Abstract
Intact etioplasts of bean (Phaseolus vulgaris) plants exhibit proteolytic activity against the exogenously added apoprotein of the light-harvesting pigment-protein complex serving photosystem II (LHCII) that increases as etiolation is prolonged. The activity increases in the membrane fraction but not in the stroma, where it remains low and constant and is mainly directed against LHCII and protochlorophyllide oxidoreductase. The thylakoid proteolytic activity, which is low in etioplasts of 6-d-old etiolated plants, increases in plants pretreated with a pulse of light or exposed to intermittent-light (ImL) cycles, but decreases during prolonged exposure to continuous light, coincident with chlorophyll (Chl) accumulation. To distinguish between the control of Chl and/or development on proteolytic activity, we used plants exposed to ImL cycles of varying dark-phase durations. In ImL plants exposed to an equal number of ImL cycles with short or long dark intervals (i.e. equal Chl accumulation but different developmental stage) proteolytic activity increased with the duration of the dark phase. In plants exposed to ImL for equal durations to such light-dark cycles (i.e. different Chl accumulation but same developmental stage) the proteolytic activity was similar. These results suggest that the protease, which is free to act under limited Chl accumulation, is dependent on the developmental stage of the chloroplast, and give a clue as to why plants in ImL with short dark intervals contain LHCII, whereas those with long dark intervals possess only photosystem-unit cores and lack LHCII.
The appearance and stabilization of the nuclear-coded LHCII protein during thylakoid biogenesis is under the control of phytochrome-regulated Lhcb transcription, the endogenous circadian clock, and the amount of Chl accumulated. LHCII is barely immunodetected in thylakoids of dark-grown leaves, and contrary to the situation found in leaves exposed to CL, in which it constitutes up to 60% of the thylakoid protein, its level in leaves exposed to ImL is very low to nil (Argyroudi-Akoyunoglou and Akoyunoglou, 1979; Dreyfuss and Thornber, 1994), despite the presence of in vitro-translatable Lhcb mRNA (Viro and Kloppstech, 1982). These plants possess small photosystem units and high PSI and PSII activity (Akoyunoglou, 1977). Furthermore, when transferred to CL after prolonged preexposure to ImL, these plants do not accumulate a high amount of additional Chl or LHCII (Akoyunoglou and Argyroudi-Akoyunoglou, 1978), even though they possess in vitro-translatable Lhcb mRNA (Argyroudi-Akoyunoglou, 1989).
The level of LHCII in ImL plants, however, depends on the duration of the dark phase in LDCs, increasing as the duration is decreased (Tzinas et al., 1987), suggesting that the rate of Chl accumulation relative to that of the other thylakoid components is an important factor in LHCII stabilization. In leaves exposed to CL for a limited time and then transferred to the dark, where Chl synthesis is completely stopped but synthesis of RC proteins and new photosynthetic units continues, or in leaves left in the light in the presence of Chl-synthesis inhibitors (e.g. levulinic acid), the preaccumulated LHCII is degraded (Argyroudi-Akoyunoglou et al., 1982; Akoyunoglou and Akoyunoglou, 1985; Anastassiou and Argyroudi-Akoyunoglou, 1995a). LHCII degradation is diminished, however, in chloramphenicol-pretreated ImL leaves (Tzinas and Argyroudi-Akoyunoglou, 1988).
Based on these results, it was previously proposed (Akoyunoglou and Argyroudi-Akoyunoglou, 1986) that the rate of Chl accumulation relative to that of the RC and LHCII apoproteins is responsible for LHCII stabilization during thylakoid biogenesis. RC and LHCII apoproteins may compete for the limited amount of Chl, which is required to anchor the proteins in the thylakoid membrane, rescuing them from degradation. It was proposed that the RC proteins, possibly having higher affinity for Chl, are the first to be stabilized under limited Chl accumulation, whereas in the absence of Chl binding, LHCII proteins are degraded.
However, in view of the recent finding that thylakoids possess proteolytic activity that is also under phytochrome and circadian control and activated under conditions inhibiting Chl accumulation (Argyroudi-Akoyunoglou et al., 1991; Anastassiou and Argyroudi-Akoyunoglou, 1995b; Bei-Paraskevopoulou et al., 1995), the question was raised whether the inability of the ImL plant to stabilize LHCII is attributable to the presence or activation of such a protease.
In the present study we examined the appearance of the thylakoid-bound proteolytic activity against LHCII apoprotein during the early stages of thylakoid biogenesis. PLBs and prothylakoids of 6-d-old etiolated bean (Phaseolus vulgaris) plants were found to possess low activity. During prolonged etiolation or exposure to ImL, the activity increased parallel to leaf tissue age; however, during exposure of etiolated plants to CL, after an initial increase, activity was gradually diminished coincident with enhanced Chl accumulation. TX-100 solubilization did not affect the activity in PLBs or primary thylakoids against exogenous LHCII apoprotein, but greatly increased the activity of thylakoids against endogenous LHCII in plants exposed to prolonged CL.
Using ImL plants exposed to LDCs with varying dark-phase durations, it was found that at equal developmental stages but different levels of Chl accumulation (i.e. similar exposure to LDCs irrespective of dark-phase duration) the proteolytic activity was similar, but at equal Chl accumulation and different developmental stages (i.e. an equal number of cycles with brief or long dark intervals) the proteolytic activity was higher in thylakoids of plants exposed to cycles with longer dark intervals (i.e. more developed leaves). The results suggest that this thylakoid-associated proteolytic activity is closely involved in the regulation of LHCII stabilization in developing thylakoids, and controls the amount of LHCII apoprotein assembled under limited Chl accumulation. These results give a clue as to why thylakoids of ImL plants exposed to cycles with long dark intervals lack LHCII.
MATERIALS AND METHODS
Plants and Handling
Red kidney bean (Phaseolus vulgaris) plants were grown on perlite in complete darkness at 22°C and 80% humidity in a growth chamber (model S10H, Conviron, Manitoba, Canada). Leaves were harvested from etiolated plants 6 to 12 d after sowing or from detached cotyledons exposed to various light treatments. In the latter case, cotyledons were harvested from 6-d-old plants, one cotyledon was removed, and the primary leaves attached to the remaining cotyledon were placed in covered Petri dishes on moist filter paper. Thereafter the leaves were either kept in the dark before or after a 2-min white-light pulse or exposed to CL (35 μmol m−2 s−1, incandescent and fluorescent lamps) or ImL cycles.
Isolation of Intact Etioplasts
Intact plastids were isolated from 15 g of etiolated bean leaves. After homogenization in a blender (Waring) with 150 mL of ice-cold buffer (0.5 m Suc, 30 mm Tricine-NaOH, pH 7.2, 1 mm MgCl2, 1 mm EDTA, 0.1% BSA) three times for 3 s at low speed, the slurry was filtered through six layers of gauze and one layer of nylon cloth (40-μm mesh pore size) and centrifuged at 1000g for 10 min. The etioplast pellet was suspended in 4 mL of homogenization buffer and layered on a Percoll gradient made from a 5-mL cushion of 80% Percoll overlayed by 20 mL of a 0% to 60% Percoll gradient for 10-d-old or older plants (Schindler and Soll, 1986) or 0% to 35% Percoll for younger, etiolated plants. The plastids collected at the interface were washed twice with homogenization buffer, and then lyophilized or lysed with 10 mm Tricine-NaOH, pH 7.3, for 30 min in an ice bath.
