Abstract
Benzodiazepines potentiate γ-aminobutyric acid type A receptor (GABAAR) activity and are widely prescribed to treat anxiety, insomnia, and seizure disorders. Unfortunately, clinical use of benzodiazepines (BZs) is severely limited by tolerance. The mechanisms leading to BZ tolerance are unknown. BZs bind at the interface between an α and γ subunit of GABAARs, preferentially enhancing synaptic receptors largely composed of α(1–3, 5), β3, and γ2 subunits. Using confocal imaging and patch-clamp approaches, we show that treatment with the BZ flurazepam decreases GABAAR surface levels and the efficacy of neuronal inhibition in hippocampal neurons. A dramatic decrease in surface and total levels of α2 subunit-containing GABAARs occurred within 24 h of flurazepam treatment, whereas GABAARs incorporating α1 subunits showed little alteration. The GABAAR surface depletion could be reversed by treatment with the BZ antagonist Ro 15-1788. Coincident with decreased GABAAR surface levels, flurazepam treatment reduced miniature inhibitory postsynaptic current amplitude, which returned to control levels with acute Ro 15-1788 treatment. GABAAR endocytosis and insertion rates were unchanged by flurazepam treatment. Treatment with leupeptin restored flurazepam lowered receptor surface levels, strongly suggesting that flurazepam increases lysosomal degradation of GABAARs. Together, these data suggest that flurazepam exposure enhances degradation of α2 subunit-containing GABAARs after their removal from the plasma membrane, leading to a reduction in inhibitory synapse size and number along with a decrease in the efficacy of synaptic inhibition. These reported subtype-specific changes in GABAAR trafficking provide significant mechanistic insight into the initial neuroadaptive responses occurring with BZ treatment.
Keywords: GABAergic, diazepam, lysosome
GABAA receptors (GABAARs) are Cl−-selective ligand-gated ion channels that mediate fast inhibition in the CNS and are important targets for benzodiazepines (BZs), drugs used to treat anxiety, insomnia, and seizure disorders and as adjunct treatments in both depression and schizophrenia. BZs enhance chloride ion conductance primarily by increasing the frequency of receptor channel opening and slowing the decay of miniature inhibitory postsynaptic currents (mIPSCs) (1–3). Structurally, GABAARs are pentameric heterooligomers that can be constructed from eight subunit classes with multiple members: α(1–6), β(1–3), γ(1–3), δ, ε, θ, π, and ρ(1–3) (4, 5). Molecular and genetic studies have revealed that synaptic BZ-sensitive GABAAR subtypes are composed of α(1–3, 5), β, and γ2 subunits, whereas GABAAR subtypes composed of α4/6, β, and δ subunits form a specialized population of extrasynaptic receptors that mediate tonic inhibition but are insensitive to modulation by BZ (reviewed in ref. 6).
BZs are highly prescribed and of major clinical significance; however, the development of tolerance restricts their usefulness and has prompted research on the mechanism for several decades. Chronic BZ treatment results in allosteric uncoupling of the GABA and BZ binding sites, suggesting changes in receptor subunit composition and/or receptor function (reviewed in ref. 7). Chronic dosing of animals with BZ leads to a reduction in GABAAR synaptic inhibition (8–10) and produces diverse changes in GABAAR transcripts across the brain (7). Direct comparison and interpretation of these and other studies assessing mRNA levels has been challenging due to differences in treatment paradigm (time and dose), brain regions assessed, and the BZ ligand used. Radioligand binding studies have reported mixed results, ranging from decreases to no change in CNS BZ binding sites, likely due to methodological limitations in assessing subtype-specific GABAAR changes.
The surface level of postsynaptic GABAARs is a critical determinant of the strength of synaptic inhibition and is largely dependent on regulated trafficking steps to and from the plasma membrane, including receptor insertion, diffusion within the membrane, receptor removal, and degradation (11). Biochemical studies show that at least 40% of the total cell surface population of GABAARs is internalized within 1 h (12). Endocytosed receptors are either rapidly recycled back to the surface for reinsertion or targeted for lysosomal degradation. In addition, GABAAR surface levels can be regulated by controlling the receptor pool available for insertion, the size of which is primarily determined by endoplasmic reticulum-associated degradation (13). Modulation of GABAAR turnover has been widely invoked to explain the development of tolerance to BZs. However, it remains to be determined whether exposure of neurons to BZs modifies the surface stability of GABAARs or the efficacy of synaptic inhibition.
We demonstrate here that 24-h treatment with the BZ flurazepam (Flz) dramatically decreases α2 subunit-containing GABAAR surface and total levels without comparable changes in levels of the α1 subunit. BZ treatment does not appear to alter receptor insertion and removal rates. However, treatment with leupeptin, an inhibitor of lysosomal proteolysis, restores BZ-lowered GABAAR levels. The observed decrease in GABAAR surface levels and synaptic inhibition occurs in a BZ binding site-dependent fashion because these alterations can be rescued by treatment with the BZ antagonist Ro 15-1788. Together, these data suggest that Flz exposure enhances degradation of α2-subtype GABAARs after endocytosis, leading to a reduction in inhibitory synapse size and number along with a decrease in the efficacy of synaptic inhibition.
Results
Acute BZ Treatment Decreases GABAAR Surface Levels.
