Abstract
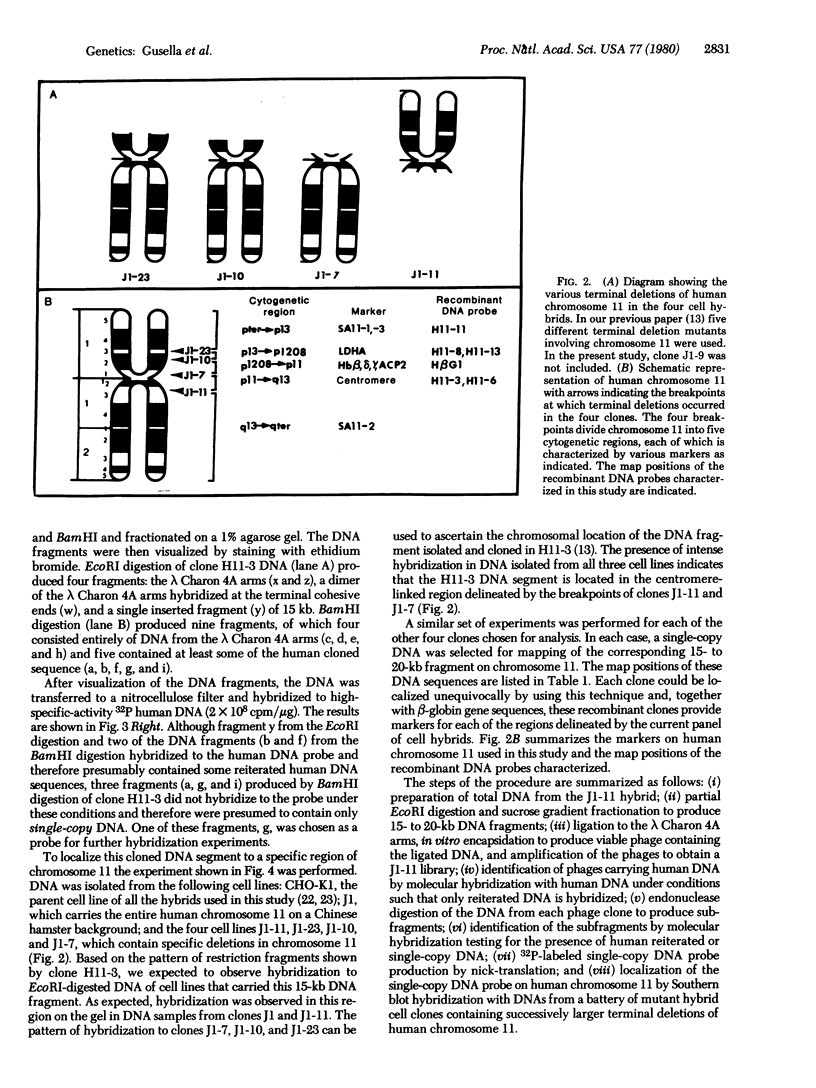
Recombinant DNA techniques have been combined with somatic cell genetic methods to identify, isolate, and amplify fragments of human DNA localized at specific regions of human chromosome 11 selected as a model system. A library of genomic DNA segments has been constructed, in λ Charon 4A bacteriophage, from the DNA of a somatic cell hybrid carrying a portion of human chromosome 11 on a Chinese hamster ovary cell background. Using a nucleic acid hybridization technique that distinguishes human and Chinese hamster interspersed, repetitive DNA, we have been able to distinguish recombinant phages carrying DNA segments of human origin from recombinant phages carrying DNA segments of Chinese hamster origin. We have isolated 50 human DNA segments thus far and have characterized 5 in detail. For each DNA segment characterized, a subsegment that carries no repetitive human DNA sequences has been identified. These segments have been used as hybridization probes in experiments that localize the DNA fragment on the chromosome. In each case an unequivocal chromosomal localization has been obtained with reference to a panel of hybrid cell clones each of which carries a deletion of a portion of the short arm of chromosome 11. At least one DNA segment has been identified which maps to each of the four regions on the short arm defined by the panel of hybrid cell clones used. The approaches described here appear to be general. They can be extended to produce a fine structure map of human chromosome 11 and other human chromosomes. This approach promises implications for human genetics generally, for the human genetic diseases, and possibly for understanding of gene regulation in normal and abnormal differentiation.