The total membrane fraction (PLBs, prothylakoids, and envelopes) was separated from the stroma by centrifugation at 100,000g for 1 h. The supernatant (stroma) was lyophilized. The membrane fraction was resuspended in 10 mm Tricine-NaOH, pH 7.3, and centrifuged at 3,000g for 10 min to recover the PLBs. The supernatant containing prothylakoids and envelope membranes was lyophilized. Intact plastids and membrane fractions were used without further washing. All samples, including the lyophilized prothylakoid-plus-envelope, and stroma fractions, were diluted with 50 mm Tris-HCl, pH 8.6, and analyzed for proteolytic activity without previous TX-100 solubilization.
Thylakoid Isolation from Broken Plastids and Solubilization
PLBs, primary thylakoids, and thylakoids were isolated from broken plastids as described previously (Argyroudi-Akoyunoglou et al., 1982) by homogenization of 4 g fresh weight of leaves in 40 mL of buffer (0.3 m Suc, 0.05 m phosphate buffer, pH 7.2, 0.01 m KCl) in a mixer (Omni, Sorvall). For etiolated and ImL leaves, mixing was for 5 s at 50% of the line voltage and another 25 s at 25%; for CL leaves mixing was for 15 s at 35% of the line voltage and another 10 s at 58%. The homogenate was centrifuged at 500g for 2 min and chloroplasts were collected from the supernatant at 1000g for 10 min (CL leaves) or 3000g for 10 min (all other leaves). All membrane pellets were washed twice with 0.05 m Tricine-NaOH, pH 7.3.
For NaBr washing, thylakoids were stirred on ice for 10 min in the presence of 2 m NaBr at 200 μg Chl mL−1, diluted thereafter with an equal volume of cold distilled water, recovered at 100,000g for 1 h (Farhaus et al., 1985), and then washed once with 50 mm Tricine-NaOH, pH 7.3. The supernatant was dialyzed against 10 mm Tricine-NaOH, pH 7.3, lyophilized, and resuspended in 50 mm Tris-HCl, pH 8.6.
For solubilization, membrane pellets were incubated at 4°C for 1 h under stirring with 1% TX-100 (TX-100/ protein = 1 [w/w]) (Anastassiou and Argyroudi-Akoyunoglou, 1995b). The solubilized thylakoid protein was recovered in the supernatant after centrifugation at 10,000g for 10 min.
Proteolytic Activity Determination
Membrane and stroma samples from etiolated, ImL, or CL plants were assayed for proteolytic activity against endogenous and/or exogenously added LHCII apoprotein during incubation at 37°C. Proteolytic activity of samples was estimated in assay mixtures containing sample protein in 40 mm Tris-HCl, pH 8.6. In assays against exogenous or exogenous-plus-endogenous LHCII, the total protein concentration (sample protein plus LHCII) in the assay mixture was 1 μg μL−1. In assays against endogenous LHCII, e.g. in CL samples, the total thylakoid protein concentration was 0.83 μg μL−1. The ratio of sample protein to purified exogenous LHCII apoprotein was 5 unless stated otherwise.
Whenever solubilized membrane samples were assayed, the final TX-100 concentration in the assay mixture was 0.5% (w/v). Whenever nonsolubilized samples were used, the assay mixtures contained 0.05% TX-100, added with exogenous LHCII apoprotein.
After incubation, equal-volume aliquots were withdrawn, added to an equal volume of sample buffer (4% SDS, 50 mm Tris-HCl, pH 7.6, 8% mercaptoethanol, 10% Suc), and kept at −20°C. Before SDS-PAGE the samples were heated for 2 min in a boiling-water bath. Aliquots containing 5 μg of protein, including the exogenous LHCII apoprotein (dark, ImL, and flash-plus-dark samples), or 2.0 to 2.5 μg of protein, including exogenous LHCII apoprotein (CL samples), were analyzed on mini gels (Hoefer Scientific, San Francisco, CA). In all cases the LHCII apoprotein in the samples loaded did not exceed 1 μg. In cases in which the activity was tested against endogenous LHCII in ImL plants, the gels were overloaded (50 μg of primary thylakoid protein). After transfer to nitrocellulose membranes, the remaining LHCII apoprotein was estimated by immunodetection with antiserum against LHCII apoprotein. Quantitative estimation was based on the amount of LHCII apoprotein remaining as a percentage of that present before the incubation at 37°C, as monitored by scanning the area under the immunodetected protein peak with a densitometer (Scan Pack II, Biometra, Goetingen, Germany). The rates obtained from thylakoids of CL plants were estimated on the basis of net thylakoid protein (after subtracting the endogenous LHCII apoprotein). Variations in assay mixtures are described in the figure legends. For D1 immunodetection on western blots, 100 μg of membrane protein solubilized in sample buffer was loaded on the gels.
Miscellaneous Methods
The LHCII apoprotein was isolated according to the method of Burke et al. (1978) and, after delipilization, was solubilized in 1% TX-100. SDS-PAGE was done according to the method of Laemmli (1970), western-blot transfer was done according to the method of Towbin et al. (1979), and immunodecoration was done according to the method of Blake et al. (1984). Chl was determined according to the method of Mackinney (1941) in 80% acetone extracts of leaves (Akoyunoglou and Argyroudi-Akoyunoglou, 1969) or thylakoid samples. Protein was estimated according to the method of Lowry et al. (1951).
