Abstract
Herein, we disclose the first metal-free synthesis of primary aromatic amines from arylboronic acids, a reaction that has eluded synthetic chemists for decades. This remarkable transformation affords structurally diverse primary arylamines in good chemical yields, including a variety of halogenated primary anilines that often cannot be prepared via transition metal-catalyzed amination. The reaction is operationally simple, requires only a slight excess of aminating agent, proceeds under neutral or basic conditions and, importantly, it can be scaled up to provide multigram quantities of primary anilines. Density functional calculations reveal that the most likely mechanism involves a facile 1,2-aryl migration and that the presence of an ortho nitro group in the aminating agent plays a critical role in lowering the free energy barrier of the 1,2-aryl migration step.
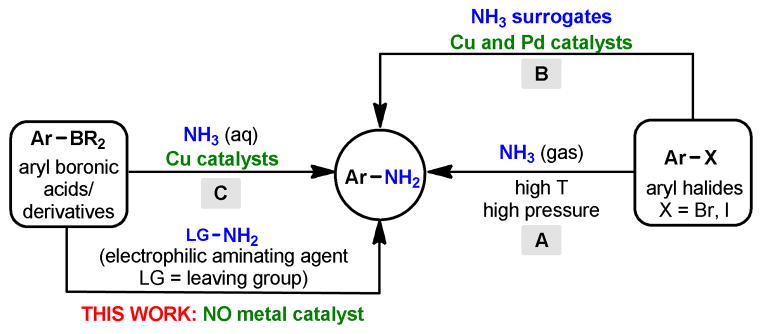
Primary aromatic amines are a prominent class of compounds found in a wide variety of natural products and as key components of pharmaceuticals, agrochemicals, dyes and polymers. 1 They are most commonly prepared via catalytic hydrogenation or metal-mediated reductions of aromatic nitro compounds,2 direct substitution of aryl halides with ammonia at high pressure and temperature3 and, transition metal catalyzed amination of aryl halides with ammonia or its surrogates (Figure 1, A & B).4 However, these transformations often have several drawbacks, for example, the concomitant formation of undesired diaryl amines, the use of strong bases, and the requirement for sometimes costly ligands. During the past decade, significant advances have been made in the area of copper-catalyzed oxidative amination of arylboronic acids which offer practical access to a wide variety of N-substituted arylamines (e.g., Chan-Lam coupling).5 More recently, the transition metal-catalyzed (Pd, Ni or Cu) electrophilic amination of various organometallic reagents6 (e.g., Li, Zn and B) as well as the direct C-H amination of aromatic rings7 have received considerable attention for the preparation of N,N-dialkyl anilines. Surprisingly, the synthesis of primary arylamines from arylboronic acids and derivatives has proven to be far more challenging.8 For example, Fu and co-workers have only recently achieved the cross-coupling of arylboronic acids with aqueous ammonia in the presence of copper(I) oxide (Figure 1, C).8a
Figure 1.
Approaches to primary aromatic amines from aryl halides and aromatic boronic acids/derivatives.
All of the aforementioned achievements are mediated by transition metals. From a practical point of view, non-metal processes are much preferred, especially in the pharmaceutical industry where the removal of undesired metal contamination can be expensive.9
Moreover, certain functional groups are incompatible with transition metals; for example, the preparation of mono- and polyhalogenated anilines6e is often not possible under transition metal catalysis due to competing cross-coupling processes.
Our recent studies10 on the hydroxylation of arylboronic acids by N-tertiary amine oxides prompted us to investigate if a similar strategy, via a leaving group-initiated 1,2-aryl migration,11 would also work for the preparation of primary arylamines (Scheme 1). However, it was clear that achieving the metal-free amination would be significantly more challenging, since it is well-known that common electrophilic aminating agents (e.g., hydroxylamine-O-sulfonic acid and alkyl azides) do not affect boronic acids and their esters.12 These aminating agents require the presence of substantially more electrophilic boron derivatives, such as borinic acids, borinic esters or dichloroboranes.13 Indeed, a thorough literature search revealed only a handful of recent methods that can convert the C(sp2)-B bond of arylboronic acids and derivatives to the corresponding C(sp2)-N bond in the absence of transition metals.14 For instance, boronic acids can be exchanged for highly electron-deficient nitrogen functional groups (i.e., nitro and nitroso groups) via an aromatic ipso substitution mechanism.14a,14d,14f There were also two reports in which electrophilic aminating agents (e.g., organic azides and N,N-dialkyl-O-benzoyl hydroxylamines) reacted with arylboronic acids14b and arylboroxines,14g respectively, albeit under very harsh reaction conditions (>130 °C), to afford the corresponding N-monosubstituted as well as N,N-disubstituted anilines. Finally, excess lithiated methoxyamine (3 equiv) was found to aminate aryl pinacol boronates, although the scope of this transformation is mostly limited to electron rich substrates and base-sensitive functional groups are apparently not compatible with the reaction conditions.14e Herein, we disclose a metal-free, mild, operationally simple and practical approach to access primary arylamines by the direct amination of arylboronic acids (Scheme 1, II).
