Abstract
Multipotent mesenchymal stromal cells (MSCs) have potential therapeutic benefit for the treatment of neurological diseases and injury. MSCs interact with and alter brain parenchymal cells by direct cell-cell communication and/or by indirect secretion of factors and thereby promote functional recovery. In this study, we found that MSC treatment of rats subjected to middle cerebral artery occlusion (MCAo) significantly increased microRNA 133b (miR-133b) level in the ipsilateral hemisphere. In vitro, miR-133b levels in MSCs and in their exosomes increased after MSCs were exposed to ipsilateral ischemic tissue extracts from rats subjected to MCAo. miR-133b levels were also increased in primary cultured neurons and astrocytes treated with the exosome-enriched fractions released from these MSCs. Knockdown of miR-133b in MSCs confirmed that the increased miR-133b level in astrocytes is attributed to their transfer from MSCs. Further verification of this exosome-mediated intercellular communication was performed using a cel-miR-67 luciferase reporter system and an MSC-astrocyte coculture model. Cel-miR-67 in MSCs was transferred to astrocytes via exosomes between 50 and 100 nm in diameter. Our data suggest that the cel-miR-67 released from MSCs was primarily contained in exosomes. A gap junction intercellular communication inhibitor arrested the exosomal microRNA communication by inhibiting exosome release. Cultured neurons treated with exosome-enriched fractions from MSCs exposed to 72 hours post-MCAo brain extracts significantly increased the neurite branch number and total neurite length. This study provides the first demonstration that MSCs communicate with brain parenchymal cells and may regulate neurite outgrowth by transfer of miR-133b to neural cells via exosomes.
Keywords: MicroRNA 133b, Exosomes, Multipotent mesenchymal stromal cells, Neurite outgrowth, Stroke
Introduction
Exosomes are membrane vesicles sized 40–100 nm in diameter and are secreted by a wide range of cell types [1–4]. These exosomes contain RNA molecules including messenger RNA (mRNA) and microRNA (miRNA), which can be transferred between cells and thus affect the protein production of recipient cells [5–7]. Increasing evidence indicates that exosomes play an important role in cell-to-cell communication [8–11].
miRNAs are evolutionarily conserved, 18–25 nucleotide, nonprotein coding transcripts that post-transcriptionally control gene expression via mRNA degradation or translational repression, or both. In eukaryotic cells, miRNAs constitute a major regulatory gene family [12–15]. They play significant roles in many regulatory mechanisms such as developmental timing and host-pathogen interactions as well as cell differentiation, proliferation, apoptosis, and tumorigenesis in various organisms [16, 17]. Studies in zebrafish and rodents demonstrate that microRNA 133b (miR-133b) is expressed in midbrain dopaminergic neurons and regulates the production of tyrosine hydroxylase and the dopamine transporter [18–20] in patients with Parkinson’s disease [20]. Furthermore, in a study of miR-133b and functional recovery after spinal cord injury in adult zebrafish, Yu et al. used morpholino antisense oligonucleotides to inhibit the expression of miR-133b and found that locomotor recovery was significantly impaired and regeneration of axons from neurons reduced by the decrease in miR-133b expression [21]. MiR-133b promotes functional recovery in Parkinson’s disease and spinal cord injury; however, its ability to do so after cerebral ischemia has not been tested.
Multipotent mesenchymal stromal cells (MSCs), isolated from various adult tissue sources, have potential therapeutic benefit in many diseases including neurological diseases and injury [22–28]. However, it is unknown how MSCs, as a model of cell-based therapy, interact with brain parenchymal cells, alter the parenchymal cells, and thereby promote functional recovery. We hypothesized that MSCs communicate with parenchymal cells via miRNA. The miRNA transfer between MSCs and parenchymal cells is mediated by exosomes, and the miRNA transfer by exosomes may contribute to the improvement of neurological function after stroke via specific gene expression regulated by miRNA. In this study, we focused on miR-133b and measured the miR-133b level in the ipsilateral hemisphere after middle cerebral artery occlusion (MCAo) and MSC treatment. In vitro, we investigated whether the miR-133b expression in MSCs and in MSC-generated exosomes is modified by ischemic conditions, and whether the miR-133b is transferred to parenchymal cells via MSC generated exosomes. Since neurons are the essential cells for the functional recovery after stroke and astrocytes are the most abundant cells and the major endogenous repair mediator in the central nervous system (CNS), in this study, we used primary cultures of neurons and astrocytes as the representative parenchymal cells.
Materials and Methods
All experimental procedures were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
MCAo Model
Adult male Wistar rats (weighing 270–300 g) purchased from Charles River Laboratories International, Inc. (Wilmington, MA) were subjected to right MCAo using a method of intraluminal vascular occlusion as modified in our laboratory [29]. Briefly, rats were initially anesthetized with 3.5% isoflurane and maintained with 1.0%–2.0% isoflurane in 70% N2O and 30% O2 using a face mask. Rectal temperature was maintained at 37°C throughout the surgical procedure using a feedback regulated water heating system. A length of 4-0 monofilament nylon suture (18.5–19.5 mm), determined by the animal weight, with its tip rounded by heating near a flame, was advanced from the external carotid artery into the lumen of the internal carotid artery until it blocked the origin of the MCA. Two hours (h) after MCAo, animals were reanesthetized with isoflurane and reperfusion was performed by withdrawal of the suture until the tip cleared the lumen of the external carotid artery. Immunosuppressants were not used in any animals in this study.
MSC Administration
At 24 hours postischemia, randomly selected rats (n = 6 per group) received MSC (derived from Wistar rats) or vehicle administration. Approximately 3 × 106 MSCs in 1 ml phosphate-buffered saline (PBS) or PBS alone was slowly injected via the tail vein over a 5-minute (min) period into each rat. All rats were sacrificed at 3 days after MSC administration. Then the ipsilateral hemispheres of the MCAo rat brain and normal rat brain as control (n = 6) were collected for total RNA extract.
Preparation of Brain Extracts
For brain extracts, the animals were sacrificed at 24, 72, or 168 hours after MCAo (n = 6 per time point), at which times the animals were reanesthetized and their brains were removed. The brain extract procedure was performed, as previously described [30]. The ipsilateral hemispheres of MCAo rat brain and normal rat brain as control (n = 6) were dissected on ice and the wet weight was rapidly measured. Subsequently, at a concentration of 100 mg/ml, the brain tissue from each time point was homogenized by adding neurobasal medium containing B27 and glutamine. The homogenate was centrifuged for 20 minute at 12,000g at 4°C. The supernatant from brain tissue extracts was collected and stored at −80°C.
MSC Culture and Treatment with MCAo Rat Brain Extracts
The MSCs used in our studies were harvested from both femur and tibia marrow of Wistar rats (2–3 m), as previously described [31, 32]. MSCs were cultured with α-modified minimum essential medium eagle (MEM) medium (Hyclone, Logan, UT) containing 20% fetal bovine serum (FBS, Gibco Laboratory, Grand Island, NY) and penicillin-streptomycin on 75 cm2 tissue culture flasks (Corning St. Louis, MO). Before treatment with brain extracts, MSCs were washed with PBS twice, and then the MSCs were cultured with neurobasal medium containing B27 and glutamine. The brain extracts from normal or MCAo rat brain was added at this time at a ratio of 1:10 (extracts/culture medium). After 24 hours in culture, the medium containing the brain extracts was removed and the MSCs were washed twice with PBS to avoid the effect of FBS on the exosomes. Fresh neurobasal medium containing B27 and glutamine was added and the MSCs were cultured for an additional 48 hours. The culture supernatants were collected and stored at 4°C temporarily, for no longer than 24 hours for subsequent MSC exosome isolation and the MSCs were also collected for RNA isolation.
