Abstract
CD8+ T cells are central to the eradication of intracellular pathogens, but they can also act to limit inflammation and immunopathology. During primary respiratory viral infection CD8+ effector T cells release the immunosuppressive cytokine IL-10, which is essential for host survival. Here we report that CD8+ T-cell–derived IL-10 is absent in a recall response. We show in mice that the lack of IL-10 is due to a persistent loss of IL-27 responsiveness in CD8+ memory T cells, caused by down-regulation of the common cytokine receptor, glycoprotein 130. CD8+ memory T cells secreted less IL-10 when activated in the presence of IL-27 than did naïve controls, and retroviral expression of glycoprotein 130 restored IL-10 and reduced IFN-γ production upon restimulation. We demonstrate that human CD8+ memory cells are also characterized by impaired IL-27 responsiveness. Our data suggest that CD8+ T-cell activation involves a persistent loss of specific cytokine receptors that determines the functional potential of these cells during rechallenge infection.
Keywords: immune regulation, T-cell differentiation, IL-10 reporter mice, influenza, Sendai virus
Immunological memory is the ability of the immune system to recognize a pathogen that it has encountered previously and to mount a faster and more effective response upon subsequent exposure (1). The strength of the recall response reflects both quantitative and qualitative changes in lymphocyte populations. Following infection with respiratory influenza or parainfluenza viruses, high frequencies of virus-specific CD8+ T cells persist many months after virus is cleared (2, 3). These CD8+ memory T cells have a lower activation threshold, more rapidly acquire effector functions, and can preferentially localize to infected tissues, compared with naïve CD8+ cells (4–6). Their presence means that, during a rechallenge infection, viral clearance is accelerated and mice are able to survive otherwise lethal doses of virus (7, 8).
In a primary infection the strength of the effector response is tempered by a need to limit collateral damage. Interleukin (IL)-10 is an important mediator of this balance (9). IL-10–deficient mice infected with Toxoplasma gondii control pathogen replication more efficiently than wild-type controls but succumb to fatal immunopathology (10, 11). The primary immune response to influenza virus also features IL-10, expressed by highly activated CD8+ effector T cells, and essential to prevent lethal pulmonary inflammation (12). In Leishmania major infection, IL-10 dampens the immune response, prevents sterile clearance of the parasite, and leads to long-term symbiosis (13, 14). IL-10 is also implicated in the persistence of chronic lymphocytic choriomeningitis virus (15, 16).
The importance of IL-10 during a recall response remains controversial. Because activated effector T cells are a prominent source of IL-10 during primary infection (12, 14, 17), the exaggerated T-cell expansion of a secondary response might be predicted to amplify IL-10 production. It could equally be argued, however, that the enhanced ability of a secondary immune response to clear the invading pathogen is evidence of reduced immunosuppression and less IL-10. To distinguish these possibilities, we analyzed IL-10 expression during primary and secondary respiratory viral infections. We observed a marked deficiency in IL-10+ CD8+ effector T cells during the recall response. The induction of IL-10 during primary viral infection was dependent on direct IL-27 signaling, and we demonstrate that its absence from the memory response is due to a persistent loss of IL-27 responsiveness caused by down-regulation of the common cytokine receptor chain, glycoprotein 130 (gp130). Together our data reveal that CD8+ T-cell differentiation involves distinct changes in cytokine responsiveness that dictate the functional characteristics of these cells during a recall response.
Results
CD8+ Memory Response to Rechallenge Is Deficient in IL-10.
To investigate changes in cytokine expression and cytokine responsiveness during T-cell differentiation in vivo, we infected WT and GFP/IL-10 reporter mice [Vert-X mice (12, 18)] with the respiratory influenza or Sendai viruses and followed the development of endogenous, antigen-specific effector and memory populations. Primary infection with either virus elicited a robust expansion of antigen-specific CD8+ effector cells in the airways (Fig. 1 A and C) and lungs (Fig.1 B and C). A subset of these effector cells expressed the immunoregulatory cytokine IL-10 (Fig. 1 A–C), consistent with data from Braciale and coworkers (12). IL-10 expression was greatest 10 d after infection with either influenza or Sendai virus, corresponding with peak expansion of antigen-specific CD8+ effector cells (Fig. 1D). At this time the majority of antigen-specific CD8+ cells, including the IL-10+ subset, also expressed the Th1-associated transcription factor T-bet (Fig. S1). IL-10 expression returned to baseline by 15 d postinfection and remained low at 45 d (Fig. 1D).
Fig. 1.
CD8+ effector T cells express IL-10 in the lung and airways during respiratory viral infection. (A) Vert-X IL-10 reporter mice were infected intranasally with influenza ×31 or Sendai virus and, 10 d later, cells in the bronchoalveolar lavage (BAL) were analyzed by flow cytometry. Infected nonreporter mice were analyzed for comparison. Data shown are gated on either total (left column) or CD8+ (right column) lymphocytes. Numbers indicate the percentage of cells within each quadrant. (B) Vert-X mice were infected as in A and, 10 d later, lymphocytes from the lung parenchyma were analyzed by flow cytometry. Sham-infected Vert-X mice were analyzed for comparison. (C) Vert-X mice were infected with Sendai virus as in A and on day 10 the indicated tissues were analyzed by flow cytometry. (D) Vert-X mice were infected with Sendai virus as in A, and on days 0, 10, 15, and 45 postinfection the indicated tissues were analyzed by flow cytometry. In all panels, graphs depict the mean ± SD of individual mice and all data are representative of at least two independent experiments using three to five mice per group.
