Abstract
Dissolution of anthropogenic CO2 increases the partial pressure of CO2 (pCO2) and decreases the pH of seawater. The rate of Fe uptake by the dominant N2-fixing cyanobacterium Trichodesmium declines as pH decreases in metal-buffered medium. The slower Fe-uptake rate at low pH results from changes in Fe chemistry and not from a physiological response of the organism. Contrary to previous observations in nutrient-replete media, increasing pCO2/decreasing pH causes a decrease in the rates of N2 fixation and growth in Trichodesmium under low-Fe conditions. This result was obtained even though the bioavailability of Fe was maintained at a constant level by increasing the total Fe concentration at low pH. Short-term experiments in which pCO2 and pH were varied independently showed that the decrease in N2 fixation is caused by decreasing pH rather than by increasing pCO2 and corresponds to a lower efficiency of the nitrogenase enzyme. To compensate partially for the loss of N2 fixation efficiency at low pH, Trichodesmium synthesizes additional nitrogenase. This increase comes partly at the cost of down-regulation of Fe-containing photosynthetic proteins. Our results show that although increasing pCO2 often is beneficial to photosynthetic marine organisms, the concurrent decreasing pH can affect primary producers negatively. Such negative effects can occur both through chemical mechanisms, such as the bioavailability of key nutrients like Fe, and through biological mechanisms, as shown by the decrease in N2 fixation in Fe-limited Trichodesmium.
Keywords: climate change, cyanobacteria, iron limitation
About one-third of the anthropogenic CO2 released into the atmosphere dissolves into the surface ocean, increasing the partial pressure of CO2, pCO2, and lowering the pH. This ocean acidification has been shown to have various consequences for marine phytoplankton (1–5). Organisms that invest a large amount of energy in the operation of a carbon-concentrating mechanism (CCM) are expected to be particularly sensitive to changes in pCO2. This is the case for marine cyanobacteria, which must elevate the CO2 concentration at the site of carbon fixation as a result of the poor affinity for CO2 of their carboxylating enzyme, ribulose bisphosphate carboxylase oxygenase (RubisCO) (6). Of particular interest is the effect of ocean acidification on the N2-fixing filamentous cyanobacterium Trichodesmium, which is responsible for a major fraction of all marine N2 fixation and thus plays a prominent role in the biogeochemical cycling of C and N (7). This bloom-forming diazotroph thrives throughout the oligotrophic tropical and subtropical oceans where P and/or Fe often limit its growth and N2 fixation (8–10).
In the past few years, the effects of ocean acidification on Trichodesmium have been studied extensively in combination with those of other environmental variables, such as temperature, light intensity, and phosphorus limitation. Stimulation of N2 fixation and growth at elevated pCO2 has been observed in both laboratory and field studies (11–19). The beneficial effect of high pCO2 has been attributed largely to the down-regulation of the CCM, which saves energetic resources for other cellular processes, such as N2 fixation (14, 15).
The positive effect of CO2 enrichment on growth and N2 fixation in marine diazotrophs may be tempered by Fe limitation, as indicated, for example, by experiments with the single-celled N2-fixing cyanobacteria Crocosphaera (20, 21). However, to study the effect of ocean acidification on Trichodesmium under low-Fe conditions requires using a medium in which the changes in Fe chemistry caused by the changing pH are known precisely (4). All previous work on the response of Trichodesmium to ocean acidification have used the artificial seawater medium YBCII (22), which contains unknown and variable concentrations of trace metals from contaminants and too low a chelator concentration (EDTA = 2 μM) to buffer free-metal concentrations properly.
Here we report the results of experiments with Trichodesmium erythraeum IMS101 (thereafter Trichodesmium) using a seawater medium with well-defined trace-metal chemistry to examine the effects of acidification under low-Fe conditions. We measured the rates of Fe uptake, growth, and N2 fixation under varying pCO2 and pH and quantified the proteins involved in N2 fixation and photosynthesis to investigate the underlying mechanisms.
Results
Effect of pH/pCO2 on Fe Uptake and Growth.
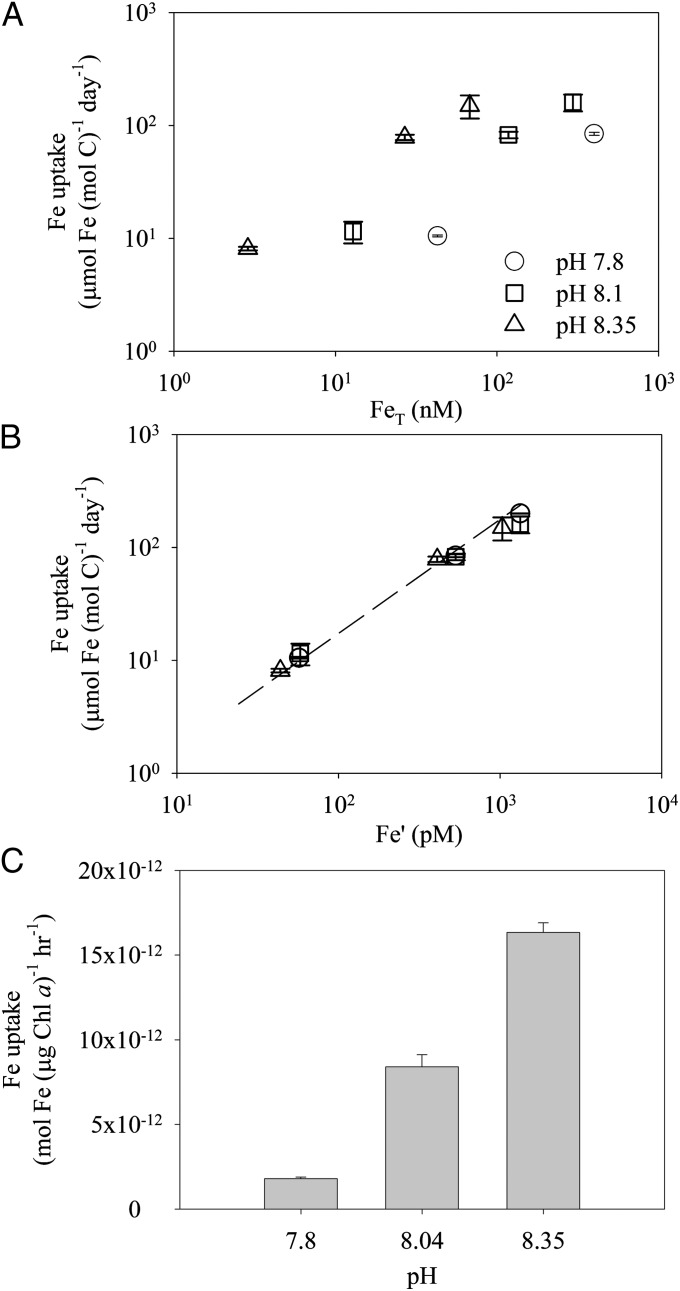
We examined the effect of pCO2 and pH on the Fe uptake and growth of Trichodesmium using an EDTA-buffered medium. At a given total Fe concentration, the steady-state Fe uptake rate of Trichodesmium growing exponentially decreased significantly with decreasing pH in the range of 8.35–7.80 (Fig. 1A). However, all the data align on the same line when plotted as a function of the calculated inorganic Fe concentration (Fe′) (Fig. 1B). The slope of the line in the logarithmic graph of Fig. 1B is 1, showing that the Fe uptake system was neither saturated nor down-regulated over the range of the Fe concentration we used.