Scheme 1.
Hydroxylation and Amination of Arylboronic Acids via Leaving-Group-Initiated 1,2-Aryl Migration
At the outset, we assessed the reactivity of various potential aminating reagents towards arylboronic acids (Table 1). We hypothesized that the ideal reagent should possess two key structural features: (a) an NH2 group that is nucleophilic enough to attack the boronic acid and electrophilic enough to accept the migrating aryl group; (b) a weak N-N or N-O bond that upon cleavage facilitates the departure of the leaving group. Attempted amination of 2-naphthylboronic acid 1a with different hydrazine derivatives (3–5) did not yield the desired primary aryl amine (entries 1–3). Surprisingly, hydroxylamine-O-sulfonic acid (HSA, 6) and O-mesitylenesulfonyl-hydroxylamine (MSH, 7), both of which are effective for the conversion of trialkylboranes to alkylamines,15 also did not transfer the NH2 group to 1a (entries 4–5). Gratifyingly, the reaction with O-(2,4-dinitrophenyl)-hydroxylamine (DPH, 8) furnished the expected primary 2-naphthylamine 2a in good isolated yield (entry 6). Importantly, aminating reagent 8 is conveniently prepared via Charette’s two-step procedure,16 and is stable at 0 °C for several months without appreciable decomposition. 17 With DPH 8 selected as the preferred aminating reagent, we next investigated the effect of solvents on the transformation 1a–2a. The choice of solvent was critical for success. Among the examined solvents, only DCE and toluene gave rise to the expected product (2a), with the latter resulting in a higher yield (70% vs 76%). Many common solvents such as THF, dioxane, methanol, acetonitrile, DMF and DMSO were apparently unsuitable for the transformation. At 25 °C, the amination was slow to complete (>2 d), however, raising the temperature to 50 °C led to the full consumption of 1a in 24 hours.
Table 1.
Potential Aminating Agents for the Conversion of 1a to 2a.

| ||
|---|---|---|
| Entrya | Aminating Agent | Yieldc (%) |
| 1 |
 3
3
|
0 |
| 2 |
 4
4
|
0 |
| 3 |
 5
5
|
0 |
| 4b |
 6
6
|
0 |
| 5b |
 7
7
|
0 |
| 6 |
 8
8
|
70 |
With the optimized reaction conditions in hand, we turned to explore the scope of substrates (Table 2). Pleasingly, these conditions were compatible with a variety of substituents, regardless of their electronic or steric properties. Toluene as the solvent worked for most substrates, however, in some cases the use of other solvents proved more advantageous (entries 10, 11, 13 & 16). Boronic acids featuring fused aromatic rings furnished the aminated products in good yields (entries 1–4). Generally, the amination of substrates with electron-donating groups (Ph, Me, i-Pr and MeO) resulted in good product yields (entries 6–10). The presence of the strongly electron-withdrawing CF3, CN and CO2Me groups in 1j–l required extended reaction times (up to 4 days) to reach full conversion (entries 11–13) and, in one case the reaction temperature had to be raised to 100 °C. Single or multiple halogen substituents (F, Cl, Br and I, 1m–t), at the ortho, meta and para-positions, were also well-tolerated in the transformation (entries 14–22). However, polyhalogenated substrates 1s and 1t required heating to 100 °C in order to undergo primary amination (entries 21 & 22). The steric bulk of ortho substituents also did not prevent the amination of substrates 1h and 1i (entries 9 & 10). In the case of the 2,4,6-trimethyl-phenylboronic acid (1i) the yield of 2i was only moderate, however, a single i-Pr group in the ortho position did not substantially impact the yield of primary aniline 2h (entry 9). Most substrates could be transformed under mild conditions without any additives. However, in a few special cases, the addition of a base, such as Cs2CO3, was beneficial for the reaction. For instance, the base slightly improved the isolated yield of 2-naphthylamine 2a (entry 2), or significantly shortened the reaction time (24 h vs 96 h) and also reduced the required reaction temperature (25 vs 50 °C) for 1r (entry 20). For unknown reasons, N-acyl substituted boronic acids do not undergo primary amination under the optimized reaction conditions. In addition, substrates containing unprotected nitrogen atoms (e.g., indole, pyrrole), sulfur atoms (e.g., thiophene) and carbonyl groups (e.g., aryl ketones) are not compatible with the reaction conditions. These findings were not very surprising since pyridines and pyrroles are known to undergo N-primary amination in the presence of aminating agent 8.16 We were pleased to find, however, that an oxygen-containing heteroaromatic boronic acid such as 1u was smoothly aminated to afford 2u in good isolated chemical yield (entry 23).
