Abstract
Rationale
Among the extracellular modulators of Bmp (bone morphogenetic protein) signaling, Bmper (Bmp endothelial cell precursor-derived regulator) both enhances and inhibits Bmp signaling. Recently we found that Bmper modulates Bmp4 activity via a concentration-dependent, endocytic trap-and-sink mechanism.
Objective
To investigate the molecular mechanisms required for endocytosis of the Bmper/Bmp4 and signaling complex and determine the mechanism of Bmper’s differential effects on Bmp4 signaling.
Methods and Results
Using an array of biochemical and cell biology techniques, we report that LRP1 (Low density lipoprotein receptor-related protein 1), a member of the LDL receptor family, acts as an endocytic receptor for Bmper and a co-receptor of Bmp4 to mediate the endocytosis of the Bmper/Bmp4 signaling complex. Furthermore, we demonstrate that LRP1-dependent Bmper/Bmp4 endocytosis is essential for Bmp4 signaling, as evidenced by the phenotype of lrp1-deficient zebrafish, which have abnormal cardiovascular development and decreased Smad1/5/8 activity in key vasculogenic structures.
Conclusions
Together, these data reveal a novel role for LRP1 in the regulation of Bmp4 signaling by regulating receptor complex endocytosis. In addition, these data introduce LRP1 as a critical regulator of vascular development. These observations demonstrate Bmper’s ability to fine-tune Bmp4 signaling at the single-cell level, unlike the spatial regulatory mechanisms applied by other Bmp modulators.
Keywords: bone morphogenetic protein, low density lipoprotein receptor-related protein 1, endocytosis, angiogenesis, endothelial cell
INTRODUCTION
Bone morphogenetic proteins (Bmps) are essential for embryonic vascular development, as illustrated by many severe, inherited vascular diseases associated with disrupted Bmp signaling1. One facet of regulation of Bmp signaling is provided by extracellular molecules that “fine-tune” Bmp signaling by modulating (usually inhibiting) Bmp’s interaction with surface receptors and subsequent downstream signaling events2. Of these extracellular modulators of Bmp signaling, Bmper (Bmp-binding endothelial cell precursor-derived regulator, the vertebrate ortholog for crossveinless-2 (Cv-2) in Drosophila) is a critical determinant of endothelial functions such as differentiation, migration and angiogenesis3–6. Bmper can both promote and inhibit Bmp activity6, 7, confounding the development of a working model to explain how Bmper impacts Bmp signaling. However, we recently found that Bmper modulates Bmp4 activity via a concentration-dependent, endocytic trap-and-sink mechanism, with low levels of Bmper promoting and high levels inhibiting Bmp4 signaling, thereby accounting for the biphasic nature of Bmper’s regulation of Bmp4 activity7. Our previous data suggested that endocytosis of the Bmper/Bmp4 complex may be critical in determining the inhibitory effect of Bmper on Bmp4 function. However, gaps remained in understanding how activation and inhibition of Bmp4 signaling by Bmper were coupled and the mechanism by which the Bmper/Bmp4 complex was endocytosed.
Here we report that LRP1 (Low density lipoprotein receptor-related protein 1) is a novel endocytic receptor for Bmper and a co-receptor of Bmp4 that is essential for mediating Bmp4 signaling. A requisite role for LRP1 in zebrafish angiogenesis is observed in that knockdown of lrp1 decreases Smad1/5/8 activity and abnormal cardiovascular development. Together, these data demonstrate that LRP1 regulates Bmp4-mediated endothelial function and vascular development in vivo and is therefore a bone fide component of the Bmp signaling pathway.
METHODS
Generation of cell lines
For stable MEC cell line construction, MECs were transduced with LRP1 or control shRNA lentiviral particles, and positive colonies were screened with puromycin and evaluated by Western blot analysis.
Chemical cross-linking in intact cells, immunoblotting, immunoprecipitation and ligand blotting
Bmper-treated MECs were crosslinked with dithiobis(succinimidylproprionate)(DSP) for immunoprecipitation and MALDI-TOF analysis. Immunoblotting, immunoprecipitation and Ligand blotting were performed following our previous protocols7–8.
FRET experiments
Experiments were performed following a previously published protocol9. Donor, acceptor and FRET images were acquired sequentially using fixed excitation and emission filters, and image processing was performed.
In vitro Matrigel tubulogenesis assay
Endothelial cell tube formation was analyzed with the Matrigel-based tube formation assay10.
Morpholino injections and zebrafish analysis
Morpholino oligonucleotides (MOs) were produced by Gene Tools (Philomath, OR). All MOs were injected into one to four cell stage embryos as previously described11. Zebrafish (Danio rerio) vascular development was analyzed following previously published methods12.
Please see the expanded Methods section, available in the Online Data Supplement for details regarding reagents, cell culture, immunoblotting, ligand blotting, immunofluorescence, and statistical analysis.
RESULTS
LRP1 associates with Bmper in MECs
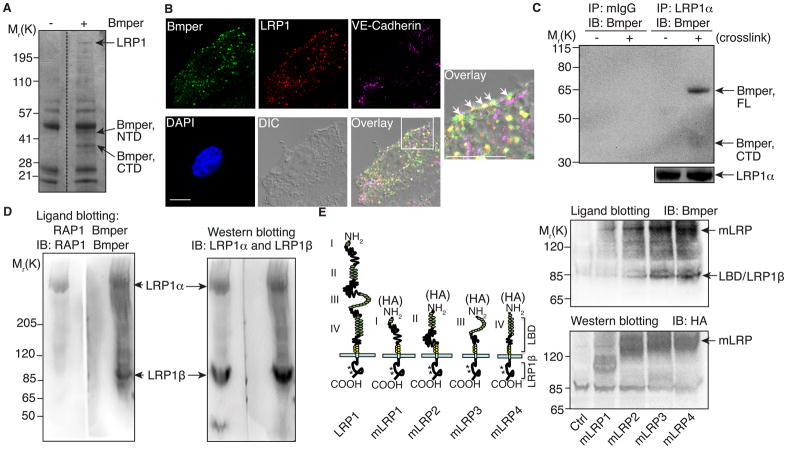
Previously we demonstrated that endocytosis of the Bmper/Bmp4 complex is critical for Bmper-mediated Bmp4 signaling7. However, the mechanism governing this endocytosis remained unclear. Hypothesizing that Bmper may interact with a partner to achieve endocytosis, we used matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry as an inductive unbiased method of identifying Bmper-associated proteins in mouse endothelial cells (MECs). LRP1, a well-defined endocytic receptor, associated with Bmper in MECs (Figure 1A; Online Figure IA and Table I). Confocal imaging of Bmper-treated MECs revealed that Bmper and LRP1 colocalize on the plasma membrane and in intracellular vesicles (Figure 1B). Immunoprecipitating for LRP1 and immunoblotting for Bmper (or the reverse, or Bmper ELISA) demonstrated that full-length Bmper associates with LRP1 in MEC lysates (Figure 1C; Online Figure IB, C), confirming our MALDI-TOF and immunofluorescence data. Notably, this association was not affected by the addition of Bmps (Online Figure ID, E). Since Bmp4 may bind Bmper through its amino-terminal domain (NTD)7, 13, its inability to disrupt the LRP1/Bmper association suggested that LRP1 may bind a different domain of Bmper and carboxyl-terminal domain (CTD) fragments of Bmper associated with LRP1 (Figure 1C; Online Figure ID), indicating that Bmper may form a ternary complex with LRP1 and Bmp4 through respective associations with its CTD and NTD. Consistent with this hypothesis, lysates of Bmp4-treated MECs immunoprecipitated with an anti-LRP1 antibody revealed the presence of LRP1/Bmp4 heterocomplexes (Online Figure IF).