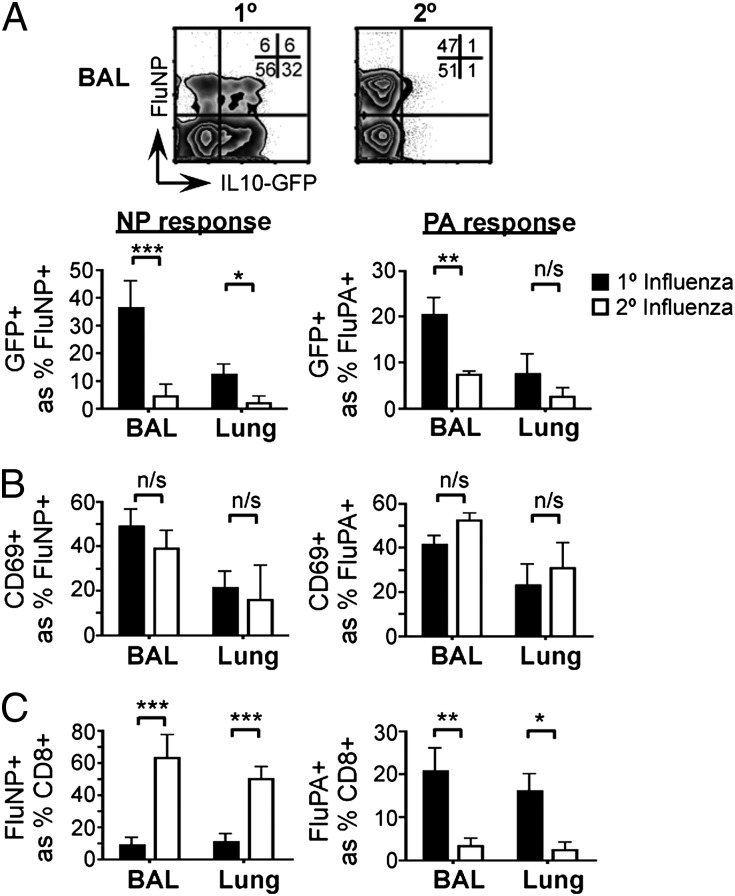
To determine whether secondary CD8+ effector T cells also express IL-10, we infected Vert-X mice with PR8 influenza virus (H1N1) and, 30–35 d later, challenged with the heterosubtypic ×31 influenza virus strain (H3N2). Control animals were initially sham-infected and subsequently received the ×31 challenge dose as a primary infection. IL-10 expression was clearly induced in primary antigen-specific CD8+ T cells in response to ×31 virus but was strikingly reduced when the same ×31 challenge was delivered as a second infection (Fig. 2A and Fig. S2). The reduced IL-10 expression during the recall response was evident on days 5, 6, and 8 after the challenge infection (Fig. S3) and was common to CD8+ cells specific for both the NP and PA epitopes of the influenza virus (Fig. 2A), suggesting that the IL-10 deficiency was independent of antigen specificity (19). Both primary and secondary CD8+ cells expressed CD69 at a similar frequency, which indicated that the loss of IL-10 in the recall response occurred despite equivalent cellular activation (Fig. 2B). The lack of IL-10 during rechallenge also occurred irrespective of the different immunodominance of primary and secondary responses (Fig. 2C) (19).
Fig. 2.
CD8+ T-cell–derived IL-10 is absent in a rechallenge infection. Vert-X mice were sham-infected (1°) or infected with PR8 influenza virus (2°) and, 30–35 d later, challenged with ×31 influenza virus. Eight days after challenge, the indicated tissues were harvested and analyzed by flow cytometry. (A) IL-10 expression; (B) CD69 expression; (C) frequency of influenza-specific populations. All data are gated on CD8+ lymphocytes. Graphs indicate the mean ± SD of individual mice and data are representative of four independent experiments with four or five mice per group.
IL-10 Expression in CD8+ T Cells Requires Direct IL-27 Signaling.
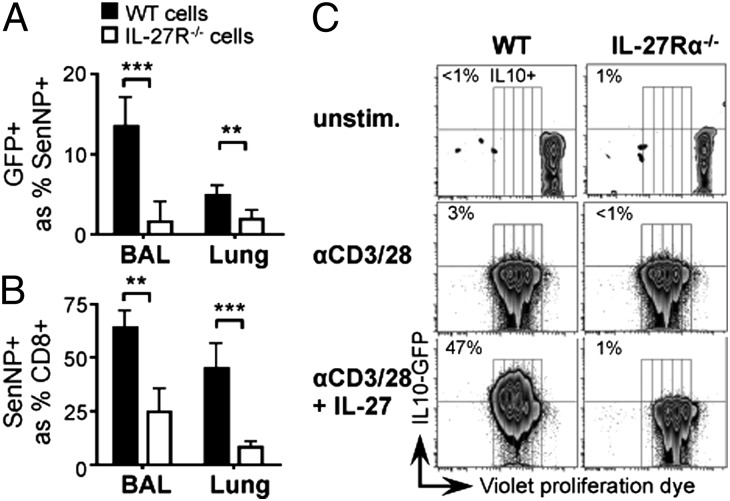
To investigate the mechanism responsible for decreased IL-10 expression in a secondary viral infection, we first sought to establish the factors required for IL-10 induction during the primary T-cell response. Several recent publications have reported a role for IL-27 in eliciting IL-10 production by CD8+ lymphocytes (20–22), and we tested whether this was also the case during respiratory viral infection. We generated mixed bone marrow chimeras reconstituted with equal parts of WT and IL-27Rα−/− (23) congenic bone marrow. This approach allowed us to compare WT and IL-27 receptor-deficient cells within the same animal and thus exposed to the same environment. When these mice were infected with Sendai virus, only the WT and not the IL-27R–deficient antigen-specific CD8+ T cells induced IL-10 (Fig. 3A). The expansion of Sendai-specific CD8+ cells upon infection was also compromised in the absence of the IL-27R (Fig. 3B), consistent with previous data (24) and amplifying the overall deficit in IL-10 expression. In vitro, the ability of IL-27 to promote IL-10 expression was independent of its effect on cell proliferation; IL-27Rα−/− cells failed to express IL-10 even at division numbers that were IL-10+ in WT cells (Fig. 3C). Collectively these data show that, during a primary respiratory viral infection, direct IL-27 signaling is required for the induction of IL-10 expression in antigen-specific CD8+ cells.