Table 2.
Scope of Metal-Free Amination Using DPH (8).

| ||||||
|---|---|---|---|---|---|---|
| Entrya | Substrate | T (°C) | Cs2CO3 (equiv) | Time (h) | Product | Yieldc (%) |
| 1 | 1a | 50 | N/A | 24 | 2a | 76 |
| 2 | 1a | 25 | 1.2 | 24 | 2a | 82 |
| 3 |
 1b |
50 | N/A | 24 |
 2b |
84 |
| 4 |
 1c |
50 | N/A | 24 |
 2c |
61 |
| 5 | Ph-B(OH)2 1d |
50 | N/A | 24 | Ph-NH2 2d |
80 |
| 6 |
1e |
50 | N/A | 24 |
2e |
62 |
| 7 |
1f |
50 | N/A | 20 |
2f |
80 |
| 8 |
1g |
50 | N/A | 4 |
2g |
72 |
| 9 |
 1h |
50 | N/A | 48 |
 2h |
67 |
| 10 |
 1i |
25 (DCE) | 1.2 | 48 |
 2i |
42 |
| 11 |
1j |
100 (THF, sealed tube) | N/A | 96 |
2j |
51 |
| 12 |
1k |
80 | N/A | 96 |
2k |
78 |
| 13 |
1l |
50 (CH3CN) | 1.2 | 24 |
2l |
88 |
| 14 |
1m |
50 | N/A | 48 |
2m |
82 |
| 15 |
1n |
50 | N/A | 24 |
2n |
83 |
| 16 |
1o |
50 (Dioxane) | N/A | 24 |
 2o |
60 |
| 17 |
 1p |
50 | N/A | 24 |
 2p |
54 |
| 18 |
 1q |
50 | N/A | 48 |
 2q |
66 |
| 19 |
 1r |
50 | N/A | 96 |
 2r |
80 |
| 20 | 1r | 25 | 1.2 | 24 | 2r | 86 |
| 21 |
 1s |
100 | N/A | 30 |
 2s |
56 |
| 22 |
 1t |
100 | N/A | 72 |
 2t |
54 |
| 23 |
 1u |
50 | 1.2 | 20 |
 2u |
66 |
Performed on 0.2 mmol scale (0.2 M solution) in toluene unless shown otherwise;
1.5 equiv of reagent 8;
Isolated yield.
To understand the mechanism and novel reactivity of reagent 8, we have used the M06-2X density functional method18 to calculate possible transition states and pathways for reaction with PhB(OH)2. We first examined possible radical pathways since the computed N-O bond dissociation free energy (BDFE) of reagent 8 is relatively small (29.9 kcal/mol). However, all pathways considered result in radical Wheland-type intermediates that have ΔG values greater than ~40 kcal/mol. We explored concerted mechanisms that begin from the B-N dative bond complex (Complex-8, Figure 2). From this slightly endergonic complex the lowest energy concerted pathway involves the 1,2-aryl migration transition state, TS-1, that has a ΔG‡ of 28.0 kcal/mol relative to separated PhB(OH)2 and 8.19 In TS-1 the Ph-B bond is stretched to 1.75 Å and the Ph-N bond is formed at 2.03 Å. There is also simultaneous N-OAr bond cleavage (1.85 Å) and shortening of the B-N bond (1.52 Å). The product resulting from TS-1 is the (PhNH2)B(OH)2(OAr) complex, which is highly exergonic with ΔGrxn = −72.0 kcal/mol.