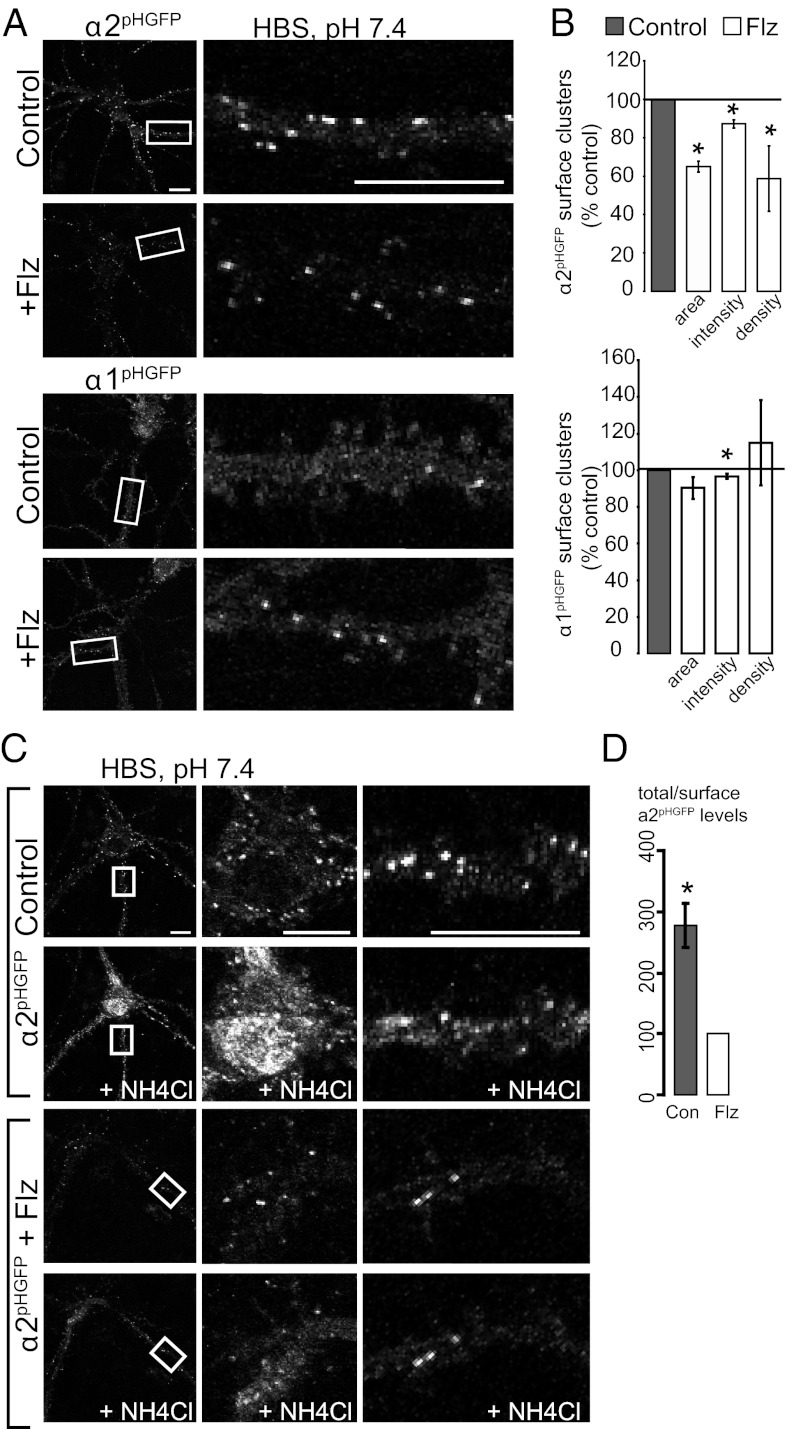
To directly address the role of BZ in regulating GABAAR surface levels, we performed live-imaging studies of pH-sensitive GFP-tagged GABAAR α1 (α1pHGFP) and α2 (α2pHGFP) subunits in hippocampal neurons. The addition of this reporter to receptor subunits is functionally silent and allows the specific visualization of surface GABAAR populations in living neurons (14, 15). Furthermore, BZ potentiation of GABA-induced currents is equivalent in HEK-293 cells expressing these tagged or untagged GABAAR α subunits cotransfected with β3γ2 subunits (Table S1). We measured the surface levels of BZ-sensitive GABAARs containing α1pHGFP or α2pHGFP subunits with 24-h 250 nM Flz or control treatment with live confocal microscopy at 37 °C in Hepes-buffered saline (HBS) (pH 7.4). Twenty-four-hour Flz treatment dramatically decreased α2 subunit-containing GABAAR surface levels on three measured parameters, expressed as percentage control: surface cluster area (65.0 ± 2.8%), average cluster fluorescence intensity (87.1 ± 2.2%), and density (58.7 ± 17%) (Fig. 1 A and B). Minor changes were observed in α1-subtype GABAAR surface levels, with a significant decrease only in average cluster fluorescence intensity (96.5 ± 1.3%) (Fig. 1 A and B).
Fig. 1.
Acute BZ treatment decreases surface and total GABAAR levels. (A) Neurons expressing α2pHGFP or α1pHGFP GABAAR subunits were incubated with or without 250 nM flurazepam (Flz) for 24 h followed by live confocal microscopy experiments (37 °C in HBS). (Right) Enlargements of dendrites in boxed areas. (B) Mean ± SEM cluster size, fluorescence intensity, and density of surface GABAAR clusters normalized to control (*significantly different from control, P < 0.05, t test; n = 15–20 neurons, 3 independent cultures). (C) Neurons were perfused at a rate of 1 mL/min with pH 7.4 HBS followed by HBS with 50 mM NH4Cl (+NH4Cl) to collapse the intracellular pH gradients and reveal intracellular GABAAR. The center panels are enlargement of cell bodies, and the right panels are dendrites in boxed areas. (D) The ratio of α2pHGFP total/surface levels was normalized to Flz-treated neurons (*P < 0.05, t test; n = 6–8 neurons per culture, 3 independent cultures; error bars represent ±SEM). (Scale bars, 10 μm.)
BZ Treatment Alters Total GABAAR Levels.
Because these initial studies revealed a major decrease in α2-containing GABAAR surface levels and a minimal change in α1-containing GABAAR surface levels with 24-h BZ treatment, subsequent experiments focused on the α2 subunit. We tested whether total BZ-sensitive GABAAR protein levels were altered in Flz-treated neurons. To do so, we visualized pHGFP-tagged GABAARs resident inside the neurons by equalizing the pH of all intracellular compartments to pH 7.4 with a brief perfusion of NH4Cl in HBS (NH4Cl in place of equimolar NaCl) (Fig. 1C). This experiment revealed that total α2-subunit levels were decreased dramatically, with the ratio of total/surface α2 being approximately threefold lower following BZ treatment (control total/surface ratio, 277 ± 36%, normalized to Flz total/surface ratio) (Fig. 1 C and D).
Decreases in GABAAR Surface Levels Occur via a BZ Binding Site-Dependent Mechanism.