MSC Exosome Isolation and Transmission Electron Microscopy Analysis
The MSC exosome isolation procedures were performed at 4°C as described in the literatures [33, 34]. Briefly, supernatants collected from cultured MSCs were first filtered through a 0.2 μm filter to remove the large debris and dead cells. Small-cell debris was removed by centrifugation at 10,000g for 30 minutes, and then the supernatants were recentrifuged at 100,000g for 3 hours. The supernatants generated at this step were stored at 4°C for future use as exosome omitted control (the average storage time was no more than 1 week). The pellets were resuspended in 30–50 μl of PBS and stored at −80°C and were used for analysis of the exosome-enriched fraction. For the transmission electron microscopy (TEM) morphology investigation, the pellets obtained above were subjected to uranyl acetate negative staining on For-mvar carbon-coated 400 mesh copper electron microscopy grids (FCF400-Cu, Electron Microscopy Sciences, Hatfield, PA). Twenty microliters of sample were applied to the grid and incubated for 5 minutes at room temperature, and then the excess solution on the grid was wicked off and dried for 30 minutes with filter papers. An equal part of 10% uranyl acetate was added to the grid for negative stain for 5 minutes. The preparations obtained were examined at 70 kV with a Philips 208 electron microscope (Philips, Bothell, WA) with an AMT digital imaging system (Advanced Microscopy Techniques Corp., Woburn, MA). The 100,000g pellets were resuspended in 0.25 M sucrose buffered with PBS and subjected to further purification analysis by sucrose gradient centrifugation. Briefly, we overlaid the resuspended pellets with a sucrose step gradient (2.5, 2.25, 2.0, 1.75, 1.5, 1.25, 1.0, 0.75, and 0.5 M). The sucrose gradient was then centrifuged at 100,000g for 5 hours, and 10 equal volume fractions were collected from the top of the gradient, diluted with PBS, and centrifuged at 100,000g for 1.5 hours. Western blot was used to identify Alix (Primary antibody, 1:1,000, sc-49268; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), a specific protein marker for exosomes, in respective fractions and the corresponding miR-133b levels were detected. Protein concentrations of exosome preparations were determined using the microbicinchoninic acid protein assay (Thermo Fisher Scientific, Lafayette, CO) and the total RNA was extracted with a miRNeasy Mini Kit (Qiagen, Valencia, CA). For neural cell treatment with MSC exosomes, we diluted the collected exosome-enriched fractions with the stored supernatant as noted above and the supernatant without exosomes was used as control. These media were then added to the cultured neural cells.
MSC Exosome Treatment of Primary Cultured Neurons and Astrocytes
Primary culture of cortical neurons was performed, as previously described [35]: pregnant Wistar rats purchased from Charles River were euthanized at embryonic day 17 under deep pentobarbital anesthesia. Embryos were removed, and the cerebral cortex was dissected out, washed in Hank’s buffered salt solution (HBSS, Gibco, Grand Island, NY), dissociated in 0.125% trypsin, washed again with HBSS, and then plated onto poly(D-lysine)-coated culture dishes at a density of 1 ×105 cells per square centimeter) in Dul-becco’s modified Eagle’s medium (DMEM) (Gibco) containing 5% FBS and antibiotics overnight. After 24 hours, cells were transferred to the serum-free neurobasal medium (Gibco) supplemented with 2% B-27 (Gibco), 500 μM L-glutamine and antibiotics. After 3 days, media were changed and further supplemented with uridine (10 nM) and 5-fluorodeoxyuridine (10 nM) to kill astrocytes. Cells were incubated for an additional 48 hours, after which the MSC exosome treatment was performed.
Primary culture of cerebral astrocytes: cortices extracted from 2-day-old neonatal Wistar rats were dissociated into a cell suspension using mechanical digestion. After digestion, cells were plated in 75 cm2 tissue culture flasks at a concentration of 15 × 106 cells in DMEM medium containing 20% FBS. The flasks were incubated at 37°C in a moist 5% CO2, 95% air atmosphere for 48–72 hours before moving. The medium was changed every 48–72 hours. After incubating the primary cultures for 7–9 days, the flasks were placed on a shaker platform in a horizontal position with the medium covering the cells and were shaken at 350 rpm for 6 hours at 35°C to separate oligodendrocytes from the astrocytes. Medium was changed and flasks were placed on the shaker for an additional 18 hours. After that, medium was again changed and shook for 24 hours more. When confluent, cells were passed and used for MSC exosome treatment.
Primary cultured neurons and astrocytes were washed twice with PBS before MSC exosome treatment, and then the media containing exosomes or media alone were added to the cultured neural cells. The neural cells were further cultured for 48 hours and collected for total RNA extract.
MiRNA Inhibitor Transfection
To knockdown miR-133b and to verify its function, we used a miR-133b inhibitor. Following the protocol provided by company, anti-rno-miR-133b miScript miRNA Inhibitor (MIN0003126; Qiagen, Valencia, CA) or a miScript Inhibitor negative control (Cat # 1027271; Qiagen) was transfected into MSCs and primary cultured neurons via HiPerFect Transfection Reagent (Cat # 301705; Qiagen).
Neurite Outgrowth Assay
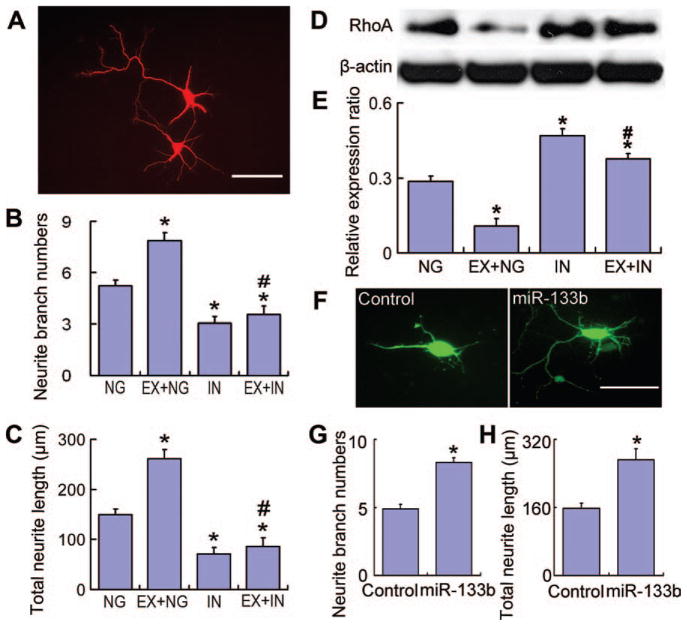
For neurite outgrowth assay, primary culture neurons were prepared as described above. Cells were transfected with miR-133b inhibitor or inhibitor negative control prior to MSC exosome treatment. As a gain of function assay, human pre-microRNA expression construct lenti-miR-133b plasmid (MI0000822, Cat #: PMIRH133bPA-1; System Biosciences, Inc., Mountain View, CA) and its corresponding control plasmid pCDH-CMV-MCS-EF1-copGFP cDNA cloning and expression vector (Cat #: CD511B-1, System Biosciences, Inc.) were transfected into primary cultured neurons. The cells were further cultured for 4 days under MSC exosome administration then fixed with 4% parafor-maldehyde and immunofluorescence staining for β-III-tubulin (Tuj1) for neurite outgrowth analysis, as previously described [36]. For analysis of the plasmid-transfected neurons, we measured the green fluorescent protein-positive neurons that are present only after successful miR-133b transfection. Since miR-133 downregulates RhoA protein expression [37, 38] and the inhibition of RhoA enhances neurite outgrowth [21] [39, 40], RhoA expression was detected by Western blot. Primary antibodies were used for RhoA (1:500, sc-418, Santa Cruz, CA) and beta actin (1:5,000, sc-1616; Santa Cruz, CA). Horseradish peroxidase-labeled secondary antibodies were applied and enhanced chemiluminescence detection was used according to the manufacturer’s instructions (Pierce, Rockford, IL).