Fig. 1.
The effect of ocean acidification on Fe uptake by Trichodesmium. (A) Steady-state Fe-uptake rates in Trichodesmium as a function of total Fe concentration (FeT) in EDTA-buffered culture medium over a range of pH/pCO2 (pH 7.8–750 ppm pCO2; pH 8.1–350 ppm pCO2; pH 8.35–180 ppm pCO2). (B) When plotted as a function of the steady-state Fe′, uptake rates in A closely follow a one-to-one line. (C) Short-term Fe uptake by Fe-limited Trichodesmium from Fe bound to EDTA (FeT = 50 nM) at three different pH/pCO2 levels (pH 7.8–750 ppm pCO2; pH 8.04–410 ppm pCO2; pH 8.35–180 ppm pCO2). Error bars represent the SD of biological replicates (n = 2).
The data in Fig. 1B also indicate that pH exerts its effect on Fe uptake by Trichodesmium through changes in Fe chemistry rather than a physiological response of the diazotroph. If so, the effect of pH should be seen in short-term uptake experiments with cells that are not acclimated to the experimental pCO2/pH levels. (We use the notation pCO2/pH to indicate that both parameters covary in most of our experiments.) Indeed, the rate of Fe uptake by Trichodesmium acclimated at Fe′ = 40 pM (and pCO2 = 352 ppm and pH = 8.1) decreased by about 50% and 90% as the pH of the uptake medium varied from 8.35 to 8.04 and 7.8, respectively (Fig. 1C). The trend and the magnitude of the pH effect on Fe uptake were comparable to those in the long-term experiment with exponentially growing cells.
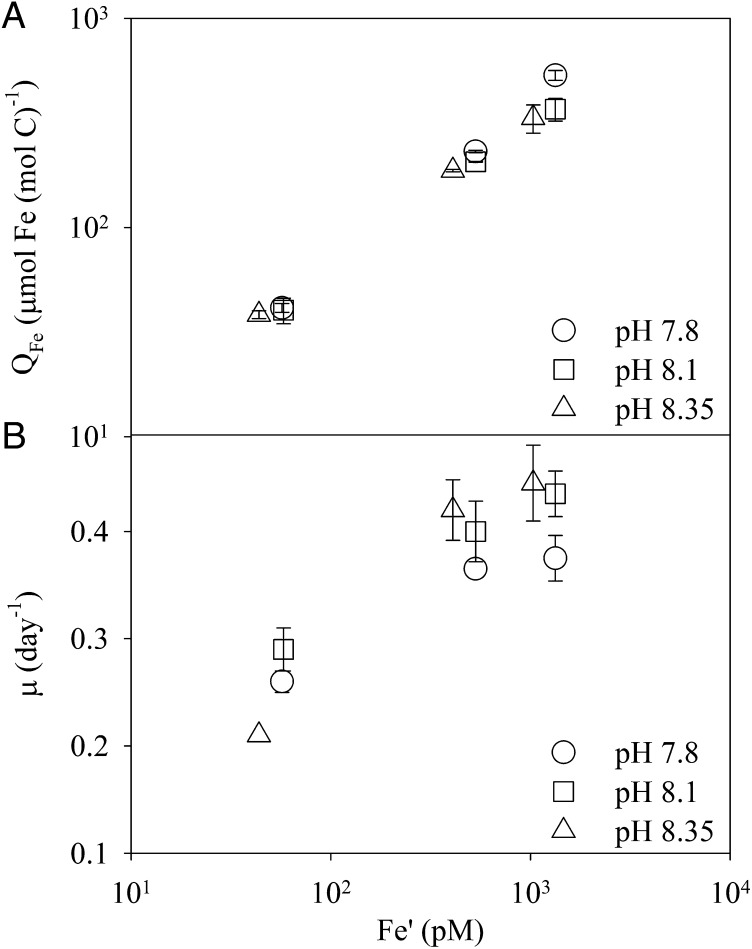
To distinguish the effects of varying pCO2/pH on growth and N2 fixation in Trichodesmium from those on Fe uptake, in all the following experiments we adjusted the total Fe concentration to maintain constant Fe availability in our EDTA-buffered medium, i.e., the total Fe concentration at low pH was increased to maintain constant Fe′. The cellular Fe concentration, QFe, of Trichodesmium and its growth rate, μ, increased with Fe′ over the range of 40–1,340 pM (Fig. 2). At a given Fe′, QFe, like the Fe-uptake rate, was unaffected by changes in pCO2/pH (Fig. 2A). However, the growth rate of Trichodesmium clearly changed with pCO2/pH: μ generally decreased along with decreasing pH, except at the lowest Fe′ tested, where μ decreased at the lowest pCO2/highest pH and the cells showed very poor growth (Fig. 2B). This latter result, which has been observed in various phytoplankton species which all exhibit an extremely low photosynthetic rate and minimal growth under very low Fe and pCO2 conditions (e.g., ref. 4), is not relevant to this study on the effects of ocean acidification. Very relevant, however, is the statistically significant decrease in Trichodesmium growth rate with decreasing pH (P = 0.023, two-way ANOVA with post hoc Tukey’s honestly significant difference test) observed under all other conditions, which is contrary to published results (e.g., 11–13, 15). In this experiment, pCO2/pH was adjusted by acid/base addition because the use of 14C for determining growth rates did not allow bubbling of the medium. In a separate experiment, in which the seawater carbonate chemistry was controlled by bubbling CO2-enriched air, the growth rate of Trichodesmium also decreased significantly at higher pCO2/lower pH for any given level of available Fe (P < 0.05, t test, for 380 ppm pCO2/pH 8.08 vs.750 ppm pCO2/pH 7.88) (Table 1).