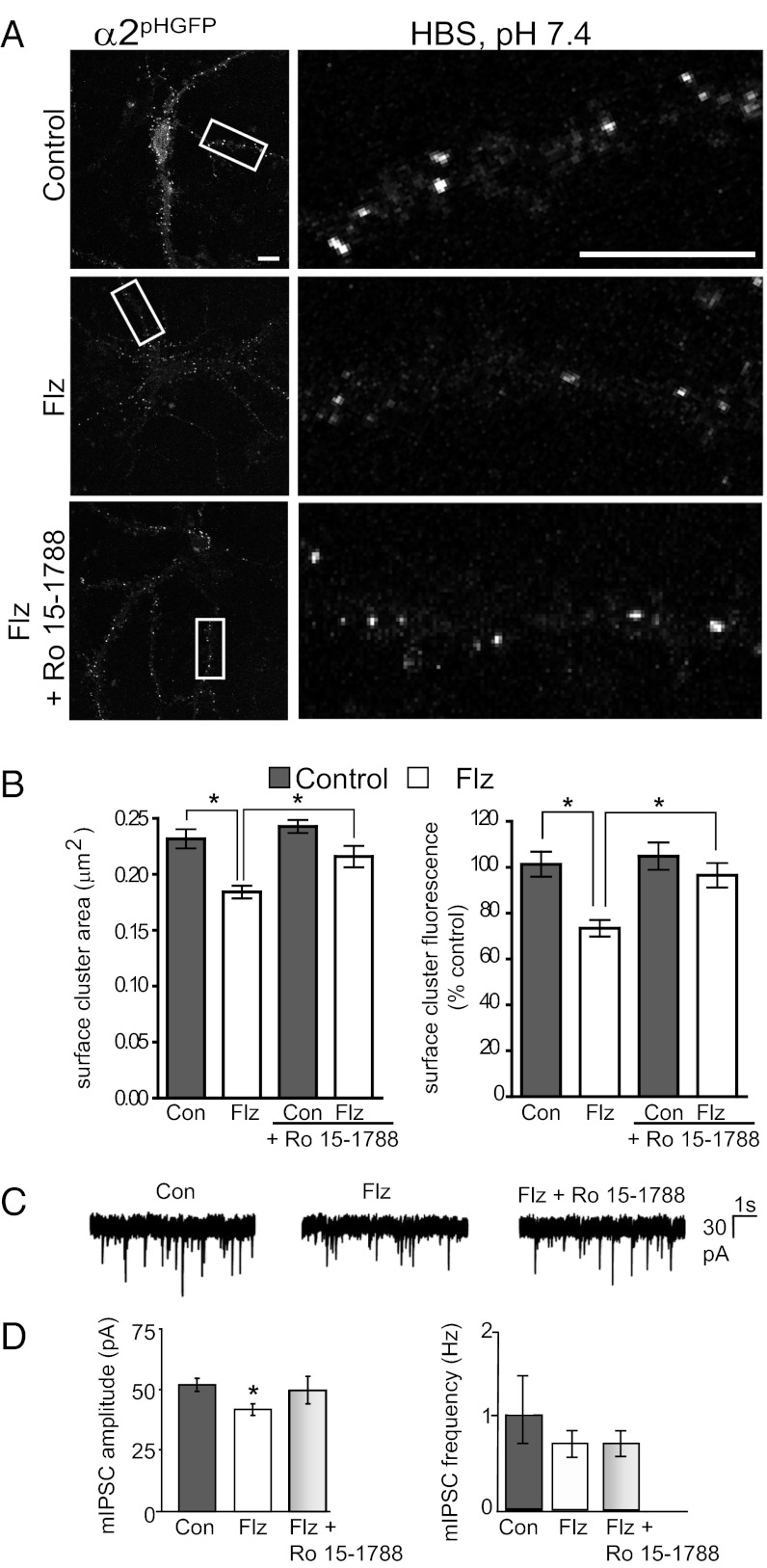
Next, we tested whether the decrease in α2-subtype GABAAR surface levels was specifically induced by BZ binding. Live-cell confocal microscopy was used to measure α2pHGFP fluorescence in neurons with 24-h Flz or control treatment followed by an acute 1-h treatment with 5 μM BZ antagonist Ro 15-1788 (Fig. 2 A and B). One-hour treatment with Ro 15-1788 restored the BZ-lowered GABAAR surface levels to control levels (cluster area in μm2: control, 0.232 ± 0.008; Flz, 0.184 ± 0.006; Flz plus Ro 15-1788, 0.216 ± 0.010; and total cluster fluorescence expressed as % control: control, 101.4 ± 5.4; Flz, 73.4 ± 3.6; Flz plus Ro 15-1788, 96.5 ± 5.3) (Fig. 2B). Control neurons treated with Ro 15-1788 showed no significant change on any measured parameter (control plus Ro 15-1788 cluster area in μm2, 0.242 ± 0.006; and total cluster fluorescence expressed as % control, 104.9 ± 5.4) (Fig. 2B). Interestingly, the BZ antagonist did not significantly increase GABAAR surface cluster density in Flz neurons, suggesting that existing inhibitory synapses are preferentially restored (clusters per 20 μm: control, 6.8 ± 2.2; Flz, 5.4 ± 1.2; control plus Ro 15-1788, 6.9 ± 1.8; Flz plus Ro 15-1788, 5.4 ± 1.6).
Fig. 2.
Decreases in GABAAR surface levels and GABAergic mIPSC amplitude occur via a BZ binding site-dependent mechanism. (A) Neurons expressing α2pHGFP were incubated with or without 250 nM Flz for 24 h and then treated for 1 h with 5 μM Ro 15-1788 (BZ antagonist) immediately before live imaging confocal microscopy experiments (37 °C in HBS). (Right) Enlargements of dendrites in boxed areas. (Scale bars, 10 μm.) (B) Surface cluster histograms showing mean area and cumulative α2pHGFP fluorescence in neurons with 24-h control (Con) or 250 nM Flz conditions with or without 1-h 5 μM Ro 15-1788 treatment (*P < 0.05, one-way ANOVA analysis and Bonferroni’s multiple-comparison test; 22–30 neurons; n = 3 cultures; error bars represent ±SEM). (C) Sample traces of mIPSCs in control (Con) neurons and those treated with 250 nM Flz for 24 h with or without 1-h 5 μM Ro 15-1788 treatment. (D) Bar graph of the mean ± SEM mIPSC amplitudes and frequency (*P < 0.05, Kolmogorov–Smirnov test; n = 7–10 cells for each condition).
Prolonged Exposure of Neurons to BZ Reduces mIPSCs.
Having determined that surface and total levels of α2-subtype GABAARs are decreased by 24-h BZ exposure, we next measured the functional effects of prolonged BZ treatment on GABAergic inhibition. It is well established that rapid application of BZ to neurons enhances GABAAR function due to an increase in the probability of channel opening. Although this results in an increased amplitude and slowed decay of mIPSCs (16, 17), the effects of prolonged initial treatment remain to be addressed. We directly compared the properties of mIPSCs in control neurons and those pretreated with Flz for 24 h before recording. BZ treatment resulted in a significant decrease in mIPSC amplitude from 52.5 ± 2.7 in control neurons to 42.2 ± 2.4 pA in those exposed to Flz for 24 h. In contrast mIPSC frequency (in Hz: control, 1.08 ± 0.34; Flz, 0.69 ± 0.12; Flz plus Ro 15-1788, 0.68 ± 0.10), 10–90% rise time (in ms: control, 1.46 ± 0.08; Flz, 1.68 ± 0.11; Flz plus Ro 15-1788, 1.59 ± 0.11), and decay times (in ms: control, 43.4 ± 1.19; Flz, 47.6 ± 2.34; Flz plus Ro 15-1788, 46.1 ± 1.66) were unaffected by prolonged exposure to BZ (Fig. 2 C and D). One-hour treatment with the BZ antagonist Ro 15-1788 restored mIPSC amplitude to control levels (50.2 ± 5.7 pA). The rapid timescale of Ro 15-1788 action is consistent with BZ-induced changes affecting receptor trafficking pathways rather than protein synthesis. Furthermore, these results are consistent with the efficacy of Ro 15-1788 reversing in vitro and in vivo BZ tolerance (18).