Construction of Luciferase Reporter System for Exosome Transfer
Cel-miR-67, which has minimal sequence identity with miRNAs in human, mouse, and rat, is often used as an inert miRNA negative control in experiments in mammalian cells [41]. For the further verification of the MSC miRNA transfer to parenchymal cells via exosomes, we constructed a cel-miR-67 luciferase reporter system and used an MSC-astrocyte coculture model to detect transfer of exogenous cel-miR-67 into the rat astrocytes. To construct the luciferase reporter system, cel-miR-67 pMIR-reporter luciferase plasmid and control plasmid (Signosis, Inc., Sun-nyvale, CA) were transfected into rat cortical astrocytes (CTX TNA2, CRL-2006, American Type Culture Collection; ATCC, Manassas, VA), respectively. Cel-miR-67 expression plasmid and cel-miR-239 (negative control) expression plasmid (GenScript USA Inc., Piscataway, NJ) were transfected into MSCs, respectively. Transfection was performed for 4 hours using Lipofect-amine 2000 (Invitrogen Corporation, Carlsbad, CA) with 4 μg of DNA per transfection. The normal culture procedure was subsequently performed for an additional 24 hours, and cells were washed three times in PBS and collected by trypsinization. Transfected astrocytes were then reseeded in 12-well plates and cocultured with cel-miR-67 or cel-miR-239 expression plasmid-transfected MSCs, respectively, via a culture insert. Thus, the cocultured astrocytes and MSCs were not in direct contact, but the communication of exosomes and free miRNAs in media was assured. Since the dimensions of the exosomes range from 40 to 100 nm, polycarbonate membrane filters with 50 or 100 nm pores (Catalog #: 111703 and 110605, Whatman, GE Healthcare Life Sciences, Piscataway, NJ) were used to separate exosomes and smaller particles in media.
The gap junction intercellular communication (GJIC) participates in molecular communications including the miRNA communication between cells [7, 42] and carbenoxolone inhibits P2X7 receptor-activated microvesicles and exosome release [43]. To test whether exosomes were transferred into astrocytes through the GJIC, carbenoxolone (3β-hydroxy-11-oxoolean-12-en-30-oic acid 3-hemisuccinate, Cat #: C4790, Sigma-Aldrich, St. Louis, MO), 150 μM, was used as an antagonist to broad-spectrum GJIC. Since soluble factors released by MSCs may also regulate the luciferase expression, we transfected the miRIDIAN cel-miR-67 heparin inhibitor (Thermo Scientific Inc., Lafayette, CO) into astrocytes that were pretransfected with cel-miR-67 pMIR-reporter luciferase plasmid to test whether luciferase expression was regulated by cel-miR-67 entering into these astrocytes. After 36 hours coculture, astrocytes were collected and lysed for luciferase assays and total RNA isolation.
Luciferase Assays
We performed the luciferase activity assay by a Luciferase Assay System kit (Promega BioSciences, LLC, San Luis Obispo, CA) [7]. Luminescence was detected on a Fusion plate reader (PerkinElmer, Inc., San Jose, CA). For all wells, each experimental group was the result of one transfection per experiment. Each luciferase experiment was performed three times, with three replicates per group. Luminescence was homogenized with the total protein weight.
Isolation of the Total RNA and Real-Time Reverse Transcribed-PCR
Brain tissues, cultured cells, and exosomes were lysed in Qiazol reagents, and the total RNA was isolated using the miRNeasy Mini kit (Qiagen, Valencia, CA). MiRNA was stem-loop reverse transcribed (RT) with the miRNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) and real-time PCR amplifying was performed with the TaqMan miRNA assay (Applied Biosystems), which is specific for mature miRNA sequences. U6 snRNA was used as the internal control for TaqMan miRNA assay to detect the expression level changes of miR-133b in cells and exosomes. To compare the miR-133b levels in the media and exosomes, as well as to determine the miR-133b levels in sucrose gradient ultracentrifuge fractions, we measured differences based on variations of the Ct values.
Statistical Analysis
Data are shown as mean ± SE. p values were calculated using one-way ANOVA or Student’s t test and post hoc for multiple comparisons.
Results
MSC Administration Significantly Increases MiR-133b Level After Stroke
We extracted the total RNA from the normal rat brain and the ipsilateral hemisphere of the MCAo rat brain with or without MSC administration. Real-time RT-PCR assay showed that compared to normal rat brain, miR-133b was significantly decreased in the ipsilateral hemisphere when rats were subjected to MCAo, and MSC administration significantly increased the miR-133b level in the ipsilateral hemisphere compared to the MCAo control (Fig. 1).
Figure 1.
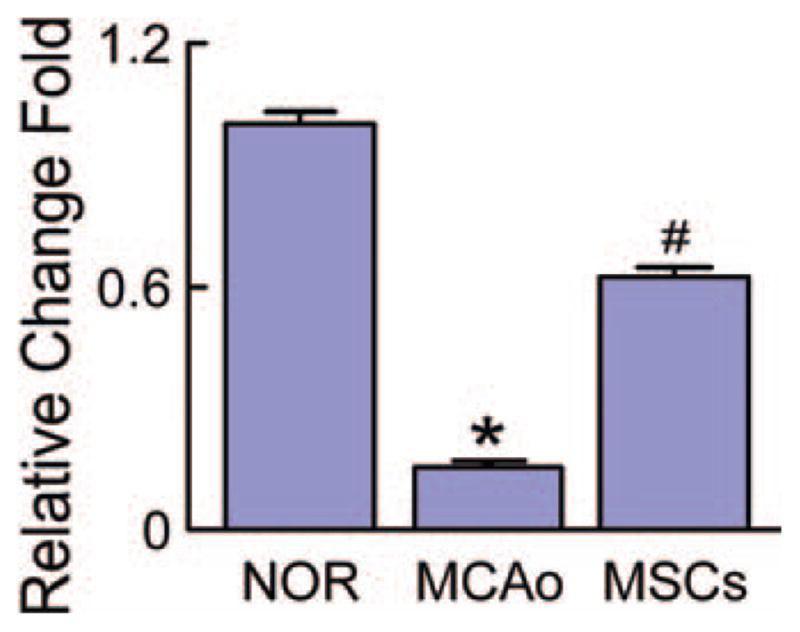
MSC administration increases the miR-133b after stroke. Real-time reverse-transcribed PCR assay showed microRNA 133b (miR-133b) significantly decreased in the ipsilateral brain tissues of rats subjected to MCAo compared to the normal rats, and MSC administration significantly increased the miR-133b level in the MCAo rat brain. NOR: normal rats, MCAo: rats subjected to MCAo, MSCs: rats subjected to MCAo with MSC administration. *, p < .05 compared with NOR. #, p < .05 compared with MCAo (n = 6 per group). Abbreviations: MCAo, middle cerebral artery occlusion; MSC, mesenchymal stromal cell.