RESULTS
Detection of Proteolytic Activity against LHCII Apoprotein in Thylakoids
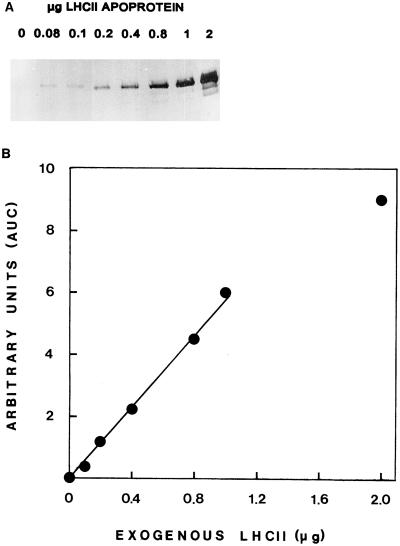
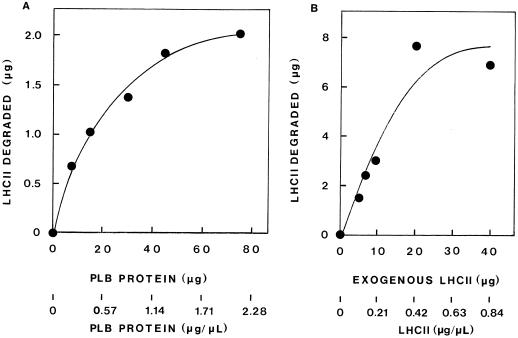
Thylakoid-associated proteolytic activity against endogenous or exogenously added LHCII apoprotein was assessed by western-blot analysis and densitometric evaluation of the immunodetected LHCII apoprotein remaining after incubation at 37°C. A linear relationship was found between the amount of LHCII apoprotein loaded on a gel and the level of immunostain on the blot for quantities up to 1 μg of LHCII apoprotein. Figure 1 shows such a representative immunoblot and a plot of the immunostain intensity as a function of the increasing quantity of LHCII apoprotein. The method allows excellent quantitation up to 1 μg of LHCII apoprotein loaded on the gel. The extent of exogenous LHCII apoprotein degradation was found to depend on the concentration of the thylakoid protein in the assay, as well as on the concentration of the substrate, LHCII, in thylakoid samples obtained from various developmental stages. Representative results are shown in Figure 2. Figure 2A shows the degradation of 5 μg of LHCII (0.14 μg mL−1) by increasing concentrations of TX-100-solubilized PLB protein. Degradation increased up to about 40 μg of PLB protein (1.14 μg PLB protein μL−1; the ratio of PLB protein to LHCII was about 9 [w/w]); thereafter, a plateau was reached. Figure 2B shows that the addition of increasing amounts of exogenous LHCII to 40 μg of primary thylakoid protein (0.83 μg mL−1) resulted in further enhancement of LHCII degradation up to about 20 μg of added LHCII (0.4 μg LHCII μL−1; the ratio of primary thylakoid protein to LHCII was 2 [w/w]).
Figure 1.
Quantitation of LHCII apoprotein immunodetection. A, Immunoblot of increasing amounts of LHCII loaded on gel. B, Standard curve of LHCII apoprotein quantity versus relative immunostain obtained from A after densitometric analysis of the immunoblot.
Figure 2.
The degradation of exogenous LHCII as affected by the concentration of thylakoid membrane protein in assay mixtures (A) or the concentration of exogenous LHCII added (B). A, Exogenous LHCII apoprotein (5 μg) was added to increasing amounts of TX-100-solubilized PLB protein obtained from 8-d-old etiolated plants in assay mixtures of 35 μL (0.5% TX-100, 40 mm Tris HCl, pH 8.6). B, To nonsolubilized primary thylakoid protein (40 μg) obtained from 6-d-old etiolated plants kept in the dark for 48 h after a 2-min light pulse, increasing amounts of exogenous LHCII apoprotein were added in assay mixtures of 48 μL (0.25% TX-100, 40 mm Tris-HCl, pH 8.6). Proteolytic activity is based on the amount of exogenous LHCII apoprotein remaining after 45 min (A) or 15 min (B) of incubation at 37°C as monitored by immunodetection on western blots.
Based on these results, and to compare rates of LHCII apoprotein degradation by thylakoid samples isolated at various stages of plastid differentiation (etioplasts, protochloroplasts, and chloroplasts) in all assay mixtures used in our study, the concentration of total protein (thylakoid plus exogenous LHCII apoprotein) was 1 μg μL−1, the ratio of thylakoid protein to exogenous LHCII apoprotein was 5 (well below the plateau in all cases), and the amount of LHCII apoprotein loaded per lane on the SDS gel for western blotting was less than 1.0 μg. In addition, estimates of degradation rates for CL thylakoids were based on thylakoid protein, from which the endogenous LHCII apoprotein was subtracted.
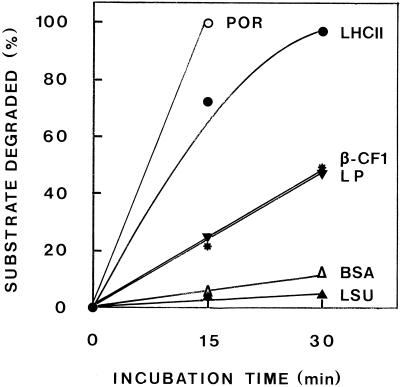
In an attempt to study the specificity of the proteolytic activity, primary thylakoid protein was incubated at 37°C for up to 30 min without addition (detection of endogenous POR and the β-subunit of coupling-factor 1) and with exogenously added proteins (LHCII, BSA, or lactoperoxidase) or stroma (detection of LSU). As shown in Figure 3, for the incubation time studied, the proteolytic activity was directed mainly against the LHCII apoprotein and POR; the β-subunit of the thylakoid-bound coupling-factor 1 and lactoperoxidase (a plastid and a nonplastid protein, respectively) were also degraded, but to a much lower extent, whereas the stromal LSU and the nonplastid BSA were almost insusceptible to proteolytic attack.
Figure 3.
Specificity of the thylakoid-bound proteolytic activity. Primary thylakoid protein (40 μg) from 6-d-old etiolated plants exposed to ImL for 16 LDCs, isolated by differential centrifugation, were incubated at 37°C with 8 μg of exogenous LHCII apoprotein (immunodetection with anti-LHCII); without addition (immunodetection with the anti-β-subunit of coupling-factor 1 [β-CF1] or anti-POR); with 8 μg of BSA or lactoperoxidase (Coomassie blue stained); or with 16 μg of protein of a stroma fraction from 10-d-old etiolated intact plastids (immunodetection with anti-LSU). All assay mixtures had a final volume of 48 μL and a final TX-100 concentration of 0.05%.
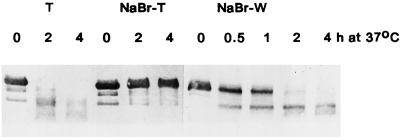
The proteolytic activity versus endogenous LHCII apoprotein in thylakoids isolated either by differential centrifugation or from lysed intact plastids was partially removed after washing of thylakoids with 50 mm Tricine-NaOH and was fully removed by NaBr treatment, suggesting that the protease is peripherally bound to thylakoids. Figure 4 shows the loss of activity from thylakoids as affected by NaBr washing and the concomitant gain of activity in the wash.
Figure 4.
The effect of NaBr washing on the proteolytic activity of thylakoids and the release of the activity in the wash. Thylakoids were obtained by differential centrifugation from 6-d-old etiolated plants exposed to CL for 4 d. After NaBr washing, the thylakoid pellet (T) was solubilized in TX-100, and the dialyzed supernatant (W) was lyophilized. The TX-100-solubilized thylakoid protein (80 μg in 80 μL) or the lyophilized wash protein (90 μg mixed with 15 μg of exogenous LHCII in 105 μL) were incubated at 37°C. All assay mixtures contained final concentrations of 0.5% TX-100 and 40 mm Tris-HCl, pH 8.6.
