Abstract
Emissions from gasoline and diesel vehicles are predominant anthropogenic sources of reactive gas-phase organic carbon and key precursors to secondary organic aerosol (SOA) in urban areas. Their relative importance for aerosol formation is a controversial issue with implications for air quality control policy and public health. We characterize the chemical composition, mass distribution, and organic aerosol formation potential of emissions from gasoline and diesel vehicles, and find diesel exhaust is seven times more efficient at forming aerosol than gasoline exhaust. However, both sources are important for air quality; depending on a region’s fuel use, diesel is responsible for 65% to 90% of vehicular-derived SOA, with substantial contributions from aromatic and aliphatic hydrocarbons. Including these insights on source characterization and SOA formation will improve regional pollution control policies, fuel regulations, and methodologies for future measurement, laboratory, and modeling studies.
Keywords: motor vehicle emission factors, photochemical oxidation, urban air quality, volatile organic compound emissions, petroleum fuel composition
Organic aerosol (OA) in the atmosphere is detrimental to human health and represents a highly uncertain forcing of climate change (1). The use of petroleum-derived fuels is an important source of reactive gas-phase organic carbon that provides key precursors to the formation of secondary OA (SOA) and tropospheric ozone (1). Controlling these emissions from gasoline and diesel vehicles is central to air quality mitigation policies in urban areas (2). Previous work has concluded that further research is necessary to elucidate all organic sources of SOA precursors (3, 4). Significant controversy exists over the contributions of precursors from gasoline and diesel vehicles, and the relative importance of each for SOA formation remains in question, in part because of insufficient chemical characterization of fuels and emissions, and the difficulty of ambient measurements of gas-phase compounds emitted from diesel sources (1, 4–8).
In the United States, diesel fuel accounts for 21% of on-road fuel use (by volume), with off-road sources increasing total use to 28% diesel. In California, the diesel share of on-road use ranges from approximately 10% in coastal cities to more than 30% in agricultural regions (SI Appendix, Table S1) (2, 9, 10). Noncombusted hydrocarbons from the fuels are emitted in the exhaust of gasoline and diesel engines, and also via evaporation from gasoline vehicles and service stations. These compounds in unburned gasoline and diesel fuel dominate vehicular emissions of reactive gas-phase carbon that have the potential to form SOA (11, 12). Previous work has shown nontailpipe emissions account for ∼30% of gasoline-related emissions in urban regions, but limited work exists constraining the emissions and SOA formation potential of gas-phase organic carbon from gasoline and diesel sources (13). By using extensive fuel analyses and field data from two sites that