MiR-133b in MSCs and Their Exosomes Are Increased After MSCs Are Exposed to MCAo Rat Brain Extracts
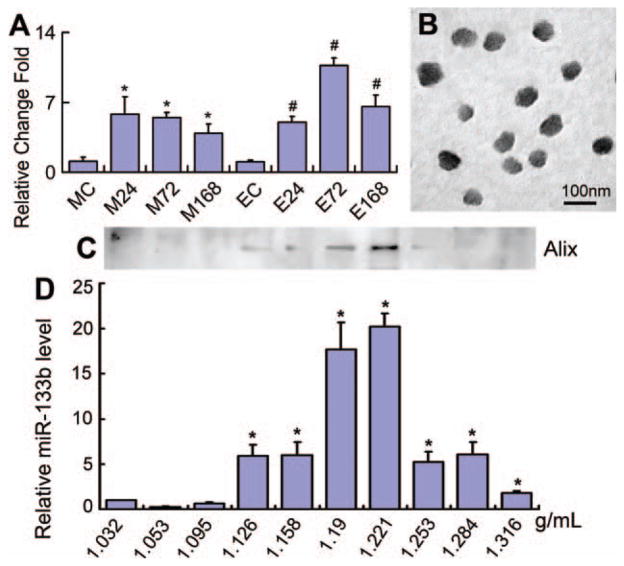
To test whether miR-133b is increased both in MSCs and their exosomes after exposure of MSCs to the cerebral tissue of rats subjected to MCAo, we harvested rat ipsilateral hemisphere extracts at 24, 72, or 168 hours after 2 hours of MCAo and separately added these extracts to the MSC cultures. We collected the total RNA and measured the miR-133b level in MSCs and their exosomes. Real-time RT-PCR data showed that compared with MSCs and their exosomes exposed to the normal rat brain tissue extract, miR-133b levels in MSCs and their exosomes exposed to 24, 72, or 168 hours MCAo brain tissue extracts were significantly increased, and the exosome miR-133b level was maximum at the 72 hours MCAo brain tissue extract exposure (Fig. 2A).
Figure 2.
Culture of mesenchymal stromal cells (MSCs) with ischemic tissue brain extracts increased the miR-133b expression in MSCs and their generated exosomes. Real-time reverse-transcribed PCR data showed that compared to the normal rat brain tissue extract treated group, miR-133b levels in MSCs exposed to middle cerebral artery occlusion (MCAo) brain tissue extracts and their exosomes were significantly increased, and the exosome miR-133b level reached a peak after MSCs cultured with the 72 hours post-MCAo brain tissue extract (A). The TEM image showed that the morphology of MSC released exosomes within a size range of 40–100 nm (B). Western blot detected that the exosome marker protein, Alix, was primary located in the range of density from 1.19 to 1.221 g/ml (C), and miR-133b also primary presented a high level in these two density fractions (D). MC, EC: MSCs and their exosomes exposed to the normal rat brain tissue extract as controls; M24, M72, M168, E24, E72, and E168: MSCs and their exosomes treated with the brain tissue extracts after MCAo at 24, 72, and 168 hours, respectively. *, p < .05, compared with MC in (A) and compared with 1.032 g/ml density fraction in (D). #, p < .05 compared with EC (n = 3 per group). Abbreviation: miR-133b, microRNA 133b.
To test whether the miR-133b is contained in exosomes released from MSC, we used TEM and sucrose gradient centrifugation methods. The TEM image showed the morphology of MSC released exosomes within a size range of 40–100 nm (Fig. 2B). Western blot demonstrated that the exosome marker protein, Alix, was primarily located in a range of density from 1.19 to 1.221 g/ml (Fig. 2C), and miR-133b was present at a high level in these two density fractions (Fig. 2D). These data indicate that miR-133b released from MSCs was contained in the exosomes.
MSC Exosomal MiR-133b Is Transferred to Astro-cytes and Neurons
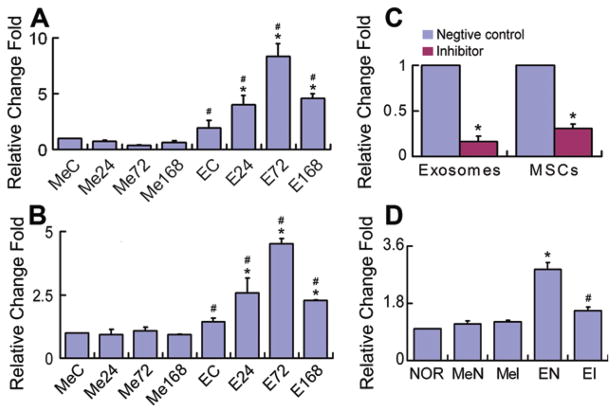
To investigate whether the miR-133b is transferred from MSCs to brain parenchymal cells, we collected the exosome-enriched fractions from MSCs exposed to the normal and MCAo brain extracts and added them to primary cultured astrocytes and neurons, respectively. Real-time RT-PCR was used to measure the miR-133b level in these astrocytes and neurons. Data show that compared to exosome-deprived media, exosome-enriched fraction treatment significantly increased the miR-133b level in astrocytes (Fig. 3A) and neurons (Fig. 3B), respectively. Compared with astrocytes and neurons treated with exosome-enriched fractions from MSCs exposed to normal rat brain tissue extracts, miR-133b levels were significantly increased in astrocytes and neurons treated with exosome-enriched fractions from MSCs exposed to 24, 72, or 168 hours post-MCAo brain tissue extracts, and the miR-133b level was maximum at the 72 hours post-MCAo brain tissue extract exposure (Fig. 3A, 3B). To further determine that miR-133b is transferred from the MSC-generated exosome-enriched fractions, we performed miR-133b knockdown in MSCs using a miR-133b inhibitor. Data showed that the miR-133b level was significantly decreased in the MSCs that were transfected with miR-133b inhibitor and in their exosome-enriched fractions (Fig. 3C). Compared with normal cultured astrocytes, treatment of astrocytes with exosome-enriched fractions from MSCs transfected with the miR-133b inhibitor negative control significantly increased the miR-133b level in these astrocytes. However, this increase in miR-133b was significantly diminished when astrocytes were treated with exosome-enriched fractions from MSCs transfected with miR-133b inhibitor. The exosome-deprived media either from MSCs transfected with miR-133b inhibitor negative control or from MSCs transfected with miR-133b inhibitor had no overt effects on the miR-133b level in astrocytes (Fig. 3D). These data suggest that exosomes from MSCs mediate the transfer of miR-133b to neural cells.
Figure 3.
