Abstract
The aetiology of seborrheic keratoses (SK), the most common benign epithelial tumours, and any relationship with malignancy are not yet known. As a protective anti-cancer mechanism, apoptosis reflects cellular loss as a reaction to proliferative activity. The objective of this study was to quantify apoptosis in different SK types (acanthotic, hyperkeratotic, reticulated, irritated and clonal) and correlate the dermoscopic picture with apoptosis rate. After a qualitative and quantitative analysis of dermoscopic images, we defined a new quantitative dermoscopic score (C3V2F, crypts, millia cysts, colours, hairpin vessels, irregular vessels, fissures) from 0 to 22, which enabled us to establish cut-offs correlating with apoptosis rates. All five SK forms were associated with lower apoptosis rates compared with normal skin. A C3V2F score >10 and greater number of crypts and colours reflected a higher apoptosis rate, which implies a benign character of evolution. In contrast, the presence of irregular vessels on more than 50% of the lesion surface implied a lower rate of apoptosis and probably associated with a risk of malignant transformation. On the basis of dermoscopic information, we used multiple regression to establish a model for estimating the rate of apoptosis with a 0.7 prediction interval (approximately 1S.D.). The new C3V2F score could be valuable for the clinical evaluation of possible SK prognosis and decisions regarding excision.
Keywords: dermoscopy, carcinogenesis, apoptosis, skin tumours, seborrheic keratoses
Introduction
Seborrheic keratoses (SK) are the most common benign epithelial tumours [1,2]. The aetiology of SK is not yet established, and the relationship with malignancy is unclear, at least for certain types, such as solitary [[3],[4],,[5]] or multiple, as in the Leser-Trélat sign, a rare paraneoplastic cutaneous syndrome [6]. Three main pathological features, which were described by Freudenthal in 1926, are present to various extents in different clinico-pathological forms: basal and squamous cell proliferation, basal cell hyperpigmentation, and lymphocytic dermal and epidermal cell infiltrate [[7],[8],,[9]]. Some SK include orthokeratotic corneous globes, which resemble the eosinophilic globes of squamous cell carcinoma (SCC) [10] in the pathological picture. An uncertain clinical or dermoscopic aspect or repetitive change in the same lesion upon different examinations requires excision of the tumour.
Apoptosis is regarded as a protective anti-cancer mechanism and reflects cellular loss as a reaction to proliferative activity [11]. In skin, as in other organs, one of the main functions of apoptosis in addition to the regulation of tissue homeostasis is the elimination of cells with damaged DNA, including premalignant cells [12,13]. Reduced apoptosis seems to be a prerequisite for melanoma formation [14]. However, the role of apoptosis in different skin lesions has not yet been elucidated because, for example, apoptosis has been documented in basal cell carcinoma (BCC), in which premalignant cells have not been described [15]. Therefore, a reasonable assumption is that the risk for malignancy in different pathological forms of solitary SK is related to cellular apoptosis rates and distribution. Correlation of standard dermoscopic features with apoptosis levels would allow rapid orientation for clinicians experienced in dermoscopy. This knowledge could be valuable for pre-operative decisions about benign lesions with possible malignant associations.
In the this study, we aimed to quantify apoptosis in different pathological types of SK and correlate the dermoscopic picture with the apoptosis rate to allow for quick guidance and a more detailed clinical diagnosis (a ‘first glance’ approach). We assumed that the pathological forms of SK might evolve differently, depending on the lesions classified by dermoscopic examination and apoptosis rates.
Materials and methods
Tissue collection
We obtained ethics approval from the Ethical Committee of ‘Victor Babes’ National Institute of Pathology, Bucharest, Romania. Incisional and excisional biopsy samples were obtained at the same centre from 34 lesions from 32 patients clinically diagnosed with SK and from four control participants (normal skin). Written consent was obtained from each patient. The age of the patients was 28–72 years and included 20 women and 12 men. The age of the controls was 31–66 years and included three women and one man.
Histology analysis
All fresh samples were fixed in 4% paraformaldehyde at 4°C for 5 days. Specimens were included in paraffin and histology analysis by routine haematoxylin & eosin (HE) staining performed at the Dermatopathology Laboratory of ‘Victor Babes’ National Institute of Pathology, Bucharest, Romania. SK specimens (n = 34) from 32 patients (30 solitary, 2 multiple SK) were histopathologically confirmed by the same dermatopathologist using HE staining.
