Abstract
Human papillomavirus (HPV) is highly prevalent in men and there is an interest in further understanding the relationship between HPV infection and disease in men, including the development of genital warts, penile intraepithelial neoplasia and invasive penile carcinomas. Genital warts are caused by HPV 6/11 and are the most common clinical manifestation of HPV in men. Though they are benign and not associated with mortality, they are a source of psychosocial distress and physical discomfort. HPV infection can also develop into invasive penile carcinoma which is associated with morbidity and mortality. Approximately 40% of invasive penile carcinomas are attributable to HPV with HPV 16, 18, and 6/11 being the genotypes most commonly detected in penile tumors. Penile carcinomas of the basaloid and warty histologic subtypes are most likely to test positive for HPV. In addition to HPV infection, the risk factors most strongly associated with penile cancer are lack of neonatal circumcision, phimosis (the inability of uncircumcised men to fully retract the foreskin), and anogenital warts. Male vaccination with the quadrivalent HPV vaccine that protects against HPV 6/11/16/18 has been shown to significantly reduce HPV-associated anogenital infection and disease in men. If the quadrivalent vaccine is successfully disseminated to large segments of the young male population, there is the potential for substantial reduction in genital HPV infection and related lesions in men.
Keywords: Human papillomavirus, Male, Genital wart, Penile cancer, Penile intraepithelial neoplasia
Human papillomavirus (HPV) is the most common sexually transmitted infection in men and women in the United States (US), with an estimated 6.2 million new cases each year (Cates, 1999). HPV is an established cause of cervical cancer, and there has been immense progress in understanding the natural history of HPV infection to disease in women. Recently there has been an interest in further understanding the relationship between HPV infection and disease in men, including the development of genital warts, penile intraepithelial neoplasia and invasive penile carcinomas.
Genital HPV infection in men
The reported prevalence of genital HPV DNA in men has ranged from 1.3% to 72.9% (with most studies reporting ≥20%) (Dunne et al., 2006). Variation in reported prevalence is likely due to differences in sampling techniques, the populations studied, genital sites sampled (e.g., scrotum, shaft, glans, etc.), and HPV DNA detection methods used. The use of a more sensitive sampling technique (i.e., a pre-wetted Dacron swab rather than a cytobrush or collecting a urine sample (Weaver et al., 2004)) can result in a higher HPV prevalence estimate. Similarly, HPV prevalence is higher when samples are collected from a greater number of anatomic sites (Nielson et al., 2007a). A multinational study recently reported that 50.5% of men tested positive for at least one known HPV genotype and an additional 14.7% of men were positive for an unknown HPV type (Giuliano et al., 2008a). There was also a high rate of men testing positive for multiple HPV genotypes (25.7%). Among men with HPV infections in that study, 12.0% had oncogenic types only, 20.7% had non-oncogenic types only, 17.8% had both oncogenic and non-oncogenic types, and 14.7% had unclassified types only (i.e., tested positive for HPV DNA using a generic probe, but negative for all of the 37 HPV genotypes tested for). Similar results were seen in a cross-sectional study of men in the US that sampled for HPV from six anogenital sites (penile shaft, glans/corona, scrotum, urethra, perianal area and anal canal) (Nielson et al., 2007a). HPV was most commonly detected in samples taken from the shaft (49.9%), glans (35.8%), and scrotum (34.2%), and less frequently in samples from the perianal area (20.0%), anal canal (17.6%), urethra (10.1%), and semen (5.3%) (Nielson et al., 2007a). HPV 16 and 6 are consistently among the most common types detected in men across studies regardless of the anatomic site sampled (as reviewed in Dunne et al., 2006). The factors independently associated with HPV detection at the shaft, glans, or scrotum in men include not being circumcised (Giuliano et al., 2009; Gray et al., 2010; Hernandez et al., 2008a; Lajous et al., 2005; Lu et al., 2009; Vaccarella et al., 2006), lack of condom use (Baldwin et al., 2003; Nielson et al., 2009a,b), a history of having ever smoked (Vaccarella et al., 2006; Nielson et al., 2009b; Nielson et al., 2007b), and a high lifetime number of sexual partners (Baldwin et al., 2003; Giuliano et al., 2009; Lu et al., 2009; Nielson et al., 2009a,b).
There does not appear to be an association between age and HPV prevalence in men. This is in contrast to the pattern observed in women, where HPV prevalence is highest among women ages 18–24 and then decreases until middle age, after which it remains steady for the remainder of the lifespan (Burchell et al., 2006). The consistently higher prevalence of HPV in men suggests that women have a stronger immune response. This is supported by serologic studies that find a higher prevalence of HPV antibodies in women than men across all ages (Kreimer et al., 2004; Markowitz et al., 2009; Svare et al., 1997). In a study of participants in the National Health and Examination Survey, the prevalence of antibodies for one or more of the HPV vaccine types (6, 11, 16, and 18) was significantly higher among women (32.5%) than men (12.2%) (Markowitz et al., 2009). A possible explanation for this difference is that when keratinized epithelium is infected with HPV it is less likely to induce an immune response than mucosal epithelium (the tissue type most commonly infected in women).