Fig. 2.
The effect of ocean acidification on cellular Fe quota and growth of Trichodesmium. (A) Cellular Fe:C ratios and (B) specific growth rates of Trichodesmium as a function of the inorganic Fe concentration, Fe′, at three different pH/pCO2 levels (pH 7.8–750 ppm pCO2; pH 8.1–350 ppm pCO2; pH 8.35–180 ppm pCO2) in EDTA-buffered culture medium. Error bars represent the SD of biological replicates (n = 2). Growth curves are shown in Fig. S1.
Table 1.
Specific growth rate, C- and N2-fixation rates, and POC:PON and Chl a:C ratios of steady-state–growing Trichodesmium under 750 ppm and 380 ppm pCO2 at 40 pM and 1,250 pM Fe′
| pCO2 (ppm) | pH | Fe′ (pM) | Growth rate (d−1) | C-fixation rate (mmol C (mol C)−1 h−1) | N2-fixation rate (mmol N (mol C)−1 h−1) | POC:PON (mol/mol) | Chl a:C (μg/μmol) |
| 380 | 8.08 | 40 | 0.26 ± 0.02a | 9.91 ± 0.59 | 2.45 ± 0.20a | 8.32 ± 0.10 | 0.095 ± 0.007 |
| 380 | 8.08 | 1,250 | 0.46 ± 0.01a | 10.64 ± 0.11 | 3.39 ± 0.21a | 7.37 ± 0.75 | 0.119 ± 0.002 |
| 750 | 7.88 | 40 | 0.19 ± 0.01b | 8.24 ± 0.61 | 1.61 ± 0.11b | 8.25 ± 0.50 | 0.101 ± 0.002 |
| 750 | 7.88 | 1,250 | 0.37 ± 0.01b | 9.58 ± 2.04 | 1.70 ± 0.10b | 6.85 ± 0.92 | 0.116 ± 0.015 |
Within each Fe′, values that were significantly different (P < 0.05, t test) between pCO2 treatments are indicated by different superscripts. Data are mean ± SD (n = 2). Growth curves are shown in Fig. S2.
C and N2 Fixation and Particulate Organic C:Particulate Organic N and Chlorophyll a:C Ratios.
To evaluate the effect of pCO2/pH on C and N2 fixation, 13C-bicarbonate and 15N2 were added simultaneously to Trichodesmium cultures growing under exponential conditions at 380 and 750 ppm pCO2 at various Fe′ (Table 1). We found that cultures grown at 750 ppm pCO2 exhibited a systematic although not statistically significant decrease in the particulate organic C (POC)-normalized C-fixation rate compared with those grown at 380 ppm pCO2 (i.e., 17% and 10% at 40 and 1,250 pM Fe′, respectively; P = 0.107 and 0.541, t test) determined during a 4-h period around midday when photosynthesis is repressed (14, 23). In addition, the N2-fixation rate, which reaches a maximum at midday (23), was considerably slower at 750 ppm than at 380 ppm pCO2 (P = 0.035 and 0.009, t test) (Table 1), with a decrease of 35% and 50% at low and high Fe′, respectively. As expected from previous studies, increasing Fe availability decreased the ratio of POC to particulate organic N (PON) (P = 0.026, t test; Table 1) and increased the chlorophyll a (Chl a):C (P = 0.01, t test) (24). However, pCO2/pH had no significant effect on either of these parameters.
Effect of pCO2 and pH on Short-Term Nitrogenase Activity and Net H2 Production.
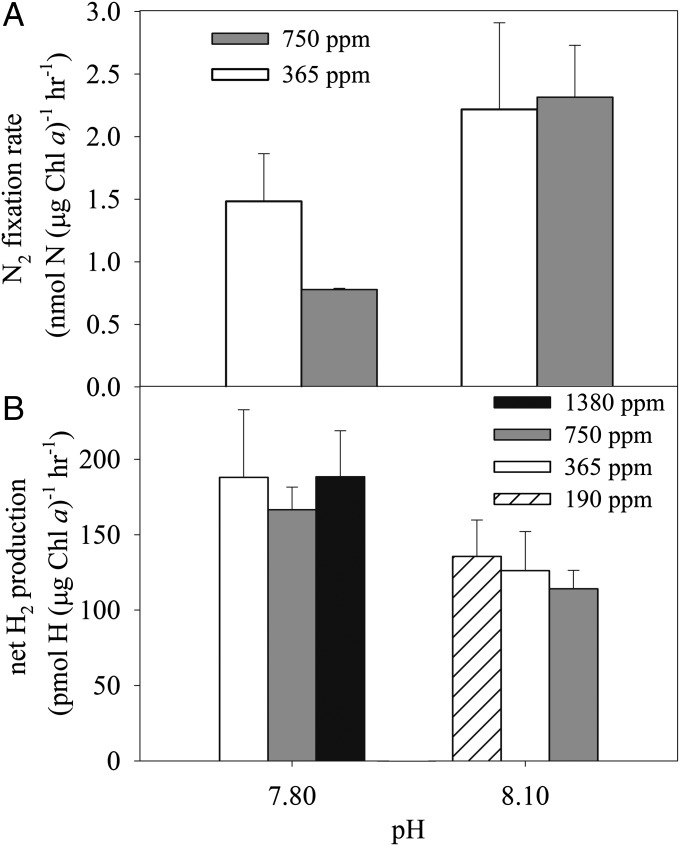
To determine whether pCO2 or pH primarily causes the reduction of N2 fixation in Trichodesmium at high pCO2/low pH, we conducted acetylene-reduction experiments with cells harvested at midday from a low-Fe culture and assayed for 2 h in Fe-limited (i.e., Fe′ = 40 pM) seawater media in which pCO2 and pH were varied independently by adjusting the dissolved inorganic C (DIC) and alkalinity (Alk). As a result, DIC and Alk of the medium increased with increasing pH for a given pCO2 and with increasing pCO2 for a given pH. We observed an adverse effect of decreasing pH (P = 0.023, two-way ANOVA) on nitrogenase activity in the low-Fe preacclimated Trichodesmium (Fig. 3A). On average, the acetylene-reduction rates at pH 7.80 dropped by 50% compared with those at pH 8.1 (P = 0.014, t test). Because the cellular enzyme concentration must remain nearly invariant in this 2-h experiment, this decrease must reflect a decrease in the efficiency of the nitrogenase enzyme, i.e., the rate of acetylene reduction per enzyme in vivo. In contrast, pCO2 had no statistically significant effect on nitrogenase activity in this short-term experiment (P = 0.391, two-way ANOVA).
Fig. 3.