Dermoscopic analysis
All lesions were dermoscopically analysed using a contact dermoscope (Heine Delta 20) attached to an Olympus E 330 camera with Immersiosal as the contact oil. Images were captured and stored electronically. Qualitative and quantitative analysis was performed for each image. Seventeen qualitative parameters were analysed, receiving a score of 0 if they were absent and a score of 1 if present: moth-eaten border, fingerprinting structures, network-like pattern, network pattern, crypts (comedo-like openings), millia cysts, pinpoint vessels, hairpin vessels, fat fingers, sharp demarcation, blue-white pigmentation, more than one colour, cerebriform structure, irregular (polymorphous) pattern, fissures, white artefacts, and irregular vessels. Six quantitative parameters (a–f) were analysed and received different scores according to an original algorithm, C3V2F (crypts, millia cysts, colours, hairpin vessels, irregular vessels, fissures, Fig. 1). The regions in (c) refer to separate groups of clustered vessels. This algorithm encompasses two different parameters of the vessels: typical hairpin vessels and irregular vessels. This difference was used to better differentiate SK from melanoma in clinical practice. The C3V2F score could range from 0 to 22.
Fig 1.
Qualitative (left panel) and quantitative (right panel, C3V2F score) dermoscopic parameters.

Apoptosis detection and quantification
Skin sections (10-μm thick) were deparaffinized, washed in PBS, and then either stained with propidium iodide (PI, 50 μg/ml) for 5 min. in the dark as described previously [16], or subjected to terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) according to the manufacturer's instructions (HRP detection, Roche, Indianapolis, IN, USA). The tissue was visualized by fluorescence or light microscopy (Nikon Eclipse TE 300, Nikon Instruments Europe B.V., Amstelveen, the Netherlands) and image analysis with Lucia software (Laboratory Imaging, Prague, Czech Republic). Cell counting was blinded and unbiased, and a minimum of 500 cells in at least five different fields (20× objective) were counted for each experimental situation. Transmission electron microscopy was used to identify distinct cellular types and confirm apoptosis. Ultrathin sections stained with uranyl acetate and Reynolds's lead citrate solutions were examined by using a Morgagni 286 TEM (FEI Company, Eindhoven, the Netherlands) at 60 kV. Digital electron micrographs were recorded with a MegaView III CCD by using iTEM-SIS software (Olympus Soft Imaging System GmbH, Muenster, Germany).
Statistical analysis
Statistical analyses consisted of one-way analysis of variance (anova) followed by a Dunnett post-hoc test, using Statistica 6.01 software (StatSoft, Inc., Tulsa, OK, USA). The results were expressed as the mean ± S.D., and a threshold of P < 0.05 was considered significant. No statistical comparison was performed for the clonal form as only one case was analysed.
Results
Definition of a new quantitative dermoscopic score
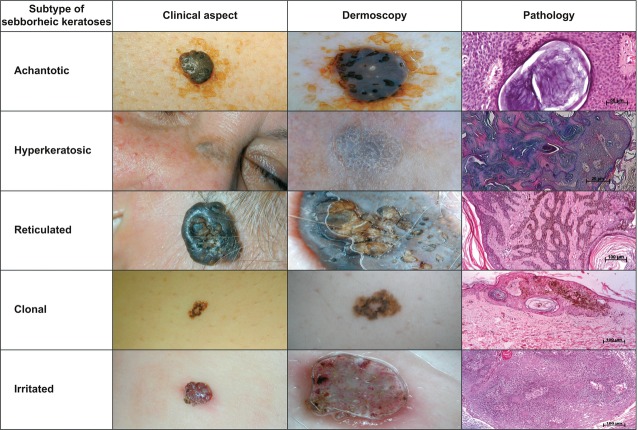
The histopathological analysis of SK samples established five anatomo-clinical forms: acanthotic (22 cases), hyperkeratotic (6 cases), reticular (3 cases), irritated (2 cases) and clonal (1 case) according to pathological criteria (Fig. 2).
Fig 2.