Keywords: DNA hybridization, human chromosome 11, reiterated DNA, recombinant DNA, bacteriophage λ Charon 4A
Full text
PDF

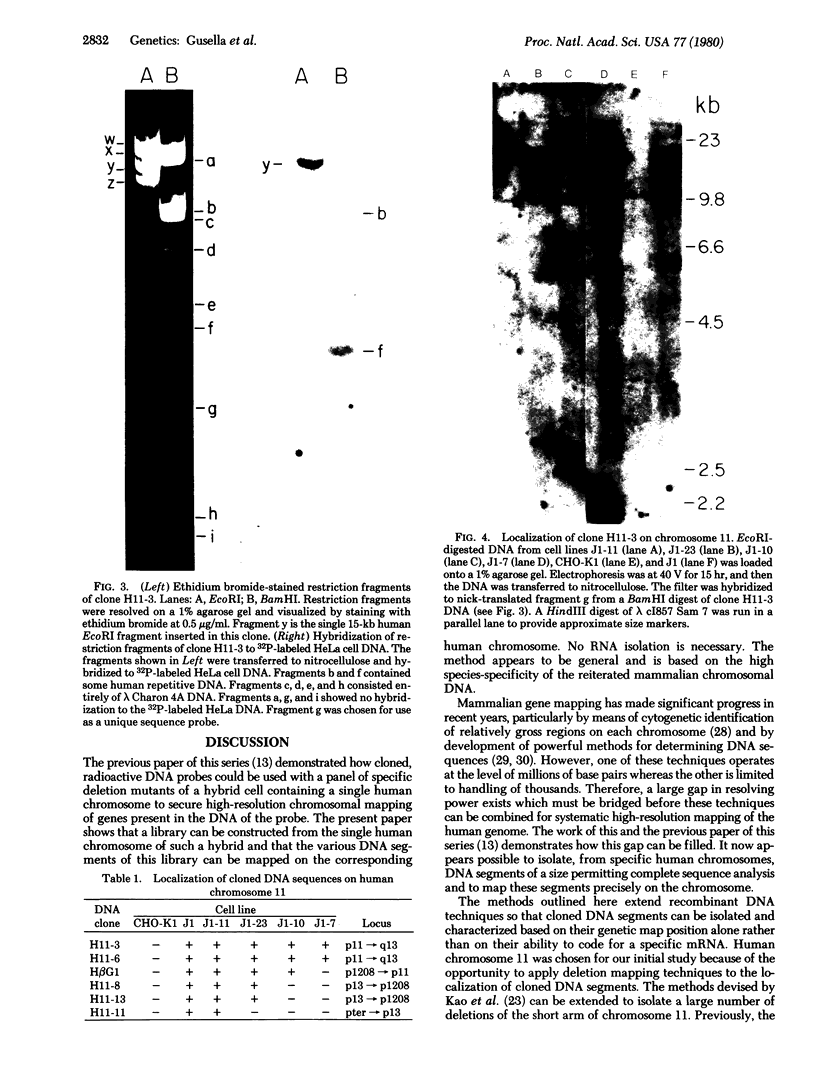

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Kooter J. M., De Boer E., Little P. F., Williamson R. Analysis of the beta-delta-globin gene loci in normal and Hb Lepore DNA: direct determination of gene linkage and intergene distance. Cell. 1978 Sep;15(1):25–41. doi: 10.1016/0092-8674(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Goss S. J., Harris H. Gene transfer by means of cell fusion I. Statistical mapping of the human X-chromosome by analysis of radiation-induced gene segregation. J Cell Sci. 1977 Jun;25:17–37. doi: 10.1242/jcs.25.1.17. [DOI] [PubMed] [Google Scholar]
- Goss S. J., Harris H. Gene transfer by means of cell fusion. II. The mapping of 8 loci on human chromosome 1 by statistical analysis of gene assortment in somatic cell hybrids. J Cell Sci. 1977 Jun;25:39–57. doi: 10.1242/jcs.25.1.39. [DOI] [PubMed] [Google Scholar]
- Goss S. J., Harris H. New method for mapping genes in human chromosomes. Nature. 1975 Jun 26;255(5511):680–684. doi: 10.1038/255680a0. [DOI] [PubMed] [Google Scholar]
- Gusella J., Varsanyi-Breiner A., Kao F. T., Jones C., Puck T. T., Keys C., Orkin S., Housman D. Precise localization of human beta-globin gene complex on chromosome 11. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5239–5242. doi: 10.1073/pnas.76.10.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C., Kao F. T. Regional mapping of the gene for human lysosomal acid phosphatase (ACP2) using a hybrid clone panel containing segments of human chromosome 11. Hum Genet. 1978 Nov 24;45(1):1–10. doi: 10.1007/BF00277567. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Jones C., Puck T. T. Genetics of cell-surface antigens: regional mapping of three components of the human cell-surface antigen complex, AL, on chromosome 11. Somatic Cell Genet. 1977 Jul;3(4):421–429. doi: 10.1007/BF01542970. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Jones C., Puck T. T. Genetics of somatic mammalian cells: genetic, immunologic, and biochemical analysis with Chinese hamster cell hybrids containing selected human chromosomes. Proc Natl Acad Sci U S A. 1976 Jan;73(1):193–197. doi: 10.1073/pnas.73.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. L., Kao F. T. Induced segregation of human syntenic genes by 5-bromodeozyuridine + near-visible light. Somatic Cell Genet. 1978 Jul;4(4):465–476. doi: 10.1007/BF01538867. [DOI] [PubMed] [Google Scholar]
- Law M. L., Kao F. T. Regional assignment of human genes TPI1, GAPDH, LDHB, SHMT, and PEPB on chromosome 12. Cytogenet Cell Genet. 1979;24(2):102–114. doi: 10.1159/000131363. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lindley M. Gene mapping at the crossroads. Nature. 1979 Sep 6;281(5726):12–12. doi: 10.1038/281012a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. Related base sequences in the DNA of simple and complex organisms. VI. The extent of base sequence divergence among the DNAs of various rodents. Biochem Genet. 