Developmentally Regulated Proteolytic Activity in Thylakoids
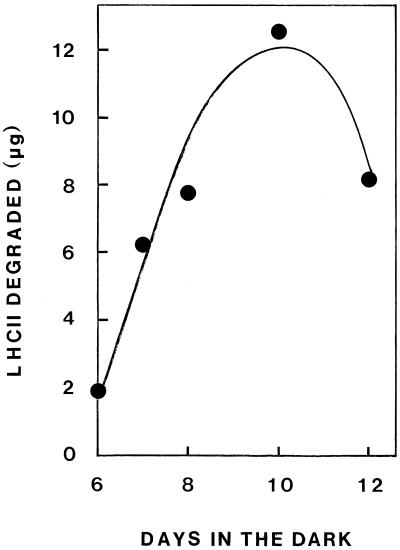
Table I shows the results of an experiment designed to demonstrate the localization of the proteolytic activity in intact etioplasts isolated from plants of various ages. In these experiments the samples were not solubilized in TX-100. In addition, the membrane fraction was not washed before the assay, since, as stated above, the protease is peripherally bound on thylakoids and can be washed off. The proteolytic activity against exogenous LHCII apoprotein was highest in etioplasts from 10-d-old plants and lower in etioplasts from plants harvested at either 7 or 11 d. The activity was mainly localized in the membrane fraction, where it gradually increased, whereas in the stroma fraction it remained low and more or less constant. Equal proteolytic activity was found in PLB and prothylakoid-plus-envelope fractions. Similar results were obtained with PLBs isolated by differential centrifugation from broken etioplasts obtained from plants at various ages. As shown in Figure 5, the activity in PLBs isolated from 6-d-old plants was very low, gradually increasing with the age of the plant up to 10 d from sowing and declining thereafter.
Table I.
Localization of proteolytic activity against exogenous LHCII apoprotein in intact etioplasts isolated from bean plants of various ages
| Sample | LHCII Degraded
|
||
|---|---|---|---|
| 7 d | 10 d | 11 d | |
| μg mg−1 protein min−1 | |||
| Intact plastids | 0.99 | 2.39 | 1.67 |
| Total membrane | 1.23 | 2.77 | 2.43 |
| PLBs | – | – | 2.37 |
| Prothylakoids | – | – | 2.55 |
| Stroma | 0.69 | 0.84 | 0.83 |
After isolation of intact etioplasts and their subfractions, the proteolytic activity of each sample was estimated. The assay mixture had 120 μg of protein from each sample and 20 μg of exogenous LHCII apoprotein (6 μg of LHCII in 10 μL of 1% TX-100) in 40 mm Tris-HCl, pH 8.6, at a final volume of 140 μL. Proteolysis was assessed by the LHCII apoprotein remaining after incubation at 37°C for 1 h, as monitored by immunodetection following western-blot analysis.
Figure 5.
The effect of the etiolated tissue age on the proteolytic activity of PLBs isolated by differential centrifugation. TX-100-solubilized protein (120 μg) was mixed with 20 μg of exogenous LHCII apoprotein in a final volume of 140 μL. The assay mixture also contained 0.5% TX-100 and 40 mm Tris-HCl, pH 8.6, and was incubated at 37°C for 1 h.
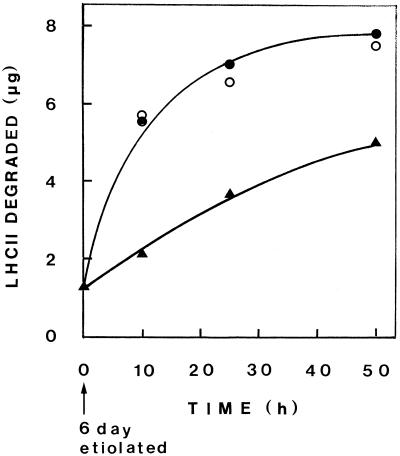
Figure 6 shows the proteolytic activity against exogenously added LHCII apoprotein in thylakoid fractions as affected by light treatment of plants. Thylakoid samples isolated by differential centrifugation from 6-d-old etiolated plants were either kept in the dark, exposed to a 2-min light pulse and kept in the dark thereafter, or exposed to ImL (2 min of light and 98 min of dark in cycles). As shown, pretreatment of etiolated plants by a light pulse resulted in enhancement of the proteolytic activity comparable with that found when these plants were exposed to repeated ImL cycles.
Figure 6.
Proteolysis of exogenous LHCII apoprotein by nonsolubilized thylakoids isolated by differential centrifugation at various stages of development. Six-day-old etiolated plants were kept in the dark (▴) or exposed to a 2-min white-light pulse and kept in the dark thereafter (○) or to ImL (2 min of light, 98 min of dark) (•). To 40 μg of PLB or primary thylakoid protein, 8 μg of exogenous LHCII apoprotein was added to assay mixtures of 48 μL (0.05% TX-100, 40 mm Tris-HCl, pH 8.6) and incubated for 30 min at 37°C.
The effect of light on protease activity was further examined by assaying thylakoids from etiolated plants exposed to CL. Because these thylakoids contain substantial amounts of endogenous LHCII, the assays were conducted in several ways (Table II). First, nonsolubilized membranes were assayed against endogenous LHCII. In this assay, apparent activity increased up to 25 h of light and then declined, as suggested by the amount of LHCII apoprotein degraded out of that present. Second, exogenous LHCII apoprotein was added to these nonsolubilized membranes and the degradation of both endogenous and exogenous LHCII was assayed. As shown in Table II, the activity in this case also increased up to about 25 h of light and then decreased; however, the decrease was less pronounced.
Table II.
Proteolytic activity of thylakoids obtained from 6-d-old etiolated plants exposed to CL
| Exposure to CL | Endo LHCII
|
Exo LHCII Added | Endo + Exo LHCII Degraded | Thylakoid Protein: LHCII (w/w) | Percent LHCII Degraded
|
||
|---|---|---|---|---|---|---|---|
| Present | Degraded | Endo | Endo + Exo | ||||
| h | μg | mg−1 protein min−1 | |||||
| Nonsolubilized | |||||||
| 5 | 0.38 | 0.01 | 8 | 1.17 | 104.0 (4.7) | 2.21 | 11.74 |
| 10 | 0.96 | 0.33 | 8 | 5.16 | 40.7 (4.4) | 29.35 | 49.17 |
| 25 | 8.25 | 5.75 | 8 | 10.46 | 3.8 (1.9) | 73.17 | 67.58 |
| 50 | 15.00 | 5.00 | 8 | 9.60 | 1.7 (1.1) | 44.44 | 55.65 |
| 95 | 24.00 | 2.90 | 8 | 8.90 | 0.7 (0.5) | 25.17 | 57.94 |
| Solubilized | |||||||
| 25 | 8.25 | 7.24 | 8 | 15.20 | 3.8 (1.9) | 92.13 | 98.20 |
| 50 | 15.00 | 15.00 | 8 | 20.50 | 1.7 (1.1) | 133.33 | 118.84 |
| 95 | 24.00 | 24.00 | 8 | 31.00 | 0.7 (0.5) | 208.33 | 201.82 |
Total thylakoid protein (40 μg) was assayed for proteolytic activity after incubation for 30 min at 37°C before or after addition of 8 μg of exogenous (Exo) LHCII apoprotein. Whenever nonsolubilized thylakoids were used, the final TX-100 was 0.05%; solubilized samples had 0.5%. Final volume was 48 μL. Estimation of the exact amount of immunodetected endogenous (Endo) LHCII apoprotein is based on standard curves obtained from increasing known amounts of exogenous LHCII apoprotein immunoblotted on the same nitrocellulose membrane. Values for the ratio of thylakoid protein to LHCII are based on endogenous LHCII present. Values in parentheses are based on total LHCII apoprotein (endogenous + exogenous). Estimation of the rate is based on thylakoid protein after subtraction of the endogenous LHCII apoprotein.