The five main subtypes of seborrheic keratosis. Pathological pictures are 10×, HE stain
Remarkably, some elementary dermoscopic lesions were always present and other lesions were never present in a specific form. In the acanthotic form, more than one colour was present in 100% of the cases, whereas a network pattern and irregular, polymorphic pattern were present in 0% of the cases. Crypts and millia cysts were present in more than 90% of acanthotic cases. In the hyperkeratotic form, more than one colour was present in 100% of the cases, whereas a network-like pattern, fat fingers, blue-white pigmentation, and irregular, polymorphic pattern were present in 0% of the cases. In the reticular form, a network-like pattern, crypts, millia cysts, more than one colour, fissures, and white artefacts were present in 100% of the cases, whereas fingerprinting structures, a network pattern, pinpoint vessels, hairpin vessels, fat fingers, sharp demarcation and irregular vessels were present in 0% of the cases. We analysed only two cases of irritated SK, but both cases displayed an identical dermoscopic picture: millia cysts, pinpoint vessels, hairpin vessels, more than one colour, white artefacts and irregular vessels.
The total C3V2F score (sum of all parameters) was 10.5 ± 1.7 for the acanthotic form, 9.3 ± 0.8 for the hyperkeratotic form, 12.0 ± 1.0 for the reticular form, 12.0 ± 0.0 for the irritated form and 9 for the clonal form.
Apoptosis rates and localization in various anatomo-clinical forms of SK
To evaluate apoptosis in different forms of SK, we performed chromatin PI staining and TUNEL for identification of apoptotic DNA fragmentation (Fig. 3). Keratinocyte apoptosis was confirmed by transmission electron microscopy, which showed typical chromatin condensation (data not shown). To quantify apoptosis, we counted only TUNEL-positive cells because PI staining could not differentiate well between apoptotic and dividing cells. The TUNEL method is primarily used to estimate apoptosis in skin for different diseases [17,18]. The percentages of apoptotic cells were 38.0 ± 3.8% for normal skin, 17.7 ± 10.4% for the acanthotic form, 19.4 ± 9.3% for the hyperkeratotic form, 21.9 ± 12.5% for the reticulated form, 5.4 ± 3.9% for the irritated form and 26.6% for the clonal case (Fig. 4A). Compared with normal skin, the acanthotic, hyperkeratotic, and irritated SK forms had significantly lower rates of apoptosis (P = 0.001, P = 0.012, and P = 0.001, respectively).
Fig 3.
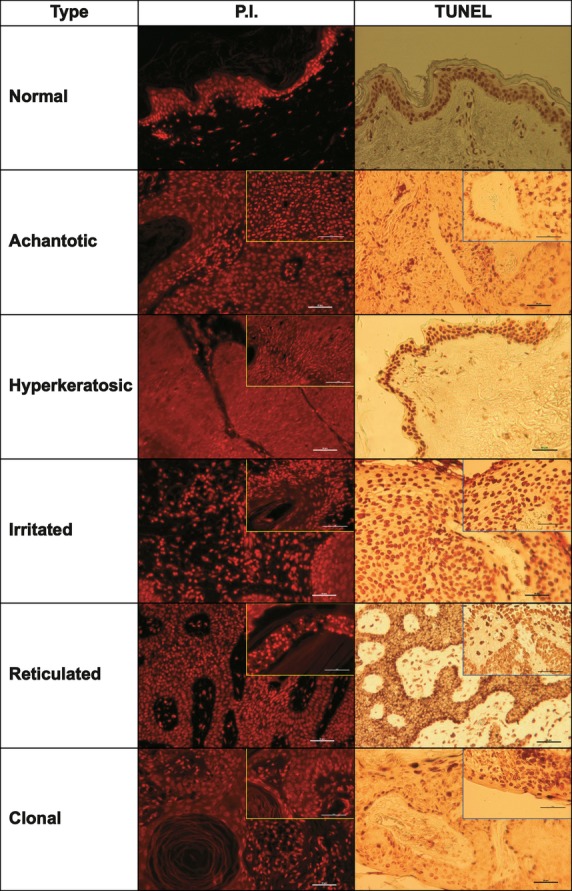
Different histopathological types of seborrheic keratosis stained for the identification of apoptotic cells. PI, propidium iodide; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling. Note the condensed and bright apoptotic nuclei stained with PI and the brownish nuclei of apoptotic, TUNEL-positive cells. The inserts show clusters of apoptotic cells around corneous globes and vessels.
Fig 4.