Fig. 3.
Direct IL-27 signaling is required for IL-10 expression. (A and B) Mixed bone marrow chimeras comprising WT and IL-27Rα−/− Vert-X cells were infected with Sendai virus and, 10 d later, cells from the bronchoalveolar lavage (BAL) and lung were analyzed by flow cytometry. (A) IL-10 expression; (B) frequency of Sendai-specific cells. (C) Naïve CD8+ T cells from WT and IL-27Rα−/− Vert-X mice were labeled with a violet proliferation dye, VPD450, and stimulated in vitro with anti-CD3 and anti-CD28 in the presence or absence of recombinant IL-27. Cells were analyzed by flow cytometry 3 d later. In all panels, data shown are gated on CD8+ lymphocytes and representative of three independent experiments. Graphs depict mean ± SD of five mice per group.
CD8+ Memory T Cells Show a Persistent Loss of IL-27 Responsiveness.
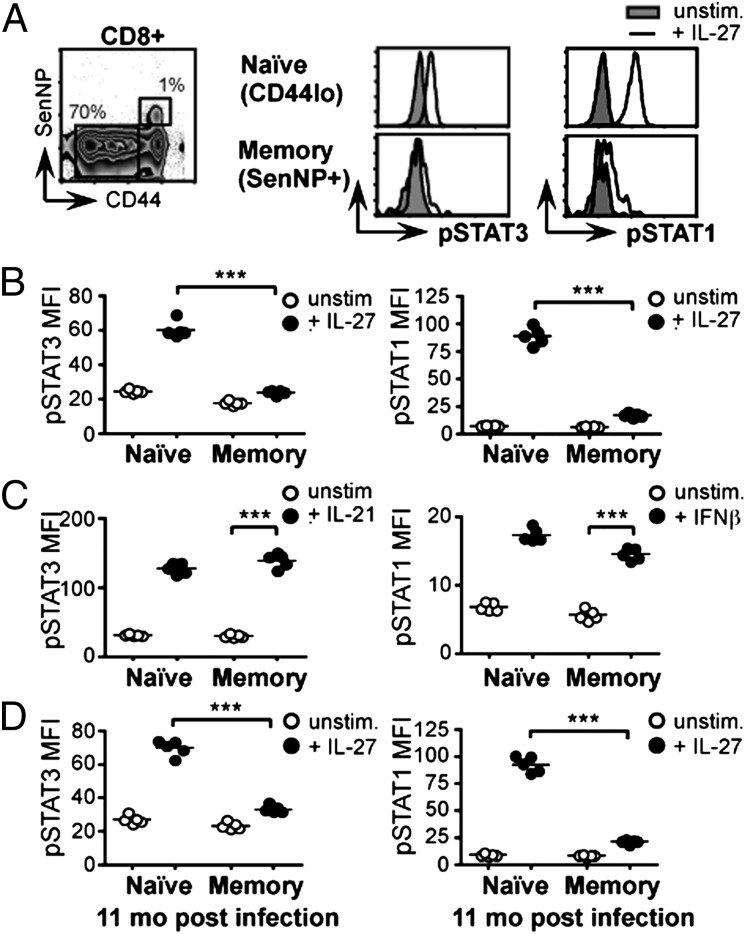
The dependence of IL-10 expression during a primary respiratory viral infection on direct IL-27 signals suggested that the absence of IL-10 in the CD8+ memory recall response could reflect a defect in the ability of CD8+ memory T cells to respond to IL-27. To test this hypothesis, we analyzed IL-27 responsiveness in resting CD8+ naïve and memory T cells. In lymphocytes, IL-27 signals through the phosphorylation of intracellular signal transducer and activator of transcription (STAT) proteins 1 and 3, of which STAT3 has been particularly associated with the induction of IL-10 (22, 25, 26). We harvested splenocytes from WT C57BL/6 mice that had been infected with either Sendai or influenza virus 30–50 d earlier, stimulated the cells with IL-27, and assessed STAT3 and STAT1 phosphorylation using flow cytometry. This approach allowed us to compare naïve and memory CD8+ T cells from the same animal, exposed to identical conditions and distinguished only by the gating strategy used in analysis (Fig. 4A). Naïve CD44lo CD8+ lymphocytes responded to IL-27 with marked phosphorylation of STAT3 and STAT1. In contrast, memory CD44hi CD8+ SenNP+ cells were strikingly impaired in their IL-27 responsiveness (Fig. 4 A and B). The reduction in STAT3 and STAT1 phosphorylation in CD8+ memory T cells reflected a specific loss of IL-27 responsiveness, because IL-21 and IFNβ elicited robust phosphorylation of the corresponding STAT proteins in both naïve and memory cells (Fig. 4C). The compromised IL-27 response in antigen-specific CD8+ memory T cells was long-lasting, evident in cells harvested from animals 11 mo after Sendai infection (Fig. 4D). Together these data reveal a profound and persistent loss of IL-27 responsiveness in resting CD8+ memory T cells.