BZ Treatment Does Not Alter the Rates of GABAAR Insertion and Removal.
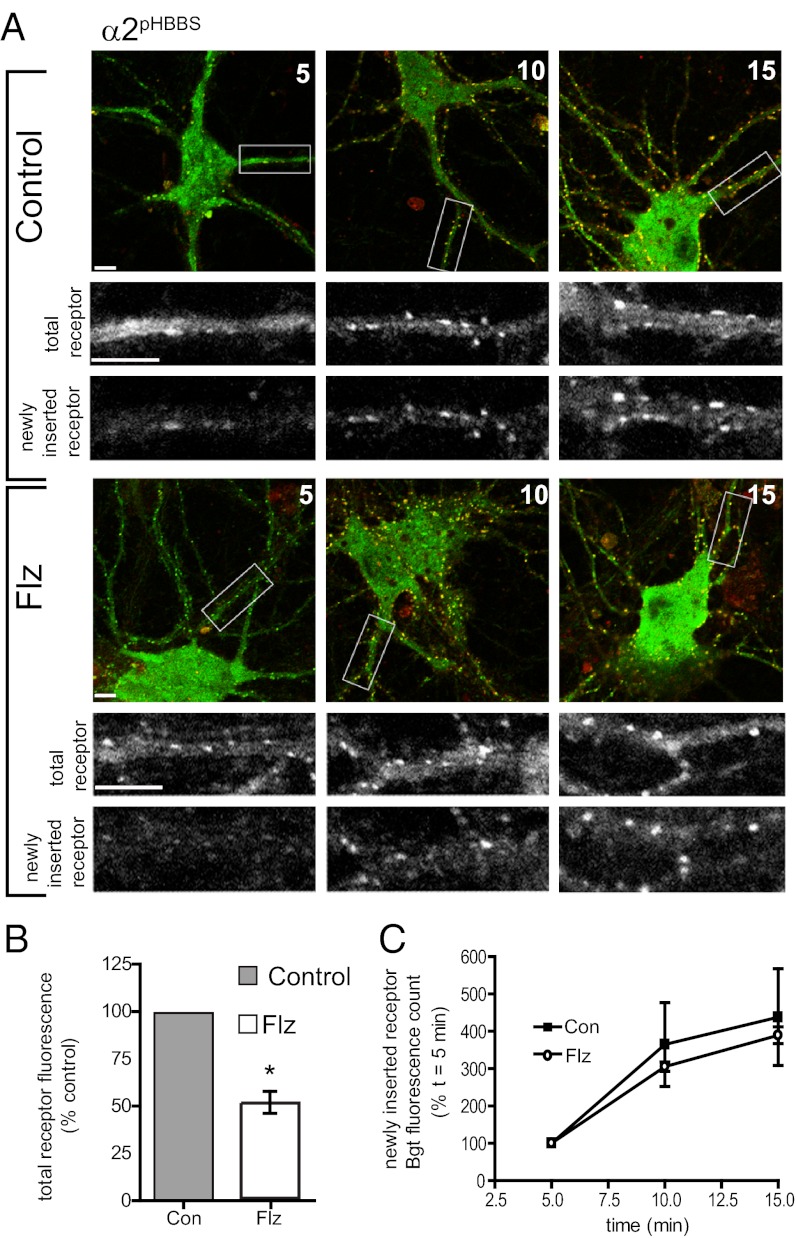
It is increasingly apparent that GABAARs on the surface of neurons are dynamic entities that undergo regulated exocytosis and constitutive clathrin-mediated, dynamin-dependent endocytosis. Changes in the rates of exocytosis or endocytosis could clearly contribute to a mechanism for BZ-dependent alteration of GABAAR surface levels. To measure the insertion and removal rate of α2-containing GABAARs, we generated a construct with a bungarotoxin (Bgt) binding site (BBS) and pHGFP encoded in the N terminus of the GABAAR subunit (α2pHBBS). The BBS allows the monitoring of receptor insertion and endocytosis with application of exogenous fluorescent Bgt and does not modify the assembly or functional properties of GABAARs (13, 19, 20). Bgt insertion assays were performed on α2pHBBS-expressing neurons undergoing control and 24-h 250 nM Flz treatments. Neurons were first incubated with unlabeled Bgt at 15 °C to block existing surface GABAAR receptor populations, and then washed followed by incubation with Alexa 594-conjugated Bgt at 37 °C to label newly inserted receptors. At 5, 10, and 15 min, samples were removed, fixed, and permeabilized, and the total receptor population was labeled with anti-GFP antibody. Quantification and analysis of surface receptor signal in control and Flz-treated neurons produced similar results to live imaging (Fig. 1), with BZ-treated neurons showing a decrease to 51.59 ± 7.3% control (Fig. 3 A and B). The fluorescence intensity of newly inserted α2pHBBS over a 15-min time course (normalized to the initial fluorescence value of each treatment) was compared in control and Flz conditions (Fig. 3 A and C), revealing that the rate of GABAAR insertion was not significantly changed by BZ treatment (Bgt fluorescence count at t = 10 min: control, 364.5 ± 112.1%; Flz, 305.3.1 ± 12.2; and at t = 15 min: control, 437 ± 129.5%; Flz, 389 ± 22.6%).
Fig. 3.
BZ treatment does not alter the insertion rate of α2 subunit-containing GABAAR. (A) Confocal imaging of GABAAR insertion in control and Flz-treated neurons. Neurons expressing α2pHBBS were treated with or without 250 nM Flz for 24 h, and then incubated with Alexa594::Bgt (red) at 37 °C for 5, 10, and 15 min to label newly inserted GABAAR, followed by fixation. Total receptor number was determined by permeabilization and staining with anti-GFP antibody (green). Panels below are enlargements of boxed dendrites. (Scale bars, 10 μm.) (B) Analysis of total receptor signal in control and Flz-treated α2pHBBS neurons. (*P < 0.01, paired t test; 10–12 neurons per condition for each culture, n = 4 cultures; error bars represent ±SEM). (C) Graph shows Alexa594::Bgt fluorescence intensity of newly inserted α2pHBBS over time in control (Con) or Flz conditions (normalized to initial fluorescence count at 5 min) (10–12 neurons per condition for each culture; n = 4 cultures; error bars represent ±SEM).