include many compounds with no previous in situ measurements, we present the most comprehensive data to date on the chemical composition, mass distribution, emissions, and SOA formation potential of nontailpipe gasoline, gasoline exhaust, and diesel exhaust. We determine the relative importance of gasoline and diesel sources for SOA formation in, and downwind of, urban regions. We assess these results in the context of other studies during the past decade and discuss their significant implications for air pollution measurement, modeling, and control.
Results and Discussion
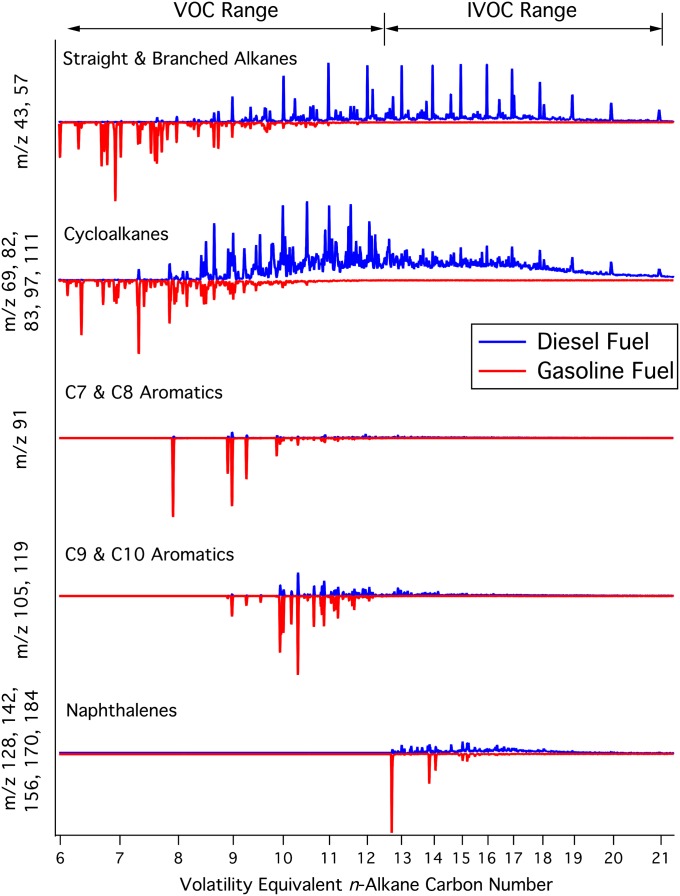
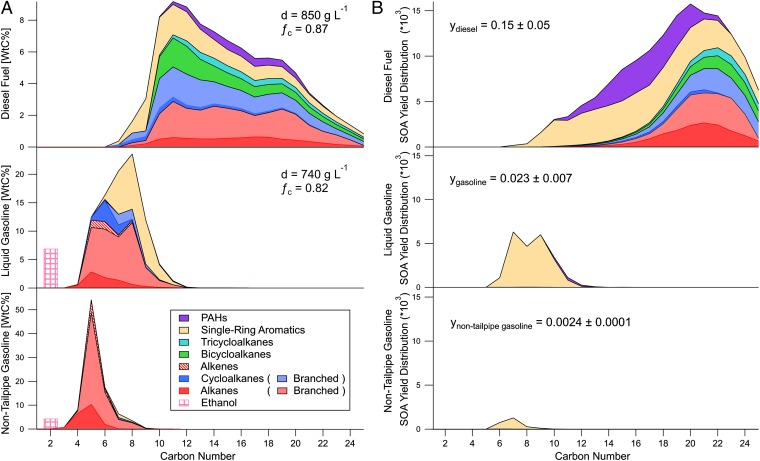
A total of 40 gasoline and 12 diesel fuel samples from California were collected (coincident with field data) and characterized by using several gas-chromatography methods, yielding comprehensive speciation of the “unresolved complex mixture” in diesel fuel. This was accomplished by using soft photoionization techniques, and provides unprecedented detail on the molecular identification and mass distribution of hydrocarbons in diesel fuel (14). Gasoline and diesel fuel, and thus their emissions of unburned hydrocarbons, can be classified by vapor pressure and span the volatile organic compound (VOC) range and the less volatile intermediate-volatility organic compound (IVOC) range (Fig. 1). Gasoline hydrocarbons fall mostly within the VOC range, with some aromatics extending into the IVOC range, whereas only 30% of diesel fuel hydrocarbons are in the VOC range. Diesel fuel is widely distributed across molecules containing 8 to 25 carbon atoms with a peak around 10 to 13 carbon atoms (Fig. 2A). This peak is a result of aromatics and cycloalkanes, as straight and branched alkanes are evenly distributed between 10 and 20 carbon atoms. Aromatic and aliphatic hydrocarbons make up 23% and 68% of diesel fuel, respectively. By comparison, gasoline contains ∼30% aromatics, with the remainder of the nonethanol fraction dominated by straight and branched alkanes with less than 10 carbon atoms (Table 1 and Fig. 2A).
Fig. 1.
Distributions of chemical classes for diesel (blue) and gasoline (red) are distinct with some overlap as shown via GC/MS for representative fuel samples. Fuels span both the VOC and IVOC volatility ranges. Chemical classes are represented by their dominant mass fragments and shown as a function of n-alkane carbon number.
Fig. 2.
Distribution of mass (A) and SOA formation potential (in μg SOA⋅μg−1; B) in diesel and gasoline fuel (representative of exhaust) and nontailpipe gasoline emissions. Distributions in A and B are colored by chemical class. Fuel properties (density, carbon fraction) and bulk SOA yields (at an organic particle loading of 10 μg⋅m−3) are superposed on A and B, respectively. Predicted SOA from gasoline exhaust is much lower than diesel and dominated solely by aromatic content, whereas diesel SOA is produced from a mix of aromatic and aliphatic compounds. A distribution of the SOA potential uncertainties is provided in SI Appendix, Fig. S5.
Table 1.