Fig. 4.
CD8+ memory T cells lose IL-27 responsiveness. (A and B) C57BL/6 mice were infected with Sendai virus and, 30–50 d later, splenocytes were harvested, cultured with or without IL-27, and analyzed by flow cytometry. (A) Representative histograms; (B) replicate data. (C) Splenocytes from C57BL/6 mice infected with Sendai virus 30–50 d earlier were pulsed with or without IL-21 or IFNβ and analyzed by flow cytometry. (D) C57BL/6 mice were infected with Sendai virus and, 11 mo later, splenocytes were harvested, cultured with or without IL-27, and analyzed by flow cytometry. In panels B–D, data are gated on CD8+ naïve (CD44lo) or Sendai-specific memory (CD44hi SenNP+) lymphocytes from the same spleen, as shown. Graphs depict the mean fluorescence intensity (MFI) of pSTAT staining, and each data point represents an individual mouse. All panels are representative of four or five mice per group in two to five independent experiments.
Human CD8+ Memory Cells Are Unresponsive to IL-27.
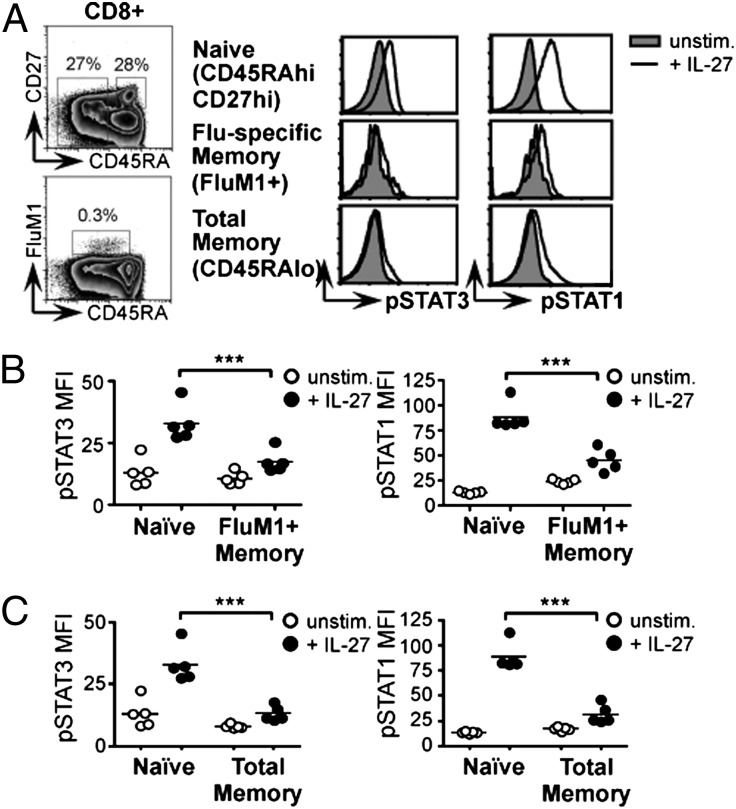
To test whether the loss of IL-27 responsiveness we observed in murine memory CD8+ T cells is also a characteristic of human cells, we obtained peripheral blood mononuclear cells (PBMC) from five healthy HLA-A2+ human donors with reported ELIspot reactivity to the influenza A M158–66 epitope (FluM1). Stimulation of these PBMC samples with recombinant human IL-27 triggered STAT3 and STAT1 phosphorylation in naïve CD45RAhi CD27hi CD8+ lymphocytes, but to a much lesser extent in influenza-specific CD45RAlo FluM1+ CD8+ memory T cells (Fig. 5 A and B). By gating on unfractionated CD45RAlo memory CD8+ T lymphocytes, we demonstrated that the loss of IL-27 responsiveness is not restricted to an influenza-specific subset but is common to the bulk memory population (Fig. 5 A and C). These data show that, like murine cells, human CD8+ memory lymphocytes display a striking reduction in their ability to transmit IL-27 signals.
Fig. 5.
Human CD8+ memory T cells show reduced responsiveness to IL-27. Human PBMCs were obtained from healthy donors with reactivity to the immunodominant influenza A M158–66 epitope (FluM1). Cells were cultured with or without recombinant human IL-27 and analyzed by flow cytometry. Naïve (CD45RAhi CD27hi), influenza virus-specific memory (FluM1+ CD45RAlo), and total memory (CD45RAlo) CD8+ lymphocyte populations were defined using the gating strategy shown. (A) Representative histograms. Graphs depict the mean fluorescence intensity (MFI) of pSTAT staining in (B) naïve vs. influenza-specific memory and (C) naïve vs. total memory CD8+ populations. Each data point represents an individual human donor.