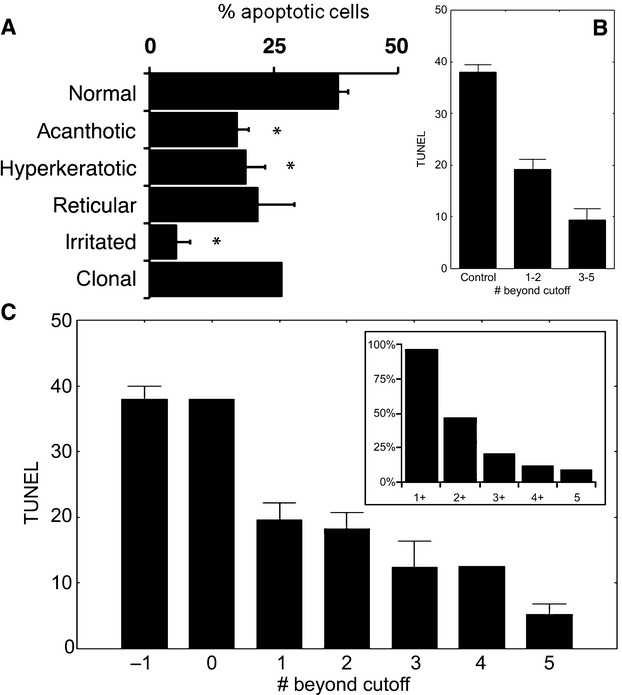
(A) Percentages of apoptotic cells identified by terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) in normal skin and different histopathological variants of seborrheic keratoses. Bars indicate the standard error of the mean (S.E.M.). *P < 0.05, anova with Dunnett post-hoc test. (B) Percentage of TUNEL-positive cells decreases as the case scores below the cut-off in an increasing number of dermoscopic scores. Error bars indicate the S.E.M.. (C) Percentage of TUNEL-positive cells grouped by dermoscopic scores predicting low rates of apoptosis (‘bad’ scores). (−1) denotes controls. Note that the presence of at least one ‘bad’ score (regardless of the score) is associated with a decreased rate of apoptosis from a normal of approximately 38–19%, whereas the case with no ‘bad score’ has an apoptosis rate in the range of controls. Note the additional drop in apoptosis rate from 2 to 3 ‘bad scores’, which justifies the grouping as presented. The insert shows the proportion of cases in our cohort with at least 1 (1+), 2 (2+), etc. ‘bad’ scores. Error bars indicate the S.E.M.
Regardless of the histopathological type, both isolated and clustered apoptotic cells were found. Isolated apoptotic cells were distributed diffusely in the tumour or in islands of dermis near the epidermis in the vicinity of the dermo-epidermal junction. Clustered apoptotic cells were located near the corneous globes in the dermis islands and around vascular lumens (Fig. 3, inserts).
Correlations between the quantitative dermoscopic score and apoptosis rates
The first observation we made was that the total dermoscopic C3V2F score significantly correlated with the crypts measure (r = 0.356, P < 0.05), but not with any other parameters of the score. Crypts significantly correlated with millia cysts (r = 0.538, P < 0.05), hairpin vessels (r = −0.433, P < 0.05) and irregular vessels (r = −0.450, P < 0.05), and millia cysts significantly correlated with hairpin vessels (r = −0.517, P < 0.05) and fissures (r = −0.385, P < 0.05). Hairpin vessels significantly correlated with fissures (r = 0.897, P < 0.05). The total dermoscopic C3V2F score did not significantly correlate with the percentage of apoptotic cells, but the above four features did (crypts, millia cysts, hairpin vessels and fissures; r = 0.441, r = 0.410, r = −0.392, and r = −0,417, respectively; P < 0.05 for all).
Next, we analysed our data to provide relevant information for clinicians. We searched for dermoscopic score cut-offs that separate cases with high and low rates of apoptosis. Figure shows that crypts >2, colour >5, or a total C3V2F score >10 reflects a high apoptosis rate, which implies a benign character of evolution. In contrast, irregular vessels >2 or total vessels (sum of hairpin vessels and irregular vessels) >4 reflect a low apoptosis rate, which is probably associated with a risk of malignant transformation.
Fig 5.