To compare samples differing in the amount of endogenous LHCII, rates were based on thylakoid protein from which the endogenous LHCII was subtracted and estimated as the percentage of LHCII degraded per milligram of protein per minute. The data show that the exogenous LHCII under all conditions was more vulnerable to degradation, and that the decrease in apparent activity against endogenous-plus-exogenous LHCII at prolonged light exposures mainly reflected the decreased degradation of the endogenous LHCII.
This is further supported by the data obtained with thylakoids after TX-100 solubilization. The activity against endogenous LHCII was greatly increased by solubilization of thylakoids. A comparable increase was observed in the rate of endogenous LHCII degradation and that of endogenous-plus-exogenous substrate. This suggests that the decrease in activity in thylakoids of plants exposed to prolonged CL exposure before their TX-100 solubilization probably reflects the inaccessibility of the substrate arising from its association with Chl (i.e. shielding of the apoprotein substrate). It should be noted here that during greening in CL the accumulation of Chl was greatly enhanced. However, as shown in Table II, since endogenous LHCII gradually accumulated during exposure to CL, the ratio of thylakoid protein to endogenous or total LHCII apoprotein present in the assays gradually decreased. This could also result in a reduction in the proteolytic rate. However, because at equal ratios no reduction in proteolytic rates was observed in the solubilized samples, the reduction in proteolytic activity of nonsolubilized samples should be attributed to shielding of the substrate.
To further check whether Chl may be directly involved in the reduction of proteolytic activity in thylakoids, experiments were designed to show whether the high proteolytic activity in Chl-deficient primary thylakoids might be reduced by Chl. In these experiments primary thylakoids were mixed with Chl-rich, mature, CL plant thylakoids. As shown in Table III, the activity in the mixture was found to be equal to the sum of the activity in the separate components. The effect of Chl, therefore, cannot be directly on the protease, but rather indirect via shielding of the substrate.
Table III.
Effect of the addition of Chl-rich mature thylakoids obtained from CL plants on the proteolytic activity of Chl-deficient primary thylakoids of etiolated plants exposed to a pulse of light and kept in the dark for 50 h thereafter
| Sample | Endo LHCII | Exo LHCII | Endo + Exo LHCII | LHCII Degradeda |
|---|---|---|---|---|
| μg | ||||
| Primary | – | 6 | 6.0 | 6.0 |
| Mature | 14.8 | – | 14.8 | 5.4 |
| Primary + mature | 14.8 | 6 | 20.8 | 12.5 |
Primary thylakoids were obtained from 6-d-old etiolated bean plants exposed to a 2-min white-light pulse and then kept in the dark for 50 h. Mature thylakoids were obtained from 6-d etiolated bean plants exposed to CL for 72 h. Primary thylakoids were not solubilized. Mature thylakoids were solubilized in TX-100. Final volume of the assay mixtures was 66 μL, and the final TX-100 concentration was 0.5%. The assays had either 30 μg of primary thylakoid protein mixed with 6 μg of exogenous (Exo) LHCII apoprotein, 30 μg of mature thylakoid protein containing 14.8 μg of endogenous (Endo) LHCII apoprotein, or a mixture of both.
Degradation for 30 min at 37°C.
The drastic difference in proteolytic activity between nonsolubilized and TX-100-solubilized thylakoids obtained from CL plants (Table II) was not observed with primary thylakoids of ImL plants or PLBs of dark-grown plants. Table IV shows representative results. The degradation of exogenous LHCII apoprotein, therefore, by PLBs or primary thylakoids is more or less complete without previous solubilization. Table IV shows rates estimated as micrograms of LHCII degraded per milligram of protein per minute, or as the percentage of LHCII degraded per milligram of protein per minute. In the former, the estimation can be used to compare rates of samples deficient in endogenous LHCII in which a constant amount of exogenous LHCII was added; in the latter, one can compare rates of samples containing added exogenous LHCII apoprotein with those that also possess endogenous LHCII.
Table IV.
Proteolytic degradation of endogenous (Endo) and exogenous (Exo) LHCII apoprotein by thylakoids obtained at different developmental stages
| Sample | Endo
LHCII
|
Exo LHCII Present | Endo + Exo LHCII Degraded | LHCII Degraded
|
||
|---|---|---|---|---|---|---|
| Present | Degraded | μg | % | |||
| μg | mg−1 protein min−1 | |||||
| 50 h of Dark | ||||||
| NS | – | – | 8 | 4.9 | 4.08 | 51.04 |
| S | – | – | 8 | 4.6 | 3.83 | 47.92 |
| 2 min of Light + 50 h of Dark | ||||||
| NS | – | – | 8 | 7.5 | 6.25 | 78.12 |
| S | – | – | 8 | 6.4 | 5.33 | 66.67 |
| 32 LDC | ||||||
| NS | – | – | 8 | 7.8 | 6.50 | 81.25 |
| S | – | – | 8 | 5.5 | 4.58 | 57.29 |
| 50 h of CL | ||||||
| NS | 15 | 5 | 8 | 9.6 | 12.80 | 55.65 |
| S | 15 | 15 | 8 | 20.5 | 27.33 | 118.84 |
Assay mixtures had 40 μg of thylakoid protein and 8 μg of exogenous LHCII apoprotein and were incubated at 37°C for 30 min. For immunoblots, 2 μg of total protein was loaded from the 48-h CL sample; 5 μg of total protein was loaded from all other samples. Nonsolubilized samples (NS) contained 0.05% TX-100; solubilized samples (S) had 0.5% TX-100. Estimation of the exact amount of immunodetected endogenous LHCII apoprotein was based on standard curves obtained from increasing known amounts of exogenous LHCII apoprotein immunoblotted on the same nitrocellulose membrane. Estimation of the rate in CL samples against endogenous + exogenous LHCII is based on total thylakoid protein after subtraction of the endogenous LHCII apoprotein.
Is the Proteolytic Activity Controlled by Chloroplast Development, Chl Accumulation, or Both?
As discussed above, thylakoids of plants exposed to ImL cycles with long dark intervals (2 min of light for every 98 min of dark) lack LHCII, Chl b, and grana, have about 14% of the Chl a of a mature-green leaf, and contain mainly cores of photosystem units (Argyroudi-Akoyunoglou and Akoyunoglou, 1970, 1979; Akoyunoglou, 1977). In contrast, thylakoids of plants exposed to ImL cycles with short dark intervals contain LHCII and larger photosystem units (Tzinas et al., 1987). Because ImL plants have limited Chl accumulation, they are ideal to test whether the thylakoid-bound protease may be responsible for the lack of LHCII in ImL plants and the reduced capacity to accumulate LHCII in CL after prolonged ImL pretreatment. Also, under these conditions Chl could not interfere with the protease activity, and the effect of development could be distinguished from that of Chl accumulation.