IL-27 Nonresponsiveness Reflects gp130 Down-Regulation.
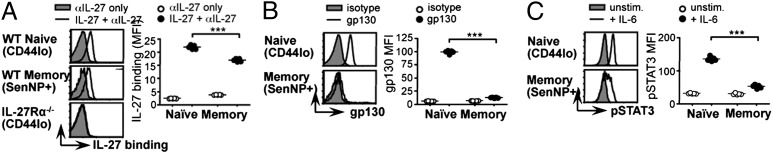
One explanation for the persistent loss of IL-27 responsiveness in CD8+ memory T cells was a down-regulation of cytokine receptor expression. The functional IL-27 receptor is a heterodimer composed of an IL-27–specific cytokine binding chain (IL-27Rα, also known as WSX-1 and TCCR) and a shared signaling component, gp130 (27). Antibodies that recognize IL-27Rα were not commercially available, so we determined its expression by pulsing ice-cold cells with recombinant IL-27 and measuring bound cytokine with a polyclonal anti–IL-27 antibody. The specificity of staining was confirmed using IL-27Rα−/− cells (Fig. 6A). Both naïve (CD44lo) and Sendai-specific memory (CD44hi SenNP+) CD8+ lymphocytes from mice infected with Sendai virus 30–45 d earlier displayed substantial levels of surface IL-27Rα, and the difference between the two populations was modest (Fig. 6A). We concluded that differential expression of IL-27Rα was unlikely to explain the loss of IL-27 responsiveness in CD8+ memory T cells.
Fig. 6.
The loss of IL-27 responsiveness in CD8+ memory T cells reflects gp130 down-regulation. (A) C57BL/6 mice were infected with Sendai virus and, 30–45 d later, splenocytes were harvested and analyzed by flow cytometry. Expression of the IL-27Rα chain was assessed by pulsing cells with recombinant IL-27 on ice and measuring bound cytokine with an anti-IL-27 Ab. Control cells were held on ice without IL-27 and otherwise stained identically. (B) Splenocytes from C57BL/6 mice infected with Sendai virus 30–45 d earlier were stained with a gp130 mAb or an isotype-matched control (rat IgG2a) and analyzed by flow cytometry. (C) Splenocytes from C57BL/6 mice infected with Sendai virus 30–45 d earlier were harvested, cultured with or without IL-6, and analyzed by flow cytometry to assess STAT3 phosphorylation. In all panels, data are gated on CD8+ naïve (CD44lo) or Sendai-specific memory (CD44hi SenNP+) lymphocytes from the same spleen. Graphs depict mean fluorescence intensity (MFI) and each data point represents an individual mouse. All panels are representative of five mice per group in three or four independent experiments.
We next examined the other component of the heterodimeric receptor, gp130. Surface gp130 was readily detectable on naïve CD8+ CD44lo splenocytes from Sendai-immune C57BL/6 mice, but not on Sendai-specific memory cells (CD44hi SenNP+) from the same mice (Fig. 6B). Because gp130 is also a critical component of the IL-6 receptor complex, the down-regulation of gp130 on CD8+ memory cells predicted that this population would also be unable to respond to IL-6. Indeed, when we incubated splenocytes from Sendai-immune mice with recombinant IL-6, STAT3 phosphorylation was markedly reduced in Sendai-specific CD8+ memory cells compared with naïve CD44lo CD8+ cells (Fig. 6C). Corresponding expression of the IL-6 receptor α chain was also reduced on virus-specific memory and effector CD8+ T cells, compared with their naïve counterparts (Fig. S4). Together these data demonstrate that the cytokine response pattern of CD8+ memory T cells reflects marked changes in their cytokine receptor repertoire, imposed during antigen-driven differentiation.
To investigate the kinetics of gp130 down-regulation, we first examined gp130 expression on CD8+ effector cells in a primary immune response. Ten days after influenza virus infection, virus-specific CD8+ cells in both the lung and the spleen showed pronounced gp130 down-regulation (Fig. S5A). Naïve cells in the same animal retained strong gp130 expression (Fig. S5A). We then examined CD8+ T cells early after activation, by stimulating OT-I T-cell receptor (TCR) transgenic cells in vitro with peptide-pulsed antigen-presenting cells (APC). Antigen-activated transgenic cells lost gp130 expression (Fig. S5B), consistent with published data (28). The reduction in gp130 expression on stimulated CD8+ T cells occurred progressively over a 3-d period, more slowly than the rapid cellular activation revealed by CD44 up-regulation (Fig. S5B). The gradual down-regulation of gp130 appears to provide a time window immediately after antigen recognition in which CD8+ T cells remain competent to receive IL-27 instruction.
Loss of IL-27 Signaling in CD8+ Memory T Cells Compromises IL-10 Production.