Short-term N2-fixation (A) and net H2-production (B) rates determined around midday in Trichodesmium grown under low-Fe conditions in the buffered Gulf stream seawater under a matrix of pCO2 and pH obtained by adjusting DIC and Alk appropriately. Error bars represent the SD of biological replicates (n = 2).
In a similar short-term incubation varying pCO2 and pH independently, we measured the net production of H2, a byproduct of N2 fixation. As shown in Fig. 3B, the net rate of H2 production increased markedly at pH 7.85 compared with pH 8.1 (an increase of 45%on average, P = 0.004, t test) but remained unchanged as pCO2 varied from 190 to 1,380 ppm (P = 0.762 and 0.496 at pH 7.85 and pH 8.1, respectively, one-way ANOVA).
Nitrogenase and Photosynthetic Proteins.
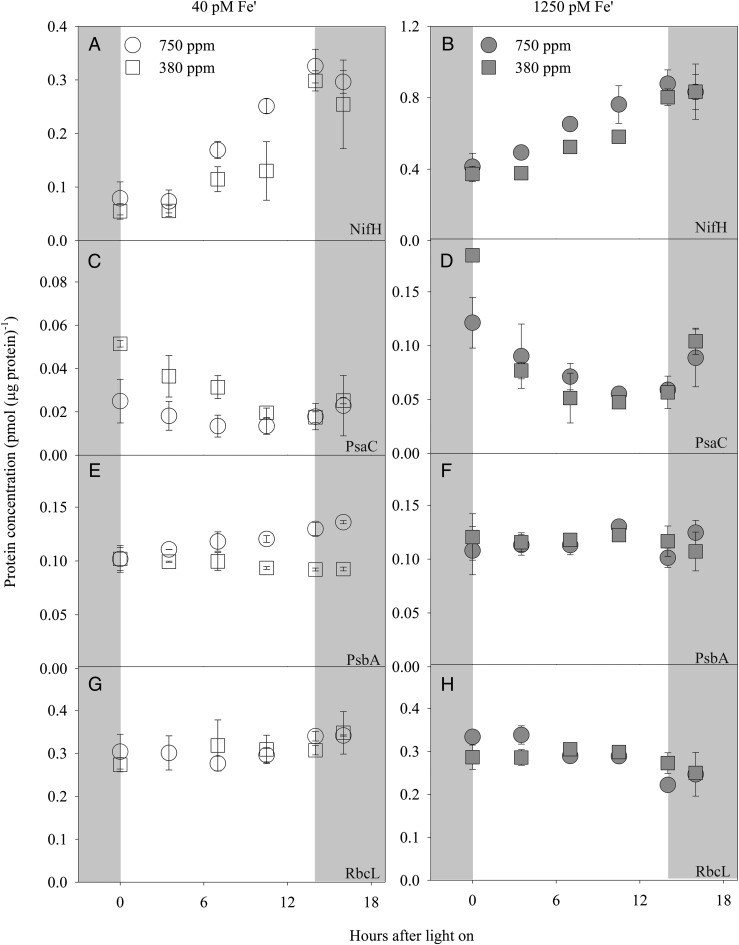
To investigate why increasing pCO2/decreasing pH unfavorably altered the rates of N2 fixation and growth in Trichodesmium, we analyzed the diel expression of key proteins involved in N2 fixation and photosynthesis: NifH, the nitrogenase reductase of the nitrogenase complex; PsaC, the core subunit of photosystem I (PSI); PsbA, the D1 protein of photosystem II (PSII); and RbcL, the large subunit of the carboxylating enzyme RubisCO. As shown in Fig. 4, NifH and PsaC were about threefold more abundant at 1,250 pM Fe′ than at 40 pM Fe′ (Fig. 4 A–D), whereas Fe′ had little effect on the abundance of PsbA and RbcL (Fig. 4 E–H). The lower PsaC and unchanged PsbA resulted in a decrease in PsaC:PsbA ratios at low Fe (Fig. 5), as has been observed previously in Trichodesmium (24) and in diatoms (25) under Fe-limited conditions.
Fig. 4.
Diel changes in the protein concentration [pmol (μg protein)−1] of (A and B) NifH, (C and D) PsaC, (E and F) PsbA, and (G and H) RbcL in steady-state–growing Trichodesmium under 750 ppm pCO2 (pH 7.88) and 380 ppm pCO2 (pH 8.08) at 40 pM (A, C, E, and G) and 1,250 pM (B, D, F and H) Fe′. The gray areas in the figures indicate the dark phase. Error bars represent the SD of biological replicates (n = 2).
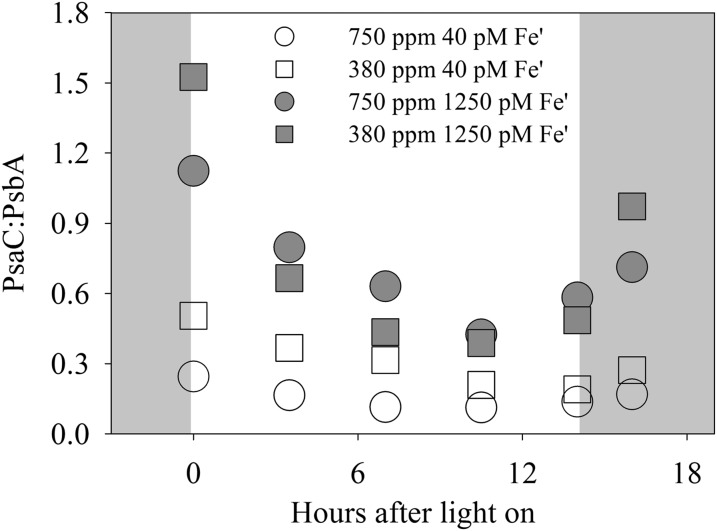
Fig. 5.
Diel changes in the PsaC:PsbA ratio in steady-state–growing Trichodesmium under 750 ppm pCO2 (pH 7.88) and 380 ppm pCO2 (pH 8.08) at 40 pM and 1,250 pM Fe′. The gray areas in the figures indicate the dark phase. Error bars represent the SD of biological replicates (n = 2).
The abundance of NifH in Trichodesmium showed a diel pattern in all treatments of our study (Fig. 4 A and B), increasing ∼3 h after the onset of light, rising until the end of the light period, and finally decreasing after 2 h in the dark. Although the rate of N2 fixation decreased (Table 1), the concentration of NifH increased at high pCO2/low pH, in particular over the 3-h period starting at midday. On average the abundance of the enzyme at 750 ppm pCO2/pH 7.88 was 1.7- and 1.3-fold that at 380 ppm pCO2/pH 8.08 under the low and high Fe′, respectively.