Distribution of mass and SOA potential by chemical class for diesel exhaust, gasoline exhaust, and nontailpipe gasoline
| Weight by carbon, wtC% |
Potential SOA formation, wt% |
|||||
| Compound class | Diesel exhaust | Gasoline exhaust | Non-tailpipe gasoline | Diesel exhaust | Gasoline exhaust | Non-tailpipe gasoline |
| Total aliphatic | 68 ± 8 | 58 ± 2 | 85 ± 4 | 47 ± 4 | 0.38 ± 0.07 | 0.9 ± 0.4 |
| Straight-chain alkanes | 7 ± 1 | 7.7 ± 0.3 | 20 ± 1 | 11 ± 2 | 0.09 ± 0.003 | 0.02 ± 0.001 |
| Branched alkanes | 23 ± 2 | 40 ± 1 | 60 ± 3 | 14 ± 2 | 0.12 ± 0.003 | 0.13 ± 0.01 |
| Cycloalkanes (single straight alkyl chain) | 2.5 ± 0.2 | 4.3 ± 0.1 | 1.03 ± 0.04 | 1.2 ± 0.3 | 0.13 ± 0.07 | 0.7 ± 0.4 |
| Cycloalkanes [branched/multiple alkyl chain(s)] | 18 ± 2 | 6.2 ± 0.3 | 5.0 ± 0.2 | 11 ± 2 | 0.04 ± 0.02 | 0.05 ± 0.03 |
| Bicycloalkanes | 13 ± 1 | 0 | 0 | 6 ± 1 | 0 | 0 |
| Tricycloalkanes | 4.8 ± 0.6 | 0 | 0 | 4 ± 1 | 0 | 0 |
| Single-ring aromatics | 19 ± 2 | 29 ± 1 | 2.7 ± 0.1 | 36 ± 9 | 96 ± 22 | 99 ± 6 |
| Polycyclic aromatic compounds | 4 ± 2 | 0.32 ± 0.02 | 0.0003 | 17 ± 8 | 3.2 ± 0.9 | 0.01 ± 0.01 |
| Alkenes (straight, branched, cyclic) | 0 | 3.6 ± 0.1 | 7.4 ± 0.3 | 0 | 0 | 0 |
| Ethanol | 0 | 6.9 ± 0.5 | 4.4 ± 0.4 | 0 | 0 | 0 |
The wt% by total mass for each source can be found in the SI Appendix, Table S2.
To examine contributions from each source to reactive gas-phase organic carbon in the ambient atmosphere and on-road emissions measured in a roadway tunnel, we used a chemical mass balance model with effective variance weighting on overconstrained least-squares regressions (SI Appendix) (15). The model uses a subset of measured compounds and capitalizes on differences in the chemical composition of sources to assess the magnitude of total noncombusted hydrocarbon emissions from each source. The source profiles used as a priori information are constructed from liquid fuel data to represent gasoline and diesel exhaust, and vapor-liquid equilibrium calculations to represent nontailpipe gasoline emissions. Equivalent chemical composition in exhaust and liquid fuel has been reported previously for gasoline and is demonstrated in this work for gasoline and diesel at both measurement sites (SI Appendix, Fig. S1) (16). Extensive diagnostics were used to assess model performance, including comparisons against independent compounds to confirm the model’s ability to predict the behavior of reactive VOCs and IVOCs emitted by gasoline and diesel sources (SI Appendix, Figs. S2–S4).
Emission factors for noncombusted gas-phase organic carbon in exhaust were determined to be 0.38 ± 0.11 gC⋅L−1 for gasoline and 0.86 ± 0.25 gC⋅L−1 for diesel vehicles, which are consistent with values calculated by using California’s emissions model for the same period (17). With respect to contributions of noncombusted hydrocarbons from gasoline and diesel exhaust, diesel accounted for 24% at the tunnel study in a coastal city, compared with 56% in the urban center of an agricultural region. Accounting for differences in emission factors and fuel densities, this is consistent with on-road fuel sales data in both regions—11% and 33% diesel fuel by volume, respectively (SI Appendix, Table S1) (10).
To assess the importance of gasoline and diesel sources for SOA in urban areas, we calculated bulk SOA yields for all three sources and compared them in context of our emission factors and source contributions. Data on SOA yields are limited for many of the hydrocarbons; the mass fraction of diesel, gasoline, and nontailpipe gasoline emissions that have unknown yields are 66%, 25%, and 7%, respectively. Thus, we modeled high-NOx SOA yields by using published data (where available) and an estimation of yields and uncertainties for unknown values based on best estimates from various plausible scenarios (Fig. 2B and SI Appendix, Fig. S5).
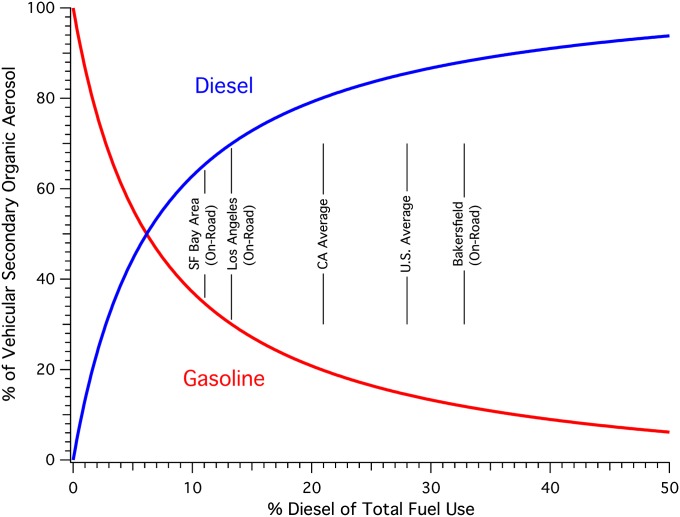
For the same mass of unburned fuel emissions reacted, diesel exhaust forms 6.7 ± 2.9 times more SOA than gasoline exhaust (bulk SOA yields of 0.15 ± 0.05 and 0.023 ± 0.007 μgSOA⋅μg−1, respectively). Considering differences in emission factors, diesel exhaust is expected to form 15 times more SOA than gasoline per liter of fuel burned. For populated regions with 10% to 30% diesel fuel use, this implies that diesel exhaust is responsible for two to seven times more SOA than gasoline exhaust (Fig. 3). Nontailpipe gasoline emissions were 39% to 77% lower than gasoline exhaust emissions and produce negligible SOA as a result of a substantially lower yield (0.0024 ± 0.0001).