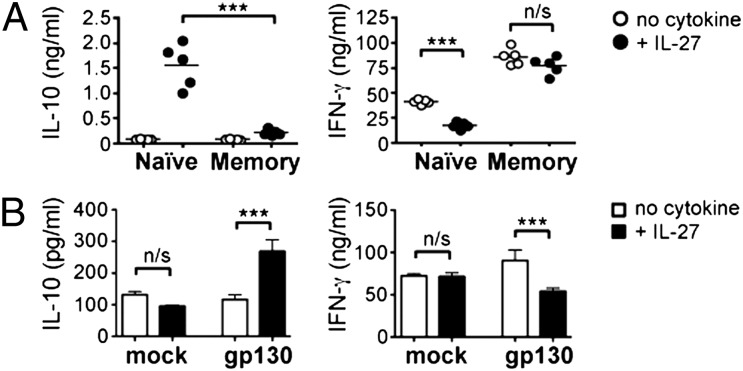
To verify the physiological significance of gp130 down-regulation and loss of IL-27 responsiveness in CD8+ memory T cells, we sorted naïve (CD44lo CD62Lhi) and memory (CD44hi CD62Llo) CD8+ lymphocytes from the spleens of individual WT C57BL/6 mice infected with Sendai virus 30–45 d earlier and stimulated each population in the presence or absence of IL-27. The loss of IL-27 responsiveness in memory cells resulted in a sharp reduction in IL-10 secretion, compared with naïve cells (Fig. 7A). The IL-10 released by naïve cells was accompanied by a decrease in IFN-γ production, whereas the lack of IL-10 expression by memory cells was consistent with equivalent levels of IFN-γ production in the presence and absence of IL-27 (Fig. 7A). Stimulation in the presence of IL-6 rather than IL-27 elicited little IL-10 even from naïve cells (Fig. S6), consistent with published data (22), and memory cells were equally muted in response to either cytokine (Fig. S6).
Fig. 7.
Altered IL-27 responsiveness in CD8+ memory T cells results in reduced IL-10 production. (A) Naïve (CD44lo CD62Lhi) and memory (CD44hi CD62Llo) CD8+ lymphocyte populations were purified from the spleens of C57BL/6 mice infected with Sendai virus 30–45 d earlier and cultured with anti-CD3 and anti-CD28 in the presence or absence of recombinant IL-27. Culture supernatants were harvested after 72 h and analyzed by cytometric bead array. Each data point represents an individual mouse and both panels are representative of five mice per group in two independent experiments. (B) TCR transgenic CD8+ cells were activated and transduced in vitro with either a gp130-expressing (gp130) or an empty retroviral vector (mock) before being rested and restimulated in the presence or absence of recombinant IL-27. Supernatants were harvested after 72 h and analyzed by cytometric bead array. Bars represent mean of triplicate cultures ± SD, and both panels are representative of three independent experiments.
To test whether the loss of IL-10 production by CD8+ memory T cells was a direct consequence of the down-regulation of gp130, we wanted to compare memory cells that either did or did not express gp130. We activated, transduced, and rested TCR transgenic CD8+ cells in vitro, before restimulating them in the presence or absence of recombinant IL-27. Preactivated transgenic cells transduced with an empty vector responded to restimulation in a manner similar to that of pathogen-induced CD8+ memory cells: The presence of IL-27 made little difference to either IL-10 or IFN-γ production (Fig. 7B). In contrast, retroviral expression of gp130 resulted in the induction of IL-10 and a corresponding reduction in IFN-γ release (Fig. 7B). These data demonstrate that gp130 expression has a direct impact on the cytokine balance shown by primary and secondary CD8+ effector T cells. Collectively our findings reveal that CD8+ T-cell differentiation involves distinct changes in cytokine responsiveness, enforced by the regulation of specific cytokine receptors, that determine the functional repertoire of these cells during a recall response.
Discussion
The differentiation of T cells into long-lasting memory populations is a defining feature of adaptive immunity, and understanding this process is fundamental to the development of effective vaccines. Our study reveals that, in comparison with their naïve counterparts, CD8+ memory T cells show a profound and sustained reduction in their ability to respond to IL-27. This loss of IL-27 signaling is caused by down-regulation of surface gp130 and results in a reduced capacity of CD8+ memory T cells to express the immunosuppressive cytokine IL-10. We propose that CD8+ memory T cells are permanently altered in their ability to receive cytokine instruction, through the differential expression of specific cytokine receptors, and that this alteration in cytokine responsiveness defines their functional potential upon rechallenge.
Our data demonstrate a sustained loss of cytokine responsiveness in memory CD8+ T cells. Cytokine receptor expression has previously been used to identify the precursors of memory T cells: Following an acute systemic viral infection, the transition from CD8+ effector cells into a long-lived memory cell has been associated with differential expression of the IL-7 receptor (29), and a similar argument has recently been made for IL-2Rα (CD25) (30). In our study, we describe changes in cytokine responsiveness that characterize steady-state CD8+ memory T cells and directly affect CD8+ T-cell effector function during a recall response. We show that the persistent loss of a functional IL-27 receptor on CD8+ memory T cells is mediated not by reduced levels of IL-27Rα but instead through down-regulation of the gp130 signal transduction chain. These results are consistent with previous observations of high IL-27Rα expression on memory cells (31) and the ability of TCR engagement to reduce gp130 expression on transgenic T cells in vitro (28). gp130 mediates cell signaling for a family of cytokines that includes IL-27, IL-6, IL-11, leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor (32). IL-6 is a proinflammatory factor that has been reported to polarize the differentiation of T helper (Th) 2, Th17, and cytotoxic T (Tc)17 T cells (33–36). T-cell polarization is thought to be established during the activation of naïve lymphocytes and then stably maintained into memory (37), suggesting that IL-6 signals may no longer be needed for this purpose in CD8+ memory cells. Interestingly, our data indicate that the reduced IL-6 responsiveness of CD8+ memory cells is less pronounced than their loss of IL-27 signaling, despite the fact that gp130 is an essential component of both receptors. We show that gp130 expression is reduced but not absent on CD8+ memory cells, and our data therefore illustrate the different thresholds of receptor expression required for signal transduction in response to discrete cytokines.