Only a few cohort studies have examined the incidence and duration of HPV infection in men (Giuliano et al., 2008b, 2011a; Kjaer et al., 2005; Lajous et al., 2005; Partridge et al., 2007; Van Doornum et al., 1994; Wikstrom et al., 2000). It is estimated that the probability of detecting a new HPV infection in men over a 12-month period is between 29% and 39% and does not significantly change across the lifespan (Giuliano et al., 2011a; Lu et al., 2009). The majority of HPV infections clear in less than 12 months; one study of men in the US reported a median time to clearance of 5.9 months (Lu et al., 2009), while a multinational study observed a median time to clearance of 7.5 months (Giuliano et al., 2011a). HPV 16 infections tend to have a longer duration and are estimated to clear at an average of 12.2 months (Giuliano et al., 2011a). Age and lifetime number of female sexual partners influence duration of infection, with increasing age and fewer number of female partners being associated with a greater probability of clearing an oncogenic infection (Giuliano et al., 2011a).
Genital warts
The majority of HPV infections are asymptomatic with an estimated 70% of incident infections clearing within 1 year (Dunne et al., 2006). If an infection does not clear however it can progress to disease. Anogenital warts are the most common clinical manifestation of HPV infection (Scheurer et al., 2005). Though they are benign and not associated with mortality, they are a source of psychosocial distress (Jeynes et al., 2009) and can cause physical discomfort including pain, bleeding and itching (Wiley et al., 2002). Genital warts are highly infectious and approximately 65% of people whose sexual partner has genital warts will develop warts themselves (Lacey, 2005). The estimated incubation period from HPV infection to genital wart development is 2 weeks to 8 months, with the majority of genital warts appearing 2–3 months after an HPV infection (Oriel, 1971). Approximately 20–30% of genital warts will spontaneously regress (Wiley et al., 2002), however, recurrence of warts is common, resulting in high medical costs for repeated treatment. An estimated $200 million is spent annually in the US for direct medical costs of genital wart treatment (Insinga et al., 2005).
In the US, 5.6% of sexually active men and women ages 18–59 years have self-reported ever being diagnosed with genital warts (Dinh et al., 2008) and 1% of US adults ages 18–45 years are estimated to have genital warts at any given time (Koutsky, 1997). Studies utilizing private health insurance claims in the US reported genital wart incidence rates ranging from 1.10 to 1.70 per 1000 person–years (Hoy et al., 2009;Insinga et al., 2003; Koshiol et al., 2004; ). These data may underestimate incidence since they exclude individuals who do not seek treatment or are not privately insured. A community sample of men ages 18–70 years observed a slightly higher incidence rate for genital warts of 2.35 per 1000 person–years (Anic et al., in press). Though age is not associated with detection of HPV in men (Giuliano et al., 2011a), the incidence of genital warts is highest among men younger than age 30 years and significantly decreases with age (Hoy et al., 2009; Insinga et al., 2003; Koshiol et al., 2004). The association with age remains significant even after adjusting for sexual behavior (Hughes et al., 2000; Jin et al., 2007; Van Den Eeden et al., 1998; Wen et al., 1999).
Testing positive for HPV types 6 and 11 is the strongest predictor of developing genital warts. In the placebo arm of an HPV vaccine trial, the women who tested positive for HPV 6/11 at baseline were 29 times more likely to develop condyloma in the first year of follow-up compared to women negative for HPV 6/11 (Garland et al., 2009). In a prospective study, men who tested positive for HPV at baseline had almost 12 times the risk of developing genital warts (Anic et al., submitted). While HPV 6 and 11 are the types most commonly found in genital warts, infections with multiple types are common including co-infection with oncogenic types. Oncogenic HPV 16 has been reported as the third most common HPV type detected after 6 and 11 in several studies examining the distribution of HPV types in male genital warts (Anic et al., in press; Aubin et al., 2008; Brown et al., 1999; Chan et al., 2009; Vandepapeliere et al., 2005).
Certain sexual behaviors are also associated with an increased risk for genital warts. A high lifetime number of female sexual partners significantly increases the risk of genital warts (Anic et al., submitted; Dinh et al., 2008; Van Den Eeden et al., 1998; Wen et al., 1999), likely by increasing a man’s chance of being exposed to HPV. Frequent condom use was protective against developing genital warts in some (Anic et al., submitted; Wen et al., 1999), but not all (Van Den Eeden et al., 1998) studies. Similarly, there have been inconsistent findings on the protective effect of condom use against HPV infection in men (Giuliano et al., 2008c). Condoms provide a protective barrier against the transmission of HPV by skin to skin contact; however, men can be infected with HPV on areas not protected by a condom.