The core subunit of PSI, PsaC, also demonstrated a strong diel variation in its abundance, decreasing immediately after the onset of light, reaching a minimum 7–10 h into the light period, and recovering in the dark period (Fig. 4 C and D). CO2 influenced the abundance of the protein, especially under the low-Fe condition. In the low-Fe′ cultures, the amount of PsaC at 750 ppm pCO2 was on average about half of that at 380 ppm pCO2 within the first 7 h of the light period (Fig. 4C). In the high-Fe cultures, the difference in protein concentration was significant only at the onset of light (34% less at high pCO2) and disappeared subsequently (Fig. 4D).
The level of the photosynthetic protein PsbA (the D1 protein of PSII) expression was similar in all treatments at the beginning of the light period (Fig. 4 E and F) and showed little change afterward except in the low-Fe/high-pCO2 treatment. At 40 pM Fe′/750 ppm pCO2, the amount of the protein increased steadily over the 16-h sampling period, eventually resulting in a 35% increase over the initial value (Fig. 4E) and suggesting an effect of CO2 under these conditions.
Generally no effect of Fe or CO2 on the concentration of the large subunit of RubisCO, RbcL, in Trichodesmium was observed in our experiments. The amount of the enzyme remained fairly constant over our sampling period under the low-Fe condition, but it seemed to decrease slightly after midday in the high-Fe treatments (Fig. 4 G and H).
Discussion
Our results show significant adverse effects of ocean acidification on Fe uptake, N2 fixation, and growth in the ecologically important cyanobacterium Trichodesmium under the low-Fe conditions typically experienced by the organism in the oceans (9). Increasing pCO2 and thus decreasing the pH in our EDTA-buffered medium decreases the Fe-uptake rate in a way that is quantitatively explained by the changes in Fe chemistry, i.e., the decrease in Fe′. Contrary to previously published results (11–13, 15–18), we observed that both N2 fixation and growth of Trichodesmium declined at high pCO2/low pH under low-Fe conditions, even though we maintained constant Fe bioavailability in our experiments. The lower rate of N2 fixation results from a decrease in the efficiency of the nitrogenase enzyme. This decrease apparently is caused by the low pH rather than the high pCO2 and is mirrored by an increase in net H2 production. The lower efficiency of nitrogenase is partly compensated by an increase in the cellular concentration of the enzyme but nonetheless results in a lower growth rate. Under low-Fe conditions, the increase in nitrogenase at low pH is accompanied by a decrease in PsaC, presumably as a result of a reallocation of the limited pool of cellular Fe, as discussed below.
Changes in Fe chemistry caused by seawater acidification have been shown to decrease the uptake rate of Fe and other metals in several neritic and oceanic phytoplankton species including centric and pennate diatoms and coccolithophores (4). Our data show a similar effect of pH on Fe uptake in the cyanobacterium Trichodesmium. Although the mechanism of Fe uptake by Trichodesmium remains to be fully elucidated, it has been shown that the uptake process can involve a bio-reduction step, as has been demonstrated in some model diatom species (26–28). Trichodesmium apparently is capable of accessing Fe from Fe oxide, aerial dust, and siderophores (29, 30) in which Fe bioavailability should be less sensitive to pH than in our EDTA-buffered medium (4). Our results clearly demonstrate that, over the range of interest, the effects of pCO2/pH on Fe uptake in Trichodesmium are caused by changes in the chemistry of the medium, not by a physiological response of the organism, in agreement with previous findings in other phytoplankton species (4).
In addition to decreasing the availability of Fe to Trichodesmium in our medium, low pH also decreases the efficiency of the nitrogenase enzyme in Fe-limited cultures, i.e., the rate of N2 fixation per enzyme in vivo. In the long-term experiment with varying pCO2 reported in Table 1 and Fig. 4A, the decrease in the N2-fixation rate and the increase in NifH concentration at pH = 7.88 compared with pH = 8.08 resulted in a decline of roughly 60% in enzymatic efficiency even though Fe′ was maintained at 40 pM. This result is similar to the reduction of 50% in nitrogenase efficiency measured in the short-term acetylene-reduction experiment (Fig. 3A) in which the reduction was shown to result from about the same variation in pH and not from a change in pCO2. This effect thus does not result from an acclimation of the cell to the acidified environment. Coincident with the decrease in N2 fixation is an increase in net H2 evolution, also driven by low pH and not by high pCO2. This effect is diagnostic of a less efficient use of reductants (31), although the reducing equivalents lost to H2 production do not account for the decrease in N2 fixation.
To compensate in part for the impaired N2 fixation, Trichodesmium synthesizes more nitrogenase at low than at high pH, with an increase by a factor of 1.7 at midday under the conditions shown in Fig. 4A. This up-regulation of nitrogenase concentration is likely limited by Fe. Because the nitrogenase complex contains 38 Fe atoms per monomer (not counting the Fe required to supply the necessary reductants), its up-regulation creates a large demand for Fe. This extra Fe must come from other Fe pools within the already Fe-limited diazotroph. Kustka et al. (32) estimated that under Fe limitation about 19–53% of the overall metabolic Fe may be bound in the nitrogenase complex of diazotrophically growing Trichodesmium, with an additional 38% present within the photosynthetic apparatus and the remainder involved either in respiratory activity or in antioxidant enzymes. In our experiments, NifH and PsaC are regulated by both the diel cycle and the pCO2/pH of the medium, providing an insight into the Fe economy of the cells.
Diel cycle.
In our low-Fe cultures (Fig. 4A) at pCO2 = 380 ppm, we calculate that the Fe in nitrogenase increased from 3.5 μmol Fe/mol C at the beginning of the light period to 21 μmol Fe/mol C at the end (assuming a 2:1 stoichiometry between the Fe-protein dimer and the MoFe-protein tetramer in the nitrogenase complex; SI Text, Cellular Nitrogenase and Fe Concentrations, Section I). This last value is about one-half of the total cellular quota in Fig. 2A and may be a bit less if the Fe-protein:MoFe-protein ratio is near 3:1 (33). The corresponding decrease in PsaC during the day can liberate only 4 μmol Fe/mol C (assuming a 1:1:1 stoichiometry for PSI, cytochrome b6f complex, and ferredoxin; SI Text, Cellular Nitrogenase and Fe Concentrations, Section II), a small fraction of the concomitant increase in nitrogenase Fe. According to Tuit et al. (34), there is no substantial change in cellular Fe in Trichodesmium during the daily cycle, so other cellular pools of Fe must be depleted. Trichodesmium may practice “hot-bunking” for Fe, as demonstrated in Crocosphaera watsonii (33). However, in Trichodesmium the exchange of Fe must occur among cells in the same trichome because the nitrogenase is localized in diazocytes that account for only 15–20% of the total cell number (35–38). It is possible that the DNA-binding protein from starved cells (Dps protein) of Trichodesmium (39) plays a role in the exchange of Fe among cells as well as in Fe storage during the cell cycle, even under Fe-limiting conditions.