1970 Jun;4(3):425–446. doi: 10.1007/BF00485758. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Ruddle F. H. The status of the gene map of the human chromosomes. Science. 1977 Apr 22;196(4288):390–405. doi: 10.1126/science.850784. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Bank A. Organization of human delta--and beta-globin genes in cellular DNA and the presence of intragenic inserts. Cell. 1978 Sep;15(1):15–23. doi: 10.1016/0092-8674(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Nakamura F., Bloom A., Konotey-Ahulu F., Bank A. Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1222–1226. doi: 10.1073/pnas.75.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. E., Jones C., Kao F. T., Oates D. C. Synteny between glycinamide ribonucleotide synthetase and superoxide dismutase (soluble). Am J Hum Genet. 1977 Jul;29(4):389–396. [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C., Mahoney M. J., Lazarus H., Hobbins J. C., Nathan D. G. Application of endonuclease mapping to the analysis and prenatal diagnosis of thalassemias caused by globin-gene deletion. N Engl J Med. 1978 Jul 27;299(4):166–172. doi: 10.1056/NEJM197807272990403. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Old J. M., Weatherall D. J., Nathan D. G. Partial deletion of beta-globin gene DNA in certain patients with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1979 May;76(5):2400–2404. doi: 10.1073/pnas.76.5.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Old J., Lazarus H., Altay C., Gurgey A., Weatherall D. J., Nathan D. G. The molecular basis of alpha-thalassemias: frequent occurrence of dysfunctional alpha loci among non-Asians with Hb H disease. Cell. 1979 May;17(1):33–42. doi: 10.1016/0092-8674(79)90292-7. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. The duplicated human alpha globin genes lie close together in cellular DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5950–5954. doi: 10.1073/pnas.75.12.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi V. M., Sujansky E., Smith A. C., Francke U. Chromosomal imbalance in the Aniridia-Wilms' tumor association: 11p interstitial deletion. Pediatrics. 1978 Apr;61(4):604–610. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Varsanyi-Breiner A., Gusella J. F., Keys C., Housman D. E., Sullivan D., Brisson N., Verma D. P. The organization of a nuclear DNA sequence from a higher plant: molecular cloning and characterization of soybean ribosomal DNA. Gene. 1979 Nov;7(3-4):317–334. doi: 10.1016/0378-1119(79)90051-9. [DOI] [PubMed] [Google Scholar]
- Waldren C., Jones C., Puck T. T. Measurement of mutagenesis in mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1358–1362. doi: 10.1073/pnas.76.3.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T., Wilson L. B., deRiel J. K., Villa-komaroff L., Efstratiadis A., Forget B. G., Weissman S. M. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucleic Acids Res. 1978 Feb;5(2):563–581. doi: 10.1093/nar/5.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]