Penile intraepithelial neoplasia
Penile intraepithelial neoplasia (PIN) is a heterogeneous condition that currently does not have standard clinical protocols for diagnosis. Because of the similarities in histologic characteristics, the classifications for PIN I, II and III have been “borrowed” from those assigned to CIN. Benign clinical conditions such as lichen sclerosus or psoriasis may appear similar to PIN on visual inspection alone; therefore, misclassification of PIN is possible without histological confirmation from a biopsy sample. High-grade PIN is considered penile carcinoma in situ and includes the clinical conditions Bowen’s disease (often found on keratinized skin) and Erythroplasia of Qeyrat (EQ) (found on the mucosal surface of the glans and foreskin) (Bleeker et al., 2009). Development of high-grade PIN is rare and the risk factors for this condition are not known. The likelihood that these lesions progress to invasive cancer is also unknown as there are currently no natural history studies that examine the proportion of PIN that progress to cancer (Fig. 1).
Fig. 1.

Penile intraepithelial neoplasm.
Studies have estimated that approximately 60–100% of PIN lesions are positive for HPV DNA (Aynaud et al., 1994; Barrasso, 1997; Cupp et al., 1995; Krustrup et al., 2009; Rubin et al., 2001; Wieland et al., 2000). One of the larger case series to date tested for the presence of 25 HPV genotypes in 30 histologically confirmed PIN lesions (Rubin et al., 2001). HPV was detected in 90% of PIN and the majority of lesions were positive for single oncogenic HPV types (59.3%). Among HPV positive samples, HPV 16 was the most common type detected (40.7%), followed by HPV 6 (22.2%,) HPV 52 (14.8%), and HPV 11 (3.7%). HPV 18 was not detected in any lesions. Results were similar in a smaller case series of 12 PIN lesions from men in the US which detected HPV in 92% of lesions (Cupp et al., 1995). HPV 16 was the most common type detected and no lesions were positive for HPV 18. Similar results were observed in a Danish study of 29 penile in-situ carcinomas that detected HPV in 90% of lesions (Krustrup et al., 2009). Most studies to date have only tested for mucosal HPV types, but there is evidence to suggest that oncogenic cutaneous HPV may also be present in PIN. Wieland et al. (2000) detected cutaneous HPV types 5 and 8 in a series of eight EQ lesions. HPV 8 DNA was detected in all lesions and co-infection with HPV 16 was observed in 88% of lesions.
Incidence and prevalence of penile cancer
Invasive penile cancer is rare and accounts for less than 0.5% of all cancers in men worldwide (Parkin & Bray, 2006). Between 1998 and 2003, the annual age-adjusted incidence rate of penile cancer in the US was 0.81 per 100,000 men and accounted for only 0.1% of male invasive cancers (Hernandez et al., 2008b). The disease most commonly affects men ages 50–70 years (Bleeker et al., 2009). Incidence of penile cancer in the US is highest among Hispanics and men who live in the Southern US or areas with high levels of poverty (Barnholtz-Sloan et al., 2007; Hernandez et al., 2008b). Worldwide, areas with a high incidence of cervical cancer also tend to have a high incidence of penile cancer (Bosch & Cardis, 1990). For example, in Brazil, reported incidence rates of penile cancer range from 2.9 to 6.8 per 100,000 men (Favorito et al., 2008). Incidence is also higher in less developed countries, where penile cancer accounts for up to 10% of all male cancers in some parts of Africa, South America and Asia (Bleeker et al., 2009) (Fig. 2).
Fig. 2.
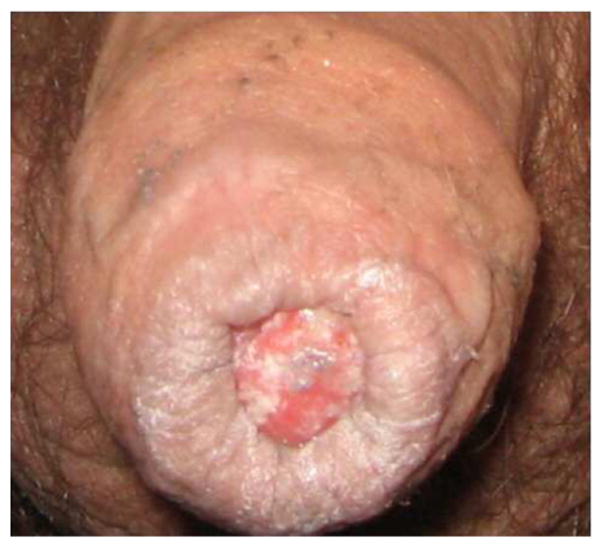
Penile cancer.