Using differential centrifugation, we isolated thylakoids of ImL plants exposed to cycles with varying dark duration, and studied the proteolysis of exogenous LHCII apoprotein after solubilization in TX-100. Thylakoids were obtained from etiolated plants exposed either to the same number of LDCs with either long or short dark intervals (i.e. the same accumulation of Chl but different developmental stage) or to such regimes of LDCs for the same period of time (i.e. the same developmental stage but different Chl accumulation).
Table V shows the proteolytic activity in primary thylakoids of 6-d-old etiolated plants exposed to 18 LDCs having short or long dark intervals (2 min of light for every 23 min of dark or 2 min of light for every 83 min of dark, respectively), or to 59 LDCs with short dark intervals (2 min of light for every 23 min of dark). The proteolytic activity was higher in plants exposed to the same number of cycles with long dark intervals than in those with short dark intervals. The accumulated Chl per gram fresh weight in the plants exposed to 18 LDCs of the two regimes was similar. Thus, at equal Chl accumulation, the thylakoids degraded low amounts of exogenous LHCII apoprotein if isolated from short-dark-interval ImL plants, but showed increased proteolytic activity if isolated from long-dark-interval ImL plants. Table V also shows that the degradation of exogenous LHCII apoprotein was similar in thylakoids of plants exposed to ImL for the same time period (24 h) irrespective of the number of LDCs of each regime (59 LDCs of 2 min of light for every 23 min of dark or 18 LDCs of 2 min of light for every 83 min of dark), and thus irrespective of the amount of Chl accumulated.
Table V.
Comparison of proteolytic activity of primary thylakoids from ImL plants having similar Chl accumulation or similar developmental stage
| Time in Cycles | No. of Cycles | LHCII
Degradeda
|
Chl a | Chl b | |
|---|---|---|---|---|---|
| % | μg | ||||
| h | μg g−1 fresh wt | ||||
| 7 | 18 (2 min of light, 23 min of dark) | 8 | 1.6 | 142 | 21 |
| 24 | 18 (2 min of light, 83 min of dark) | 38 | 7.6 | 187 | 7 |
| 24 | 59 (2 min of light, 23 min of dark) | 33 | 6.7 | 463 | 89 |
Six-day-old etiolated plants were exposed to LDCs with short or long dark intervals. Primary thylakoids were isolated by differential centrifugation, washed twice with 50 mm Tricine, pH 7.3, and solubilized in TX-100. TX-100-solubilized primary thylakoids (120 μg) were mixed with 20 μg of exogenous LHCII apoprotein in a final volume of 140 μL (0.5% TX-100, 40 mm Tris-HCl, pH 8.6). Ten micrograms of protein (thylakoid protein plus exogenous LHCII apoprotein) was applied to gels.
Degradation at 37°C for 30 min.
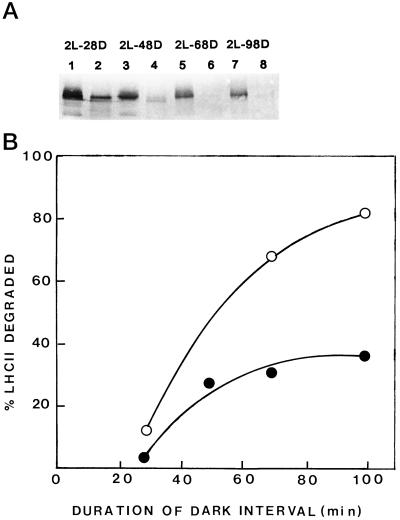
Figure 7 shows the effect of the dark-interval duration in the ImL regime on the proteolytic activity of nonsolubilized (Fig. 7A) or solubilized (Fig. 7B) primary thylakoids obtained from 6-d-old etiolated plants exposed to 20 LDCs. All samples before or after TX-100 solubilization exhibited proteolytic activity, which increased as the duration of the dark phase in LDCs was increased. Figure 7A shows an immunoblot of a gel overloaded with nonsolubilized primary thylakoid protein (50 μg), so that the barely immunodetected endogenous LHCII apoprotein can be seen; the LHCII level was higher in the short-dark-interval plants, being reduced at long dark intervals. In addition, the degradation of exogenous LHCII apoprotein by solubilized primary thylakoids increased as the duration of the dark interval increased (Fig. 7B).
Figure 7.
The effect of the dark-interval duration on the proteolytic activity of primary thylakoids versus endogenous (A) or exogenous (B) LHCII apoprotein. Primary thylakoids were obtained by differential centrifugation from plastids. A, Immunoblot of a gel loaded with 50 μg of nonsolubilized primary thylakoid protein obtained from etiolated plants exposed to 20 LDCs of: 2 min of light (2L) 28 min of dark (28D) (lanes 1 and 2); 2 min of light, 48 min of dark (48D) (lanes 3 and 4); 2 min of light, 68 min of dark (68D) (lanes 5 and 6); or 2 min of light, 98 min of dark (98D) (lanes 7 and 8). The first sample of each set is the one before incubation and the second is after 90 min at 37°C. B, Proteolysis of exogenous LHCII apoprotein by primary thylakoids of ImL plants exposed to 20 LDCs of various dark-interval durations. Solubilized primary thylakoid protein (120 μg) was mixed with 20 μg of exogenous LHCII apoprotein in assay mixtures containing 40 mm Tris-HCl, pH 8.6, and 0.5% TX-100 in a final volume of 140 μL. ○, Incubation for 60 min at 37°C; •, incubation for 30 min at 37°C.
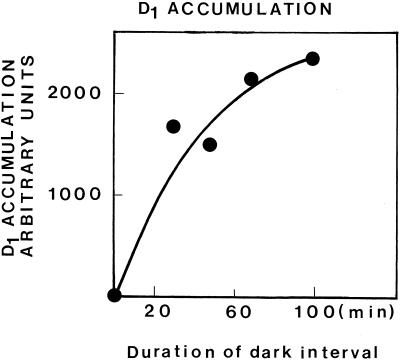
Parallel to the increase in proteolytic activity, which occurred as the dark-phase duration in ImL cycles was prolonged, it was found that the formation of new photosystem units, as monitored by the accumulation of the D1 RC protein, was also enhanced. As shown in Figure 8, the level of D1 accumulated in plants exposed to 20 LDCs was progressively increased parallel to the duration of the dark phase. These results are comparable with those concerning the proteolytic activity.
Figure 8.
Accumulation of D1 in primary thylakoids of 6-d etiolated plants exposed to 20 LDCs isolated by differential centrifugation, as affected by the dark-phase duration in ImL. One-hundred micrograms of nonsolubilized thylakoid protein was loaded per slot. Accumulation is estimated on the basis of the area under the D1 curve, as monitored by scanning of immunoblots.