MSC exosomes containing microRNA 133b (miR-133b) are transferred to neural cells. Real-time reverse-transcribed PCR revealed that compared to exosome-enriched fraction deprived media, exosome-enriched fractions collected from MSCs exposed to middle cerebral artery occlusion (MCAo) brain extracts significantly increased the miR-133b level in astrocytes (A) and neurons (B), respectively. Compared with astrocytes and neurons treated with exosome-enriched fractions from MSCs exposed to normal rat brain tissue extracts, miR-133b levels were significantly increased in astrocytes and neurons treated with exosome-enriched fractions from MSCs exposed to 24, 72, or 168 hours post-MCAo brain tissue extracts, and the miR-133b level was maximum at the 72 hours post-MCAo brain tissue extract exposure (A, B). To further confirm that the increased parenchymal cell miR-133b is attributed to the transfer of exosomes generated by MSCs, MSCs were transfected with a miR-133b inhibitor and the miR-133b level was significantly decreased in the MSCs and their exosome-enriched fractions (C). Compared with normal cultured astrocytes, treatment with exosome-enriched fractions from MSCs transfected with the miR-133b inhibitor negative control significantly increased the miR-133b level in the astrocytes, but this increase in miR-133b was significantly diminished when astrocytes were treated with exosome-enriched fractions from MSCs transfected with miR-133b inhibitor. The exosome-enriched fraction deprived media either from MSCs transfected with miR-133b inhibitor negative control or from MSCs transfected with miR-133b inhibitor had no obvious effects on the miR-133b level in astrocytes (D). MeC, EC: media and exosome-enriched fractions from MSCs treated with the normal rat brain tissue extracts as controls, respectively; Me24, Me72, Me168, E24, E72, and E168: media- and exosome-enriched fractions from MSCs treated with the MCAo rat brain tissue extracts for 24, 72, and 168 hours, respectively; NOR: normal cultured astrocytes; MeN, MeI: astrocytes treated with exosome-enriched fraction deprived media from MSCs transfected with miR-133b inhibitor negative control and miR-133b inhibitor, respectively; EN, EI: astrocytes treated with exosome-enriched fractions from MSCs transfected with miR-133b inhibitor negative control and miR-133b inhibitor, respectively. *, p < .05 compared with EC in (A) and (B); compared with negative control, respectively, in (C); compared with NOR in (D). #, p < .05 compared with MeC in (A) and (B); compared with EN in (D) (n = 3 per group). Abbreviation: MSC, mesenchymal stromal cell.
Exosomes Mediate the miRNA Communication Between MSCs and Astrocytes
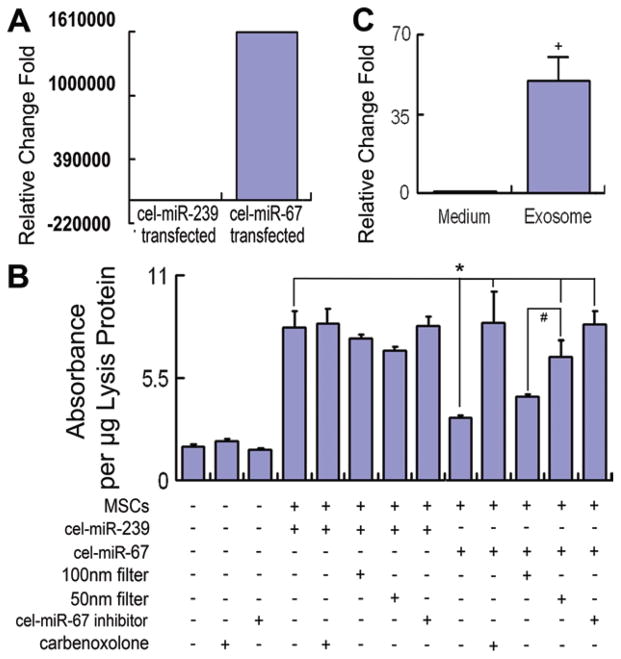
To test the miRNA communication between MSCs and astrocytes, with the MSCs not in direct contact with the astrocytes, the cel-miR-67 pMIR-reporter luciferase system and the MSC-astrocyte coculture model were used. Cel-miR-67 was expressed in cel-miR-67 expression plasmid-transfected MSCs (Fig. 4A). Figure 4B shows that luciferase expression in the astrocytes was significantly increased after noncontact coculture with MSCs. The luciferase expression significantly decreased in astrocytes cocultured with cel-miR-67 expression plasmid-transfected MSCs compared with negative control cel-miR-239 expression plasmid-transfected MSCs. The 50-nm filter but not the 100-nm filter significantly inhibited the decrease of luciferase expression in astrocytes cocultured with cel-miR-67 expression plasmid-transfected MSCs, suggesting cel-miR-67 is transferred between noncontact cells via the microvesicles between 50 and 100 nm in diameter. The MiRIDIAN miRNA inhibitor of cel-miR-67 counteracted the effects of cel-miR-67 expression plasmid-transfected MSC on the decrease of luciferase expression in astrocytes, implying that the cel-miR-67 from MSCs entered the astrocytes and regulated the luciferase expression. Furthermore, 150 μM of the GJIC inhibitor carbenoxolone also significantly inhibited the downregulation of luciferase expression in astrocytes cocultured with cel-miR-67 expression plasmid-transfected MSCs (Fig. 4B). Total RNA samples from the exosome-enriched fractions and the medium derived from MSCs transfected with the cel-miR-67 plasmid were collected separately, and real-time RT-PCR was used to measure the exosomal cel-miR-67 and free cel-miR-67 in the medium released from MSCs. Data showed that the cel-miR-67 was primarily located in the exosomes (Fig. 4C). Collectively, these data indicate that the GJIC participates in the exosome-mediated miRNA communication between noncontacted MSCs and astrocytes.
Figure 4.
Exosomes mediate the microRNA (miRNA) communication between noncontacting MSCs and astrocytes mainly through the gap junction intercellular communication. Data show that cel-miR-67 is expressed in cel-miR-67 expression plasmid-transfected MSCs (A). Luciferase expression in the astrocytes was significantly increased after coculture with MSCs. The luciferase expression significantly decreased in astrocytes cocultured with cel-miR-67 expression plasmid-transfected MSCs compared with negative control cel-miR-239 expression plasmid-transfected MSCs. Fifty nanometer filter but not the 100 nm filter significantly inhibited the decrease of luciferase expression in astrocytes cocultured with cel-miR-67 expression plasmid-transfected MSCs. miRIDIAN miRNA inhibitor of cel-miR-67 counteracted the effect of cel-miR-67 on the decrease of luciferase expression in astrocytes. Furthermore, 150 μM carbenoxolone also substantially inhibited the downregulation of luciferase expression in astrocytes cocultured with cel-miR-67 expression plasmid-transfected MSCs (B). The cel-miR-67 was primarily located in exosome-enriched fractions with very low levels in the media (C).+, p < .01 compared with cel-miR-67 transfected medium group. *, p < .05 compared with the astrocytes cocultured with cel-miR-67 expression plasmid-transfected MSCs; #, p < .05, compared with the 100 nm filter applied.+, p < .01 compared with cel-miR-67 transfected medium group (n = 3 per group). Abbreviation: MSC, mesenchymal stromal cell.
