Abstract
Previews
Recent research suggests that obesity may be influenced not only by dietary and genetic risk factors, but also by the trillions of microorganisms inhabiting our gastrointestinal tract. Consistent with this notion, Cho et al. (2012) use mice to demonstrate that subtherapeutic antibiotic treatment promotes adiposity.
Humans and other mammals have co-evolved with trillions of microorganisms that thrive in and on our bodies. The ensuing host-microbial interactions are often beneficial; however, disturbance of this delicate balance is thought to lead to an increased risk of disease (Dethlefsen et al., 2007). Recent efforts have extensively characterized the composition of the microbial communities found in multiple body habitats (Consortium, 2012), but we are only just beginning to discover the unintended consequences of a Western lifestyle on our microbial partners (Blaser and Falkow, 2009). In particular, orally administered broad-spectrum antibiotics have a clear potential to influence the microbes inhabiting our gastrointestinal tract (the gut microbiota).
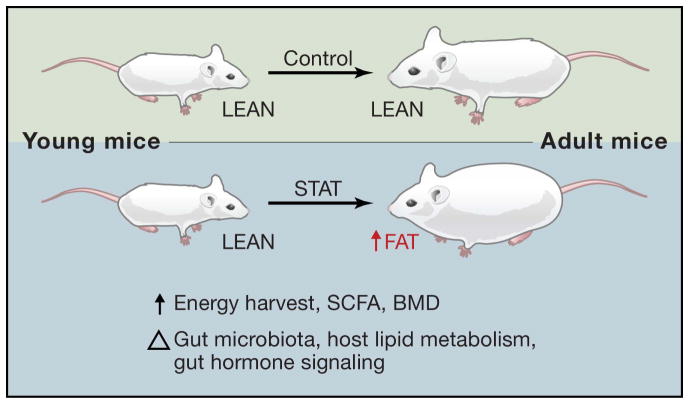
But what are the functional consequences of the widespread use of antibiotics? One possible ramification is altered host energy balance. A number of studies have linked the gut microbiota to obesity (Backhed et al., 2004; Turnbaugh et al., 2006), and low dose antibiotics have been used for decades to enhance growth and feed efficiency in farm animals (Jukes, 1971). The mechanisms through which antibiotics promote this phenomenon and whether the increasing use of antibiotics in children contributes to a predisposition to obesity later in life are currently unknown. Tractable model systems are needed to tease apart the many confounding factors potentially influencing host energy balance. Recently, work done by Martin Blaser and colleagues (Cho et al., 2012) has made an important first step towards addressing these issues, exposing young mice to various low dose antibiotic treatments for 7 weeks, resulting in increased adiposity (Figure 1).
Figure 1. The Many Effects of Subtherapeutic Antibiotic Treatment (STAT) on Young Mice.
Relative to control mice, STAT results in similar total body weight but increased adiposity, dietary energy harvest, bone mineral density (BMD), and short chain fatty acid (SCFA) levels. These phenotypic differences were associated with changes to the gut microbial community, host lipid metabolism, and gut hormone signaling. Future studies promise to elucidate the mechanistic basis for these changes.
The microbial response to subtherapeutic antibiotic treatment (STAT) was evidenced by an altered community structure rather than total abundance. A shift towards an increased Firmicutes to Bacteroidetes ratio has been associated with an obese state (Ley et al., 2005; Turnbaugh et al., 2006). Surprisingly, this shift was evident after co-administration of penicillin and vancomycin, two primarily gram-positive (i.e. Firmicutes) targeting compounds, and not after administration of either antibiotic alone. Chlortetracycline, another antimicrobial that acts through a different mechanism, also caused this shift. Given the different modes of action and range of these antimicrobials, this calls into question how these various antibiotics affect the microbial community and to what degree individual microbial taxonomic groups, or multiple groups working in concert, might directly contribute to the development of adiposity.
One possibility is that the disrupted gut microbiota following STAT has an enhanced ability to harvest energy from the diet, through microbial production of short chain fatty acids (SCFAs), to provide substrates for storage into adipose tissue, as proposed for obesity (Turnbaugh et al., 2006). The authors observed that STAT led to increased overall caloric extraction from the diet, together with increased SCFA concentrations. Although the four treatment options had variable effects on microbial community structure and SCFA levels, all of these treatments resulted in some degree of increased adiposity, suggesting that there may be a common host response to multiple types of perturbations to the gut microbiota. To determine whether a link could be made between the gut microbiota, SCFA levels, and adiposity, changes in downstream host genes for hepatic lipid metabolism were quantified by microarray and qPCR. Some genes coding for lipogenesis and triglyceride synthesis were upregulated, but no phenotypic signs of hepatic steatosis or differences in metabolic activity of visceral adipose tissues were observed. Increased serum levels of a secreted gastrointestinal hormone (GIP) may be an alternate contributor to adiposity. Further experiments to clarify these putative mechanisms and experiments in germ-free mice exposed to STAT are necessary to rule out any direct effects these compounds may have on host physiology.
Interestingly, unlike what is seen in livestock, STAT had a minimal effect on overall body weight and feed efficiency in mice. The authors did report an accelerated growth rate within the first week of STAT, and an increase in bone mineral density after 3 weeks. It is possible that this initial accelerated growth towards maturity is sufficient to promote fat deposition.
This work raises many questions as to the potential links between antibiotic exposure and adiposity. Given that antibiotic use has a greater effect on growth in young animals than mature animals (Jukes, 1971), to what degree does the effect of STAT depend on the age of the mice? Is this phenomenon unique to early life? How much, if at all, does the effect depend on the specific antibiotics used and their relative proportions? Do antibiotics influence host energy balance through direct effects on the host or are the observed phenotypes entirely dependent on the gut microbiota? To what degree does the prevalence and horizontal transfer of antibiotic resistance genes influence the increase in adiposity? How dependent are these effects on other factors known to influence the gut microbiota and host energy balance; for example, would consumption of a high-fat/high-sugar “Western” diet exacerbate the effects of STAT? Also, while much of the attention devoted to the role of the microbiota in regulating host metabolism is focused on an increase in dietary energy harvest, what impact could the microbiota have on the other side of the energy balance equation? Is there an effect of the gut microbiota on energy expenditure?
Perhaps most importantly, this work provides a tractable animal model to investigate these, and many other, burning questions regarding the energetic consequences of perturbations to the gut microbiota. Of note, another recent study performed by Blaser and colleagues supports the relevance of these findings to humans: infants exposed to antibiotics within the first 6 months of life had increased body mass later in life (Trasande et al., 2012). Together, these results underscore a view of human metabolism as a composite of our human and microbial genomes, and the critical need for a better understanding of how these host-microbial interactions contribute to health and disease in the presence or absence of a wide range of orally administered therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nature reviews Microbiology. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium HM. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes TH. The present status and background of antibiotics in the feeding of domestic animals. Annals of the New York Academy of Sciences. 1971;182:362–379. doi: 10.1111/j.1749-6632.1971.tb30672.x. [DOI] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]