Fig. 3.
The percent contribution of gasoline and diesel exhaust to SOA over 0% to 50% diesel fuel use demonstrates the predominance of diesel sources for SOA formation. SOA contributions form the two sources are equivalent at 6% diesel fuel use. The United States and California state averages shown are based on total on- and off-road use. The urban areas in California shown are for on-road fuel use only; off-road contributions will increase the diesel fraction of total use by several percent, but are not available at this scale.
Our methods also allowed us to examine the most important chemical classes and mass distribution of SOA formation. The vast majority of SOA from gasoline sources is a result of its aromatic content, whereas diesel SOA is predicted to be 47 ± 7% from aliphatics, with the remainder from aromatics (Fig. 2B and Table 1).
Regional estimates of daytime SOA concentrations from diesel and gasoline using our model results and calculated SOA yields are consistent with independent positive matrix factor analysis results for aromatic and aliphatic SOA from fossil fuel combustion in the San Joaquin Valley using aerosol MS (AMS) and Fourier transform IR spectroscopy (FTIR) measurements. Based on our model results, we expect an average of 1.3 ± 0.4 μgOA⋅m−3 from motor vehicles compared with average 1.0-μm particulate matter concentrations of 1.8 to 2.1 μg OA⋅m−3 from FTIR and AMS data, respectively (18). These independent data also support the predominance of diesel SOA in the San Joaquin Valley, as young aerosol (O:C ratios of 0.27–0.36) was 58% aliphatic and 42% aromatic (18).
SOA models have made considerable progress by using a parameterization known as the volatility basis set to estimate contributions from unmeasured intermediate and semivolatile compounds (5, 19). Together with traditional explicit models for individual hydrocarbons in the VOC range, models are better able to predict the magnitude of observed SOA, but not all temporal patterns or physical/chemical characteristics (3, 19, 20). Here we evaluate the inclusion of SOA precursors in these models and their distribution in gasoline and diesel exhaust. Aromatics with single or multiple rings have rightfully received considerable attention historically, but their distribution between gasoline and diesel emissions has been relatively unexplored. Gasoline exhaust dominates emissions of C7 and C8 aromatics. C9 aromatic content is four times greater in gasoline than in diesel, and there are nearly equivalent amounts of C10 aromatics. For an urban region with 15% diesel fuel use, this implies that gasoline emits more than 90% of the C9 aromatics and 75% of the C10 aromatics. Gasoline SOA from C9 and C10 aromatics represent 26% and 14% of total SOA from gasoline, respectively, and C9–11 aromatics represent 5% of SOA from diesel exhaust (Table 1). Emissions of naphthalene and similar small polycyclic aromatic hydrocarbons (PAHs) are shared by gasoline and diesel vehicles, but represent only a minor contribution to potential SOA formation as a result of their minor weight fractions in the fuels (Fig. 2 and SI Appendix, Tables S9 and S10).
We examined the compounds included in SOA models and found that 20% to 30% of the SOA formed from gasoline exhaust was not included in recent urban studies (21–23) (SI Appendix). Given the contributions of C9–11 aromatics to SOA formation from gasoline and diesel vehicles, it is important that they are better represented in explicit traditional SOA models or the extension of volatility basis set modeling to include the 107 and 108 μg⋅m−3 effective saturation concentration (C°) bins that fall in the VOC range (SI Appendix, Fig. S6) (5, 19, 22). For recent urban studies, we scaled up traditional compound-explicit SOA models (without the volatility basis set) to include the missing 20% to 30% of gasoline SOA and contributions from diesel (assuming 15% diesel fuel use) and calculated a fivefold increase in modeled SOA from vehicular exhaust. Such an inclusion dramatically improves model closure, which has typically underestimated SOA in urban regions by 80% to 90% (19), but additional contributions from other sources of SOA precursors remain critical to model all observed SOA. Further chamber and modeling studies on SOA yields of aromatics with nine or more carbon atoms are important to reduce uncertainties in the SOA-forming potential of gasoline and diesel exhaust emissions and their overall contribution to SOA in urban regions. Additional studies on the SOA yields of cyclic alkanes with five- and six-membered rings are also of interest because they are unstudied and represent 37% of diesel and 11% of gasoline fuel.