GJIC Affects the Exosome Release from MSCs But Not Their Intake into Astrocytes
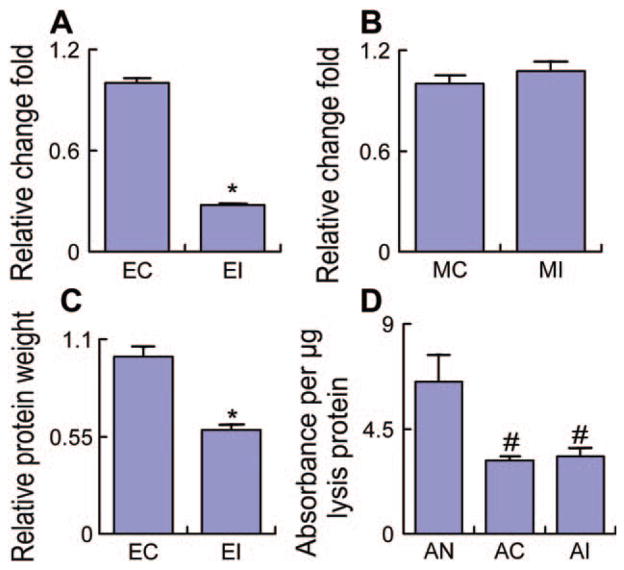
To further test whether the GJIC affects the exosome release from MSCs or their intake into astrocytes, we separately treated the cel-miR-67 pMIR-reporter luciferase plasmid-transfected astrocytes and the cel-miR-67 expression plasmid-transfected MSCs with 150 μM carbenoxolone. The cel-miR-67 level in the exosomes and the total protein of the exosomes were measured as an indicator of quantity of exosomes released from MSCs. The cel-miR-67 level was significantly decreased in the exosomes released from MSCs treated with 150 μM carbenoxolone (Fig. 5A); however, the cel-miR-67 level inside MSCs was not altered (Fig. 5B). Concomitantly, the total exosome protein significantly decreased after administration of 150 μM carbenoxolone (Fig. 5C). The exosomes collected from the cel-miR-67 expression plasmid-transfected MSCs were added to cel-miR-67 pMIR-reporter luciferase plasmid-transfected astrocytes to test whether carbenoxolone affects the cel-miR-67 intake. Data showed that the exosomes downregulated the luciferase expression in the astrocytes. However, treatment of these astrocytes with 150 μM carbenoxolone had no obvious effect on the downregulation of luciferase expression by the exosomes released from cel-miR-67 expression plasmid-transfected MSCs (Fig. 5D). These data indicate that the GJIC mediates the exosomal miRNA transfer by affecting exosome release from MSCs but not intake into astrocytes.
Figure 5.
GJIC mediates the exosomal microRNA transfer via affecting the exosome release from MSCs but not their intake into astrocytes. Data showed that the cel-miR-67 level was significantly decreased in the exosome-enriched fractions released from MSCs treated with 150 μM carbenoxolone (A), but the cel-miR-67 level in MSCs was not changed (B). Concomitantly, after 150 μM carbenoxolone, the total protein that indicates exosome quantity significantly decreased (C). The exosome-enriched fractions from cel-miR-67 expression plasmid-transfected MSCs downregulated the luciferase expression in cel-miR-67 pMIR-reporter luciferase plasmid-transfected astrocytes, but treatment of astrocytes with 150 μM carbenoxolone had no obvious effect on the luciferase expression downregulation by the exosome released from cel-miR-67 expression plasmid-transfected MSCs (D). EC: exosome-enriched fractions from non-carbenoxolone-treated MSCs, EI: exosome-enriched fractions from carbenoxolone-treated MSCs, MC: non-carbenoxolone-treated MSCs, MI: carbenoxolone-treated MSCs, AN: non-carbenoxolone-treated astrocytes without exosome-enriched fractions treatment, AC: non-carbenoxolone-treated astrocytes with exosome-enriched fractions treatment, AI: carbenoxolone-treated astrocytes with exosome-enriched fractions treatment. *, p < .05 compared with EC. #, p < .05 compared with AN (n = 3 per group).
MiR-133b in the Exosomes Released by MSCs Promotes Neurite Outgrowth
To investigate whether the stroke-increased miR-133b in MSCs transfers to neural cells and affects neurite outgrowth, we transfected primary cultured neurons with a miR-133b inhibitor plasmid or a blank vector (as negative control). Treatment of control neurons (transfected with the blank vector) with exosome-enriched fractions from MSCs exposed to the 72 hours post-MCAo brain extract significantly increased the neurite branch number (Fig. 6A, 6B) and total neurite length (Fig. 6A, 6C) compared to nonexosome-treated control neurons. MiR-133b inhibitor transfected neurons exhibited significantly decreased neurite branch number and total neurite length compared with control neurons, including neurons with exosome-enriched fraction treatment. Western blot (Fig. 6D) data showed RhoA expression was decreased after MSC exosome-enriched fraction treatment in control neurons, and miR-133b inhibitor transfected neurons exhibited a significant increase in RhoA level (Fig. 6E). miR-133b direct transfection (Fig. 6F) data showed that the increase of miR-133b expression in neurons enhanced the neurite branch number (Fig. 6G) and total neurite length (Fig. 6H). These data suggest that the increased exosome miR-133b from MSCs exposed to MCAo brain extract transfers to neural cells and promotes neurite outgrowth, at least partly by inhibiting the RhoA expression.
Figure 6.
Exosomal miR-133b from middle cerebral artery occlusion (MCAo)-modified mesenchymal stromal cells (MSCs) increases neurite outgrowth. Cortical neuron neurite outgrowth is shown using fluorescence microscopy (A). Compared with the normal cultured (nonexosome treatment) group, administering exosome-enriched fractions from MSCs exposed to the 72 hours post-MCAo brain extract significantly increased the neurite branch number (B) and total neurite length (C). The miR-133b inhibitor substantially decreased the neurite branch number and total neurite length compared with normal cultured or exosome-enriched fraction treated neurons. Western blot (D) data showed that the RhoA expression significantly decreased after MSC exosome treatment in control neurons, and the RhoA level significantly increased in miR-133b inhibitor transfected neurons (E). Data also showed that increased miR-133b expression by miR-133b direct transfection (F) in neurons significantly increased the neurite branch number (G) and the total neurite length (H), respectively. NG: the miR-133b inhibitor negative control-transfected neurons, EX+NG: the miR-133b inhibitor negative control-transfected neurons treated with exosome-enriched fractions from MSCs exposed to MCAo brain extracts, IN: the miR-133b inhibitor-transfected neurons, EX+IN: the miR-133b inhibitor-transfected neurons treated with exosome-enriched fractions from MSCs exposed to MCAo brain extracts. Scale bars = 50 μm. *, p < .01, compared with NG; #, p < .01, compared with EX+NG. Data are presented as mean ± SE (neurons n = 50 per group). Abbreviation: miR-133b, microRNA 133b.