Figure 1. LRP1 associates with Bmper.
(A) Lysates of Bmper-treated MECs were immunoprecipitated with an anti-Bmper antibody and stained with Coomassie blue to identify proteins. (B) Bmper-treated MECs (5 nmol/L for 1 minute) were stained with anti-Bmper and LRP1 antibodies and examined using confocal imaging. Scale bar: 10 μm. (C) Lysates of MECs were immunoprecipitated with anti-LRP1 antibody or mouse control IgG and analyzed by Western blotting with an biotinylated anti-Bmper antibody. (D) Non-denatured lysates of MECs were analyzed for ligand blotting. The membrane was incubated with recombinant Bmper and immunoblotted with anti-Bmper antibody (left panel). LRP1 and RAP1 interaction8 is included as a positive control. (E) Non-denatured lysates of HEK 293 cells transfected with mLRP1-4 were analyzed for ligand blotting with an anti-Bmper antibody (right top panel). Right bottom panel is included as a loading control. Left panel is a schematic representation of LRP1 and each mLRP1-4 construct.
Like other members of the LDL receptor family, LRP1 is a heterodimer composed of a 515-kDa α chain possessing four extracellular ligand binding domains (LBDs) and an 85-kDa membrane-anchored β chain14. Ligand blotting analysis with full-length LRP1 revealed that Bmper associates with both the α and β chains of LRP1 (Figure 1D). To determine which LBD of LRP1 is required for binding to Bmper, cells were transfected with membrane-containing mini-LRP1 receptors (mLRP1~415; Figure 1E, left panel) and analyzed via ligand blotting. LBDIII/IV exhibited the strongest association with Bmper, whereas LBDII had only a weak association and LBDI did not associate with Bmper at all (Figure 1E, right top panel), consistent with what is found with other LRP1 ligands such as RAP115. However, Bmper also binds to the β chain of LRP1, making Bmper unique among other known LRP1 ligands. The significance of Bmper binding to LRP1’s β chain remains unknown; however, internalized CTD fragments of Bmper may bind LRP1 and modulate its association with scaffold proteins that participate in LRP1-dependent signaling mechanisms16. Together, these data identify that LRP1, a recognized endocytic receptor, associates with Bmper in MECs and therefore could be responsible for endocytosis of Bmper/Bmp4 complexes.
LRP1 is required for physiologic Bmper internalization
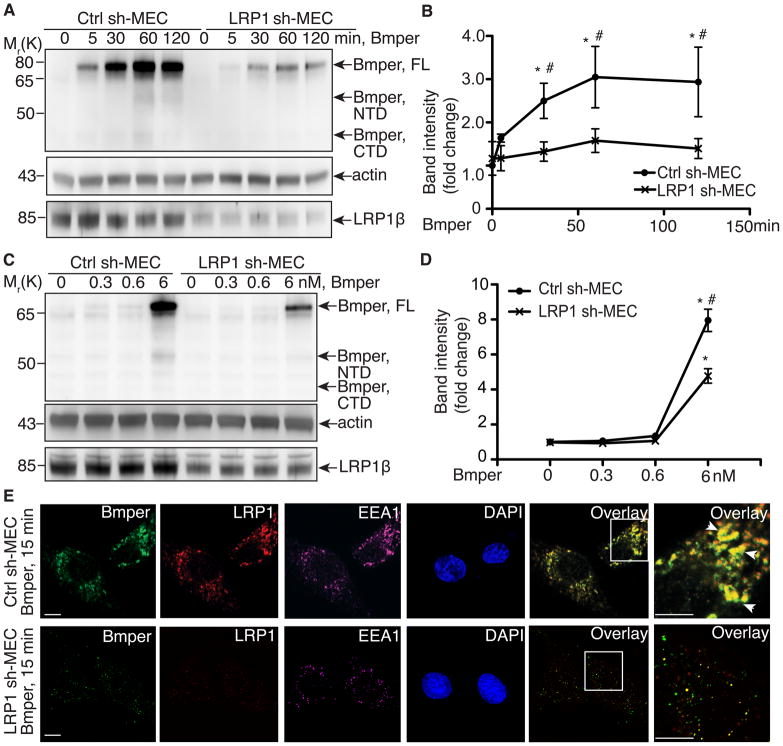
Previously, we found that Bmper undergoes endocytosis via an unknown mechanism that modulates downstream Bmp4 signaling7. Given our observation of a direct interaction between Bmper and the endocytic receptor-LRP1, we hypothesized that LRP1 may act as an endocytic receptor for Bmper and examined cell lysates from LRP1-knockdown (or control) MECs treated with Bmper. The level of internalized Bmper in control MECs increased over time, peaking at 60 min and subsequently decreased, probably due to degradative processing, as previously demonstrated7 (Figure 2A, B). LRP1-knockdown MECs also demonstrated a time-dependent increase in Bmper internalization; however, internalization was substantially less than in control MECs (Figure 2B). When cells were treated with increasing doses of Bmper, the presence of cytoplasmic Bmper in control MECs increased, an effect that was significantly decreased in LRP1-knockdown cells (Figure 2C, D). Similar results were observed in MEFs (Online Figure IIA-D). These data suggest that the majority, but not all, of Bmper is via an LRP1-dependent mechanism and we further explored the role that LRP1 plays in Bmper/Bmp4 endocytosis.
Figure 2. LRP1 is required for Bmper endocytosis.
(A) Time course of non-denatured cell lysates from Bmper-treated MECs (6 nmol/L) were analyzed by Western blotting. (B) Quantification of Bmper protein band intensity in A. *, compared to non-treated control sh-MECs, P<0.02, n=3; #, compared to LRP1-knockdown MECs treated similarly, P<0.05, n=3. (C) Non-denatured cell lysates from MECs treated with increasing doses of Bmper were analyzed by Western blotting. (D) Quantification of Bmper protein band intensity in C. *, compared to non-treated control sh-MECs, P<0.05; #, compared to LRP1-knockdown MECs treated similarly, P<0.02, n=3. (E) Bmper-treated MECs (15 minutes) were analyzed by confocal imaging for the co-localization of Bmper (green), LRP1 (red) and EEA1 (purple), indicated by the arrows. Scale bars: 5 μm.
We used confocal microscopy to investigate whether LRP1 is involved in the previously reported internalization and transport of Bmper to endosomes7. Following Bmper internalization, both LRP1 and Bmper were detected in a subset of vesicles expressing EEA-1 and Rab-7 (endosome markers; Online Figure IIE, F). LRP1-knockdown MECs contained far fewer Bmper-containing EEA-1 and Rab-7 positive endosomal vesicles compared to control MECs (Figure 2E and data not shown). Similar results were found in LRP1 null MEFs (Online Figure IIG). Further subcellular fractionation experiments confirmed this localization (Online Figure IIH). These data collectively indicate that LRP1 influences the endosomal localization of Bmper and acts as an endocytic receptor for Bmper. We have previously shown that Bmper is internalized in a complex together with Bmp4 and Bmp receptors and that endocytosis of this holocomplex is critical for modulating Bmp4 signaling7. Therefore, we next investigated whether the interaction between LRP1 and Bmp receptors could influence Bmp4-dependent Smad1/5/8 signaling in endothelial cells.