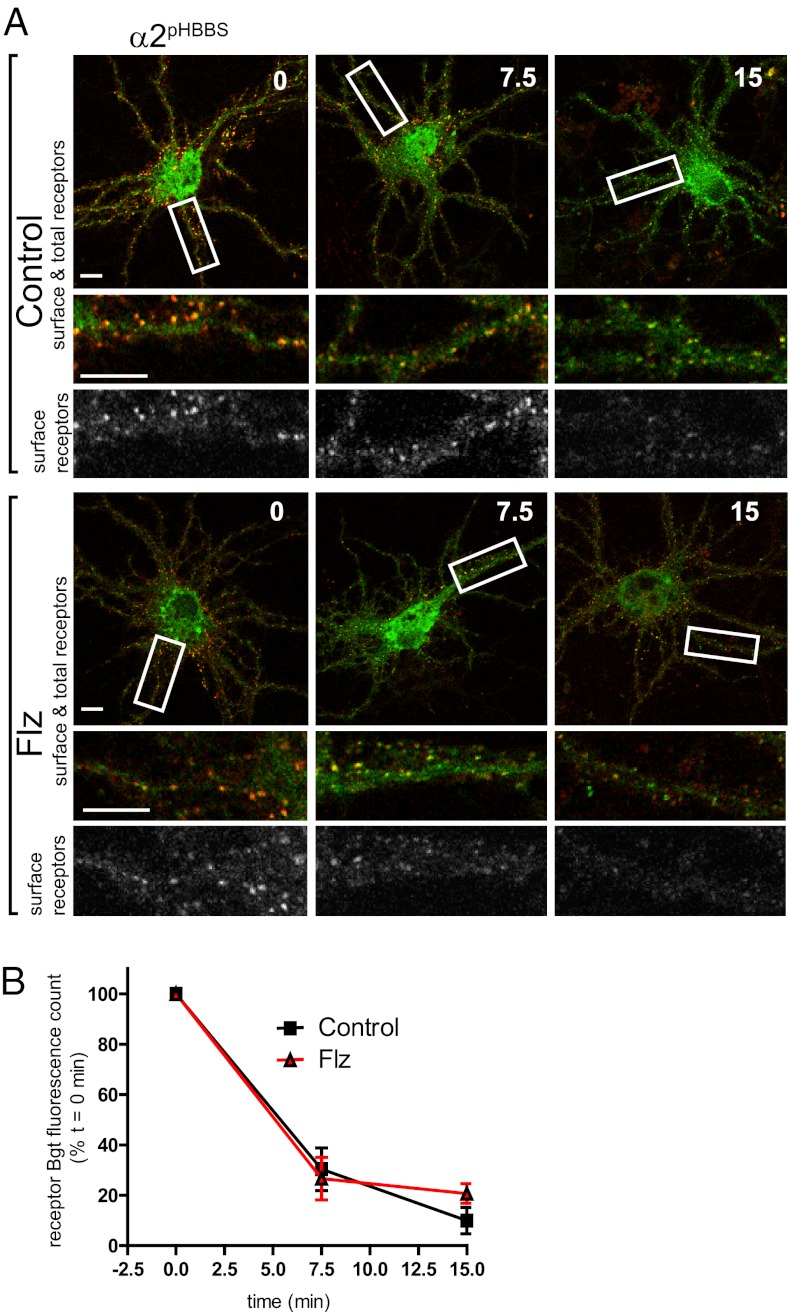
We next measured the rate of endocytosis in control versus Flz-treated neurons expressing α2pHBBS using an established fluorescent endocytosis assay (19). Surface GABAAR were live-labeled with Alexa594::Bgt for 5 min at 37 °C, followed by washing to remove unbound Alexa594::Bgt, and then incubated at 37 °C. At t = 0, 7.5, and 15 min, neurons were removed from the incubator and fixed. Total receptor number was visualized by permeabilization and staining with anti-GFP antibody. Quantification of confocal microscopy images showed loss of Alexa594::Bgt fluorescence over time with endocytosis of surface GABAAR (Fig. 4 A and B). The Alexa594::Bgt total fluorescence count for control and Flz-treated neurons at each time point was compared with the signal at 0 time, set to a value of 100% (Fig. 4B). Analysis of the endocytosis assay time course indicated that the rate of GABAAR endocytosis was also not changed by BZ treatment (remaining surface Bgt fluorescence: at t = 7.5 min, control, 30.3 ± 8.4%, and Flz, 26.7 ± 8.4%, and at t = 15 min, control, 10.0 ± 5.3%; Flz, 20.7 ± 3.9%). The lack of change in exocytosis and endocytosis rates, combined with the observed dramatic decrease in total GABAAR levels, suggested that BZ treatment promotes GABAAR degradation, likely through increased lysosomal degradation of endocytosed receptors or increased endoplasmic reticulum-associated protein degradation via the proteasome.
Fig. 4.
GABAAR endocytosis rate is not increased by BZ treatment. (A) Confocal imaging of GABAAR endocytosis in control and Flz-treated neurons. Surface GABAAR in α2pHBBS neurons were live-labeled with Alexa594::Bgt (red), and then unbound Alexa594::Bgt was removed by washing, followed by incubation at 37 °C. At t = 0, 7.5, and 15 min, samples were removed and fixed. Total receptor number was assayed by permeabilization and staining with anti-GFP antibody (green). Panels below are enlargements of boxed dendrites. (Scale bars, 10 μm.) (B) Graph represents the surface GABAAR Alexa594::Bgt fluorescence loss over time with endocytosis in control (Con) and Flz-treated neurons (normalized to the t = 0 Alexa594::Bgt surface signal for each treatment, respectively) (10–12 neurons per condition; n = 3 cultures; error bars represent ±SEM).
