Abstract
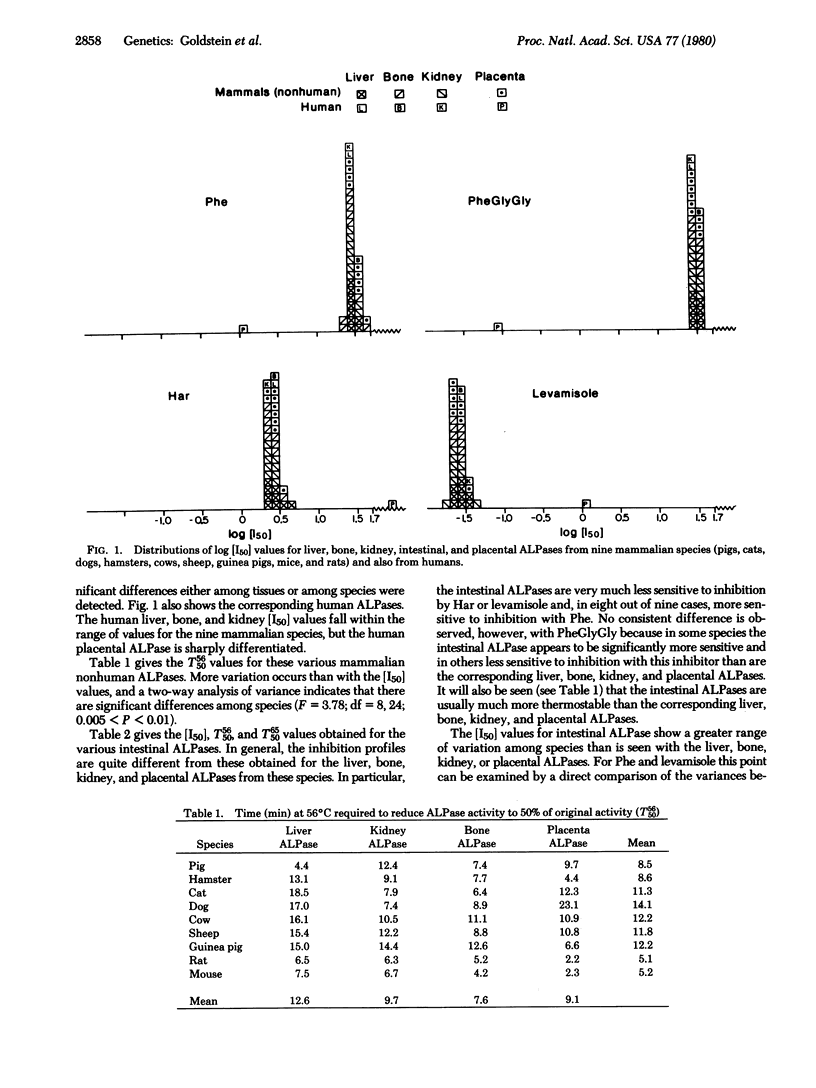
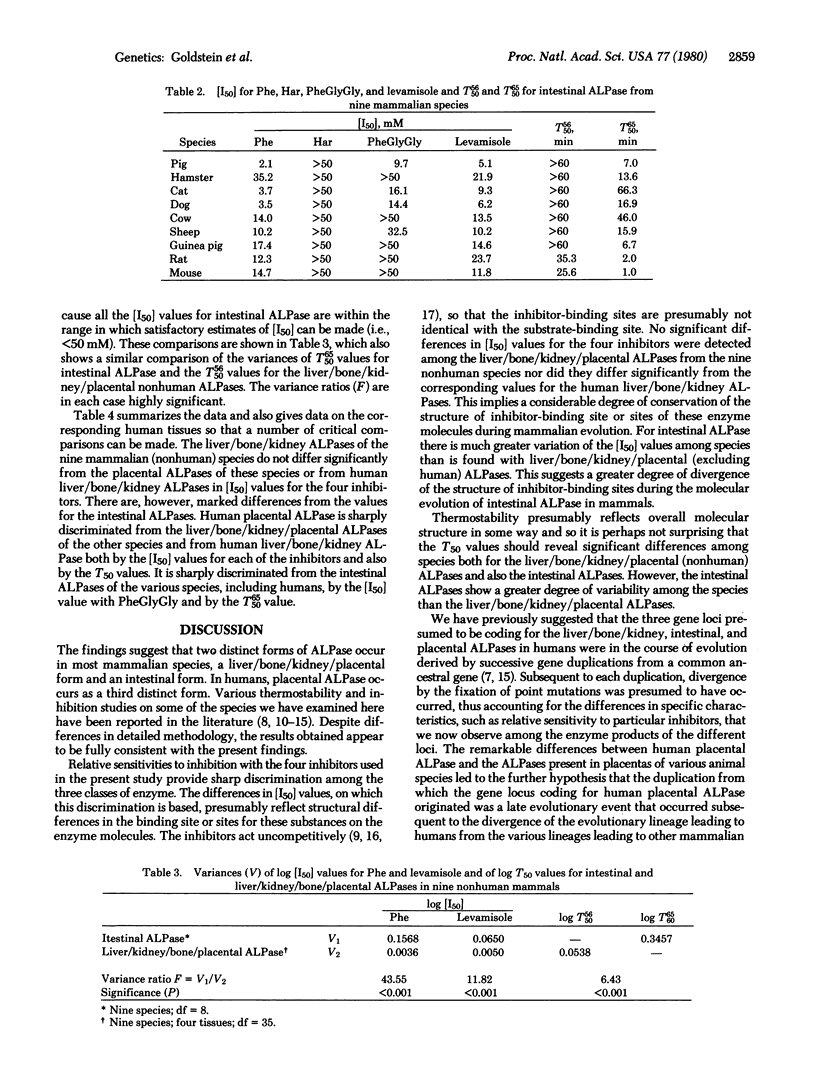
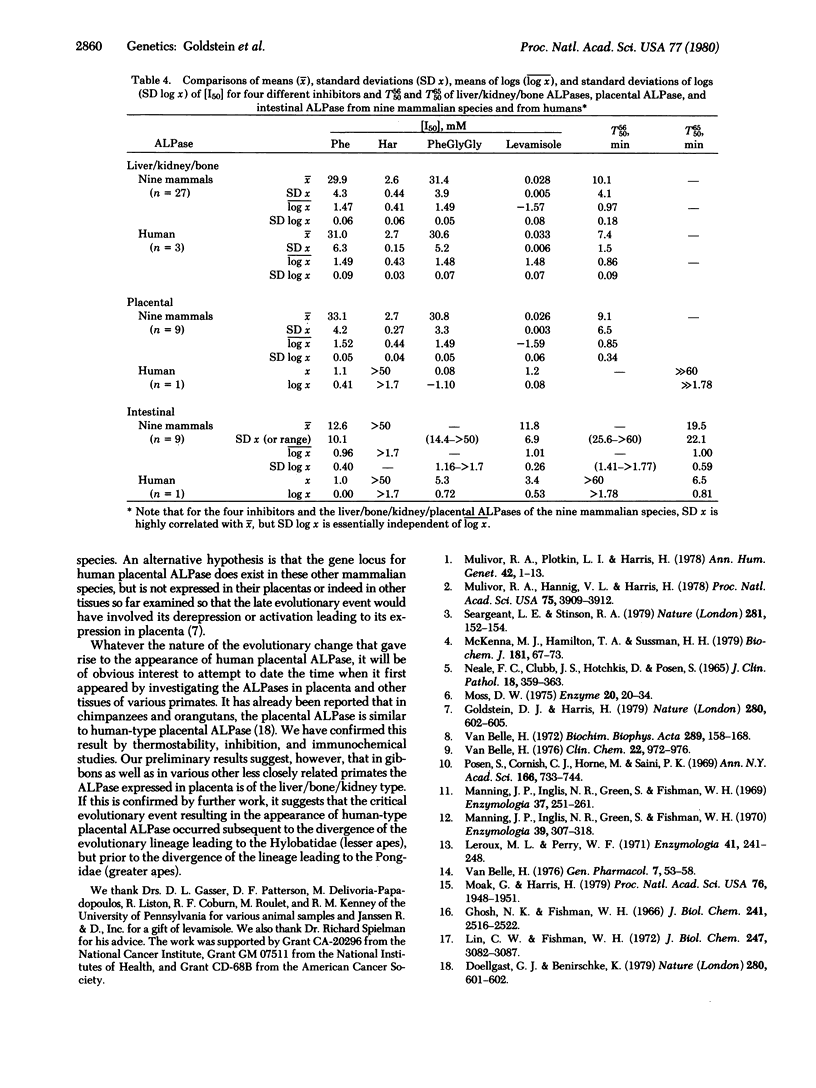
Alkaline phosphatases [orthophosphoric-monoester phosphohydrolase (alkaline optimum), EC 3.1.3.1] have been examined in liver, bone, kidney, intestine, and placenta from nine mammalian species by quantitative inhibition and thermostability studies and compared with alkaline phosphatases in the corresponding human tissues. In humans, three kinds of alkaline phosphatase can be sharply differentiated by these methods, one occurring in liver, bone, and kidney, one in intestine, and one in placenta. They are evidently determined by separate gene loci. In the mammals only two sorts of alkaline phosphatase were found: one, which occurs in liver, bone, kidney, and also placenta, corresponds to the human liver/bone/kidney enzyme and the other corresponds to the human intestinal enzyme. The findings support our earlier proposal that the expression of a distinctive type of alkaline phosphatase in human placenta is the consequence of a late evolutionary event which occurred subsequent to the divergence of the evolutionary lineage leading to humans from the various lineages leading to other mammalian species. The concentrations of the inhibitors, phenylalanine, homoarginine, phenylalanylglycylglycine, and levamisole, required to give 50% inhibition, [I50], of the liver/bone/kidney/placental (nonhuman) alkaline phosphatases showed no significant variation among the species. However, the [I50] values for the intestinal enzyme varied among species to a much greater extent. This implies that in the liver/bone/kidney/placental (nonhuman) alkaline phosphatase the structures of the binding sites for these inhibitors have been highly conserved during mammalian evolution, but there has been much greater divergence of these structures in the evolution of intestinal alkaline phosphatases.