LRP1 is associated with Bmp receptor type IB/ALK6
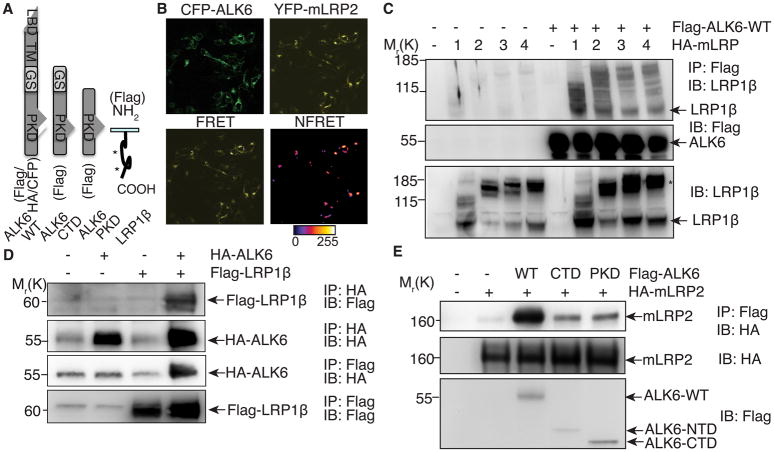
LRP1 functions as a co-receptor for ligands such as transforming growth factor (TGF) β and NMDA17, 18. Given our data demonstrating that LRP1 is an endocytic receptor for Bmper, we hypothesized that LRP1 may act as a co-receptor of Bmp receptors. There are multiple Bmps (Bmp2, 4, 6) and Bmp type I receptors (ALK1, 2, 3 and 6) in MECs and specific siRNA knockdown of ALK2, 3, and 6 (but not ALK1) inhibited Bmp4-induced Smad1/5/8 phosphorylation (Online Figure IIIA-C). Given our previous data identified ALK6 as a Bmp4 type I receptor that mediates Bmp-dependent endothelial migration and angiogenesis10, we used ALK6 as a representative Bmp type I receptor in the following experiments. Fluorescence energy transfer (FRET) imaging analysis demonstrated the close proximity of CFP-tagged ALK6 (Figure 3A) and YFP-tagged mLRP2 in cytoplasmic vesicles and plasma membranes of transfected cells (Figure 3B), indicating that LRP1 and ALK6 could physically interact in cells. Co-immunoprecipitation of lysates from cells transfected with a wild-type ALK6 plasmid (ALK6-WT; Figure 3A) and each mLRP construct (Figure 1E) confirmed this association (Figure 3C). Additionally, when cells were transfected with a LRP1β construct (Figure 3A), the LRP1 and ALK6 association persisted (Figure 3D), indicating that this association likely occurs through the LRP1β chain. Because Bmper could bind to this region of LRP1 (Figure 1D), this interesting observation suggests that both Bmper and Bmps influence LRP1 signaling, thereby initiating cross-talk between the LRP1 and Bmp signaling pathways.
Figure 3. The association of LRP1 and ALK6.
(A) A schematic representation of wild type ALK6, its deletion mutants- ALK6-CTD and ALK6-PKD, and LRP1β constructs. (B) HEK 293 cells were transfected with CFP-ALK6 and YFP-mLRP2 and analyzed by FRET. The calibration bar indicates fluorescence signal level with white being highest intensity in normalized FRET channel (NFRET). Scale bar: 10 μm. (C) Lysates of HEK 293 cells transfected with plasmids encoding Flag-ALK6-WT and HA-mLRP1~4 were immunoprecipitated with an anti-Flag antibody and analyzed by Western blotting. * indicates precursor ‘ER’ and fully glycosylated ‘Golgi’ forms of mLRPs15. (D) Lysates of HEK293 cells transfected with HA-ALK6-WT and Flag-LRP1β were immunoprecipitated with anti-HA or Flag antibody and analyzed by Western blotting. (E) Lysates of HEK 293 cells transfected with Flag-ALK6-CTD/PKD and HA-mLRP2 were immunoprecipitated with an anti-Flag antibody and analyzed by Western blotting.
To determine the domains of ALK6 involved in the interaction with LRP1, ALK6 deletion mutants were constructed (Figure 3A). Both ALK6-CTD and ALK6-PKD co-immunoprecipitated with mLRP2, indicating that the cytoplasmic region of ALK6 is required for the association of ALK6 with mLRP2 (Figure 3E). However, the association of mLRP2 with ALK6-CTD and PKD was decreased in comparison to mLRP2 binding to ALK6-WT, suggesting that CTD of ALK6 is required but not sufficient for the association with mLRP2. Similar results were observed with the interaction of ALK6 and mLRP4 (Online Figure IIID). Collectively, we conclude that the intracellular domains and possible membrane region of ALK6 are required for the association with LRP1. Because the PKD of ALK6 is critical for downstream Bmp-mediated Smad1/5/8 phosphorylation19, next we tested whether the ALK6 and LRP1 interaction was involved in Bmp4-regulated Smad1/5/8 signaling induced by Bmper.