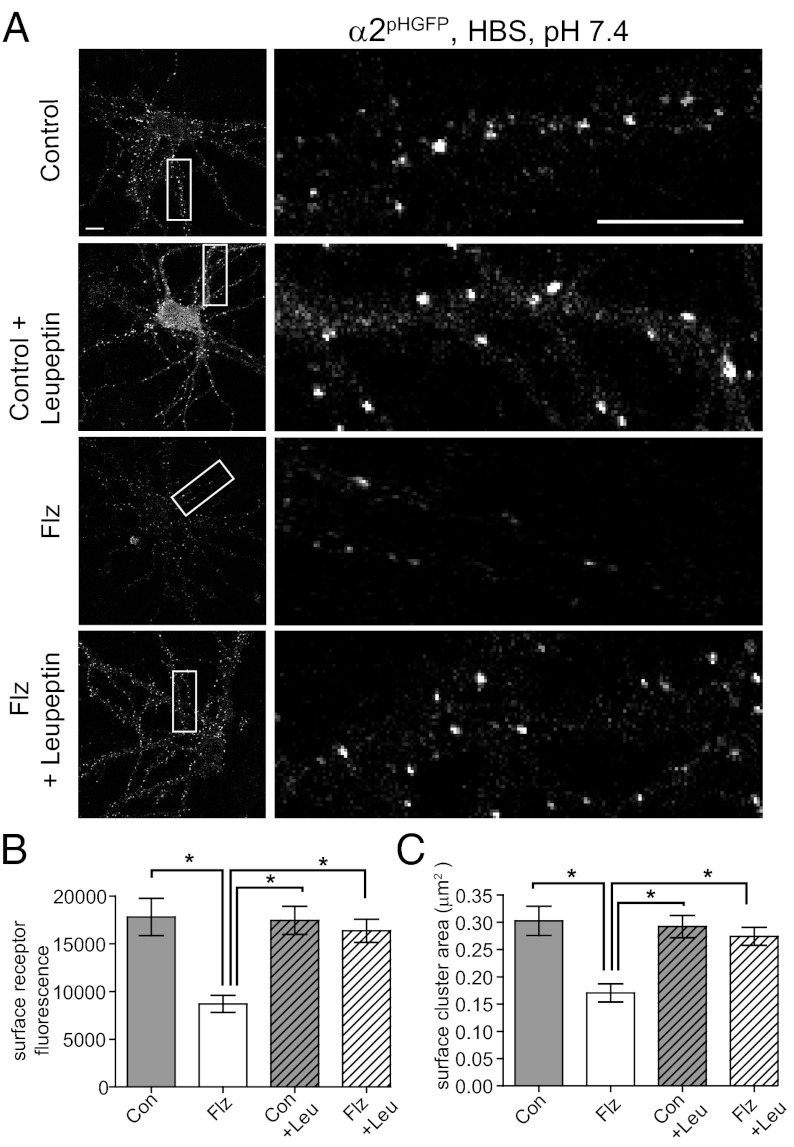
GABAAR Surface Levels Are Restored in BZ-Treated Neurons by Leupeptin Treatment.
Cell surface GABAARs undergo constitutive endocytosis and can be recycled or targeted for degradation in the lysosome (11). We next analyzed the role of lysosomal degradation in the decrease in surface and total GABAAR levels observed with BZ exposure. Neurons were treated with or without 250 nM Flz for 24 h followed by control or 2-h 200-μM treatment with the lysosomal protease inhibitor leupeptin. Live confocal microscopy experiments were used to measure surface α2pHGFP-subtype GABAAR levels (Fig. 5 A and B). Quantification of receptor surface fluorescence showed a dramatic increase in Flz-conditioned neurons treated with leupeptin (control, 17,808 ± 1,954; Flz, 8,697 ± 891; Flz plus Leu, 16,359 ± 1,219). Leupeptin caused no significant changes in surface GABAAR cluster fluorescence for control neurons (control plus Leu, 17,447 ± 1,473). A similar rescue of GABAAR surface cluster area was observed (in μm2): control, 0.30 ± 0.11; Flz, 0.17 ± 0.05; Con plus Leu, 0.29 ± 0.08; Flz plus Leu, 0.27 ± 0.08 (Fig. 5C). Analysis of α2-containing GABAAR cluster density showed a trend toward higher values with leupeptin treatment, although these did not reach statistical significance (expressed as % control: control, 101 ± 34; Flz, 60 ± 22; Con plus Leu, 138 ± 67; Flz plus Leu, 100 ± 41; n = 8–15 neurons from two to three independent cultures). Together, these data suggest that exposure of neurons to Flz results in enhanced lysosomal degradation of α2-subtype GABAARs after their removal from the plasma membrane, reducing the total available GABAAR pool, decreasing inhibitory synapse size and number along with the efficacy of synaptic inhibition.
Fig. 5.
GABAAR surface levels are restored in BZ-treated neurons by leupeptin treatment. (A) Live confocal imaging of neurons treated with or without 250 nM Flz for 24 h followed by control (Con) or 2-h 200 μM treatment with the lysosomal inhibitor leupeptin (Leu). (Right) Enlargements of boxed dendrites. (Scale bars, 10 μm.) (B and C) Mean ± SEM surface receptor α2pHGFP fluorescence and cluster area [*P < 0.01, one-way ANOVA (Bonferonni post test); 10–20 neurons per condition; n = 3 cultures].
Discussion
We have begun to dissect the molecular mechanism underlying the neuronal adaptations in GABAergic inhibition induced by BZ treatment. We used live confocal imaging to identify subtype-specific changes in GABAAR neuronal surface levels and electrophysiology to measure BZ-dependent changes in inhibition. A comparison of α1- and α2-subtype GABAARs showed α2-containing GABAAR surface levels were greatly diminished by 24-h BZ treatment. Further analysis showed that control neurons had nearly threefold higher total α2 levels compared with Flz-treated neurons. Concurrent with the decrease in GABAAR surface levels, Flz treatment resulted in a significant decrease in mIPSC amplitude. We demonstrated that the decrease in GABAAR surface levels occurs in a BZ binding site-dependent manner, as acute treatment with the BZ antagonist Ro 15-1788 restored the BZ-lowered GABAAR surface levels to control levels. Furthermore, acute Ro 15-1788 treatment returned mIPSC amplitude to control levels. Interestingly, BZ treatment did not alter the basal insertion and endocytosis rates of α2-containing GABAAR. Rather, BZ treatment promoted the degradation of α2-containing GABAARs, decreasing the total available α2 GABAAR pool. Finally, blockade of lysosomal degradation was able to return α2 GABAAR surface levels in BZ-treated neurons to control levels. We anticipate that the sustained loss in surface and total GABAARs during initial 24-h BZ treatment initiates a series of adaptations that develop into long-term changes in inhibition coincident with the development of BZ tolerance.
There is substantial evidence that a number of molecular processes are altered by short- and long-term BZ treatment, but comparatively little is understood regarding the initiation of these events. BZ treatment in vivo, in cultured neurons and in GABAAR-transfected cell lines, leads to a reduction in the allosteric coupling of BZ and GABA binding sites (7), suggesting that homeostatic down-regulation occurs to diminish BZ-dependent enhancement of GABAAR activity. Although earlier experiments suggested that uncoupling manifested over several weeks, BZ allosteric uncoupling has been shown to occur after a single dose of diazepam, with peak attenuation occurring between 4 and 12 h and returning to normal after 24 h (21). This rapid uncoupling is consistent with reports in mammalian (22) and chick neuronal culture: Flz treatment of cultured chick neurons showed uncoupling of the BZ and GABA sites with a t1/2 of ∼18 h (23). Combined with the rapid 24–48 h recovery from uncoupling both in animals chronically dosed or singly treated with BZ (21, 24), the current evidence suggests that BZs trigger an acute neuronal adaption modifying GABAergic inhibition. Studies of recombinant GABAAR expressed in Sf9 insect cells reported diazepam-induced uncoupling of both α1(α1β2γ2) and α2 (α2β3γ2), with greater uncoupling of α2-containing receptors (25). This observation in a nonneuronal system suggests that individual subunits are uncoupled by distinct mechanisms, likely to be a posttranslational modification such as phosphorylation of GABAARs or an interacting protein (26).