Effect of pCO2/pH.
The up-regulation of nitrogenase at pCO2 = 750 ppm/pH = 7.88 seen in Fig. 4A results in a faster increase in NifH 3 h after the onset of light, so that a large fraction of the cellular Fe must be allocated to nitrogenase shortly after midday, leaving little possibility for further up-regulation of the enzyme. The earlier down-regulation of PsaC brings its concentration and the PSI/PSII ratio down by a factor of two at the onset of light (Figs. 4C and 5). These values are likely near the minimum that allows significant photosynthesis, and they show little further decrease at midday, when photosynthesis is nearly shut down (21).
Under high-Fe conditions, the maximum NifH concentration measured at the end of the light period (Fig. 4B) corresponds to a nitrogenase mass of about 7% of the total protein mass (assuming again a 2:1 stoichiometry between the Fe-protein dimer and the MoFe-protein tetramer; SI Text, Cellular Nitrogenase and Fe Concentrations, Section III). The nitrogenase is localized in diazocytes that have the same size as other cells in a trichome but account for only 15–20% of the total cell number, so that 30–50% of the protein mass in diazocytes must be in nitrogenase. Given that these cells possess complete photosynthetic machinery, this high nitrogenase content likely is near the maximum attainable. The up-regulation of nitrogenase at pCO2 = 750 ppm/pH = 7.88 seen in Fig. 4B brings its concentration near this maximum shortly after midday, so that there is little opportunity for further up-regulation. The decrease in growth rate seen at high Fe in Fig. 2B thus may be caused by the decrease in N2-fixing efficiency at low pH.
The diel variations in NifH, PsaC, and PsbA seen in Fig. 4 are surprising in view of the current understanding of the links between photosynthetic activity and N2 fixation in Trichodesmium (23, 40). Our results indicate an increase in nitrogenase and a decrease in PSI during daylight, but PSII remains essentially invariant. The resulting diel cycle in the PSI/PSII ratio exhibits a minimum at midday (Fig. 5), when photosynthetic activity is at its minimum and N2 fixation at its maximum. This result seemingly is inconsistent with the proposition that pseudocyclic electron transport after PSI serves to increase O2 consumption and provide ATP for N2 fixation (40).
Our results under high-Fe conditions are at odds with previous experiments that demonstrated an increase in N2 fixation and growth at high pCO2/low pH (e.g., 11–13, 15). All those experiments used the medium YBCII, which has a poorly defined trace metal chemistry as a result of the uncontrolled concentration of contaminants in the artificial seawater salts and the insufficient level of buffering afforded by the low EDTA concentration (2 μM) (22). For example, the calculated Fe′, >190 nM, is 140 times greater than in our medium and much above saturation of Fe(III) in seawater. Other metals, such as nickel and vanadium which are cofactors of important enzymes in Trichodesmium (41), are not part of the YBCII recipe, but the concentration of molybdenum, a key constituent of the nitrogenase tetramer, is about 1/10 that in seawater. The variability in metal contamination, potentially resulting in either limitation or toxicity to Trichodesmium, is likely responsible for the large variations in growth rates observed among studies with the medium YBCII under supposedly identical conditions and even within a single study (11, 13, 14).
In view of the large effect of pH variations on metal uptake demonstrated here and in other studies (4, 42), it is possible that the positive effect of high pCO2 commonly observed on Trichodesmium cultured in YBCII may be caused by changes in the bioavailability of essential or toxic metals. This positive effect also likely reflects the energy savings afforded by a down-regulation of the CCM, as usually has been inferred (43). Such energy savings should partly offset the reduced nitrogenase efficiency seen at low pH in our experiments, depending on the energetic demands of the cells as influenced by conditions of nutrient limitation or toxicity. In particular, the availability of inorganic C, which directly influences the energetic demand of the CCM, must be one of the dominant factors that affect the net response of Trichodesmium to high pCO2 and low pH. For example, when Trichodesmium trichomes are organized in colonies with sizes approaching 1 mm (“puffs” and “tufts” visible by the naked eye), the rate of molecular diffusion to these aggregates can be slow and limit the rate of C uptake (15), thus requiring a particularly active CCM. In this situation, an increase in CO2 and bicarbonate concentration can result in a substantial energetic savings for the cells. Such savings probably explain the beneficial effects of high pCO2 on N2 and C fixation observed in P- and Fe-replete incubations of Trichodesmium thiebautii colonies collected from the field (19).
Trichodesmium dwells in oligotrophic tropical and subtropical regions of the oceans where Fe concentrations in surface waters are very low. It has been suggested that N2 fixation by marine diazotrophs including Trichodesmium in these regions often is limited by Fe availability (9, 44, 45). According to our results, as the ocean acidifies, the dual effects of decreasing pH on Fe availability and on N2 fixation by Trichodesmium may act synergistically to result in a sizable decline in new N input to oligotrophic waters, given that this organism is estimated to contribute a large fraction of contemporary oceanic N2 fixation (7). This decline may be partly offset by the beneficial effect of increasing CO2 on the energetics of the cells whose importance under ambient conditions depends on the relative contribution of single trichomes and colonies to the overall N2 fixation (46–48).
Materials and Methods
Methods are described in more detail in SI Materials and Methods.
Cultures and Growth.
The marine cyanobacterium Trichodesmium erythraeum (IMS101) was obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton and was incubated in Gulf Stream seawater at 27 °C and ∼90 μmol quanta m−2 s−1 (14-h:10-h light-dark cycle). The seawater was buffered with 20 μM EDTA and enriched in vitamins and trace metals with various concentrations of Fe added. Cultures were maintained under exponential growth conditions, and pCO2/pH in media were manipulated either by addition of ultrapure HCl/NaOH or by bubbling with humidified air/CO2-mixes (carbonate chemistry for the different experiments is shown in Tables S1 and S2). Specific growth rates were determined from linear regressions of the natural log of either fixed POC or Chl a vs. time.
Cellular Fe:C Ratios.