Endocytosis of the Bmper/Bmp4 complex is required for both stimulatory and inhibitory regulation of Bmp4 signaling by Bmper
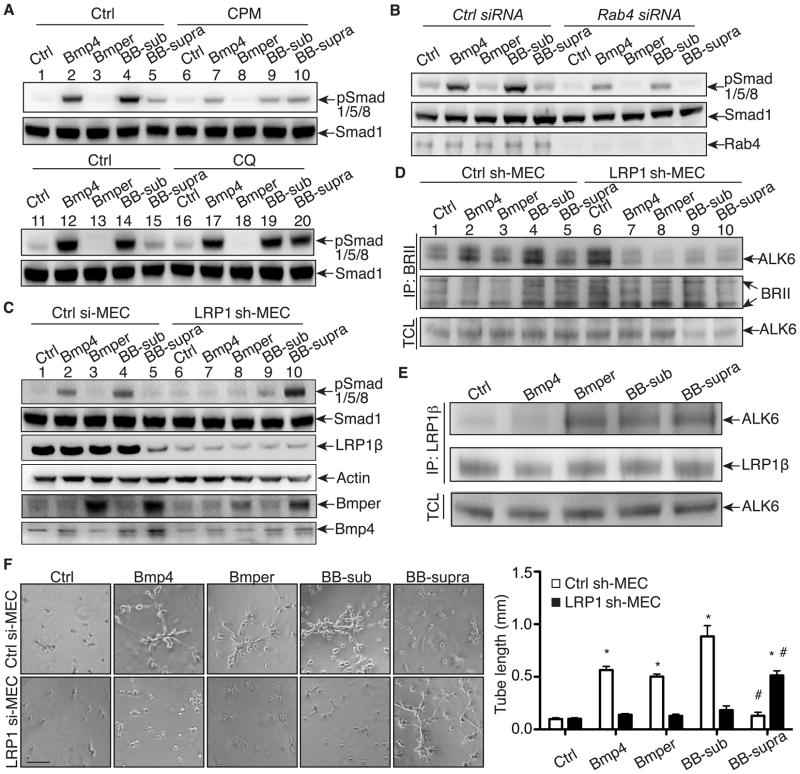
We previously demonstrated that endocytosis of the Bmper/Bmp4 complex is required for the inhibitory effect of Bmper on Bmp4 signaling7, but whether endocytosis of the Bmper/Bmp4 complex is also required more generally for Bmper-dependent Bmp4 signaling regulation remained unclear. Therefore, we used three endocytosis inhibitors - chlorpromazine (CPM), chloroquine (CQ), bafilomycin A1 (BafA1) - in MECs and evaluated the subsequent effect on Bmp4-dependent phosphorylation of Smad1/5/8. Treatment of MECs with Bmp4 alone or in the presence of substoichiometric concentrations of Bmper increased Smad1/5/8 phosphorylation (Figure 4A, lane 2, 4, 12 and 14; Online Figure IVA), consistent with our previous observations that substoichiometric ratios of Bmper to Bmp4 enhances Bmper-dependent Bmp4 signaling7. However, when cells were pretreated with CPM to prevent clathrin-mediated endocytosis, the level of Smad1/5/8 phosphorylation induced by either Bmp4 alone or Bmp4 plus Bmper in substoichiometric concentrations was substantially decreased (Figure 4A, lane 7, 9; Online Figure IVB), indicating that Bmper-mediated enhancement of Bmp4 signaling requires endocytosis of the Bmper/Bmp4 complex. Next we investigated whether Rab4-mediated rapid recycling is required for Bmper’s promoting effect on Smad1/5/8 phosphorylation. Rab4 siRNA knockdown resulted in a significant decrease in Smad1/5/8 phosphorylation induced by Bmp4 alone or Bmp4 plus Bmper in substoichiometric concentrations (Figure 4B, Online Figure IVC). This suggests that the Rab4-mediated rapid recycling route is required for both Bmp4 activity and the promoting effect of Bmper at stoichiometric concentrations. This observation, combined with our previous data demonstrating that endocytosis is also necessary for the inhibition of Bmp4 signaling caused by suprastoichiometric concentrations of Bmper in the Bmper/Bmp4 complex7, indicates that endocytosis is required for all aspects of Bmper-mediated regulation of Bmp4 signaling.
Figure 4. LRP1-mediated endocytosis is required for the Bmper-dependent regulation of Bmp4 downstream signaling.
(A) Endocytosis is required for both promoting- and inhibiting Bmp function of Bmper. MECs were pretreated with CPM or CQ and then treated with Bmp4 (0.6 nmol/L) and Bmper for 15 minutes (CPM) or 120 minutes (CQ) at 10 nmol/L (Bmper alone); 0.3 nmol/L (“BB-sub” = sub-stoichiometric Bmper); 10 nmol/L (“BB-supra” = Bmper suprastoichiometric). Cell lysates were analyzed by Western blotting. (B) Rab4 is required for Smad1/5/8 phosphorylation induced by Bmp4 and substoichiometric Bmper. Lysates of MECs transfected with control or Rab4 siRNA and treated with Bmp4 and Bmper (15 minutes) were analyzed by Western blotting. (C) LRP1 is required for Bmper/Bmp internalization and Bmper-dependent Bmp downstream signaling. Lysates of LRP1-knockdown or control MECs treated with Bmp4 and Bmper (30 minutes) were analyzed by Western blotting. (D) Lysates of MECs treated with Bmp4 and Bmper for 15 minutes were immunoprecipitated with an anti-BMPRII antibody and analyzed by Western blotting. (E) The association of LRP1 and ALK6 is regulated upon the different treatment of Bmp4 and Bmper. Lysates of MECs following Bmp4 and Bmper treatments for 15 minutes were immunoprecipitated with the mouse anti-LRP1β antibody and analyzed by Western blotting. (F) MECs were subjected to the in vitro Matrigel angiogenesis assay. *, compared to the same MECs at control condition, P<0.05. #, compared to the same MECs treated with Bmp4, P<0.01. n=3. Scale bar: 100 μm.
We next examined the effect that CQ and BafA1 had on Bmper-mediated Bmp4 signaling. Treatment of cells with CQ did not affect the ability of Bmp4 alone or Bmp4 plus Bmper in substoichiometric concentrations to induce Bmp4-mediated Smad1/5/8 phosphorylation (Figure 4A, lane 17, 19; Online Figure IVD). However, CQ treatment relieved the inhibitory effect of Bmp4 in the presence of suprastoichiometric concentrations of Bmper, resulting in augmented Smad1/5/8 phosphorylation (Figure 4A, compare lanes 15 and 20). A similar effect on Smad1/5/8 phosphorylation was obtained with BafA1 pretreatment (Online Figure IVE). Since CQ and BafA1 prevent endosomal acidification and inhibit endosome fusion and lysosomal degradation, our data suggest that the inhibition of Bmp signaling by suprastoichiometric concentrations of Bmper involves the lysosomal degradation of the Bmper/Bmp4/BMPR complex, and not simply endocytosis of the signaling complex as previously thought7. Collectively, these data demonstrate that endocytosis is a crucial process linked to Bmper’s ability to both activate and inhibit Bmp4 signaling. In addition, the inhibitory effect of suprastoichiometric concentrations of Bmper on Bmp4 signaling may involve the lysosomal degradation of the Bmper/Bmp4 signaling complex.
LRP1 is required for Bmper/Bmp4-dependent signaling
We next examined the role that LRP1 plays in Bmper-dependent Bmp4 signaling. Following treatment with Bmp4 and Bmper, Smad1/5/8 phosphorylation was evaluated in LRP1-knockdown MECs. Not surprisingly, LRP1-knockdown inhibited Bmper and Bmp4 internalization in these cells (Figure 4C) and decreased Smad1/5/8 phosphorylation induced by Bmp4 alone and by Bmp4 plus Bmper in substoichiometric concentrations (Figure 4C, lane 7, 9; Online Figure IVF), supporting that endocytosis is required for Bmper-dependent enhancement of Bmp4 signaling. Interestingly, LRP1-knockdown in MECs relieved the inhibitory effect of suprastoichiometric concentrations of Bmper on Bmp4-dependent Smad1/5/8 phosphorylation (Figure 4C, lane 10), proving that LRP1 mediates the inhibitory effect of suprastoichiometric concentrations of Bmper on Bmp4 signaling. These data demonstrate the pivotal role that LRP1 plays in Bmper-mediated Bmp4 signaling and support the theory that endocytosis of the Bmper/Bmp4 complex is essential for both the stimulatory and inhibitory actions that Bmper exerts on Bmp4 signaling.