Importantly, many groups have shown that uncoupling occurs in vivo and in cultured systems without any contemporaneous changes in transcription or steady-state mRNA levels (21, 27, 28). These observations and reports of various, differing changes in mRNA levels (and often inverse changes in the same subunit) between different brain regions with chronic BZ treatment suggest that observed transcriptional changes may not directly correspond with functional changes in GABAAR protein levels, activity, and BZ tolerance (7). Although downstream signaling events after BZ administration are largely unknown, a recent microarray-based analysis following a single dose of diazepam identified a decrease in transcripts for CaMKIIα, BDNF, MKP-1, GIF, c-fos, and NGFI-A (29). Interestingly, the transcript levels had returned to baseline by 40 h for all of the genes except for CaMKIIα, further suggesting that key events may be posttranslational in nature.
Alterations in the regulated assembly, delivery, and removal of GABAARs have been proposed as mechanisms for BZ treatment-induced changes of GABAAR activity, although they have not been thoroughly explored. Mice chronically treated with lorazepam show an increase in flunitrazepam binding in clathrin-coated vesicles and a decrease in synaptic membranes, suggesting sequestration of GABAARs with BZ treatment (30). Studies of uncoupling in recombinant expression systems have also suggested BZ-induced internalization of GABAARs into an acidic compartment that could retain BZ binding capability (26). Here, we show a mechanism for rapid neuronal adaptation to BZ treatment that occurs via enhanced degradation of α2-containing GABAARs, rather than alterations in receptor insertion or removal. The quick recovery of synaptic inhibition and GABAAR surface levels with 1 h of BZ antagonist Ro 15-1788 treatment is consistent with BZ-induced changes affecting receptor stability rather than protein synthesis. We surmise that the BZ antagonist Ro 15-1788 acts to restore GABAAR surface levels by blockade of BZ potentiation of GABA, thereby pushing GABAergic inhibition to low levels that are subsequently compensated for by increased GABAAR trafficking and reduced degradation.
Acute BZ tolerance has been reported with a single BZ dose (31–33), suggesting a link between rapid allosteric uncoupling and tolerance development. The underlying neuronal adaptations that modulate GABAergic signaling may be a highly evolutionarily conserved mechanism because treatment with the GABAA agonist muscimol in the nematode Caenorhabditis elegans results in selective removal of GABAAR from synapses during adaptation to muscimol, a process that occurs on a 24-h timescale and can largely be blocked in a mutant with defective lysosomal degradation (34). By providing a mechanism linking BZ treatment of neurons to changes in surface and total levels of GABAAR subtypes and the efficacy of neuronal inhibition, our results provide a mechanism for the initial adaptations after BZ treatment. A greater understanding of these events and the subsequent long-lasting changes with chronic BZ treatment at an individual GABAAR subtype level will advance current treatment paradigms, potentially producing less tolerance and potential for abuse of one of the most widely prescribed medications in the Western world.
Materials and Methods
Cell Culture, Transfection, and Expression Constructs.
Hippocampal neurons were prepared from embryonic day 18 rats, and constructs (14) were nucleofected at plating (Amaxa). The α2pHBBS construct was generated by standard molecular biology techniques and sequenced. All studies were conducted in accordance with the institutional review boards of Tufts University School of Medicine and University of Pittsburgh School of Medicine.
Chemicals and Antibodies.
Flz and Ro 15-1788 (Sigma) treatments were performed with 250 nM Flz and 5 μM Ro 15-1788. Anti-GFP antibodies were used as previously reported (13).
Live Imaging.
Measurements were made on 14–17 d in vitro (DIV) hippocampal neurons at 37 °C in a closed heated chamber continuously perfused with extracellular Hepes-buffered saline, containing the following (in mM): 135 NaCl, 4.7 KCl, 10 Hepes, 11 glucose, 1.2 MgCl2, and 2.5 CaCl2 (adjusted to pH 7.4 with NaOH). Images were acquired using a Nikon A1 confocal microscope with a 60× oil objective (N.A., 1.4) at 2× zoom. Data were analyzed in Metamorph (Molecular Devices) as previously described (20), blind to experimental condition.
Insertion and Endocytosis Assays.
Hippocampal neurons (14-17 DIV) expressing α2pHBBS were assayed for GABAAR insertion (35) and endocytosis (20) with Bgt (Invitrogen) as previously and described in Results. All incubations were performed in the presence of 150 μM tubocurarine (Sigma) to block Bgt binding to endogenous acetylcholine receptors (36). See SI Materials and Methods for additional information.
Electrophysiology.
Neurons were plated on 12-mm glass coverslips (German glass; VWR) coated with poly-l-lysine (0.5 mg/mL; Sigma) and cultured for 2–3 wk before the recordings. To measure mIPSCs, coverslips were placed in a recording chamber mounted on the stage of an inverted microscope and continually perfused with the following (in mM): 140 NaCl, 4.7 KCl, 10 Hepes, 11 glucose, 2 MgCl2, and 2.5 CaCl2 (adjusted to pH 7.4 with NaOH, 295–315 mOsm). For mIPSC recording, the extracellular solution was supplemented with 200 nM TTX, 10 μM CNQX, and 20 μM d-AP5 (Sigma). Borosilicate pipettes (3-6 MΩ) were filled with the following (in mM): 150 CsCl, 10 Hepes, 1.1 EGTA, 2 MgCl2, 0.1 CaCl2, 2 Mg2+-ATP (adjusted to pH 7.2 with CsOH). Recordings were started 5–10 min after a stable whole-cell access was obtained. See SI Materials and Methods for additional information.
Supplementary Material
Acknowledgments
The work was in part supported by National Institute of Neurological Disorders and Stroke Grants 046478, 048045, 051195, 056359, and NS054900. T.C.J. is in part supported by National Institute of Mental Health Grant R03 MH90253-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204994109/-/DCSupplemental.