In 1993, with the goal of mitigating emissions of particulates and nitrogen oxides, California regulated diesel fuel to have less than 10% single-ring aromatics and 1.4% polycyclic aromatic hydrocarbons, but concerns about engine performance and the cost of fuel production led the state to allow higher aromatic levels in diesel fuel (24) (SI Appendix). It is evident from our data (Table 1) that the vast majority of diesel fuels sold in California are certified alternative formulations that contain nearly double the aromatic content than initial regulations intended. Although the fuel regulations were designed to help control primary particulate emissions (i.e., black carbon), this enhancement of aromatic content in diesel fuel increases the SOA potential of diesel emissions, especially for hydrocarbons with 9 to 17 carbon atoms. Significant progress is being made to improve heavy-duty diesel engine performance with postcombustion control technology, which may affect emissions of gas-phase organic carbon, but it is clear that attention to gasoline and diesel fuel composition and emissions of reactive organic gases is necessary to control SOA precursor contributions from all vehicle classes. Furthermore, this work has focused on organic carbon emissions originating from fuels, but emissions of unburned motor oil from both gasoline and diesel vehicles represent an additional source of organic carbon. Although total consumption of oil is minor relative to fuel, oil contributes gas and particle-phase compounds with lower volatilities than diesel fuel and should continue to be monitored in field, laboratory, and modeling studies.
Comparing observed concentrations of OA to carbon monoxide has been insightfully used in numerous past studies to examine primary emissions of vehicular (and other anthropogenic) OA and track the formation of SOA in the atmosphere (6, 21, 25–30). ΔOA/ΔCO ratios, and slopes determined from the regression of these values, originate at smaller values following emission before any atmospheric processing. Over the course of 1 to 2 d, ratios change dynamically and increase with the formation of SOA and further atmospheric processing, as the lifetime of CO is relatively long compared with the other processes. A diverse range of ΔOA/ΔCO slopes has been observed across the studies in which this technique has been used (30). In the context of this method, we review data from numerous field campaigns with our observations and SOA yield modeling results to assess the behavior of vehicular OA and the contribution of nonvehicular SOA to total observed OA. We calculate predicted ΔOA/ΔCO ratios from oxidized gasoline and diesel emissions, and the combined effect of emissions from both these sources and photochemical processing in the atmosphere. We also evaluate differences in ΔOA/ΔCO ratios between the week and weekend, and assess the suitability of using solely ΔOA/ΔCO slopes to determine the contributions of gasoline and diesel sources to SOA.
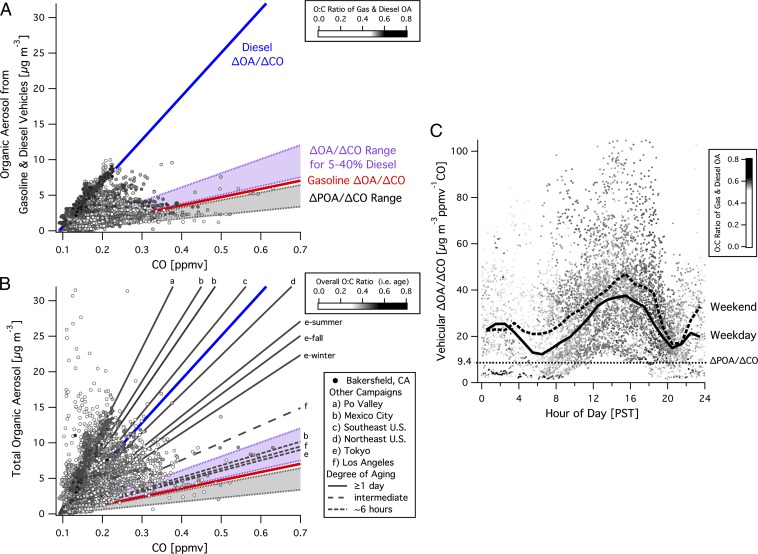
By using derived SOA yields and emission factors for reactive gas-phase organic carbon and CO, we predict ΔOA/ΔCO ratios for a mixture of gasoline and diesel fuel use for comparison with our observations in the San Joaquin Valley (Bakersfield, CA) and other urban studies during the past decade (Fig. 4 and SI Appendix). Predicted ΔOA/ΔCO slopes for a range of typical fuel use are consistent with observed ΔOA/ΔCO values in Los Angeles, Tokyo, and Mexico City after initial SOA formation occurring in the first 6 h of processing (Fig. 4B) (6, 25, 26). We predict “young” ΔOA/ΔCO ratios well, but as air masses develop from a relatively young photochemical age of ∼6 h to ∼1 d, ΔOA/ΔCO ratios increase. A three- to fourfold increase was observed in Mexico City, and the effect of increased processing can also be observed in Tokyo, where ΔOA/ΔCO slopes for multiple seasons depict a clear seasonal trend, with the greatest slope occurring in the summer for processed air parcels, whereas less-processed parcels remain consistent with expected ratios for a mix of gasoline and diesel emissions (19, 25, 26).