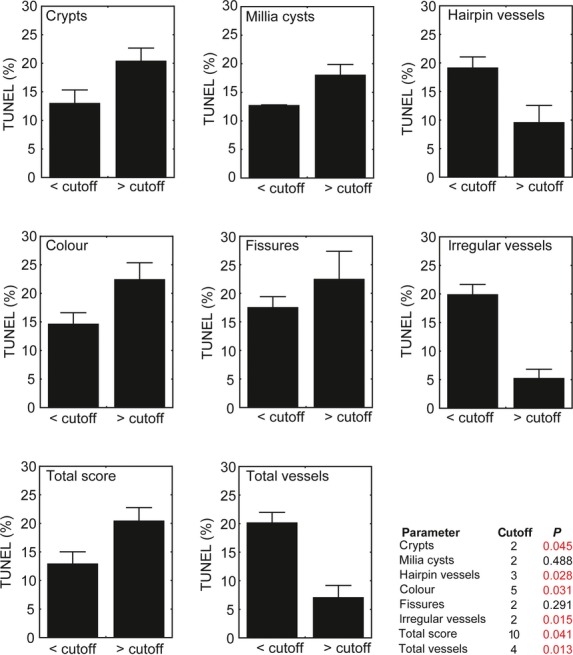
Significance (P value) of quantitative parameter cut-offs included in the C3V2F score. ‘Total score’ (C3V2F) refers to the added values of parameters a–f (crypts, millia-cysts, hairpin vessels, colour, fissures and irregular vessels). ‘Total vessels’ refers to added values of parameters quantifying vessels (hairpin vessels and irregular vessels). In the graphs, the average percentage of apoptotic cells in the populations separated by the cut-offs is represented. Bars indicate the S.E.M. and significance is determined as P < 0.05 (anova with Dunnett post-hoc test).
Although we were able to identify cut-off values that provide significant differences between the mean percentages of TUNEL-positive cells for most dermoscopic parameters, our cohort included only one case (2.8%) with no dermoscopic score predictive of low apoptotic rates, which had a percentage of TUNEL-positive cells within the range of controls. The TUNEL-positive rates decreased dramatically (from 38% to 19%) when at least one dermoscopic score was below the cut-off (Fig. 4B). In our cohort, 50% of the cases had one, and 47.1% had at least two, scores predictive of a low percentage of TUNEL-positive cells (i.e. ‘bad’ scores) (Fig. 4C).
Using multiple regression, we looked for a model that is suitable for our data. Because of an opposed correlation of the ‘total vessels’ score and the sum of all other parameters with the rate of apoptosis, we analysed a composite score (total without vessels – total vessels). Figure shows that the percentage of apoptotic cells identified by TUNEL is estimated by the formula: 4.84 + 1.66 × composite score, with a 0.7 prediction interval (r = 0.57, P < 0.05). All significant correlations between the percentage of apoptotic cells and total without vessels and total vessels scores are also shown in Fig. 6.
Fig 6.
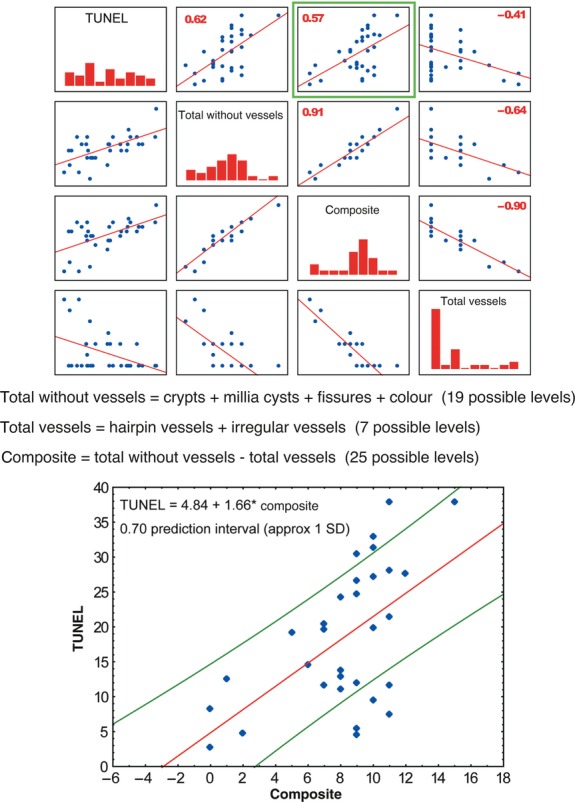
Correlation graphs of the percentage of apoptotic cells (TUNEL-positive), ‘composite’, ‘total without vessels’ and ‘vessels’ scores. The numbers in the upper panels represent the Pearson correlation coefficient, r. The ‘total without vessels’ score is defined as the sum of parameters a (crypts), b (millia cysts), d (colours) and e (fissures) of the C3V2F score. The ‘total vessels’ score is defined as the sum of parameters c (hairpin vessels) and f (irregular vessels) of the C3V2F score. The ‘composite score’ is defined as the difference between ‘total without vessels’ and ‘vessels’ scores. The bottom panel shows the green framed upper panel in detail, the formula of the multiple regression model, and the prediction interval of 0.7.