References
- 1.Poncer JC, Dürr R, Gähwiler BH, Thompson SM. Modulation of synaptic GABAA receptor function by benzodiazepines in area CA3 of rat hippocampal slice cultures. Neuropharmacology. 1996;35(9–10):1169–1179. doi: 10.1016/s0028-3908(96)00055-x. [DOI] [PubMed] [Google Scholar]
- 2.Study RE, Barker JL. Diazepam and (−)-pentobarbital: Fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci USA. 1981;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otis TS, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49(1):13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- 4.Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2(8):795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 5.Uusi-Oukari M, Korpi ER. Regulation of GABAA receptor subunit expression by pharmacological agents. Pharmacol Rev. 2010;62(1):97–135. doi: 10.1124/pr.109.002063. [DOI] [PubMed] [Google Scholar]
- 6.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6(3):215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 7.Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8(1):5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- 8.Poisbeau P, Williams SR, Mody I. Silent GABAA synapses during flurazepam withdrawal are region-specific in the hippocampal formation. J Neurosci. 1997;17(10):3467–3475. doi: 10.1523/JNEUROSCI.17-10-03467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng XJ, Tietz EI. Benzodiazepine tolerance at GABAergic synapses on hippocampal CA1 pyramidal cells. Synapse. 1999;31(4):263–277. doi: 10.1002/(SICI)1098-2396(19990315)31:4<263::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Gallager DW, Lakoski JM, Gonsalves SF, Rauch SL. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature. 1984;308(5954):74–77. doi: 10.1038/308074a0. [DOI] [PubMed] [Google Scholar]
- 11.Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9(5):331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kittler JT, et al. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci USA. 2004;101(34):12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saliba RS, Michels G, Jacob TC, Pangalos MN, Moss SJ. Activity-dependent ubiquitination of GABAA receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27(48):13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tretter V, et al. The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28(6):1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee J, et al. The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J Neurosci. 2011;31(41):14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozrzymas JW, et al. GABA transient sets the susceptibility of mIPSCs to modulation by benzodiazepine receptor agonists in rat hippocampal neurons. J Physiol. 2007;585(Pt 1):29–46. doi: 10.1113/jphysiol.2007.143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumpel E, Behrends JC. Postsynaptic receptor occupancy during evoked transmission at striatal GABAergic synapses in vitro. J Neurophysiol. 2000;84(2):771–779. doi: 10.1152/jn.2000.84.2.771. [DOI] [PubMed] [Google Scholar]
- 18.Barnes EM., Jr Use-dependent regulation of GABAA receptors. Int Rev Neurobiol. 1996;39:53–76. doi: 10.1016/s0074-7742(08)60663-7. [DOI] [PubMed] [Google Scholar]
- 19.Bogdanov Y, et al. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25(18):4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob TC, et al. GABAA receptor membrane trafficking regulates spine maturity. Proc Natl Acad Sci USA. 2009;106(30):12500–12505. doi: 10.1073/pnas.0903943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt RA, Bateson AN, Martin IL. Decreased GABA enhancement of benzodiazepine binding after a single dose of diazepam. J Neurochem. 1999;72(5):2219–2222. doi: 10.1046/j.1471-4159.1999.0722219.x. [DOI] [PubMed] [Google Scholar]
- 22.Hu XJ, Ticku MK. Chronic benzodiazepine agonist treatment produces functional uncoupling of the gamma-aminobutyric acid-benzodiazepine receptor ionophore complex in cortical neurons. Mol Pharmacol. 1994;45(4):618–625. [PubMed] [Google Scholar]
- 23.Roca DJ, Rozenberg I, Farrant M, Farb DH. Chronic agonist exposure induces down-regulation and allosteric uncoupling of the gamma-aminobutyric acid/benzodiazepine receptor complex. Mol Pharmacol. 1990;37(1):37–43. [PubMed] [Google Scholar]
- 24.Tietz EI, Chiu TH, Rosenberg HC. Regional GABA/benzodiazepine receptor/chloride channel coupling after acute and chronic benzodiazepine treatment. Eur J Pharmacol. 1989;167(1):57–65. doi: 10.1016/0014-2999(89)90747-4. [DOI] [PubMed] [Google Scholar]
- 25.Primus RJ, et al. Allosteric uncoupling after chronic benzodiazepine exposure of recombinant gamma-aminobutyric acid(A) receptors expressed in Sf9 cells: Ligand efficacy and subtype selectivity. J Pharmacol Exp Ther. 1996;276(3):882–890. [PubMed] [Google Scholar]
- 26.Ali NJ, Olsen RW. Chronic benzodiazepine treatment of cells expressing recombinant GABA(A) receptors uncouples allosteric binding: Studies on possible mechanisms. J Neurochem. 2001;79(5):1100–1108. doi: 10.1046/j.1471-4159.2001.00664.x. [DOI] [PubMed] [Google Scholar]
- 27.Wong G, Lyon T, Skolnick P. Chronic exposure to benzodiazepine receptor ligands uncouples the gamma-aminobutyric acid type A receptor in WSS-1 cells. Mol Pharmacol. 1994;46(6):1056–1062. [PubMed] [Google Scholar]
- 28.Kang I, Miller LG. Decreased GABAA receptor subunit mRNA concentrations following chronic lorazepam administration. Br J Pharmacol. 1991;103(2):1285–1287. doi: 10.1111/j.1476-5381.1991.tb09781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huopaniemi L, Keist R, Randolph A, Certa U, Rudolph U. Diazepam-induced adaptive plasticity revealed by alpha1 GABAA receptor-specific expression profiling. J Neurochem. 2004;88(5):1059–1067. doi: 10.1046/j.1471-4159.2003.02216.x. [DOI] [PubMed] [Google Scholar]
- 30.Tehrani MH, Barnes EM., Jr Sequestration of gamma-aminobutyric acidA receptors on clathrin-coated vesicles during chronic benzodiazepine administration in vivo. J Pharmacol Exp Ther. 1997;283(1):384–390. [PubMed] [Google Scholar]
- 31.File SE, Wilks LJ, Mabbutt PS. Withdrawal, tolerance and sensitization after a single dose of lorazepam. Pharmacol Biochem Behav. 1988;31(4):937–940. doi: 10.1016/0091-3057(88)90408-x. [DOI] [PubMed] [Google Scholar]
- 32.Lister RG, Nutt DJ. Mice and rats are sensitized to the proconvulsant action of a benzodiazepine-receptor inverse agonist (FG 7142) following a single dose of lorazepam. Brain Res. 1986;379(2):364–366. doi: 10.1016/0006-8993(86)90791-2. [DOI] [PubMed] [Google Scholar]
- 33.Wong PT, Yoong YL, Gwee MC. Acute tolerance to diazepam induced by benzodiazepines. Clin Exp Pharmacol Physiol. 1986;13(1):1–8. doi: 10.1111/j.1440-1681.1986.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 34.Davis KM, et al. Regulated lysosomal trafficking as a mechanism for regulating GABAA receptor abundance at synapses in Caenorhabditis elegans. Mol Cell Neurosci. 2010;44(4):307–317. doi: 10.1016/j.mcn.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Saliba RS, Pangalos M, Moss SJ. The ubiquitin-like protein Plic-1 enhances the membrane insertion of GABAA receptors by increasing their stability within the endoplasmic reticulum. J Biol Chem. 2008;283(27):18538–18544. doi: 10.1074/jbc.M802077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekine-Aizawa Y, Huganir RL. Imaging of receptor trafficking by using alpha-bungarotoxin-binding-site-tagged receptors. Proc Natl Acad Sci USA. 2004;101(49):17114–17119. doi: 10.1073/pnas.0407563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.