Figure 2.
1,2-Aryl migration transition state. (Bond lengths in Å)
To understand why reagent 8 provides a facile 1,2-migration pathway, we investigated the impact of the NO2 groups in stabilizing TS-1. Removal of only the para-NO2 group increases ΔG‡ by 4.4 kcal/mol. Surprisingly, removal of only the ortho-NO2 group increases ΔG‡ by 12.2 kcal/mol. This shows that the ortho-NO2 group is key in providing a low barrier for 1,2-aryl migration because it both stabilizes the developing aryl oxide anion and participates in a hydrogen bonding interaction with one of the amino NH bonds.
In accordance with no product formation, reagents 3–5 have ΔG‡ values that range from 54–66 kcal/mol for aryl migration. Reagents 6 and 7 have ΔG‡ values of 31.1 and 31.6 kcal/mol, respectively. In general there is a connection between these barrier heights and the N-N and N-O bond strengths of the aminating agents. For reagents 3, 4, and 5 the N-N BDFEs are 82.1, 74.1, and 68.4 kcal/mol, respectively. Reagents 6 and 7 with N-O bonds have BDFEs of 52.7 and 49.4 kcal/mol. All of these BDFEs are significantly larger than the N-O BDFE of reagent 8. However, there is clearly no direct linear correlation between barriers and BDFEs.
We have already established that electron deficient amination reagents lower the 1,2-aryl migration barrier. To examine the electronic effects of the aryl boronic acid we calculated ΔG‡ values for para substituted versions. This analysis showed that electron-donating groups lower the barrier for migration. This is likely due to facilitating intramolecular attack of the N-O antibond and stabilization of the electron deficient nitrogen as the anionic OAr group is displaced.
Next, we explored if representative commercially available boronic acid derivatives would also undergo primary amination using 8 (Table 3). To our delight, both 2-naphthyltrifluoroborate 3a as well as 2-naphthylboronic ester 3b were converted to the corresponding primary 2-aminonaphthalene 2a when water was used as a co-solvent in the reaction. In both cases, use of Cs2CO3 as an additive improved the isolated yield of 2a.
Table 3.
Arylboronic Acid Derivatives as Substrates Using Aminating Agent DPH (8).
| Entrya | Substrate | T (°C) | Cs2Co3 (equiv) | Time (h) | Product | Yieldb (%) |
|---|---|---|---|---|---|---|
| 1 |
 3a |
50 (toluene:H2O = 5:1) | 1.2 | 30 |
 2a |
74 |
| 2 |
 3b |
100 (dioxane:H2O = 5:1) | 1.2 | 3 |
 2a |
73 |
Performed on 0.2 mmol scale (0.2 M solution) in the solvent shown;
Isolated yield.
Encouraged by the results above, the amination reaction was subsequently conducted on a gram scale, demonstrating its practicality (Scheme 2). When substrates 1a and 1r were primary aminated either in the presence or absence of Cs2CO3, the corresponding anilines were obtained in similar chemical yields.
Scheme 2.
Primary Amination of Arylboronic Acids on a Gram-Scale Using Aminating Agent DPH (8).
In summary, the first metal-free amination of aromatic boronic acids leading to primary arylamines, a reaction that has eluded synthetic chemists for decades, was demonstrated. The transformation is operationally simple, proceeds under mild conditions and, affords structurally diverse primary aniline products in good chemical yield. It is especially noteworthy that a variety of halogenated primary anilines, that often cannot be prepared via the transition metal-catalyzed amination of the corresponding arylboronic acids and aryl halides, are also readily produced using this method while obviating the formation of undesired N-polyarylated products. In addition, the reaction can be readily scaled up to gram-scale, thereby offering a practical route for the production of structurally diverse primary arylamines. The exploration of related transformations and several powerful aminating agents is currently under way in our laboratories.