Discussion
MSCs have potential therapeutic benefit in the CNS [23, 24, 28]. They interact with brain parenchymal cells and enhance neurite outgrowth that contributes to functional recovery [36, 44–48]. This interaction is direct via cell-cell communication [49] and/or is indirect being mediated by the secretion of factors by MSCs and/or parenchymal cells [25, 30, 50–52]. In this study, we observed that MSCs communicate with astrocytes and neurons via release of exosomes containing miRNA, and the transfer of their increased miR-133b to neurons and astrocytes enhances neurite outgrowth. As exosomes cannot experimentally be separated from microvesicles, we used TEM to measure the vesicle size and the sucrose gradient centrifugation method to separate the fractions by density combined with Western blot to detect an exosome-specific protein marker. Using these procedures, our data suggest that miR-133b released from MSCs was primarily contained in the exosomes. Although we only observed the direct transfer of miR-133b from MSCs to neurons and astrocytes, we cannot exclude the possibility that the increased miR-133b in one parenchymal cell population, for example, astrocytes, may also be transferred to another, for example, neurons.
miRNAs are exported from cells both within and outside the exosomes [53, 54]. Exosomes act as mediators of cell-cell communication [55–58] and are carriers for functional miRNA delivery [5, 6, 59]. There is considerable interest in using exosomes in clinical applications as biomarkers and/or as potential therapeutic tools [5, 60]. In this study, we found that the miR-133b level is increased in exosomes released from MSCs exposed to MCAo brain extracts. When primary-cultured neurons and astrocytes were treated with these exosomes, the miR-133b level in the neurons and astrocytes were increased, suggesting that the exosomes mediate the miR-133b transfer from MSCs to the neurons and astrocytes. Using the cel-miR-67 pMIR-reporter luciferase system and an MSC-astrocyte coculture model, we demonstrated that the cel-miR-67 is transferred from MSCs to astrocytes via exosomes. Knockdown of miR-133b in MSCs directly confirmed that the increased miR-133b level in astrocytes is attributed to their transfer from MSCs to neural cells. P2X7 receptor-activated microvesicles and exosome release are inhibited by carbenoxolone [6], and miRNA passes between breast cancer cells and stroma through the GJIC [7]. In our previous study, we found that the GJIC mediated the functional miRNA communication between cells and noted that miRNAs may be transported directly through the intercellular channels or through other processes such as microvesicle release that is influenced by gap junctions [8]; therefore, in this study, we investigated the role of the GJIC in exosomal miRNAs communication between noncontacted cells. Our data showed that the GJIC inhibitor, carbenoxolone, affects the miRNA containing exosome release from MSCs but does not affect exosome intake by astrocytes. This study provides the first demonstration that MSCs communicate with astrocytes and neurons and regulate neurite outgrowth by transfer of miR-133b via exosomes.
miR-133a/b is downregulated in myocardial infarction [61, 62] and ischemia reperfusion [63], suggesting that miR-133a/b plays a role in modulating myocardial ischemia-reperfusion injury [64]. We now demonstrate that miR-133b is substantially downregulated in rat brain after MCAo, and MSC administration significantly increased the miR-133b level. Although previous studies showed that miRNA communication occurs between MSCs and breast cancer cells when in contact culture [42], the number of administered MSCs recruited in the ischemic brain after treatment is far too small [28] to be in contact with all the parenchymal cells [65, 66]. Our data show that MSCs release miRNA contained in their secreted exosomes. Thus, the increase in miR-133b in brain tissue induced by MSC treatment of stroke likely occurs via exosome transfer from MSCs to parenchyma.
MiR-133a/b is active in several regulatory processes, for example, miR-133a/b regulates cell proliferation and apoptosis by inhibiting caspase-9 in myocardial infarction and cancer [63, 64, 67–72]. Connective tissue growth factor (CTGF), a major inhibitor of axonal growth at injury sites in the CNS in mammals, is also regulated by miR-133 [73, 74]. The transfer of miR-133b from MSCs to astrocytes via exosomes may downregulate CTGF expression, thin the glial scar, and benefit neurite outgrowth. MiR-133 also downregulates RhoA protein expression [37, 38], and since the inhibition of RhoA enhances regrowth of the corticospinal tract after spinal cord injury [39, 40], miR-133b appears essential for neurite outgrowth and functional recovery after spinal cord injury in adult zebrafish [21]. Increasing miR-133b transfer from MSC exosomes may regulate target genes like RhoA in neurons that stimulate neurite outgrowth and thereby improve functional recovery after stroke. Further in vivo studies on how miR133-b affects neurite outgrowth after stroke are needed.
The identification of exosomes released from MSCs as a shuttle that carries miR-133b to astrocytes and neurons after cerebral ischemia helps to explain, at least in part, how the exogenous MSCs contribute to neurological recovery after stroke. MSCs exhibit a degree of “immune privilege” due to their ability to suppress T-cell-mediated responses for tissue rejection [75–80]. MSCs escape immune system surveillance and survive in the CNS even after transplantation of allogeneic [81] or xenogeneic MSCs [52]. Thus, exogenous application of MSCs transfected with miR-133b warrants investigation as a treatment for stroke. Exosome delivery of functional miRNAs, like miR-133b, that promote neurite outgrowth may show benefit in other neurological diseases, in addition to stroke.
Conclusion
Ischemic conditions increase the miR-133b level in MSCs, and MSCs regulate neurite outgrowth at least partly by transfer of the miR-133b to neurons and astrocytes via the release of exosomes.
Acknowledgments
This work was supported by NINDS Grants R01 AG037506 (MC), R01 NS66041 (YL), and R01 NS75156 (ZGZ). We thank Tom Christopherson for technical assistance on TEM, Cindi Roberts and Qinge Lu for technical assistance on histology.
Footnotes
Disclosure of potential conflicts of interest is found at the end of this article.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author contributions: H.X.: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; Y.L.: conception and design, data analysis and interpretation, financial support, and manuscript writing; B.B., M.K., Y.Z., X.W., and X.S.: collection and/or assembly of data; Z.G.Z.: conception and design, data analysis and interpretation, and financial support; M.C.: conception and design, data analysis and interpretation, financial support, manuscript writing, and final approval of manuscript.