Discussion
After a qualitative and quantitative analysis of dermoscopic images, we defined a new quantitative dermoscopic score, C3V2F, which ranges from 0 to 22 and enabled us to establish cut-offs correlated with the rate of apoptosis in different types of SK. A C3V2F score higher than 10 or a score higher than 2 for crypts, or higher than 4 for colours reflects a higher apoptosis rate, which probably implies a benign character of evolution. In contrast, the presence of irregular vessels on more than 50% of the lesion surface or a total vessel score of more than 4 implies a lower rate of apoptosis, which could be associated with a risk of malignant transformation. Moreover, if more than four colours are present, the lack of melanocytic criteria for diagnosis implies benignity, in contrast to the colour significance for melanoma.
Compared with SCC, the biological behaviour of SK is benign. However, in solitary forms of SK, an association with malignancy by tumoural collision or malignant transformation has been described [3,5,19,20], with the incriminated cancers being mostly epithelial in origin (keratinocytic in BCC, SCC, and Bowen, from hair follicles in keratoacanthoma, and melanocytic in melanoma). How benign tumours derived from keratinocytes (i.e. SK) can transform or be associated with a malignant tumour derived from melanocytes (melanoma) is unknown. This concept is controversial because some authors have indicated biopsy of any modified SK and argued for a possible neoplastic transformation [[1],[3],,18], but others regarded this association as a coincidental process (tumoural collision) [21].
In our study, all pathological forms of SK studied (acanthotic, hyperkeratotic, reticulated, irritated and clonal) were associated with lower apoptosis rates compared with normal skin. This result shows that an altered balance between cell proliferation and cell loss is probably present in SK, which could lead to tumour growth without activation of compensatory apoptosis. Compared with SCC, a reduced rate of apoptosis [15] and increased bcl-2 expression [22] have been demonstrated in SK. In addition, significantly higher rates of apoptosis have been detected in rapidly growing neoplasms compared with slow-growing neoplasms [15]. A previous study suggested that, in SK, inflammation or irritation may be related to a shift in the cell cycle and bcl-2 may be increased in SK as an anti-apoptotic mechanism [22].
In the present work, we showed that the aspect of orthokeratotic globes and other forms of ‘onion leaf arrangement’ in SK is reminiscent of the pathological picture of SCC. In the vicinity of such globes and blood vessels, we found clusters of apoptotic cells, which could be the starting point of a phenotypic change and initiation of malignancy, with apoptosis activated as a defence mechanism in these areas.
Dermoscopy, as a non-invasive method, has an important value for differentiating between benign and malignant skin lesions [23]. In a study of 203 lesions, 15 morphological diagnostic criteria for pigmented SK were summarized [24] with an emphasis on classic dermoscopic criteria, such as millia-like cysts and comedo-like openings, but also on additional dermoscopic criteria, such as fissures, hairpin blood vessels, sharp demarcation, moth-eaten borders, network-like structures and network structures, which improve diagnostic accuracy. ‘Fat fingers’ were described later as a clue for dermoscopic diagnosis of SK, especially when the classic features are absent [25]. Standard dermoscopic features were correlated with histopathological forms of SK in a few reports [26,27,,28] but, to the best of our knowledge, an extensive updated study, including correlations with apoptosis, has not yet been performed.
The qualitative analysis of dermoscopic information showed the frequency of elementary lesion distribution in our SK series. As distinctive features that may orientate the dermoscopist with the histopathological type, we mention the network pattern (found only in the hyperkeratotic form), fat fingers (found only in the acanthotic form) and the irregular, polymorphic pattern (found only in the reticular form). The most unspecific dermoscopic parameter was more than one colour, which was found in 100% of the cases irrespective of histopathological type. However, because of the limited number of cases, these findings should be regarded as informative only.
