn C, Zou L, Hullinger RL, Andrisani OM. Hepatitis B virus X protein activates the p38 mitogen-activated protein kinase pathway in dedifferentiated hepatocytes. J Virol. 2002;76:9763–9772. doi: 10.1128/JVI.76.19.9763-9772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh JC, Jeong DL, Kim IK, Oh SH. Activation of calcium signaling by hepatitis B virus-X protein in liver cells. Exp Mol Med. 2003;35:301–309. doi: 10.1038/emm.2003.41. [DOI] [PubMed] [Google Scholar]
- 24.Lara-Pezzi E, Armesilla AL, Majano PL, Redondo JM, López-Cabrera M. The hepatitis B virus X protein activates nuclear factor of activated T cells (NF-AT) by a cyclosporin A-sensitive pathway. EMBO J. 1998;17:7066–7077. doi: 10.1093/emboj/17.23.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chami M, Ferrari D, Nicotera P, Paterlini-Bréchot P, Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem. 2003;278:31745–31755. doi: 10.1074/jbc.M304202200. [DOI] [PubMed] [Google Scholar]
- 26.Geng X, et al. (2012) Proc Natl Acad Sci USA, 10.1073/pnas.1204652109.
- 27.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 28.Kimura T, et al. New enzyme immunoassay for detection of hepatitis B virus core antigen (HBcAg) and relation between levels of HBcAg and HBV DNA. J Clin Microbiol. 2003;41:1901–1906. doi: 10.1128/JCM.41.5.1901-1906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan AJ, Jacob R. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J. 1994;300:665–672. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang LR, Wu HL, Chen PJ, Chen DS. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2006;103:17862–17867. doi: 10.1073/pnas.0608578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollicino T, Terradillos O, Lecoeur H, Gougeon ML, Buendia MA. Pro-apoptotic effect of the hepatitis B virus X gene. Biomed Pharmacother. 1998;52:363–368. doi: 10.1016/s0753-3322(99)80003-1. [DOI] [PubMed] [Google Scholar]
- 32.Guo JT, et al. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J Virol. 2000;74:1495–1505. doi: 10.1128/jvi.74.3.1495-1505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrish J, Metters H, Chen L, Xue D. Demonstration of the in vivo interaction of key cell death regulators by structure-based design of second-site suppressors. Proc Natl Acad Sci USA. 2000;97:11916–11921. doi: 10.1073/pnas.210391597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhivotovsky B, Galluzzi L, Kepp O, Kroemer G. Adenine nucleotide translocase: A component of the phylogenetically conserved cell death machinery. Cell Death Differ. 2009;16:1419–1425. doi: 10.1038/cdd.2009.118. [DOI] [PubMed] [Google Scholar]
- 35.Bernardi P, Rasola A. Calcium and cell death: The mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 36.Breckenridge DG, Xue D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol. 2004;16:647–652. doi: 10.1016/j.ceb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai T, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce RH, Vail ME, Ralph L, Campbell JS, Fausto N. Bcl-2 expression inhibits liver carcinogenesis and delays the development of proliferating foci. Am J Pathol. 2002;160:1555–1560. doi: 10.1016/S0002-9440(10)61101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Chen CJ, et al. REVEAL-HBV Study Group Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 41.Yuen MF, et al. Risk for hepatocellular carcinoma with respect to hepatitis B virus genotypes B/C, specific mutations of enhancer II/core promoter/precore regions and HBV DNA levels. Gut. 2008;57:98–102. doi: 10.1136/gut.2007.119859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.