Abstract
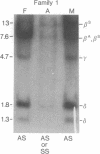
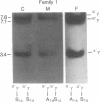
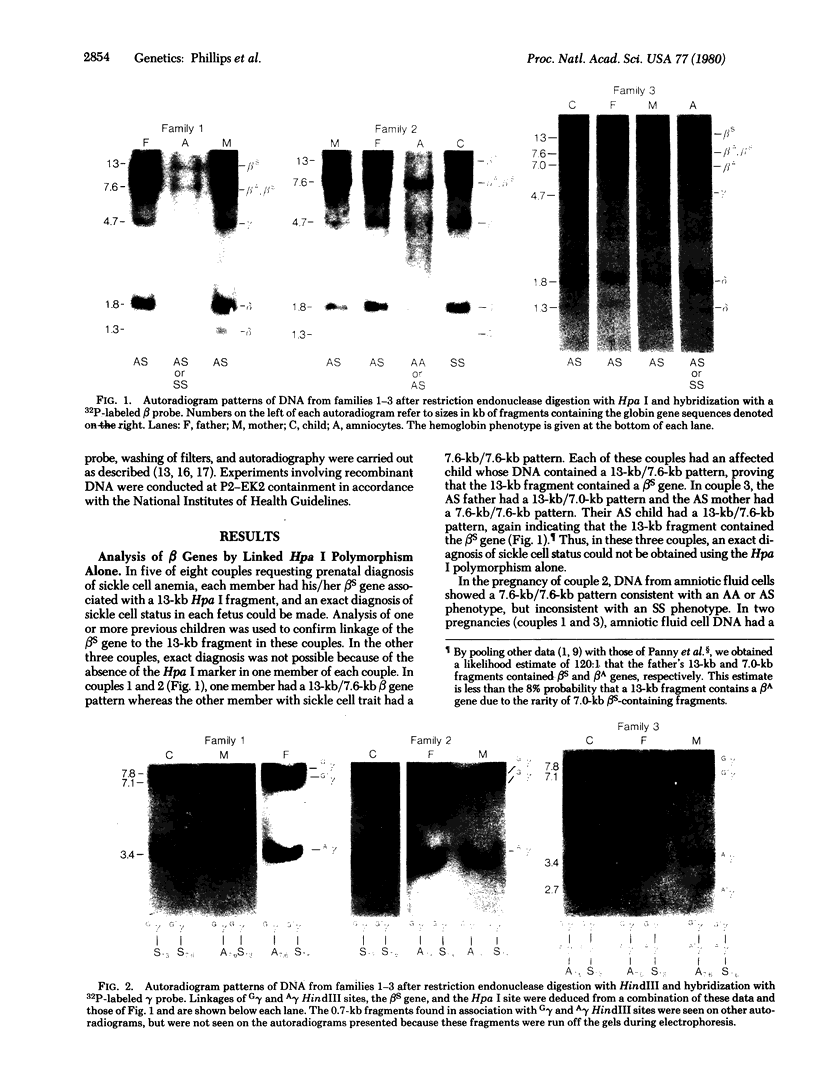
Polymorphism for a Hpa I restriction endonuclease site associated with about 60% of beta S genes in American Blacks allows exact prenatal diagnosis of sickle cell anemia by amniocentesis in 36% of couples at risk. In three families in whom exact diagnosis by Hpa I sites was impossible, we found analysis for the presence of polymorphic HindIII sites in the G gamma and A gamma intervening sequences would allow an exact prenatal diagnosis of sickle cell status in all three. In one of these families, the presence of an A gamma HindIII site in amniocyte DNA confirmed the diagnosis (sickle cell trait) made by synthetic studies using fetal erythrocytes obtained at fetoscopy. Studies of other Black families and individuals provide evidence for linkage disequilibrium in the G gamma-A gamma-delta-beta gene complex involving the four sites, G gamma HindIII, A gamma HindIII, beta S, and Hpa I, which span 33 kilobases (kb). Ten of 14 chromosomes bearing a beta S gene in a 7.6-kb Hpa I fragment contained a G gamma but not an A gamma HindIII site, whereas 16 of 16 chromosomes bearing a beta S gene in a 13-kb Hpa I fragment lacked both the G gamma and A gamma HindIII sites. Two-thirds of beta A-bearing chromosomes lacked both G gamma and A gamma sites, whereas one-third contained either the G gamma or both G gamma and A gamma sites. These data demonstrate that combined analysis of both Hpa I and HindIII polymorphisms and verification of their linkage phase should increase the fraction of couples for whom amniocentesis can provide an exact diagnosis of sickle cell status from 36% to greater than 80%.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Modell C. B., Fairweather D., Hobbins J. C., Mahoney M. J., Frigoletto F. D., Sherman A. S., Nathan D. G. Prenatal diagnosis of hemoglobinopathies. A review of 15 cases. N Engl J Med. 1976 Dec 23;295(26):1437–1443. doi: 10.1056/NEJM197612232952601. [DOI] [PubMed] [Google Scholar]
- Alter B. P. Prenatal diagnosis of hemoglobinopathies and other hematologic diseases. J Pediatr. 1979 Oct;95(4):501–503. doi: 10.1016/s0022-3476(79)80753-2. [DOI] [PubMed] [Google Scholar]