References
- 1.Stoorvogel W, Kleijmeer MJ, Geuze HJ, et al. The biogenesis and functions of exosomes. Traffic (Copenhagen, Denmark) 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 2.Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 4.Heijnen HF, Schiel AE, Fijnheer R, et al. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 5.Zomer A, Vendrig T, Hopmans ES, et al. Exosomes: Fit to deliver small RNA. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katakowski M, Buller B, Wang X, et al. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–8263. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smalheiser NR. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct. 2007;2:35. doi: 10.1186/1745-6150-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Record M, Subra C, Silvente-Poirot S, et al. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Fiore R, Siegel G, Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim Biophys Acta. 2008;1779:471–478. doi: 10.1016/j.bbagrm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Wang Q, Pan X. MicroRNAs and their regulatory roles in animals and plants. J Cell Physiol. 2007;210:279–289. doi: 10.1002/jcp.20869. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Pan X, Cobb GP, et al. Plant microRNA: A small regulatory molecule with big impact. Dev Biol. 2006;289:3–16. doi: 10.1016/j.ydbio.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Cai Y, Yu X, Hu S, et al. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim PK, Patel SA, Gregory LA, et al. Neurogenesis: Role for micro-RNAs and mesenchymal stem cells in pathological states. Curr Med Chem. 2010;17:2159–2167. doi: 10.2174/092986710791299894. [DOI] [PubMed] [Google Scholar]
- 18.Dreyer JL. New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med. 2010;2:92. doi: 10.1186/gm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Simon FM, Zhang XX, Loh HH, et al. Morphine regulates do-paminergic neuron differentiation via miR-133b. Mol Pharmacol. 2010;78:935–942. doi: 10.1124/mol.110.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Inoue K, Ishii J, et al. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu YM, Gibbs KM, Davila J, et al. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci. 2011;33:1587–1597. doi: 10.1111/j.1460-9568.2011.07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul D, Samuel SM, Maulik N. Mesenchymal stem cell: Present challenges and prospective cellular cardiomyoplasty approaches for myocardial regeneration. Antioxid Redox Signal. 2009;11:1841–1855. doi: 10.1089/ars.2009.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456:120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Chopp M, Li Y, Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Abdallah BM, Kassem M. Human mesenchymal stem cells: From basic biology to clinical applications. Gene Ther. 2008;15:109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 27.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Li Y, Wang L, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 31.Phinney DG, Kopen G, Isaacson RL, et al. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: Variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 32.Mahmood A, Lu D, Yi L, et al. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J Neurosurg. 2001;94:589–595. doi: 10.3171/jns.2001.94.4.0589. [DOI] [PubMed] [Google Scholar]
- 33.Khatua AK, Taylor HE, Hildreth JE, et al. Exosomes packaging APO-BEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol. 2009;83:512–521. doi: 10.1128/JVI.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Cesca F, Loers G, et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31:7275–7290. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buller B, Liu X, Wang X, et al. MicroRNA-21 protects neurons from ischemic death. FEBS J. 2010;277:4299–4307. doi: 10.1111/j.1742-4658.2010.07818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xin H, Li Y, Shen LH, et al. Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS One. 2010;5:e9027. doi: 10.1371/journal.pone.0009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Care A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 38.Chiba Y, Tanabe M, Goto K, et al. Down-regulation of miR-133a contributes to up-regulation of Rhoa in bronchial smooth muscle cells. Am J Respir Crit Care Med. 2009;180:713–719. doi: 10.1164/rccm.200903-0325OC. [DOI] [PubMed] [Google Scholar]
- 39.Dergham P, Ellezam B, Essagian C, et al. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holtje M, Djalali S, Hofmann F, et al. A 29-amino acid fragment of Clostridium botulinum C3 protein enhances neuronal outgrowth, connectivity, and reinnervation. FASEB J. 2009;23:1115–1126. doi: 10.1096/fj.08-116855. [DOI] [PubMed] [Google Scholar]
- 41.Ge Y, Sun Y, Chen J. IGF-II is regulated by microRNA-125b in skeletal myogenesis. J Cell Biol. 2011;192:69–81. doi: 10.1083/jcb.201007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim PK, Bliss SA, Patel SA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 43.Qu Y, Franchi L, Nunez G, et al. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, Zhang RL, Li Y, et al. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40:2546–2551. doi: 10.1161/STROKEAHA.109.547265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen LH, Xin H, Li Y, et al. Endogenous tissue plasminogen activator mediates bone marrow stromal cell-induced neurite remodeling after stroke in mice. Stroke. 2011;42:459–464. doi: 10.1161/STROKEAHA.110.593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z, Li Y, Zhang ZG, et al. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295. doi: 10.1038/jcbfm.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Li Y, Zhang X, et al. Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Z, Li Y, Zhang RL, et al. Bone marrow stromal cells promote skilled motor recovery and enhance contralesional axonal connections after ischemic stroke in adult mice. Stroke. 2011;42:740–744. doi: 10.1161/STROKEAHA.110.607226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Q, Katakowski M, Chen X, et al. Human marrow stromal cells enhance connexin43 gap junction intercellular communication in cultured astrocytes. Cell Transplant. 2005;14:109–117. doi: 10.3727/000000005783983205. [DOI] [PubMed] [Google Scholar]
- 50.Xin H, Li Y, Chen X, et al. Bone marrow stromal cells induce BMP2/ 4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006;83:1485–1493. doi: 10.1002/jnr.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Katakowski M, Li Y, et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: Growth factor production. J Neurosci Res. 2002;69:687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 53.Wang K, Zhang S, Weber J, et al. Export of microRNAs and micro-RNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iguchi H, Kosaka N, Ochiya T. Secretory microRNAs as a versatile communication tool. Commun Integr Biol. 2010;3:478–481. doi: 10.4161/cib.3.5.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rani S, O’Brien K, Kelleher FC, et al. Isolation of exosomes for subsequent mRNA, microRNA, and protein profiling. Methods Mol Biol. 2011;784:181–195. doi: 10.1007/978-1-61779-289-2_13. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed KA, Xiang J. Mechanisms of cellular communication through intercellular protein transfer. J Cell Mol Med. 15:1458–1473. doi: 10.1111/j.1582-4934.2010.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thery C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmed KA, Xiang J. Mechanisms of cellular communication through intercellular protein transfer. J Cell Mol Med. 2011;15:1458–1473. doi: 10.1111/j.1582-4934.2010.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bobrie A, Colombo M, Raposo G, et al. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 61.Bostjancic E, Zidar N, Stajer D, et al. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115:163–169. doi: 10.1159/000268088. [DOI] [PubMed] [Google Scholar]
- 62.Bostjancic E, Zidar N, Glavac D. MicroRNA microarray expression profiling in human myocardial infarction. Disease Markers. 2009;27:255–268. doi: 10.3233/DMA-2009-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He B, Xiao J, Ren AJ, et al. Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci. 2011;18:22. doi: 10.1186/1423-0127-18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye Y, Perez-Polo JR, Qian J, et al. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- 65.Korbo L, Pakkenberg B, Ladefoged O, et al. An efficient method for estimating the total number of neurons in rat brain cortex. J Neurosci Methods. 1990;31:93–100. doi: 10.1016/0165-0270(90)90153-7. [DOI] [PubMed] [Google Scholar]
- 66.Pakkenberg B, Pelvig D, Marner L, et al. Aging and the human neo-cortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 67.Kuwabara Y, Ono K, Horie T, et al. Increased microRNA-1 and micro-RNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4:446–454. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 68.Akcakaya P, Ekelund S, Kolosenko I, et al. miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int J Oncol. 2011;39:311–318. doi: 10.3892/ijo.2011.1043. [DOI] [PubMed] [Google Scholar]
- 69.Hu G, Chen D, Li X, et al. miR-133b regulates the MET protooncogene and inhibits the growth of colorectal cancer cells in vitro and in vivo. Cancer Biol Ther. 2010;10:190–197. doi: 10.4161/cbt.10.2.12186. [DOI] [PubMed] [Google Scholar]
- 70.Kano M, Seki N, Kikkawa N, et al. miR-145, miR-133a and miR-133b: Tumor suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127:2804–2814. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 71.Bostjancic E, Zidar N, Stajner D, et al. MicroRNA miR-1 is up-regulated in remote myocardium in patients with myocardial infarction. Folia Biol. 2010;56:27–31. doi: 10.14712/fb2010056010027. [DOI] [PubMed] [Google Scholar]
- 72.Han M, Toli J, Abdellatif M. MicroRNAs in the cardiovascular system. Curr Opin Cardiol. 2011;26:181–189. doi: 10.1097/HCO.0b013e328345983d. [DOI] [PubMed] [Google Scholar]
- 73.Duisters RF, Tijsen AJ, Schroen B, et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of micro-RNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. 176p following 178. [DOI] [PubMed] [Google Scholar]
- 74.White RE, Jakeman LB. Don’t fence me in: Harnessing the beneficial roles of astrocytes for spinal cord repair. Restor Neurol Neurosci. 2008;26:197–214. [PMC free article] [PubMed] [Google Scholar]
- 75.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 76.Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 77.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 78.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 79.Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 80.Beggs KJ, Lyubimov A, Borneman JN, et al. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- 81.Li Y, McIntosh K, Chen J, et al. Allogeneic bone marrow stromal cells promote glialaxonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313–325. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]