To activate Bmp signaling, Bmp receptor (BMPR) I/II heterodimers must form before Smad1/5/8 phosphorylation occurs20. Having established that LRP1 is required for Bmper-dependent regulation of Bmp4 signaling, we tested the involvement of LRP1 in the regulation of ALK6/BMPRII heterodimerization. ALK6/BMPRII heterodimers were detected in control MECs under basal conditions (Figure 4D, lane 1) with the level increasing steadily with the addition of Bmp4, or Bmp4 plus substoichiometric concentrations of Bmper (Figure 4D, lane 2 & 4; Online Figure IVG). In contrast, when cells were treated with Bmper alone or suprastoichiometric ratios of Bmper to Bmp4, the level of ALK6/BMPRII heterodimerization was similar to that seen under basal conditions (Figure 4D, lane 5). Surprisingly, in LRP1-knockdown MECs, the decrease of LRP1 protein level resulted in a greater abundance of ALK6/BMPRII heterodimers in the basal state (Figure 4D, comparing lane 6 to 1), suggesting that LRP1 competes with BMPRII for association with ALK6. In contrast, when LRP1-knockdown MECs were treated with Bmp4, Bmper, or Bmp4 and both low and high concentrations of Bmper, ALK6/BMPRII heterodimers were decreased in abundance (Figure 4D, lanes 7–10, compared to lane 6). These data suggest that LRP1 regulates the interaction of ALK6 with BMPRII under both basal and stimulated conditions – blocking the association of the receptors in the absence of Bmp4 or Bmper and promoting receptor interaction once stimulation with Bmp4 and/or Bmper at substoichiometric concentrations occurs. Other Bmp type II receptors (e.g., activin receptor type II) showed different behavior in response to LRP1 (Online Figure IVH). In addition, the binding of ALK6 and BMPRII was similar but not same in MEFs (Online Figure IVI). Because exogenous mLRPs and ALK6 were associated in HEK293 cells (Figure 3), we wanted to know whether they form a complex in MECs and, if so, how Bmper/Bmp4 affect this association. Immunoprecipitation assays using MEC lysates confirmed that ALK6 and LRP1 were associated in MECs. Whereas their interaction was inhibited by Bmp4, it was increased by treatment with Bmper alone, or Bmp4 and Bmper at both sub- and suprastoichiometric concentrations (Figure 4E, Online Figure IVJ), suggesting that the LRP1 and ALK6 association is dynamically regulated by Bmp4 and Bmper, which may explain the differential effects of Bmper at sub-or suprastoichiometric concentrations on Smad1/5/8 activation.
LRP1 regulates Bmper/Bmp4-dependent endothelial migration and angiogenesis
Our data demonstrating that LRP1 is required for the Bmper-mediated Bmp4 signaling module suggests that LRP1 could influence physiological outcomes of Bmper-mediated Bmp4 signaling such as endothelial migration and angiogenesis. In Boyden chamber migration assays, both Bmp4 and Bmper enhanced endothelial migration in MECs whereas a Bmp4 neutralizing antibody blocked Bmp4-induced cell migration (Online Figure IVK, L), consistent with previous reports3, 21. However, LRP1 knockdown in MECs completely inhibited migration induced by Bmp4, Bmper alone or Bmp4 plus a substoichiometric concentration of Bmper. Furthermore, LRP1 knockdown in MECs relieved the inhibition on cell migration caused by the combined treatment of Bmp4 plus suprastoichiometric concentrations of Bmper, consistent with our finding that the inhibition of Smad1/5/8 phosphorylation induced by high concentrations of Bmper was also relieved in the absence of LRP1 (Figure 4C). To study the role of LRP1 in angiogenesis, we performed an in vitro Matrigel tubulogenesis assay. Similar to the effects on endothelial migration, LRP1 knockdown in MECs blocked tube formation induced by Bmp4, Bmper or the cotreatment of Bmp4 plus Bmper at the substoichiometric concentrations (Figure 4F). However, LRP1-knockdown MECs demonstrated increased tube formation upon the treatment of Bmp4 and Bmper at suprastoichiometric concentrations (Figure 4F). These data establish that the biochemical model we constructed through in vitro analysis holds true in a physiologically relevant cellular setting, demonstrating that LRP1 is a critical determinant of Bmper-mediated Bmp4 signaling events.
LRP1 is necessary for cardiovascular development in zebrafish
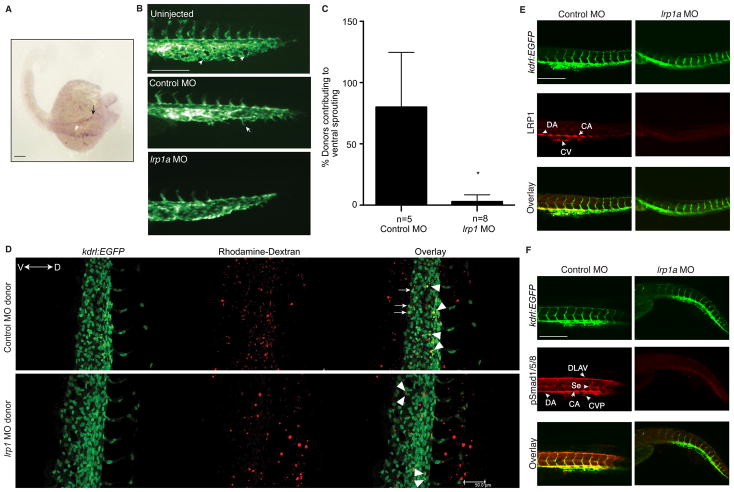
The fact that Bmper/Bmp signaling pathways are essential for vascular development in zebrafish5, 12, along with our observations of a clear reliance of Bmper-mediated Bmp4 signaling on LRP1, prompted us to test whether LRP1 may also play an important role in Bmp4-dependent cardiovascular development. The spatiotemporal expression of lrp1 during zebrafish embryonic development was examined. Weak lrp1a expression was observed at 12 hours post fertilization (hpf), whereas a stronger, symmetrical expression signal could be detected at the lateral dorsal aorta (LDA) at 24 hpf and other vascular structures at later time points (Figure 5A, Online Figure VA). Interestingly, the expression pattern of lrp1a closely paralleled that of bmper5, in that lrp1a was expressed in structures that have Bmp and vasculogenic activity, such as LDA and dorsal longitudinal anastomotic vessel (DLAV).
Figure 5. LRP1 is required for cardiovascular development in zebrafish.
(A) RNA expression of lrp1a in a whole-mount zebrafish embryo, analyzed by in-situ hybridization using an lrp1a-specific antisense probe. The arrow represents the lateral dorsal aorta (LDA). (B) Loss of lrp1a results in a disrupted vascular phenotype. Images are lateral views of Tg(kdrl:EGFP)S843 zebrafish embryo tails at 24 hpf. The arrowheads represent the caudal vein plexus (CVP) with branches; the arrows represent filopodia located on the front edge of vessel plexus. (C) Quantitative analysis of the donor cells located at the tip cell region of cardinal vein plexus and contributing to ventral sprouting. *, compared to control MO-injected donor cells, P≤0.001. n is the number of recipient embryos. (D) Representative lateral views of wild-type recipient embryos of Tg(kdrl:EGFP)s843 fish tail at 34 hpf. Confocal imaging was performed. In the control MO-donor cells-transplanted embryo, three donor cells (white arrows) participated in active ventral sprouting. However, in the lrp1 MO-donor cells-transplanted embryo, donor cells remained in dorsal or axial vasculature (white arrowheads). (E, F) Confocal imaging analysis of whole mount Tg(kdrl:EGFP)s843 fish embryo tails at 48 hpf, with immunostaining for LRP1 (E) and phospho-Smad1/5/8 (F). Scale bars: 100 μm (A, B, E, F); 50 μm (D).