We show here that the loss of IL-27 responsiveness conferred by gp130 down-regulation on CD8+ memory T cells abrogates their IL-10 production during recall. Several recent reports have described the ability of IL-27 to induce IL-10 in both autoimmune and infection models (20–22, 38–40). This has been proposed to be an indirect effect, mediated via IL-21 (41), but our comparison of WT and IL-27Rα−/− cells in chimeric animals demonstrated an intrinsic requirement for IL-27 signaling in eliciting IL-10 expression during respiratory virus infection. IL-27 is released by APC in response to pathogen stimulation (38, 42). We reported recently that cytokine signaling in vivo can be pervasive, affecting the vast majority of cells within a reactive lymph node (43). Such a model of indiscriminate cytokine signaling emphasizes the importance of receptor regulation in enabling a cell to control its response to the cytokine environment. The loss of a functional IL-27 receptor would override any variation in exposure to IL-27.
In a primary infection the strength of the effector response is tempered by a need to limit collateral damage, and in influenza and other acute infections IL-10 plays an essential role in preventing lethal immunopathology (10, 12). IL-10 can inhibit both T-cell proliferation and IFN-γ production (9), and the reduced IL-10 expression in the lung during a secondary influenza infection that we and others have observed (44) is therefore consistent with the prolific expansion and rapid effector differentiation that characterize a recall response. During a primary influenza infection, however, IL-10 signaling is essential for survival, and without it mice succumb to extensive pulmonary inflammation (12). We report a marked reduction of IL-10 expression in CD8+ effector T cells during a rechallenge infection with influenza virus, and yet the mice survive. Our model is that the increased frequency and enhanced anti-viral potential of antigen-specific CD8+ memory T cells enables a secondary immune response, unimpeded by IL-10, to become effective more quickly and to clear the pathogen at lower viral titers than those of a naïve host (7, 8). The shorter duration of the secondary immune response decreases the risk of immunopathology, negates the need for IL-10, and is reinforced by reduced IL-10 expression. This model predicts that whereas CD8-derived IL-10 is host-protective in a primary infection (12), it is detrimental in a recall response. We have shown that forced expression of gp130 in CD8+ memory T cells restores IL-10 production, and it will be interesting to test whether this change in cytokine potential will inhibit the protective capacity of these cells in vivo.
Our data and those of Braciale and coworkers (12) illustrate the dominance of CD8+ effector T cells within the IL-10+ population elicited by primary respiratory viral infection. Other groups have also reported the ability of CD8+ T cells to express IL-10 (45, 46) and, as in CD4+ lymphocytes (17), IL-10 production correlates with CD8+ effector cells displaying the most activated phenotype (12). This is consistent with the predominant localization of IL-10+ CD8+ cells in the infected tissues and their concomitant expression of high levels of IFN-γ (12, 47). Many other cell types have the capacity to produce IL-10 during viral infection, including dendritic cells and CD4+ T cells (16, 44). CD8+ memory T cells remain IL-10–responsive (48) and therefore able to be influenced by IL-10 from other sources. It will be interesting to determine whether IL-10 production by non-CD8+ cell populations is also decreased during a secondary recall response.
Together our data illustrate distinct patterns of cytokine responsiveness in naïve and memory T cells that are imposed by persistent changes in cytokine receptor expression and that define the functional characteristics of each population. We demonstrate that a sustained modulation of cytokine responsiveness is a feature of both murine and human CD8+ T-cell differentiation, and thus our findings are particularly relevant to the design of medical strategies to manipulate effector and memory cell function via cytokine signals, such as vaccination or cytokine therapy.
Materials and Methods
Mice and Infections.
Vert-X (C57BL/6 IL-10/eGFP) (18), C57BL/6, CD45.1+ congenic (B6.SJL-PtprcaPep3b/BoyJ), Il27ra−/− (23), TCRβδ−/− (B6.129P2-Tcrbtm1MomTcrdtm1Mom/J), and OTI [C57BL/6-Tg(TcraTcrb)1100Mjb/J] mice were bred and housed at the Trudeau Institute. Animals were kept under specific pathogen-free conditions in filter-top cages and used at 6–12 wk of age. Mice were infected intranasally with either Sendai or influenza virus. Virus strains and doses are given in SI Materials and Methods. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Trudeau Institute and the University of British Columbia.
Tissue Sampling and Flow Cytometry.
Details of antibodies used are given in SI Materials and Methods. Samples were acquired on a FACSCanto or LSRII cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
Cytokine Stimulation and Phospho-STAT Analysis.
To assess cytokine responsiveness, cells were labeled with tetramers, stimulated for 15 min at 37 °C with 20 ng/mL recombinant murine IL-6, IL-21, IL-27, or IFNβ (Peprotech), and immediately fixed and permeabilized using PhosFlow reagents (BD Biosciences). Phosphorylated STAT1 (pY701) and STAT3 (pY705) were detected by intracellular staining (clones 4a and 4/P-Stat3 respectively; BD Biosciences).
Human PBMC.