- Feldenzer J., Mears J. G., Burns A. L., Natta C., Bank A. Heterogeneity of DNA fragments associated with the sickle-globin gene. J Clin Invest. 1979 Sep;64(3):751–755. doi: 10.1172/JCI109519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Characterisation of deletions which affect the expression of fetal globin genes in man. Nature. 1979 Jun 14;279(5714):598–603. doi: 10.1038/279598a0. [DOI] [PubMed] [Google Scholar]
- Golbus M. S., Loughman W. D., Epstein C. J., Halbasch G., Stephens J. D., Hall B. D. Prenatal genetic diagnosis in 3000 amniocenteses. N Engl J Med. 1979 Jan 25;300(4):157–163. doi: 10.1056/NEJM197901253000402. [DOI] [PubMed] [Google Scholar]
- Hobbins J. C., Mahoney M. J. In utero diagnosis of hemoglobinopathies. Technic for obtaining fetal blood. N Engl J Med. 1974 May 9;290(19):1065–1067. doi: 10.1056/NEJM197405092901908. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. DNA sequence variants in the G gamma-, A gamma-, delta- and beta-globin genes of man. Cell. 1979 Sep;18(1):1–10. doi: 10.1016/0092-8674(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Antenatal diagnosis of sickle-cell anaemia by D.N.A. analysis of amniotic-fluid cells. Lancet. 1978 Oct 28;2(8096):910–912. doi: 10.1016/s0140-6736(78)91629-x. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. A., 3rd, Scott A. F., Kazazian H. H., Jr, Smith K. D., Stetten G., Thomas G. H. Prenatal diagnosis of hemoglobinopathies by restriction endonuclease analysis: pregnancies at risk for sickle cell anemia and S--O Arab disease. Johns Hopkins Med J. 1979 Aug;145(2):57–60. [PubMed] [Google Scholar]
- Schachat F. H., Hogness D. S. Repetitive sequences in isolated Thomas circles from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1974;38:371–381. doi: 10.1101/sqb.1974.038.01.040. [DOI] [PubMed] [Google Scholar]
- Scott A. F., Phillips J. A., 3rd, Migeon B. R. DNA restriction endonuclease analysis for localization of human beta- and delta-globin genes on chromosome 11. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4563–4565. doi: 10.1073/pnas.76.9.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tuan D., Biro P. A., deRiel J. K., Lazarus H., Forget B. G. Restriction endonuclease mapping of the human gamma globin gene loci. Nucleic Acids Res. 1979 Jun 11;6(7):2519–2544. doi: 10.1093/nar/6.7.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. T., Wilson L. B., deRiel J. K., Villa-komaroff L., Efstratiadis A., Forget B. G., Weissman S. M. Insertion of synthetic copies of human globin genes into bacterial plasmids. Nucleic Acids Res. 1978 Feb;5(2):563–581. doi: 10.1093/nar/5.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]