On the basis of this quantification of dermoscopic information, we were able to determine a composite score (total score without vessels – total vessels score) that estimates the apoptosis rate with a 0.7 prediction interval (approximately 1S.D.), which was confirmed in a multiple regression model.
However, our study has several limitations. First, TUNEL positive skin cells do not always express other markers of apoptosis, such as Fas antigen or Bcl-2 family proteins [29] and therefore this technique might overestimate the apoptosis rate. Another limitation is given by the limited number of control cases analysed in our work (n = 3). This was due to difficulty in obtaining skin probes from healthy participants. Moreover, to validate an SK prognosis based on the newly defined dermoscopic score, a large-case follow-up analysis would be needed. This will be difficult to achieve, as a part of SK lesions are surgically removed anyway.
In conclusion, to the best of our knowledge, this report is the first to correlate dermoscopic features, histopathological types and apoptosis in SK. For the clinician, this newly proposed algorithm based on dermoscopic information allows an ‘at first glance’ rapid and accurate pre-operative decision. Our findings might contribute to the elucidation of the pathogenesis of a dermoscopically polymorphous lesion.
Acknowledgments
This study was supported by the Sectorial Operational Programme Human Resources Development (SOP HRD), financed by the European Social Fund and the Romanian Government under contract number POSDRU/89/1.5/S/64109.
Conflict of interest
None declared.
References
- Thomas VD, Swanson NA, Lee KK. Benign epithelial tumours, hamartomas, and hyperplasias. In: Wolff K, Goldsmith LA, Katz SI, editors. Fitzpatrick's dermatology in general medicine. New York, USA: McGraw-Hill; 2008. pp. 1054–7. [Google Scholar]
- Cockerell CJ, Larsen F. Benign epidermal tumours and proliferations. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. Mosby: Elsevier; 2008. pp. 1661–4. [Google Scholar]
- Blotch PH. Transformation of seborrheic keratosis into Bowen's disease. J Cutan Pathol. 1978;5:361–7. doi: 10.1111/j.1600-0560.1978.tb00966.x. [DOI] [PubMed] [Google Scholar]
- Monteagudo JC, Jorda E, Terencio C, et al. Squamous cell carcinoma in situ (Bowen's disease) arising in seborrheic keratosis: three lesions in two patients. J Cutan Pathol. 1989;16:348–52. doi: 10.1111/j.1600-0560.1989.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Lindelöf B, Sigurgeirsson B, Melander S. Seborrheic keratoses and cancer. J Am Acad Dermatol. 1992;26:947–50. doi: 10.1016/0190-9622(92)70139-7. [DOI] [PubMed] [Google Scholar]
- Schwartz RA. Sign of Leser-Trélat. J Am Acad Dermatol. 1996;35:88–95. doi: 10.1016/S0190-9622(96)90502-2. [DOI] [PubMed] [Google Scholar]
- Kirkham N. Tumours and cysts of the epidermis. In: Elder DE, Johnson BL Jr, Murphy G, Xu X, editors. Lever's histopathology of the skin. Philadelphia, PA, USA: Lippincott Williams&Wilkins; 2009. pp. 795–8. [Google Scholar]
- Boyd AS. Tumours of the epidermis. In: Barnhill RL, editor. Textbook of dermatopathology. New York, USA: McGraw-Hill; 1998. pp. 501–3. [Google Scholar]
- McKee PH. Pathology of the skin with clinical correlations. London, UK: Mosby-Wolfe; 1997. Tumours of the surface epithelium; pp. 14.1–4. [Google Scholar]
- Noiles K, Vender R. Are all seborrheic keratoses benign? Review of the typical lesion and its variants. J Cutan Med Surg. 2008;12:203–10. doi: 10.2310/7750.2008.07096. [DOI] [PubMed] [Google Scholar]
- Eberle J, Fecker LF, Forschner T, et al. Apoptosis pathways as promising targets for skin cancer therapy. Br J Dermatol. 2007;156:18–24. doi: 10.1111/j.1365-2133.2007.07855.x. [DOI] [PubMed] [Google Scholar]