DISCUSSION
LHCII, a protein encoded in nDNA after synthesis on cytoplasmic ribosomes as a hydrophilic precursor, is imported into the chloroplast and inserted into the thylakoid membrane as a lipophilic protein via its three transmembrane helices and the help of Chl. Mg-GTP and a stromal protein factor believed to be homologous to the srp54 subunit (a 54-kD nuclear-encoded protein) are considered to be required for a “transit-complex” formation that aids LHCII apoprotein insertion in the thylakoid (Cline, 1986; Chitnis et al., 1987; Li et al., 1995). During development in CL the inserted protein is gradually trimerized, as suggested from “green” gel analysis of leaf pigment-protein complexes (Kalosakas et al., 1981; Dreyfuss and Thornber, 1994). According to in vitro studies this process largely protects the protein from proteolytic degradation (Hoober et al., 1990; Kuttkat et al., 1995). Successfully inserted LHCII apoprotein in thylakoids is resistant to exogenous proteases (Schmidt et al., 1981), whereas shielding of the protein by its 15 Chl a and b molecules protects it from degradation (Paulsen et al., 1993). In vitro studies show that for reconstitution of a functional monomeric complex, the combination of at least two xanthophylls (lutein, violaxanthin, or neoxanthin) is required in addition to Chl (Plumley and Schmidt, 1987).
In the present study the involvement of a membrane-bound, but only peripherally attached, protease in the degradation of LHCII apoprotein has been established. The protease was found to act mainly against POR and LHCII, suggesting that it constitutes a regulatory mechanism, the main targets of which are Chl-binding proteins. Under all conditions studied, the level of thylakoid-associated proteolytic activity was found to be dependent on the plastid developmental stage. Based on the finding that NaBr washing of thylakoids removes all proteolytic activity, and on the earlier observations that the association of the protease with thylakoids may be under cation control (Tziveleka and Argyroudi-Akoyunoglou, 1995), one could speculate that the cation concentration in the stroma or the concentration of a developmentally regulated factor (which might also be required for binding of the protease to thylakoids, as in the case of LHCII apoprotein itself [Cline, 1986]), might be responsible for the gradual appearance of the proteolytic activity in thylakoids. If this were so, one would expect to find constant proteolytic activity in isolated intact plastids during development, but a change in the distribution of activity between stroma and prothylakoid fractions. Our results showed that in intact etioplasts the proteolytic activity increased parallel to tissue age and that the activity in the stroma fraction remained low and constant. Therefore, the gradual appearance of the proteolytic activity in thylakoids during development probably reflects the synthesis of the proteolytic system.
The proteolytic activity was increased by a pulse of white light applied to etiolated plants; the increase was similar to that observed after a number of LDCs, or after brief exposure to CL (up to 25 h), suggesting that the protease is light activated. However, exposure of etiolated plants to prolonged CL resulted in reduced proteolytic activity, mainly versus endogenous LHCII, in a manner inversely proportional to Chl accumulation. This reduction in activity was not observed in TX-100-solubilized thylakoids, suggesting that it probably does not reflect a direct effect of Chl on the activity of the protease. Furthermore, the addition of TX-100-solubilized, Chl-rich, mature-green thylakoids to Chl-deficient primary thylakoids did not reduce the activity of the latter. Chl, therefore, seems to act by shielding the LHCII apoprotein, rescuing the protein from proteolytic attack.
The reduction in proteolytic activity of thylakoids obtained from CL plants might also be attributed to the gradual decrease in the ratio of thylakoid protein to endogenous LHCII protein (which occurs during greening) caused by the enhanced accumulation of LHCII. However, this does not seem to be the case, because solubilized thylakoids show high activity at equal ratios. It is interesting to note also that the rate of exogenous LHCII degradation by a constant amount of primary thylakoid protein reaches a plateau at ratios of primary thylakoid protein to LHCII of about 2, a value not far from that in mature-green plant thylakoids (about 1). This suggests that mature-green plants have the potential to degrade their endogenous LHCII apoprotein; however, they confront this activity via Chl-protein complex formation. Therefore, our earlier findings that administration of Chl-synthesis inhibitors to plants results in enhanced thylakoid-bound proteolytic activity against exogenous azocoll substrate (Anastassiou and Argyroudi-Akoyunoglou, 1995a) cannot be attributed to a direct effect of Chl on the protease. One could speculate that Chl may affect the organization of the thylakoid, the protease itself, or free access of the protease to the substrate. Further work is needed to answer this question.
Our data also suggest that the inability of the long- versus the short-dark-interval ImL plant to accumulate LHCII in thylakoids was caused by the increased proteolytic activity in the former. For a similar level of Chl accumulation, the time offered for protease accumulation was higher in the long-dark-interval ImL. In addition, since under these conditions Chl accumulation was limited, the newly synthesized LHCII apoprotein could not be rescued and was completely degraded. In contrast, in the short-dark-interval ImL, a similar level of Chl was accumulated in a much shorter time, during which the protease accumulation was limited. However, the accumulation of D1 monitoring of new photosystem-unit formation was also found to depend on the duration of the dark interval in ImL, increasing with prolonged duration of the dark phase. This suggests that under the latter conditions the formation of new photosystem units is enhanced. The limited amount of Chl in the long-dark-interval ImL cycles, therefore, is expected to be bound in photosystem cores, and the LHCII apoprotein in the absence of Chl binding is expected to be easily degraded. This proteolytic mechanism seems to be involved in LHCII-apoprotein stabilization during thylakoid biogenesis and to be responsible for the lack of LHCII from thylakoids of plants exposed to long-dark-interval LDCs (Tzinas et al., 1987).
ACKNOWLEDGMENTS
We are grateful for the generous gifts of antibodies from Prof. Dr. K. Kloppstech, Hannover University, Germany (anti-LHCII and anti-LSU); from Prof. I. Ohad, Jerusalem University, Israel (anti-D1); from Prof. Dr. W. Ruediger, Munich University, Germany (anti-β-subunit of coupling-factor 1); and from Prof. T. Griffiths, Bristol University, UK (anti-POR).
Abbreviations:
- Chl
chlorophyll
- CL
continuous light
- D1
the reaction-center protein of PSII
- ImL
intermittent light
- LDC
light-dark cycle
- LHCII
light-harvesting pigment-protein complex serving PSII
- LSU
large subunit of Rubisco
- PLB
prolamellar body
- POR
protochlorophyllide oxidoreductase
- RC
chloroplast-encoded reaction center
- TX-100
Triton X-100
Footnotes
This work was partly supported by grants from the European Union (no. BIO2CT930400) and the Greek General Secretariat of Research and Technology (no. PENED 1996–1998).