Fig. 4.
Comparisons of OA vs. CO show the behavior of primary and secondary OA in the atmosphere and are used to examine vehicular OA and total OA in the San Joaquin Valley (Bakersfield, CA) and numerous other urban sites (6, 18, 21, 25–29). Photochemical aging increases ΔOA/ΔCO ratios and is represented by increased O:C ratios shaded in each panel. (A) Best estimates for ΔOA/ΔCO ratios expected for pure gasoline and diesel emissions are added to a ΔPOA/ΔCO value of 9.4 μg⋅m−3⋅ppmv−1 CO to account for primary OA and shown with a range of ΔPOA/ΔCO values (21, 30). Vehicular OA is determined from AMS factor analysis and observations are well constrained at Bakersfield with the exception of the most aged air parcels, whose ΔOA/ΔCO ratios are greater than expected for the mix of gasoline and diesel use. (B) Predicted ΔOA/ΔCO slopes for a range of fuel mixtures ranging from 5% to 40% diesel agree with observations of relatively young aerosol in urban areas and vehicular OA at Bakersfield. Observed ΔOA/ΔCO ratios increase with degree of aging and/or the influence of other SOA precursor sources that do not emit CO, which are prominent at Bakersfield and sites a–c. (C) Weekday and weekend diurnal averages of vehicular ΔOA/ΔCO show greater ratios in the afternoon and over the weekend as a result of increased photochemical aging. Ratios are calculated with a 90 ppbv CO background, and SDs are shown in SI Appendix, Fig. S8.
In the San Joaquin Valley, the increase in ΔOA/ΔCO ratios appears to be coincident with the transition of young semivolatile aerosols to more aged aerosols with lower volatility as shown by the increase in O:C ratios that peaks with ΔOA/ΔCO ratios in the afternoon (Fig. 4) (3, 19, 26). Similarly, a greater fraction of low-volatility OA was observed in the summer in Tokyo (3). Aged ΔOA/ΔCO ratios exceed our predictions despite the consistency demonstrated earlier in this work between average daytime observed vehicular OA concentrations measured by AMS with predicted SOA from motor vehicles. This suggests that the comprehension of all OA transformation processes is incomplete and further work remains to understand the development of low-volatility OA observed in urban plumes globally, a conclusion supported by recent observations and consideration of other mechanisms (3, 20, 31, 32).
Examining differences between weekdays and weekends is another common and insightful metric for assessing emissions and chemical processes. We observed no weekday/weekend difference in the distribution of emissions between gasoline and diesel exhaust in Bakersfield, as daytime values of both decreased by ∼40% over the weekend (SI Appendix, Fig. S7). However, weekend OA concentrations (total and vehicular) were greater as a result of increased photochemical aging evidenced by higher ΔOA/ΔCO ratios (Fig. 4C and SI Appendix, Fig. S8). Recent work focused on Los Angeles reported that gasoline is vastly more important than diesel as a source of SOA precursors based on the observation that weekend ΔOA/ΔCO slopes were marginally similar to weekday slopes, with similar photochemical ages despite large differences in diesel activity (6). Similar to Los Angeles, OA concentrations and ΔOA/ΔCO ratios are higher in Bakersfield over the weekend, but this occurs despite no change in the relative use of gasoline and diesel, suggesting that increased OA at both locations over the weekend is a function of decreased diesel NOx emissions leading to faster photochemical processing, and is independent of changes in the mix of fuel use (33). The ubiquitous increase in ΔOA/ΔCO ratios with increased processing for vehicular and total OA is independent of the mixture of gasoline and diesel, and ΔOA/ΔCO slopes alone are insufficient to discern organic SOA precursor contributions from gasoline vs. diesel given the variability in Los Angeles measurements (SI Appendix, Fig. S9) (6).
Nonvehicular anthropogenic and biogenic sources also lead to elevated ΔOA/ΔCO ratios with higher slopes occurring in regions with large nonvehicular sources, such as Mexico City, the Southeast United States, and the Po Valley of Italy (Fig. 4B). ΔOA/ΔCO ratios in the San Joaquin Valley span a broad range of values observed at other sites and the importance of other SOA sources is supported by elevated ΔOA/ΔCO ratios in aged air masses and episodic contributions of low O:C OA from other sources (Fig. 4B and SI Appendix, S10) (6, 21, 25–29).