- Erb P, Ji J, Kump E, et al. Apoptosis and pathogenesis of melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:283–95. doi: 10.1007/978-0-387-77574-6_22. [DOI] [PubMed] [Google Scholar]
- Rodust PM, Stockfleth E, Ulrich C, et al. UV-induced squamous cell carcinoma-a role of antiapoptotic signaling pathways. Br J Dermatol. 2009;161(Suppl. 3):107–15. doi: 10.1111/j.1365-2133.2009.09458.x. [DOI] [PubMed] [Google Scholar]
- Palmieri G, Capone M, Ascierto ML, et al. Main roads to melanoma. J Transl Med. 2009;7:86. doi: 10.1186/1479-5876-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori O, Hachisuka H, Kiyokawa C, Sasai Y. Apoptosis identified by DNA fragmentation in epidermal neoplasms. J Dermatol. 1995;22:917–20. doi: 10.1111/j.1346-8138.1995.tb03945.x. [DOI] [PubMed] [Google Scholar]
- Popescu AT, Vidulescu C, Stanciu CL, et al. Selective protection by phosphatidic acid against staurosporine-induced neuronal apoptosis. J Cell Mol Med. 2002;6:433–8. doi: 10.1111/j.1582-4934.2002.tb00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Izhak O, Laster Z, Akrish S, et al. TUNEL as a tumour marker of tongue cancer. Anticancer Res. 2008;28:2981–6. [PubMed] [Google Scholar]
- Pesce C, Scalora S. Apoptosis in the areas of squamous differentiation of irritated seborrheic keratosis. J Cutan Pathol. 2000;27:121–3. doi: 10.1034/j.1600-0560.2000.027003121.x. [DOI] [PubMed] [Google Scholar]
- Clemmensen OJ, Sjølin KE. Malignancy in seborrheic keratoses. Acta Derm Venereol. 1986;66:158–61. [PubMed] [Google Scholar]
- Endoh K, Ohara M, Kosegawa G, Akasaka T. Occult basal cell carcinoma arising in seborrheic keratosis. J Dermatol. 1998;25:374–8. doi: 10.1111/j.1346-8138.1998.tb02417.x. [DOI] [PubMed] [Google Scholar]
- Birnie AJ, Varma S. A dermatoscopically diagnosed collision tumour: malignant melanoma arising within a seborrhoeic keratosis. Clin Exp Dermatol. 2008;33:512–3. doi: 10.1111/j.1365-2230.2008.02715.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Yamamura K, Maeda S, Ichihashi M. bcl-2 expression in epidermal keratinocytic diseases. Cancer. 1994;74:1720–4. doi: 10.1002/1097-0142(19940915)74:6<1720::aid-cncr2820740613>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Ko CJ, Shintaku P, Binder SW. Comparison of benign keratoses using p53, bcl-1, and bcl-2. J Cutan Pathol. 2005;32:356–9. doi: 10.1111/j.0303-6987.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679–93. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- Braun RP, Rabinovitz HS, Krischer J, et al. Dermoscopy of pigmented seborrheic keratosis: a morphological study. Arch Dermatol. 2002;138:1556–60. doi: 10.1001/archderm.138.12.1556. [DOI] [PubMed] [Google Scholar]
- Kopf AW, Rabinowitz H, Marghoob A, et al. “Fat fingers:” a clue in the dermoscopic diagnosis of seborrheic keratoses. J Am Acad Dermatol. 2006;55:1089–91. doi: 10.1016/j.jaad.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Wang SQ, Rabinovitz H, Oliviero MC. Dermoscopic patterns of solar lentigines and seborrheic keratoses. In: Marghoob AA, Braun RP, Kopf AW, editors. Atlas of dermoscopy. New York, USA: Taylor & Francis Group; 2004. pp. 60–70. [Google Scholar]
- Soyer HP, Kenet RO, Wolf IH, et al. Clinicopathological correlation of pigmented skin lesions using dermoscopy. Eur J Dermatol. 2008;10:22–8. [PubMed] [Google Scholar]
- Zaballos P, Blazquez S, Puig S, et al. Dermoscopic pattern of intermediate stage in seborrhoeic keratosis regressing to lichenoid keratosis: report of 24 cases. Br J Dermatol. 2007;152:266–72. doi: 10.1111/j.1365-2133.2007.07963.x. [DOI] [PubMed] [Google Scholar]
- Baima B, Sticherling M. How specific is the TUNEL reaction? An account of a histochemical study on human skin. Br J Dermatol. 2001;144:958–66. doi: 10.1097/00000372-200204000-00004. [DOI] [PubMed] [Google Scholar]