59Fe (as 59FeCl3) and 14C (NaH14CO3) were added to pCO2/pH-adjusted experimental media and allowed to equilibrate overnight before the cells were inoculated (∼0.5 μmol C L−1). Exponentially growing cells were harvested by filtration at a density of ∼20 μmol C L−1. To remove extracellular Fe, cells were washed with an oxalate-EDTA solution (49). 59Fe was measured with a gamma counter and 14C by liquid scintillation counting. Intracellular Fe:C ratios were determined (50), and steady-state Fe-uptake rates were calculated from cellular Fe:C ratios and specific growth rates.
Short-Term Fe Uptake.
The uptake medium was prepared from 0.22 μm filtered Gulf Stream seawater containing 50 nM Fe and 20 μM EDTA. Twenty-milliliter aliquots from each treatment were removed at 1-h intervals for a total period of 3 h for analyzing intracellular Fe.
C and N2 Fixation and H2 Evolution.
Rates of NaH13CO3 uptake and 15N2 incorporation by exponentially growing cells were determined over a 4-h period (between 5 and 9 h after onset of the light) (51, 52). In the 2-h short-term experiments, to distinguish the effect of pCO2 from that of pH on N2 fixation and H2 evolution, the acetylene-reduction assay was used to determine the N2-fixation rate (53), and H2 production was measured with a Peak Performer gas chromatograph (Peak Laboratories, LLC).
Quantification of Total Protein and Western Blotting.
Trichodesmium was collected by filtration onto 5-μm polycarbonate membrane filters, which were flash-frozen in liquid N and then immediately stored at −80 °C until total protein concentration was determined via bicinchoninic acid (BCA) standard procedure (Pierce, Thermo Scientific). The expression of NifH, PsaC, PsbA, and RbcL in Trichodesmium cells was quantified by immunoblot analyses.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation, a grant to the Princeton Environmental Institute from British Petroleum and Ford Motor Co., the Walbridge Fund Graduate Award, and a start-up fund from the State Key Laboratory of Marine Environmental Science (Xiamen University).
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 18255 (volume 109, number 45).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216012109/-/DCSupplemental.
References
- 1.Riebesell U, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407(6802):364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- 2.Riebesell U, et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature. 2007;450(7169):545–548. doi: 10.1038/nature06267. [DOI] [PubMed] [Google Scholar]
- 3.Tortell PD, et al. CO2 sensitivity of Southern Ocean phytoplankton. Geophysical Research Letters. 2008;35(4):L04605. [Google Scholar]
- 4.Shi DL, Xu Y, Hopkinson BM, Morel FMM. Effect of ocean acidification on iron availability to marine phytoplankton. Science. 2010;327(5966):676–679. doi: 10.1126/science.1183517. [DOI] [PubMed] [Google Scholar]
- 5.Hopkinson BM, Xu Y, Shi DL, McGinn PJ, Morel FMM. The effect of CO2 on the photosynthetic physiology of phytoplankton in the Gulf of Alaska. Limnology and Oceanography. 2010;55(5):2011–2024. [Google Scholar]
- 6.Badger MR, et al. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Canadian Journal of Botany. 1998;76(6):1052–1071. [Google Scholar]
- 7.Karl D, et al. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature. 1997;388(6642):533–538. [Google Scholar]
- 8.Webb EA, Jakuba RW, Moffett JW, Dyhrman ST. Molecular assessment of phosphorus and iron physiology in Trichodesmium populations from the western Central and western South Atlantic. Limnology and Oceanography. 2007;52(5):2221–2232. [Google Scholar]
- 9.Moore CM, et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nature Geoscencei. 2009;2(12):867–871. [Google Scholar]
- 10.Chappell PD, Moffett JW, Hynes AM, Webb EA. Molecular evidence of iron limitation and availability in the global diazotroph Trichodesmium. International Society for Microbial Ecology Journal. 2012;6:1728–1739. doi: 10.1038/ismej.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levitan O, et al. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Global Change Biology. 2007;13(2):531–538. [Google Scholar]
- 12.Hutchins DA, et al. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: Implications for past, present, and future ocean biogeochemistry. Limnoloogy and Oceanography. 2007;52(4):1293–1304. [Google Scholar]
- 13.Ramos JBE, Biswas H, Schulz KG, LaRoche J, Riebesell U. 2007. Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Global Biogeochemical Cycles 21(2): GB2028.
- 14.Kranz SA, Sultemeyer D, Richter KU, Rost B. Carbon acquisition by Trichodesmium: The effect of pCO2 and diurnal changes. Limnology and Oceanography. 2009;54(2):548–559. [Google Scholar]
- 15.Kranz SA, et al. Combined effects of CO2 and light on the N2-fixing cyanobacterium Trichodesmium IMS101: Physiological responses. Plant Physiology. 2010;154(1):334–345. doi: 10.1104/pp.110.159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levitan O, Sudhaus S, LaRoche J, Berman-Frank I. The influence of pCO2 and temperature on gene expression of carbon and nitrogen pathways in Trichodesmium IMS101. PLoS ONE. 2010;5(12):e15104. doi: 10.1371/journal.pone.0015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levitan O, et al. Combined effects of CO2 and light on the N2-fixing cyanobacterium Trichodesmium IMS101: A mechanistic view. Plant Physiology. 2010;154(1):346–356. doi: 10.1104/pp.110.159285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitan O, et al. Regulation of nitrogen metabolism in the marine diazotroph Trichodesmium IMS101 under varying temperatures and atmospheric CO2 concentrations. Environmental Microbiology. 2010;12(7):1899–1912. doi: 10.1111/j.1462-2920.2010.02195.x. [DOI] [PubMed] [Google Scholar]
- 19.Lomas MW, et al. Effect of ocean acidification on cyanobacteria in the subtropical North Atlantic. Aquatic Microbial Ecology. 2012;66:211–222. [Google Scholar]
- 20.Fu FX, et al. Interactions between changing pCO2, N2 fixation, and Fe limitation in the marine unicellular cyanobacterium Crocosphaera. Limnology and Oceanography. 2008;53(6):2472–2484. [Google Scholar]
- 21.Law CS, et al. 2012. No stimulation of nitrogen fixation by non-filamentous diazotrophs under elevated CO2 in the South Pacific. Global Change Biology 18(10):3004–3014.