Our expanded measurement capabilities for gasoline and diesel compounds in the liquid fuels and the ambient atmosphere produce a more complete picture of SOA formation from motor vehicles. We provide the ability to predict emissions of SOA precursors and SOA formation that is consistent with fuel use data and ambient measurements. SOA from diesel sources outweighs gasoline contributions, and other sources provide significant precursors in many urban regions. The inclusion of our insights will allow for the development of more effective pollution control policies and inform the design of future studies in the ambient atmosphere, laboratory experiments, and modeling efforts.
Materials and Methods
In situ gas-phase organic carbon measurements were made by using an automated sampling system coupled to a gas chromatograph and mass spectrometer at the Caldecott Tunnel in Oakland, CA, and in Bakersfield, CA, as part of the California at the Nexus of Air Quality and Climate Change (CalNex) campaign. Offline analyses used gas chromatography and MS to analyze the composition of gasoline and diesel fuels. Estimates of bulk SOA yields for each source and SOA yields for precursors without established yields were calculated via a Monte Carlo analysis by using all appropriate laboratory and modeling data. OA in Bakersfield was measured in situ with AMS and offline via FTIR analysis of filter samples. SI Appendix provides further detail on the instrumentation, analytical methods, and results presented in this work.
Supplementary Material
Acknowledgments
We thank Izadyar Dalvand, Kelsey Boulanger, Brian McDonald, Raymond Lo, and Trevor Ford, as well as staff of the California Air Resources Board, University of California Cooperative Extension (Kern County), and Caldecott Tunnel for their help on various aspects of the data collection and analysis. This work was supported by California Air Resources Board Grant 08-316, US Environmental Protection Agency (EPA) Grant RD834553, EPA Science To Achieve Results Grant FP-91781901-0, US Department of Energy Laboratory-Directed Research and Development Program of Lawrence Berkeley National Laboratory Grant DE-AC02-05CH11231, and National Oceanic and Atmospheric Administration Grant NA10OAR4310104.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212272109/-/DCSupplemental.
References
- 1.Hallquist M, et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos Chem Phys. 2009;9:5155–5236. [Google Scholar]
- 2. US Environmental Protection Agency (2008) U.S. Clean Air Act. (And subsequent amendments/rulings). Available at http://www.gpo.gov/fdsys/pkg/USCODE-2008-title42/pdf/USCODE-2008-title42-chap85.pdf.
- 3.Jimenez JL, et al. Evolution of organic aerosols in the atmosphere. Science. 2009;326(5959):1525–1529. doi: 10.1126/science.1180353. [DOI] [PubMed] [Google Scholar]
- 4.Worton DR, Gentner DR, Isaacman G, Goldstein AH. Embracing complexity: Deciphering origins and transformations of atmospheric organics through speciated measurements. Environ Sci Technol. 2012;46(10):5265–5266. doi: 10.1021/es301199y. [DOI] [PubMed] [Google Scholar]
- 5.Robinson AL, et al. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science. 2007;315(5816):1259–1262. doi: 10.1126/science.1133061. [DOI] [PubMed] [Google Scholar]
- 6.Bahreini R, et al. Gasoline emissions dominate over diesel in formation of secondary organic aerosol mass. Geophys Res Lett. 2012;39:L06805. [Google Scholar]
- 7.Weitkamp EA, Lambe AT, Donahue NM, Robinson AL. Laboratory measurements of the heterogeneous oxidation of condensed-phase organic molecular makers for motor vehicle exhaust. Environ Sci Technol. 2008;42(21):7950–7956. doi: 10.1021/es800745x. [DOI] [PubMed] [Google Scholar]
- 8.Pye HOT, Pouliot GA. Modeling the role of alkanes, polycyclic aromatic hydrocarbons, and their oligomers in secondary organic aerosol formation. Environ Sci Technol. 2012;46(11):6041–6047. doi: 10.1021/es300409w. [DOI] [PubMed] [Google Scholar]
- 9.US Energy Information Administration 2010. Prime Supplier Sales Volumes. Available at http://www.eia.gov/dnav/pet/pet_cons_prim_dcu_nus_a.htm. Accessed May 2012.
- 10.California Department of Transportation California Motor Vehicle Stock Travel, and Fuel Forecast (MVSTAFF) 2008 Report. 2008. Available at http://www.dot.ca.gov/hq/tsip/otfa/tab/documents/mvstaff/mvstaff08.pdf. Accessed April 2012.