Cryopreserved human PBMCs were purchased from Cellular Technology Ltd. Cells were labeled with a Pro5 Recombinant Murine MHC Pentamer specific for the influenza A M1 epitope (Proimmune), stimulated for 15 min at 37 °C with 20 ng/mL recombinant human IL-27 (R&D Systems), and stained for pSTAT1 and pSTAT3 as above. Surface antigens were assessed using fluorochrome-labeled antibodies against CD8 (clone RPA-T8), CD19 (HIB19), CD27 (O323), and CD45RA (HI100) (eBioscience).
In Vitro Culture and Retroviral Transduction.
Sort-purified naïve or memory CD8+ T cells were activated in vitro by coculture with live APC, anti-CD3, and anti-CD28. OTI transduction was performed by infecting activated OTI cells with a MIG retrovirus. Cells were transferred to medium containing IL-15 to rest on day 3 after activation, before being restimulated and assayed on day 7. Details are given in SI Materials and Methods.
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism 5. Significant differences between sample groups were determined using a Student t test or a one-way analysis of variance with Bonferroni’s posttest to compare two preselected groups (*P < 0.05; **P < 0.01; ***P < 0.001; n/s, not significant).
Supplementary Material
Acknowledgments
We thank Nico Ghilardi (Genentech) for Il27ra−/− mice, P. Scottie Adams and the Trudeau Institute Molecular Biology Core Facility for production of Sendai- and influenza-specific tetramers, and Ron LaCourse and Brandon Sells for cell sorting. This work was supported by a BD Biosciences Research Grant (G.P.-W.); start-up funds from the University of British Columbia (G.P.-W.), National Institutes of Health Grants AI83610 (to J.E.K.), AI32573 (to E.J.P.), AI67967 and AI76499 (to D.L.W.), and AI072296 (to M.M.); and funds from the Trudeau Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.M.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119133109/-/DCSupplemental.
References
- 1.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4(9):835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 2.Hogan RJ, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166(3):1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- 3.Marshall DR, et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98(11):6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hikono H, et al. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204(7):1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohlmeier JE, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29(1):101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 7.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994;152(4):1653–1661. [PubMed] [Google Scholar]
- 8.Flynn KJ, et al. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8(6):683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 9.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli RT, et al. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157(2):798–805. [PubMed] [Google Scholar]
- 11.Suzuki Y, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol. 2000;164(10):5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15(3):277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420(6915):502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204(2):285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203(11):2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic D, et al. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204(2):273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madan R, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183(4):2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe SR, et al. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J Exp Med. 2003;198(3):399–410. doi: 10.1084/jem.20022151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batten M, et al. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180(5):2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8(12):1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 22.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407(6806):916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 24.Mayer KD, et al. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol. 2008;180(2):693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 25.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 26.Takeda A, et al. Cutting edge: Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170(10):4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 27.Pflanz S, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172(4):2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 28.Betz UA, Müller W. Regulated expression of gp130 and IL-6 receptor alpha chain in T cell maturation and activation. Int Immunol. 1998;10(8):1175–1184. doi: 10.1093/intimm/10.8.1175. [DOI] [PubMed] [Google Scholar]
- 29.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 30.Kalia V, et al. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32(1):91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Villarino AV, et al. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174(12):7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Lupardus P, Laporte SL, Garcia KC. Structural biology of shared cytokine receptors. Annu Rev Immunol. 2009;27:29–60. doi: 10.1146/annurev.immunol.24.021605.090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rincón M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185(3):461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Hamada H, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182(6):3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perona-Wright G, et al. Concurrent bacterial stimulation alters the function of helminth-activated dendritic cells, resulting in IL-17 induction. J Immunol. 2012;188(5):2350–2358. doi: 10.4049/jimmunol.1101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panus JF, McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific T helper cell function: differential cytokine expression in primary and memory responses. J Exp Med. 2000;192(9):1301–1316. doi: 10.1084/jem.192.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8(12):1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 39.Anderson CF, Stumhofer JS, Hunter CA, Sacks D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol. 2009;183(7):4619–4627. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: A novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179(7):4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 41.Pot C, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183(2):797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molle C, et al. IL-27 synthesis induced by TLR ligation critically depends on IFN regulatory factor 3. J Immunol. 2007;178(12):7607–7615. doi: 10.4049/jimmunol.178.12.7607. [DOI] [PubMed] [Google Scholar]
- 43.Perona-Wright G, Mohrs K, Mohrs M. Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nat Immunol. 2010;11(6):520–526. doi: 10.1038/ni.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKinstry KK, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182(12):7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarawar SR, Doherty PC. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68(5):3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumgarth N, Brown L, Jackson D, Kelso A. Novel features of the respiratory tract T-cell response to influenza virus infection: Lung T cells increase expression of gamma interferon mRNA in vivo and maintain high levels of mRNA expression for interleukin-5 (IL-5) and IL-10. J Virol. 1994;68(11):7575–7581. doi: 10.1128/jvi.68.11.7575-7581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer KD, et al. The functional heterogeneity of type 1 effector T cells in response to infection is related to the potential for IFN-gamma production. J Immunol. 2005;174(12):7732–7739. doi: 10.4049/jimmunol.174.12.7732. [DOI] [PubMed] [Google Scholar]
- 48.Biswas PS, et al. Pathogen-specific CD8 T cell responses are directly inhibited by IL-10. J Immunol. 2007;179(7):4520–4528. doi: 10.4049/jimmunol.179.7.4520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.