Keywords: inhibitors, thermostability, evolution
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doellgast G. J., Benirschke K. Placental alkaline phosphatase in Hominidae. Nature. 1979 Aug 16;280(5723):601–602. doi: 10.1038/280601a0. [DOI] [PubMed] [Google Scholar]
- Ghosh N. K., Fishman W. H. On the mechanism of inhibition of intestinal alkaline phosphatase by L-phenylalanine. I. Kinetic studies. J Biol Chem. 1966 Jun 10;241(11):2516–2522. [PubMed] [Google Scholar]
- Goldstein D. J., Harris H. Human placental alkaline phosphatase differs from that of other species. Nature. 1979 Aug 16;280(5723):602–605. doi: 10.1038/280602a0. [DOI] [PubMed] [Google Scholar]
- Leroux M. L., Perry W. F. Some characteristics of placental alkaline phosphatase of a variety of mammals. Enzymologia. 1971 Oct;41(4):241–248. [PubMed] [Google Scholar]
- Lin C. W., Fishman W. H. L-Homoarginine. An organ-specific, uncompetitive inhibitor of human liver and bone alkaline phosphohydrolases. J Biol Chem. 1972 May 25;247(10):3082–3087. [PubMed] [Google Scholar]
- Manning J. P., Inglis N. R., Green S., Fishman W. H. Characterization of placental alkaline phosphatase from the rabbit, guinea pig, mouse and hamster. Enzymologia. 1970;39(5):307–318. [PubMed] [Google Scholar]
- Manning J. P., Inglis N. R., Green S., Fishman W. H. Characterization of placental alkaline phosphatase from three primates: African green and rhesus monkey and baboon. Enzymologia. 1969 Oct 31;37(4):251–261. [PubMed] [Google Scholar]
- McKenna M. J., Hamilton T. A., Sussman H. H. Comparison of human alkaline phosphatase isoenzymes. Structural evidence for three protein classes. Biochem J. 1979 Jul 1;181(1):67–73. doi: 10.1042/bj1810067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moak G., Harris H. Lack of homology between dog and human placental alkaline phosphatases. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1948–1951. doi: 10.1073/pnas.76.4.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. W. Alkaline phosphatase isoenzymes. Technical and clinical aspects. Enzyme. 1975;20(1):20–34. doi: 10.1159/000458916. [DOI] [PubMed] [Google Scholar]
- Mulivor R. A., Hannig V. L., Harris H. Developmental change in human intestinal alkaline phosphatase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3909–3912. doi: 10.1073/pnas.75.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulivor R. A., Plotkin L. I., Harris H. Differential inhibition of the products of the human alkaline phosphatase loci. Ann Hum Genet. 1978 Jul;42(1):1–13. doi: 10.1111/j.1469-1809.1978.tb00927.x. [DOI] [PubMed] [Google Scholar]
- NEALE F. C., CLUBB J. S., HOTCHKIS D., POSEN S. HEAT STABILITY OF HUMAN PLACENTAL ALKALINE PHOSPHATASE. J Clin Pathol. 1965 May;18:359–363. doi: 10.1136/jcp.18.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posen S., Cornish C. J., Horne M., Saini P. K. Placental alkaline phosphatase and pregnancy. Ann N Y Acad Sci. 1969 Oct 14;166(2):733–744. doi: 10.1111/j.1749-6632.1969.tb46431.x. [DOI] [PubMed] [Google Scholar]
- Seargeant L. E., Stinson R. A. Evidence that three structural genes code for human alkaline phosphatases. Nature. 1979 Sep 13;281(5727):152–154. doi: 10.1038/281152a0. [DOI] [PubMed] [Google Scholar]
- Van Belle H. Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans. Clin Chem. 1976 Jul;22(7):972–976. [PubMed] [Google Scholar]
- Van Belle H. Kinetics and inhibition of alkaline phosphatases from canine tissues. Biochim Biophys Acta. 1972 Nov 10;289(1):158–168. doi: 10.1016/0005-2744(72)90118-0. [DOI] [PubMed] [Google Scholar]
- van Belle H. Kinetics and inhibition of rat and avian alkaline phosphatases. Gen Pharmacol. 1976;7(1):53–58. doi: 10.1016/0306-3623(76)90033-1. [DOI] [PubMed] [Google Scholar]