- 22.Chen YB, Zehr JP, Mellon M. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: Evidence for a circadian rhythm. Journal of Phycology. 1996;32(6):916–923. [Google Scholar]
- 23.Berman-Frank I, et al. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science. 2001;294(5546):1534–1537. doi: 10.1126/science.1064082. [DOI] [PubMed] [Google Scholar]
- 24.Berman-Frank I, Cullen JT, Shaked Y, Sherrell RM, Falkowski PG. Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnology and Oceanography. 2001;46(6):1249–1260. [Google Scholar]
- 25.Strzepek RF, Harrison PJ. Photosynthetic architecture differs in coastal and oceanic diatoms. Nature. 2004;431(7009):689–692. doi: 10.1038/nature02954. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado MT, Price NM. Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae) Journal of Phycology. 2001;37(2):298–309. [Google Scholar]
- 27.Shaked Y, Kustka AB, Morel FMM. A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnology and Oceanography. 2005;50(3):872–882. [Google Scholar]
- 28.Kustka AB, Allen AE, Morel FMM. Sequence analysis and transcriptional regulation of iron acquisition genes in two marine diatoms. Journal of Phycology. 2007;43(4):715–729. [Google Scholar]
- 29.Achilles KM, Church TM, Wilhelm SW, Luther GW, Hutchins DA. Bioavailability of iron to Trichodesmium colonies in the western subtropical Atlantic Ocean. Limnology and Oceanography. 2003;48(6):2250–2255. [Google Scholar]
- 30.Rubin M, Berman-Frank I, Shaked Y. Dust- and mineral- iron utilization by the marine dinitrogen-fixer Trichodesmium. Nature Geoscience. 2011;4(8):529–534. [Google Scholar]
- 31.Schubert KR, Evans HJ. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci USA. 1976;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kustka AB, et al. Iron requirements for dinitrogen- and ammonium-supported growth in cultures of Trichodesmium (IMS 101): Comparison with nitrogen fixation rates and iron: carbon ratios of field populations. Limnology and Oceanography. 2003;48(5):1869–1884. [Google Scholar]
- 33.Saito MA, et al. Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph Crocosphaera watsonii. Proc Natl Acad Sci USA. 2011;108(6):2184–2189. doi: 10.1073/pnas.1006943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuit C, Waterbury J, Ravizzaz G. Diel variation of molybdenum and iron in marine diazotrophic cyanobacteria. Limnology and Oceanography. 2004;49(4):978–990. [Google Scholar]
- 35.Fredriksson C, Bergman B. Nitrogenase quantity varies diurnally in a subset of cells within colonies of the nonheterocystous cyanobacteria Trichodesmium spp. Microbiology. 1995;141:2471–2478. [Google Scholar]
- 36.Durner J, Böhm I, Knörzer OC, Böger P. Proteolytic degradation of dinitrogenase reductase from Anabaena variabilis (ATCC 29413) as a consequence of ATP depletion and impact of oxygen. Journal of Bacteriology. 1996;178(3):606–610. doi: 10.1128/jb.178.3.606-610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SJ, Henze S, Lundgren P, Bergman B, Carpenter EJ. Whole-cell immunolocalization of nitrogenase in marine diazotrophic cyanobacteria, trichodesmium spp. Applied and Environmental Microbiology. 1998;64(8):3052–3058. doi: 10.1128/aem.64.8.3052-3058.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman-Frank I, Lundgren P, Falkowski P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Research in Microbiology. 2003;154(3):157–164. doi: 10.1016/S0923-2508(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 39.Castruita M, et al. Overexpression and characterization of an iron storage and DNA-binding Dps protein from Trichodesmium erythraeum. Applied and Environmental Microbiology. 2006;72(4):2918–2924. doi: 10.1128/AEM.72.4.2918-2924.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milligan AJ, Berman-Frank I, Gerchman Y, Dismukes GC, Falkowski PG. Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection. Journal of Phycology. 2007;43(5):845–852. [Google Scholar]
- 41.Nuester J, Vogt S, Newville M, Kustka AB, Twining BS. The unique biogeochemical signature of the marine diazotroph trichodesmium. Frontiers in Microbiology. 2012;3:150. doi: 10.3389/fmicb.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Shi DL, Aristilde L, Morel FMM. The effect of pH on the uptake of zinc and cadmium in marine phytoplankton: Possible role of weak complexes. Limnology and Oceanography. 2012;57(1):293–304. [Google Scholar]
- 43.Kranz SA, Eichner M, Rost B. Interactions between CCM and N2 fixation in Trichodesmium. Photosynth Res. 2011;109(1-3):73–84. doi: 10.1007/s11120-010-9611-3. [DOI] [PubMed] [Google Scholar]
- 44.Rueter JG. Iron stimulation of photosynthesis and nitrogen fixation in Anabaena-7120 and Trichodesmium (Cyanophyceae) Journal of Phycology. 1988;24(2):249–254. [Google Scholar]
- 45.Campbell L, Carpenter EJ, Montoya JP, Kustka AB, Capone DG. Picoplankton community structure within and outside a Trichodesmium bloom in the southwestern Pacific Ocean. Vie et Milieu. 2005;55(3-4):185–195. [Google Scholar]
- 46.Carpenter EJ, Subramaniam A, Capone DG. Biomass and primary productivity of the cyanobacterium Trichodesmium spp. in the tropical N Atlantic ocean. Deep Sea Research Part I Oceanography Research Papers. 2004;51(2):173–203. [Google Scholar]
- 47.Davis CS, McGillicuddy DJ., Jr Transatlantic abundance of the N2-fixing colonial cyanobacterium Trichodesmium. Science. 2006;312(5779):1517–1520. doi: 10.1126/science.1123570. [DOI] [PubMed] [Google Scholar]
- 48.Taboada FG, Gil RG, Hofer J, Gonzalez S, Anadon R. Trichodesmium spp. population structure in the eastern North Atlantic subtropical gyre. Deep Sea Research Part I Oceanography Research Papers. 2010;57(1):65–77. [Google Scholar]
- 49.Tang DG, Morel FMM. Distinguishing between cellular and Fe-oxide-associated trace elements in phytoplankton. Marine Chemistry. 2006;98(1):18–30. [Google Scholar]
- 50.Sunda WG, Huntsman SA. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Marine Chemistry. 1995;50(1-4):189–206. [Google Scholar]
- 51.Orcutt KM, et al. A seasonal study of the significance of N2 fixation by Trichodesmium spp. at the Bermuda Atlantic Time-series Study (BATS) site. Deep Sea Research Part II Topical Studies in Oceanography. 2001;48(8-9):1583–1608. [Google Scholar]
- 52.Mohr W, Grosskopf T, Wallace DWR, LaRoche J. Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE. 2010;5(9):e12583. doi: 10.1371/journal.pone.0012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capone DG. Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure. In: Kemp PF, Sherr B, Sherr E, Cole J, editors. Handbook of Methods in Aquatic Microbial Ecology. New York: Lewis Publishers; 1993. pp. 621–631. [Google Scholar]