To determine the importance of lrp1a in vasculogenesis, we utilized lrp1a-specific morpholino oligonucleotides (MOs) to knock down lrp1a during zebrafish embryonic development. lrp1a knockdown efficiently decreased embryonic levels of lrp1a RNA as determined by RT-PCR (Online Figure VB) and resulted in an abnormal vascular phenotype, illustrated by delayed dorsal and intersegmental vessel formation, fewer vascular branches within the caudal vein plexus, and a large swollen vascular lumen with ectopically-placed Kdr+ cells (Figure 5B), which have also been described for bmper morphants5. Additionally, lrp1a morphants demonstrated disrupted blood flow and a slower or stopped heart beat (dsRed images in Online Figure VC and Table III). Increased doses of lrp1a MOs resulted in a higher percentage of affected embryos (increasing from 75% to 100%) at 24 hpf (Online Table II). This dose-dependent effect of the lrp1a MO was specific to the knockdown of lrp1a RNA and not due to activation of the p53-dependent cell death pathway22(Online Figure VC–F, Table II–III and Movie I–III). Knockdown of the second lrp1 gene (lrp1b, ENSDART00000088208, chr. 23), either alone or with lrp1a resulted in a similar vascular phenotype, suggesting that the lrp1 genes possess redundant functions (Online Figure VG).
Next, we investigated whether the vascular defect of lrp1 morphant fish is cell-autonomous by performing cell transplantation assays. Control or lrp1 MO-injected ‘donor’ cells were transplanted into wild-type recipient embryos. We observed that both control and lrp1 MO-injected cells contributed to blood, endothelial structures (dorsal aorta (DA), cardinal vein, CV plexus (CVP) and intersegmental vessel (Se)) and other structures (somite, notochord, etc.) similarly (data not shown), suggesting that lrp1 MO did not affect cell differentiation during development. lrp1 MO-injected cells were excluded from the tip cell position within venous network located in the CVP and participated in fewer ventral sprouting events (Figure 5C, D, Online Figure VH). However, there was no obvious defect in the injected cell’s ability to contribute to the tip cell within the Se, which is predominantly arterial in nature at this developmental stage. Bmp signaling was recently reported to regulate the ventral sprouting from the axial vein12. This finding, together with our data, suggests that LRP1, similar to Bmp, regulates vein development in a cell-autonomous fashion. The cardiovascular defects observed in LRP1 morphant fish might be due to non-autonomous effects of LRP1 and reflect the autonomous requirement of LRP1 in venous endothelial function.
Bmper knockdown in zebrafish leads to diminished levels of Smad1/5/8 phosphorylation and a dorsalized phenotype consisting of defects in hematopoiesis and vascular patterning, reflecting the role that Bmper-mediated Bmp signaling plays during embryo gastrulation and vascular development23,5. Since the pattern of lrp1a in the developing zebrafish embryo mirrors that of bmper, we examined the effect of lrp1a knockdown on Bmper-mediated Bmp signaling in lrp1a MO-injected Tg(kdrl:EGFP)s843 fish using immunohistochemical localization of Smad1/5/8 phosphorylation. In wild-type fish, LRP1 expression was localized to the DA, CV and caudal artery (CA), whereas the expression of LRP1 protein was significantly reduced in lrp1a morphant fish (Figure 5E). In wild-type fish, the signal for phosphorylated Smad1/5/8 was mainly localized to the DLAV, DA, CA, and some at Se and CV (Figure 5F). In contrast, lrp1a knockdown decreased Smad1/5/8 phosphorylation in these regions (Figure 5F), indicating that LRP1, similar to Bmper, is required for Bmp-dependent events in vascular development.
DISCUSSION
The data presented in this report is a continuation of our previous work designed to elucidate the molecular mechanisms involved in Bmper regulation of Bmp signaling. Here we present data supporting a model in which LRP1 acts as an endocytic receptor for Bmper, facilitating the formation and internalization of the Bmp4/BMPR signaling complex. Receptor endocytosis plays a critical role not only in the control of receptor protein levels at the cell surface and in the regulation of signaling pathways24. Similar to the case of endocytosis of TGFβ and EGF24, we believe that, in the case of Bmp4 signaling, clathrin-coated pits and early endosomes are signaling compartments, whereas late endosomes and lysosomes are sites where signaling is eventually blocked. Furthermore, our data support a model in which the magnitude and rate of LRP1-dependent endocytosis and the association of LRP1 and Bmp receptors, regulated by the Bmper:Bmp4 stoichiometric ratio, are critical factors determining the endocytic route of the Bmp4 signaling complex.
Previous studies have demonstrated that regulators of Bmp signaling modulate Bmp signaling using a spatial gradient effect that covers the distance of many cells25. In contrast, our data suggest that the mechanism for Bmper’s regulation of Bmp4 signaling operates at the single cell level and involves a negative feedback loop within the same cell. For example, when Bmp4 is released it is bound by extracellular Bmper that initially is substoichiometric. Bmp4 together with Bmper binds to BMPRs and is subsequently endocytosed and recycled via an LRP1-dependent mechanism, which promotes the activation of Bmp4 signaling. Bmp signaling results in a plethora of cellular responses, including upregulation of Bmper expression26. As Bmp4 signaling continues, more Bmper is released into the extracellular environment until the intercellular concentration of Bmper eventually exceeds that of Bmp4. When this happens, the endocytosed Bmper/Bmp4/ALK6 complex is routed to lysosomes where it is degraded, thereby resulting in inhibition of Bmp4 signaling. In this way, each cell involved in the Bmp4 signaling event responds in a tightly controlled manner.
Although the focus of this report has been the effect of LRP1 on Bmp4 signaling, it is entirely possible that Bmper/Bmp4 may also have an effect on LRP1 signaling. More than 40 ligands have been identified for LRP1, encompassing multiple cellular functions such as the regulation of lipid metabolism, cell migration, blood-brain barrier integrity and neuronal homeostasis14. Our observation that both Bmper and ALK6 bind to the β chain of LRP1, which is responsible for ligand uncoupling14, raises the interesting possibility that Bmper and Bmps may influence the signaling mechanisms carried out by LRP1. This is an intriguing thought, especially given the evidence that LRP1 regulates atherosclerosis via modulation of PDGF and TGF–β receptor functions14. A potential interaction or competition between previously identified LRP1 signaling pathways and Bmp-mediated pathways remains a topic of future research.
We have demonstrated that LRP1, through its effect on Bmp signaling, is essential for cardiovascular development in zebrafish. The expression pattern of lrp1 in developing embryos is remarkable for several reasons. lrp1 is expressed in regions known for their high Bmper/Bmp activity and areas of known vasculogenesis5. This pattern places lrp1 at a time and location in which it could interact with Bmper and therefore mediate Bmper/Bmp signaling. This observation is confirmed by the similar phenotype of lrp1- and bmper-deficient fish. Knockdown of either lrp1 or bmper leads to a similar abnormality in vascular development, such as the compromised caudal vein plexus and aberrant intersegmental vessels. However, bmper morphants also exhibit a reduced number of gata1 expressing hematopoietic precursor cells and circulating blood cells5. Whereas these cell types were distributed in a disorganized pattern in lrp1 morphants, the actual cell number remained similar to wild-type embryos. The subtle differences in the expression pattern and knockdown phenotype of LRP1, compared to Bmper, could be attributed to the activation of LRP1 by its other ligands.