LITERATURE CITED
- Akoyunoglou A, Akoyunoglou G. Reorganization of thylakoid components during chloroplast development in higher plants after transfer to darkness. Changes in photosystem I and cytochromes. Plant Physiol. 1985;79:425–431. doi: 10.1104/pp.79.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoyunoglou G. Development of the photosystem II unit in plastids of bean leaves greened in periodic light. Arch Biochem Biophys. 1977;183:571–577. doi: 10.1016/0003-9861(77)90392-7. [DOI] [PubMed] [Google Scholar]
- Akoyunoglou G, Argyroudi-Akoyunoglou JH. Effect of intermittent and continuous light on Chl formation in etiolated bean plants of various ages. Physiol Plant. 1969;22:228–235. [Google Scholar]
- Akoyunoglou G, Argyroudi-Akoyunoglou JH. Control of thylakoid growth in Phaseolus vulgaris. Plant Physiol. 1978;61:834–837. doi: 10.1104/pp.61.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoyunoglou G, Argyroudi-Akoyunoglou JH (1986) Post translational regulation of chloroplast differentiation. In G Akoyunoglou, H Senger, eds, Regulation of Chloroplast Differentiation. Alan R. Liss, New York, pp 571–581
- Anastassiou R, Argyroudi-Akoyunoglou JH. Thylakoid-bound proteolytic activity versus exogenous azocoll during chloroplast development in bean. J Plant Physiol. 1995a;145:24–30. [Google Scholar]
- Anastassiou R, Argyroudi-Akoyunoglou JH. Thylakoid-bound proteolytic activity against LHC-II apoprotein in bean. Photosynth Res. 1995b;43:241–250. doi: 10.1007/BF00029937. [DOI] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou JH (1989) Photoregulation of LHCII accumulation in thylakoids during chloroplast development. In J Barber, R Malkin, eds, Techniques and New Developments in Photosynthesis Research. NATO ASI A168. Plenum Press, New York, pp 99–110
- Argyroudi-Akoyunoglou JH, Akoyunoglou A, Kalosakas K, Akoyunoglou G. Reorganization of photosystem II unit in developing thylakoids of higher plants after transfer to darkness. Changes in chlorophyll b, light harvesting content and grana stacking. Plant Physiol. 1982;70:1242–1248. doi: 10.1104/pp.70.5.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou JH, Akoyunoglou G. Photoinduced changes in the chlorophyll a to chlorophyll b ratio in young bean plants. Plant Physiol. 1970;46:247–249. doi: 10.1104/pp.46.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou JH, Akoyunoglou G. The chlorophyll-protein complexes of the thylakoid in greening plastids of Phaseolus vulgaris. FEBS Lett. 1979;104:78–84. [Google Scholar]
- Argyroudi-Akoyunoglou JH, Bei-Paraskevopoulou T, Triantaphyllopoulos K, Anastassiou R (1991) Regulation of photosynthetic unit assembly. In JH Argyroudi-Akoyunoglou, ed, Regulation of Chloroplast Biogenesis. NATO ASI A226. Plenum Press, New York, pp 313–321
- Bei-Paraskevopoulou T, Anastassiou R, Argyroudi-Akoyunoglou JH. Circadian expression of the light-harvesting protein of photosystem II in etiolated bean leaves following a single red light pulse: coordination with the capacity of the plant to form chlorophyll and the thylakoid-bound protease. Photosynth Res. 1995;44:93–106. doi: 10.1007/BF00018300. [DOI] [PubMed] [Google Scholar]
- Blake MS, Johnston KH, Russel GJ, Gotschlich EC. A rapid sensitive method for detection of alkaline phosphatase conjugated anti-antibody on western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Burke JJ, Ditto CL, Arntzen CJ. Involvement of the light-harvesting complex in cation-regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys. 1978;187:252–263. doi: 10.1016/0003-9861(78)90031-0. [DOI] [PubMed] [Google Scholar]
- Chitnis PR, Nechushtai R, Thornber JP. Insertion of the precursor of the light-harvesting chlorophyll a/b-protein into the thylakoid requires the presence of a developmentally regulated stromal factor. Plant Mol Biol. 1987;10:3–11. doi: 10.1007/BF00014181. [DOI] [PubMed] [Google Scholar]
- Cline K. Import of proteins into chloroplasts. J Biol Chem. 1986;261:14804–14810. [PubMed] [Google Scholar]
- Dreyfuss BW, Thornber JP. Assembly of the light-harvesting complexes (LHCs) of photosystem II. Monomeric LHC IIb complexes are intermediates in the formation of oligomeric LHC IIb complexes. Plant Physiol. 1994;106:829–839. doi: 10.1104/pp.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhaus J, Dilley RA, Cramer WA. Selective inhibition of the spinach thylakoid LHCII protein kinase. Biochim Biophys Acta. 1985;809:17–26. [Google Scholar]
- Hoober JK, Maloney MA, Asbury LR, Marks DB. Accumulation of chlorophyll a/b-binding polypeptides in Chlamydomonas reinhardtii y-1 in the light or dark at 38°C. Plant Physiol. 1990;92:419–426. doi: 10.1104/pp.92.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalosakas K, Argyroudi-Akoyunoglou JH, Akoyunoglou G (1981) The formation of pigment-protein complexes in thylakoids of Phaseolus vulgaris during chloroplast development. In G Akoyunoglou, ed, Photosynthesis, Vol V. Balaban International Scientific Services, Philadelphia, pp 569–580
- Kuttkat A, Grimm R, Paulsen H. Light-harvesting chlorophyll a/b-binding protein inserted into isolated thylakoids binds pigments and is assembled into trimeric light-harvesting complex. Plant Physiol. 1995;109:1267–1276. doi: 10.1104/pp.109.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li X, Henry R, Yuan J, Cline K, Hoffman NE. A chloroplast homologue of the signal recognition particle subunit SRP54 is involved in the posttranslational integration of a protein into thylakoid membranes. Proc Natl Acad Sci USA. 1995;92:3789–3793. doi: 10.1073/pnas.92.9.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–322. [Google Scholar]
- Paulsen H, Finkenzeller B, Kuehlein N. Pigments induce folding of light-harvesting chlorophyll a/b-binding protein. Eur J Biochem. 1993;215:809–816. doi: 10.1111/j.1432-1033.1993.tb18096.x. [DOI] [PubMed] [Google Scholar]
- Plumley FG, Schmidt GW. Reconstitution of chlorophyll a/b light harvesting complexes: xanthophyll-dependent assembly and energy transfer. Proc Natl Acad Sci USA. 1987;84:146–150. doi: 10.1073/pnas.84.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Soll J. Protein transport in intact, purified pea etioplasts. Arch Biochem Biophys. 1986;247:211–220. doi: 10.1016/0003-9861(86)90550-3. [DOI] [PubMed] [Google Scholar]
- Schmidt GW, Bartlett SG, Grossman AR, Cashmore AR, Chua NH. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981;91:468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzinas G, Argyroudi-Akoyunoglou JH. Chloramphenicol-induced stabilization of light-harvesting complexes in thylakoids during development. FEBS Lett. 1988;229:135–141. [Google Scholar]
- Tzinas G, Argyroudi-Akoyunoglou JH, Akoyunoglou G. The effect of dark interval in intermittent light on thylakoid development: photosynthetic unit formation and light-harvesting protein accumulation. Photosynth Res. 1987;14:241–258. doi: 10.1007/BF00032708. [DOI] [PubMed] [Google Scholar]
- Tziveleka LA, Argyroudi-Akoyunoglou JH (1995) Cations control the association of a stromal protease to thylakoids In P Mathis, ed, Photosynthesis: From Light to Biosphere, Vol III. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 835–838
- Viro M, Kloppstech K. Expression of genes for plastid membrane proteins in barley under intermittent light conditions. Planta. 1982;154:18–24. doi: 10.1007/BF00385491. [DOI] [PubMed] [Google Scholar]