- 11.Kirchstetter TW, Singer BC, Harley RA. Impact of oxygenated gasoline use on california light-duty vehicle emissions. Environ Sci Technol. 1996;30:661–670. [Google Scholar]
- 12.Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 2. C1 through C30 organic compounds from medium duty diesel trucks. Environ Sci Technol. 1999;33:1578–1587. [Google Scholar]
- 13.Gentner DR, Harley RA, Miller AM, Goldstein AH. Diurnal and seasonal variability of gasoline-related volatile organic compound emissions in Riverside, California. Environ Sci Technol. 2009;43(12):4247–4252. doi: 10.1021/es9006228. [DOI] [PubMed] [Google Scholar]
- 14.Isaacman G, et al. Improved resolution of hydrocarbon structures and constitutional isomers in complex mixtures using gas chromatography-vacuum ultraviolet-mass spectrometry. Anal Chem. 2012;84(5):2335–2342. doi: 10.1021/ac2030464. [DOI] [PubMed] [Google Scholar]
- 15.Watson JG, Cooper JA, Huntzicker JJ. The effective variance weighting for least squares calculations applied to the mass balance receptor model. Atmos Environ. 1984;18:1347–1355. [Google Scholar]
- 16.Leppard WR, et al. Effects of Gasoline Composition on Vehicle Engine-Out and Tailpipe Hydrocarbon Emissions. Technical Paper Series no. 920329. Warrendale, PA: Society of Automotive Engineers; 1992. [Google Scholar]
- 17. California Air Resources Board (2011) Motor Vehicle Emission Factor/Emission Inventory Model - EMFAC 2011. Available at http://www.arb.ca.gov/msei/msei.htm. Accessed April 2012.
- 18.Liu S, et al. Secondary organic aerosol formation from fossil fuel sources contribute majority of summertime organic mass at Bakersfield. J Geophys Res. 2012 [Google Scholar]
- 19.Hodzic A, et al. Modeling organic aerosols in a megacity: Potential contribution of semi-volatile and intermediate volatility primary organic compounds to secondary organic aerosol formation. Atmos Chem Phys. 2010;10:5491–5514. [Google Scholar]
- 20.Cappa CD, Wilson KR. Multi-generation gas-phase oxidation, equilibrium partitioning, and the formation and evolution of secondary organic aerosol. Atmos Chem Phys Discuss. 2012;12:3295–3356. [Google Scholar]
- 21.de Gouw JA, et al. Sources of particulate matter in the northeastern United States: 1. Direct emissions and secondary formation of organic matter in urban plumes. J Geophys Res. 2008;113:D08301. [Google Scholar]
- 22.Dzepina K, et al. Modeling the multiday evolution and aging of secondary organic aerosol during MILAGRO 2006. Environ Sci Technol. 2011;45(8):3496–3503. doi: 10.1021/es103186f. [DOI] [PubMed] [Google Scholar]
- 23.Johnson D, et al. Simulating regional scale secondary organic aerosol formation during the TORCH 2003 campaign in the southern UK. Atmos Chem Phys. 2006;6:403–418. [Google Scholar]
- 24. California Air Resources Board California Diesel Fuel and Reformulated Gasoline Regulations. [with subsequent amendments (2004, 2008) and certified alternative diesel formulations (1993)]. Available at http://www.arb.ca.gov/fuels/diesel/081404dslregs.pdf, http://www.arb.ca.gov/fuels/gasoline/082908CaRFG_regs.pdf, http://www.arb.ca.gov/fuels/diesel/diesel_alt.htm. Accessed April 2012.
- 25.Takegawa N, et al. Seasonal and diurnal variations of submicron organic aerosol in Tokyo observed using the Aerodyne aerosol mass spectrometer. J Geophys Res. 2006;111:D11206. [Google Scholar]
- 26.Kleinman KI, et al. The time evolution of aerosol composition over the Mexico City plateau. Atmos Chem Phys. 2008;8:1559–1575. [Google Scholar]
- 27.Weber RJ, et al. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. J Geophys Res. 2007;112:D13302. [Google Scholar]
- 28.DeCarlo PF, et al. Fast airborne aerosol size and chemistry measurements above Mexico City and central Mexico during the MILAGRO Campaign. Atmos Chem Phys. 2008;8:4027–4048. [Google Scholar]
- 29.Crosier J, et al. Chemical composition of summertime aerosol in the Po Valley (Italy), northern Adriatic and Black Sea. Q J R Meteorol Soc. 2007;113:61–75. [Google Scholar]
- 30.de Gouw JA, Jimenez JL. Organic aerosols in the Earth’s atmosphere. Environ Sci Technol. 2009;43(20):7614–7618. doi: 10.1021/es9006004. [DOI] [PubMed] [Google Scholar]
- 31.Ervens B, Turpin BJ, Weber RJ. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos Chem Phys. 2011;11:11069–11102. [Google Scholar]
- 32.Rollins AW, et al. Nighttime growth of particulate organic nitrates: A significant source of atmospheric secondary organic aerosols. Science. 2012;337(6099):1210–1212. [Google Scholar]
- 33.Russell AR, Valin LC, Bucsela EJ, Wenig MO, Cohen RC. Space-based constraints on spatial and temporal patterns of NO(x) emissions in California, 2005-2008. Environ Sci Technol. 2010;44(9):3608–3615. doi: 10.1021/es903451j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.