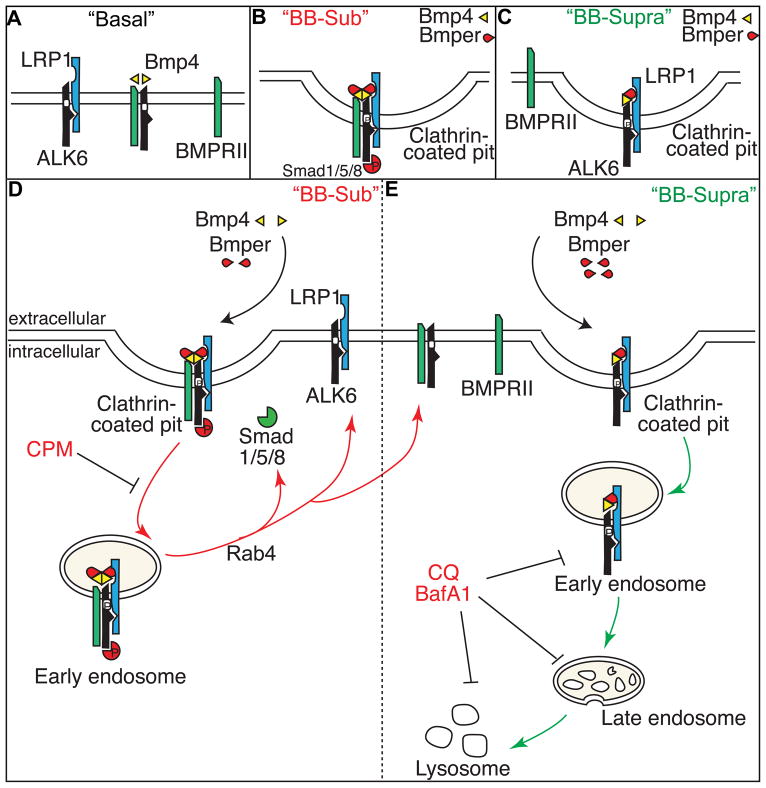
Collectively, our data suggest that LRP1 plays a requisite role in Bmper-mediated regulation of Bmp4 signaling by acting as an endocytic receptor for Bmper and mediating the endocytosis of the Bmper/Bmp4/BMPR complex. Based on these observations, we propose the following working model to explain the role of LRP1 in the regulation of Bmper-mediated Bmp4 signaling (Figure 6). In the absence of ligand, ALK6 and LRP1 are associated, blocking the assembly of an active BMPRII/ALK6 complex (Figure 6A, left). However, in the presence of Bmp4 (Figure 6A, middle), ALK6 dissociates from LRP1 and heterodimerizes with BMRPII, The receptor complex is then endocytosed via clathrin-coated pits in an LRP1-independent manner and sequestered within endosomes where Bmp4-dependent Smad1/5/8 activation occurs20. When the concentration of Bmper is substoichiometric, Bmper/LRP1 forms a transient holocomplex with Bmp4/ALK6/BMPRII (Figure 6B), promoting the Rab4-dependent endocytic fast recycling and therefore enhancing downstream signaling (Figure 6D). The signaling reaction continues to completion, possibly by termination of Smad1/5/8 phosphorylation via phosphatase activation. We speculate that, at the completion of the signaling reaction, the various components of the Bmper/Bmp4 signaling complex are recycled back to the cell membrane (Figure 6D, red route), where they would be available for future signaling events. This pathway would explain the ability of substoichiometric concentrations of Bmper to activate Bmp4 signaling. When the concentration of Bmper is suprastoichiometric, the association of LRP1 with ALK6 increases, but that of ALK6 with BMPRII decreases (Figure 6C). The LRP1-dependent endocytosis of a transient Bmper/Bmp4/ALK6/LRP1 holocomplex leads to the degradation of the Bmper/Bmp4 signaling complex and early termination of Bmp4 signaling activity (Figure 6E, green route).
Figure 6. A schematic model shows how LRP1 is required for Bmp4/Bmper signaling.
(A, left) In the absence of ligand, ALK6 and LRP1 are associated, blocking the assembly of an active BMPRII/ALK6 complex. In the presence of Bmp4 (A, middle), ALK6 dissociates from LRP1 and heterodimerizes with BMPRII20. This Bmp4/BMPRII/ALK6 receptor complex is sequestered within endosomes where Bmp signaling occurs30. (B) When the concentration of Bmper is substoichiometric, Bmper/LRP1 forms a transient holocomplex of Bmp/ALK6/BMPRII, which promotes Rab4-dependent endocytic fast recycling and enhances downstream Bmp signaling (D, red route). (C) When the concentration of Bmper is suprastoichiometric, the association of LRP1 with ALK6 increases, but that of ALK6 with BMPRII decreases. The LRP1-dependent endocytosis of a transient Bmper/Bmp/ALK6/LRP1 holocomplex leads to the degradation of the Bmper/Bmp signaling complex and termination of Bmp signaling activity (E, green route).
We therefore propose that the different components of the transient Bmp4/Bmper receptor holocomplex may be the determining factor in deciding which endocytic sorting routes are used in the presence of sub- versus suprastoichiometric concentrations of Bmper. Bmper was recently reported to preferentially regulate Bmp9/ALK1 signaling in endothelial cells27. Whether LRP1 is required for Bmper-modulated Bmp9/ALK1 signaling and how Bmp9 regulates Bmp4/Bmper/BMPR/LRP1 complex formation needs future investigation. Moreover, the mechanisms behind the receptor-dependent endocytic sorting described remain unknown. The different components of the transient holocomplex may recruit different scaffolding proteins, thereby influencing the intracellular route of receptor complex processing. Alternatively, intracellular routing of the Bmper/Bmp4 signaling complex may be regulated by post-translational modification of LRP1, which may occur differentially depending on which components comprise the Bmper/Bmp4 receptor complex. For example, LRP1 contains an NPxY motif in its cytoplasmic tail that lies proximal to the plasma membrane. This NPxY motif is a sorting nexin 17 (Snx17) -binding motif that help sort LRP1-contained endosomes during the receptor recycling process. If this motif is mutated, LRP1-containing endosomes cannot be recycled and become targets of lysosomal degradation28. LRP1 endocytosis can also be regulated by a cyclic AMP-dependent protein kinase A-mediated serine phosphorylation on its cytoplasmic tail29. It is possible that the different Bmper/Bmp4 receptor complexes formed in the presence of high and low concentrations of Bmper may influence either the phosphorylation of the LRP1 cytoplasmic tail or the recruitment of endosome sorting proteins such as Snx17, which in turn may result in differential intracellular sorting routes. Although additional work is needed to fully elucidate the exact mechanism for the different sorting processes by which LRP1 regulates Bmper/Bmp4 signaling, LRP1-dependent endocytosis is clearly critical for all aspects of Bmper-mediated Bmp4 signaling, and the stoichiometric ratio of Bmper to Bmp4 is a key to determine whether Bmp signaling is activated or inhibited.