Supplementary Material
Acknowledgments
L.K. gratefully acknowledges the generous financial support of the UT Southwestern Endowed Scholars in Biomedical Research Program (W.W. Caruth, Jr, Endowed Scholarship in Biomedical Research), the Robert A. Welch Foundation (Grant I-1764), the ACS Petroleum Research Fund (Doctoral New Investigator Grant 51707-DNI1) and the American Cancer Society & Simmons Cancer Center Institutional Research Grant (New Investigator Award in Cancer Research, ACS-IRG 02-196). J.R.F. thanks the NIH (GM31278) and the Robert A. Welch Foundation (GL-625910) for financial support. D.H.E. thanks BYU and the Fulton Supercomputing Lab for support of this work.
Footnotes
Notes
The authors declare no competing financial interest.
Complete experimental procedures and characterization data including 1H and 13C NMR spectra, further computational details and full reference 18b. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Lawrence SA, editor. Amines: Synthesis, Properties and Applications. Cambridge University Press; Cambridge: 2004. [Google Scholar]; (b) Rappoport Z, editor. The Chemistry of Anilines, Parts 1–2. John Wiley & Sons; 2007. [Google Scholar]; (c) Scholz U, Schlummer B. Sci Synth. 2007;31b:1565. [Google Scholar]; (d) Ricci A, editor. Amino Group Chemistry: From Synthesis to the Life Sciences. Wiley-VCH; 2008. [Google Scholar]
- 2.(a) Blaser HU, Siegrist U, Steiner H, Studer M. Fine Chem Heterog Catal. 2001:389. [Google Scholar]; (b) Mallat T, Baiker A, Kleist W, Koehler K. Handb Heterog Catal. (2) 2008;7:3548. [Google Scholar]; (c) Blaser HU, Steiner H, Studer M. ChemCatChem. 2009;1:210. [Google Scholar]
- 3.Aubin Y, Fischmeister C, Thomas CM, Renaud JL. Chem Soc Rev. 2010;39:4130. doi: 10.1039/c003692g. [DOI] [PubMed] [Google Scholar]
- 4.(a) Klinkenberg JL, Hartwig JF. Angew Chem, Int Ed. 2011;50:86. doi: 10.1002/anie.201002354. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rao H, Fu H. Synlett. 2011:745. [Google Scholar]
- 5.(a) Qiao JX, Lam PYS. In: Boronic Acids. 2. Hall DG, editor. Vol. 1. Wiley-VCH; 2011. p. 315. [Google Scholar]; (b) Qiao JX, Lam PYS. Synthesis. 2011:829. [Google Scholar]
- 6.(a) Berman AM, Johnson JS. J Am Chem Soc. 2004;126:5680. doi: 10.1021/ja049474e. [DOI] [PubMed] [Google Scholar]; (b) Barker TJ, Jarvo ER. J Am Chem Soc. 2009;131:15598. doi: 10.1021/ja907038b. [DOI] [PubMed] [Google Scholar]; (c) Barker TJ, Jarvo ER. Synthesis. 2011:3954. [Google Scholar]; (d) Olson DE. Mini-Rev Org Chem. 2011;8:341. [Google Scholar]; (e) Rucker RP, Whittaker AM, Dang H, Lalic G. Angew Chem, Int Ed. 2012;51:3953. doi: 10.1002/anie.201200480. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kawano T, Hirano K, Satoh T, Miura M. J Am Chem Soc. 2010;132:6900. doi: 10.1021/ja101939r. [DOI] [PubMed] [Google Scholar]; (b) Yoo EJ, Ma S, Mei TS, Chan KSL, Yu JQ. J Am Chem Soc. 2011;133:7652. doi: 10.1021/ja202563w. [DOI] [PubMed] [Google Scholar]
- 8.(a) Rao H, Fu H, Jiang Y, Zhao Y. Angew Chem, Int Ed. 2009;48:1114. doi: 10.1002/anie.200805424. [DOI] [PubMed] [Google Scholar]; (b) Liesen AP, Silva AT, Sousa JC, Menezes PH, Oliveira RA. Tetrahedron Lett. 2012;53:4240. [Google Scholar]
- 9.(a) Garrett CE, Prasad K. Adv Synth Catal. 2004;346:889. [Google Scholar]; (b) Welch CJ, Albaneze-Walker J, Leonard WR, Biba M, DaSilva J, Henderson D, Laing B, Mathre DJ, Spencer S, Bu X, Wang T. Org Process Res Dev. 2005;9:198. [Google Scholar]; (c) Qiu F, Norwood DL. J Liq Chromatogr Relat Technol. 2007;30:877. [Google Scholar]