Supplementary Material
Novelty and Significance.
What Is Known?
Bmp (bone morphogenetic protein) is an essential regulator of endothelial function.
Bmper (Bmp endothelial cell precursor-derived regulator) is an important extracellular modulator of Bmp and regulates Bmp4 activity via a concentration-dependent, endocytic trap-and-sink mechanism.
What New Information Does This Article Contribute?
LRP1 (low density lipoprotein receptor-related protein 1) acts as an endocytic receptor for Bmper and a co-receptor of Bmp4.
LRP1-dependent endocytosis governs the signaling output of the Bmper/Bmp4 system in endothelial cells and in angiogenesis in vivo.
Bmper, and possibly other Bmp modulators, functions both extracellularly and intracellularly to regulate Bmp4 function, allowing regulation of Bmp4 at the single cell level.
The Bmper/Bmp signaling axis is an important signaling pathway that regulates endothelial function in both health and disease. In this study, we identified LRP1 as a novel endocytic receptor of Bmper and a co-receptor of Bmp4 and demonstrated that the LRP1-dependent endocytosis of Bmper/Bmp4 receptor complex is a crucial molecular mechanism for both promoting and inhibiting Bmp4 activity by Bmper. Previous studies have demonstrated that regulators of Bmp signaling, such as Chordin, Noggin and Gremlin, modulate Bmp signaling using a spatial gradient effect that covers the distance of many cells. In contrast, our data indicates that Bmper, and possibly these other Bmp modulators, can work at the single cell level; not only binding Bmp4 and influencing its interaction with receptors, but also internalizing with the receptor complex to regulate intracellular signaling. In addition, our data introduce LRP1 as a novel regulator of endothelial function and vessel development. The knowledge gained from this study will provide crucial mechanistic information concerning blood vessel formation in health and disease, and offers novel therapeutic targets and strategies against angiogenesis-related diseases such as blood vessel ischemic disease, cancer and other related diseases.
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by NIH grant R01-HL061656 (to C.P.).
We thank the UNC Michael Hooker Proteomics Center for help with protein characterization, the UNC Zebrafish Aquaculture core facility for help with zebrafish experiments, and the UNC Microscopy Services Laboratories for help with immunohistochemistry and in-situ hybridization experiments.
Non-standard Abbreviations
- Bmp
bone morphogenetic protein
- Bmper
Bmp endothelial cell precursor-derived regulator
- LRP1
Low density lipoprotein receptor-related protein 1
- pSMAD1/5/8
phosphorylated SMAD1/5/8
- Cv-2
crossveinless-2
- MEC
mouse endothelial cell
- MEF
mouse embryonic fibroblast
- ALK
activin-like kinase receptor
- BMPR
Bmp receptor
- NTD
amino-terminal domain
- CTD
carboxyl-terminal domain
- MALDI-TOF
matrix-assisted laser desorption/ionization-time of flight
- CPM
chlorpromazine
- BafA1
bafilomycin A1
- CQ
chloroquine
- hpf
hours post-fertilization
- MO
morpholino oligonucleotides
- TGF
transforming growth factor
- EGF
epidermal growth factor
- LDA
lateral dorsal aorta
- Se
intersegmental vessell
- CA
caudal artery
- CV
caudal vein
- CVP
caudal vein plexus
- DLAV
dorsal longitudinal anastomotic vessel
- DA
dorsal aorta
Footnotes
DISCLOSURES
None.
References
- 1.Lowery JW, de Caestecker MP. BMP signaling in vascular development and disease. Cytokine Growth Factor Rev. 2010;21:287–298. doi: 10.1016/j.cytogfr.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinke J, Wehofsits L, Zhou Q, Zoeller C, Baar KM, Helbing T, Laib A, Augustin H, Bode C, Patterson C, Moser M. BMPER is an endothelial cell regulator and controls bone morphogenetic protein-4-dependent angiogenesis. Circ Res. 2008;103:804–812. doi: 10.1161/CIRCRESAHA.108.178434. [DOI] [PubMed] [Google Scholar]
- 4.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moser M, Yu Q, Bode C, Xiong JW, Patterson C. BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J Mol Cell Cardiol. 2007;43:243–253. doi: 10.1016/j.yjmcc.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Miralles I, Ren R, Moser M, Hartnett ME, Patterson C. Bone morphogenetic protein endothelial cell precursor-derived regulator regulates retinal angiogenesis in vivo in a mouse model of oxygen-induced retinopathy. Arterioscler Thromb Vasc Biol. 2011;31:2216–2222. doi: 10.1161/ATVBAHA.111.230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bu G, Geuze HJ, Strous GJ, Schwartz AL. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 1995;14:2269–2280. doi: 10.1002/j.1460-2075.1995.tb07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J. 2001;81:2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 12.Wiley DM, Kim JD, Hao J, Hong CC, Bautch VL, Jin SW. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat Cell Biol. 2011;13:687–693. doi: 10.1038/ncb2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JL, Qiu LY, Kotzsch A, Weidauer S, Patterson L, Hammerschmidt M, Sebald W, Mueller TD. Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev Cell. 2008;14:739–750. doi: 10.1016/j.devcel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obermoeller-McCormick LM, Li Y, Osaka H, FitzGerald DJ, Schwartz AL, Bu G. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J Cell Sci. 2001;114:899–908. doi: 10.1242/jcs.114.5.899. [DOI] [PubMed] [Google Scholar]
- 16.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–1893. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Huang SS, Huang JS. Function of the type V transforming growth factor beta receptor in transforming growth factor beta-induced growth inhibition of mink lung epithelial cells. J Biol Chem. 1997;272:18891–18895. doi: 10.1074/jbc.272.30.18891. [DOI] [PubMed] [Google Scholar]
- 18.May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, Noebels JL, Beffert U, Sweatt JD, Weeber EJ, Herz J. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 20.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 21.Southwood M, Jeffery TK, Yang X, Upton PD, Hall SM, Atkinson C, Haworth SG, Stewart S, Reynolds PN, Long L, Trembath RC, Morrell NW. Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J Pathol. 2008;214:85–95. doi: 10.1002/path.2261. [DOI] [PubMed] [Google Scholar]
- 22.Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–811. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- 24.Di Fiore PP, De Camilli P. Endocytosis and signaling. an inseparable partnership. Cell. 2001;106:1–4. doi: 10.1016/s0092-8674(01)00428-7. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O’Connor MB, Blair SS. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14:940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, Bostrom KI. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119:5037–5047. doi: 10.1182/blood-2011-10-385906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Kerkhof P, Lee J, McCormick L, Tetrault E, Lu W, Schoenfish M, Oorschot V, Strous GJ, Klumperman J, Bu G. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J. 2005;24:2851–2861. doi: 10.1038/sj.emboj.7600756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, van Kerkhof P, Marzolo MP, Strous GJ, Bu G. Identification of a major cyclic AMP-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: implication for receptor-mediated endocytosis. Mol Cell Biol. 2001;21:1185–1195. doi: 10.1128/MCB.21.4.1185-1195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol. 2006;26:7791–7805. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.