- 10.Zhu C, Wang R, Falck JR. Org Lett. 2012;14:3494. doi: 10.1021/ol301463c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Jiang N, Qu Z, Wang J. Org Lett. 2001;3:2989. doi: 10.1021/ol016324p. [DOI] [PubMed] [Google Scholar]; (b) Pei T, Chen C-y, Dormer PG, Davies IW. Angew Chem, Int Ed. 2008;47:4231. doi: 10.1002/anie.200705804. [DOI] [PubMed] [Google Scholar]; (c) Pei T, Tellers DM, Streckfuss EC, Chen CY, Davies IW. Tetrahedron. 2009;65:3285. [Google Scholar]
- 12.Hall DG. In: Boronic Acids. 2. Hall DG, editor. Vol. 1. Wiley- VCH; 2011. p. 1. [Google Scholar]
- 13.(a) Brown HC, Heydkamp WR, Breuer E, Murphy WS. J Am Chem Soc. 1964;86:3565. [Google Scholar]; (b) Rathke MW, Inoue N, Varma KR, Brown HC. J Am Chem Soc. 1966;88:2870. [Google Scholar]; (c) Brown HC, Kim KW, Cole TE, Singaram B. J Am Chem Soc. 1986;108:6761. [Google Scholar]; (d) Brown HC, Salunkhe AM, Singaram B. J Org Chem. 1991;56:1170. [Google Scholar]
- 14.(a) Prakash GKS, Panja C, Mathew T, Surampudi V, Petasis NA, Olah GA. Org Lett. 2004;6:2205. doi: 10.1021/ol0493249. [DOI] [PubMed] [Google Scholar]; (b) Ou L, Shao J, Zhang G, Yu Y. Tetrahedron Lett. 2011;52:1430. [Google Scholar]; (c) Wu XF, Schranck J, Neumann H, Beller M. Chem Commun. 2011;47:12462. doi: 10.1039/c1cc15484b. [DOI] [PubMed] [Google Scholar]; (d) Manna S, Maity S, Rana S, Agasti S, Maiti D. Org Lett. 2012;14:1736. doi: 10.1021/ol300325t. [DOI] [PubMed] [Google Scholar]; (e) Mlynarski SN, Karns AS, Morken JP. J Am Chem Soc. 2012;134:16449. doi: 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Molander GA, Cavalcanti LN. J Org Chem. 2012;77:4402. doi: 10.1021/jo300551m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Xiao Q, Tian L, Tan R, Xia Y, Qiu D, Zhang Y, Wang J. Org Lett. 2012;14:4230. doi: 10.1021/ol301912a. [DOI] [PubMed] [Google Scholar]
- 15.Tamura Y, Minamikawa J, Fujii S, Ikeda M. Synthesis. 1974:196. [Google Scholar]
- 16.Legault C, Charette AB. J Org Chem. 2003;68:7119. doi: 10.1021/jo034456l. [DOI] [PubMed] [Google Scholar]
- 17.Aminating agent 8 is commericially available from Corvinus Chemicals, LLC in multi-gram quantities with >99% purity (corvinuschemicals@gmail.com).
- 18.(a) All calculations were performed in Gaussian 09 with the M06-2X/6-31G(d,p) functional and basis set in conjunction with the SMD solvent model for toluene. All structures were confirmed as minima or saddle points through normal mode analysis and free energies are reported at 298 K Frisch MJ, et al. Gaussian 09, revision A.02. Gaussian, Inc; Wallingford, CT: 2009. Zhao Y, Truhlar DG. Theor Chem Acc. 2008;120:215.Zhao Y, Truhlar DG. Acc Chem Res. 2008;41:157. doi: 10.1021/ar700111a.Marenich AV, Cramer CJ, Truhlar DG. J Phys Chem B. 2009;113:6378. doi: 10.1021/jp810292n.
- 19.(a) As a comparison, the ΔG‡ values computed for TS-1 using the B3LYP, ωB97-XD, and BMK functionals with the 6-31G(d,p) basis set are 26.1, 28.7 and 30.2 kcal/mol, respectively. Becke AD. J Chem Phys. 1993;98:5648.Boese AD, Martin JML. J Chem Phys. 2004;121:3405. doi: 10.1063/1.1774975.Chai JD, Head-Gordon M. Phys Chem Chem Phys. 2008;10:6615. doi: 10.1039/b810189b.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.