HPV prevalence in penile cancer
Though the etiology of penile cancer is still unknown, approximately 40% of all penile tumors are thought to be attributable to HPV infection (Human papillomaviruses, 2007). HPV DNA has been detected in 14%–100% of invasive penile carcinomas (Bezerra et al., 2001a; Chan et al., 1994; Cupp et al., 1995; Ferreux et al., 2003; Gregoire et al., 1995; Heideman et al., 2007; Humbey et al., 2003; Iwasawa et al., 1993; Krustrup et al., 2009; Levi et al., 1998; Lont et al., 2006; Maden et al., 1993; McCance et al., 1986; Nasca et al., 1999; Pascual et al., 2007; Picconi et al., 2000; Rubin et al., 2001; Salazar et al., 2005; Sarkar et al., 1992), with higher prevalence estimates among case series with small sample sizes. Differences in methods used for DNA detection and tumor tissue storage (fresh vs. paraffin embedded) and the inclusion of tumors with different histologic subtypes may contribute to the variation in HPV prevalence across studies. A quantitative review of studies that used PCR methods for HPV DNA detection found HPV present in 45.4% of invasive penile tumors after adjusting for PCR primer, histology sub-type, and year and geographical location of the study (Backes et al., 2009). Another review of 31 studies examining the prevalence of HPV in invasive penile tumors found that among those with HPV, HPV 16 was the most common type detected (60.2%), followed by HPV 18 (13.3%) and HPV types 6/11 (8.13%) (Miralles-Guri et al., 2009). Unclassified infections have been observed in penile tumors even in studies that use assays that test for many genotypes. Rubin et al. (2001) tested for 25 mucosal HPV types and observed unclassified infections in 12% of the HPV positive tumors. Given that the majority of studies only test for mucosal types, it is possible that cutaneous HPV types account for some unclassified infections. In two small case series, oncogenic cutaneous HPV types 5 and 8 were detected in 6% (Humbey et al., 2003) and 11% (Heideman et al., 2007) of penile tumors, respectively.
The difference in the rate of HPV detection is highly dependent on the histologic type of penile cancer, similar to vulvar cancers. Approximately 95% of invasive penile cancers are squamous cell carcinomas (SCC) (Bleeker et al., 2009) and the most common penile SCC histologic sub-types are keratinizing (49%), mixed warty-basaloid (17%), verrucous (8%), warty (6%), and basaloid (4%) (Bleeker et al., 2009). HPV is most commonly detected in basaloid and warty tumors (Bezerra et al., 2001a; Ferreux et al., 2003; Gregoire et al., 1995; Rubin et al., 2001). One of the larger case series to stratify by histologic subtype found HPV present in 100% of warty and 80% of basaloid tumors, but only about one-third of keratinizing and verrucous tumors (Rubin et al., 2001). The same study found HPV 16 to be the most common type, detected in 100% of warty and 83% of basaloid tumors (Fig. 3).
Fig. 3.
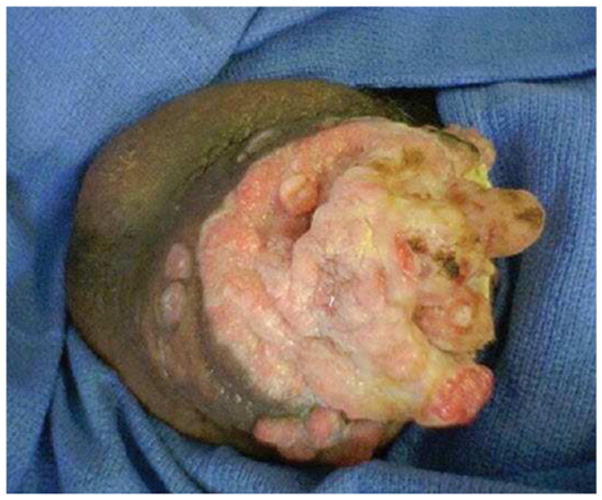
Penile cancer affecting the glans.
In serologic studies there is a high prevalence of HPV 16 antibodies among penile cancer cases. A study from the Netherlands found men with invasive penile cancer had a significantly higher seroprevalence of HPV 16 (38%) compared to controls with gastric cancer (18%) and hospital-based controls with no cancer (18%) (Van Doornum et al., 2003). Another study also observed a significantly higher seroprevalence of HPV 16 in penile cancer cases (28%) compared to population-based controls (13%) (Carter et al., 2001). This study also tested for HPV 18 antibodies, but did not observe any penile cancer cases seropositive for HPV 18. Only HPV 16 and 18 have been examined in serologic studies of penile cancer thus far and no studies to date have assessed the seroprevalence of HPV by histologic sub-type.
Risk factors for penile cancer
The risk factors most strongly associated with penile cancer are lack of neonatal circumcision (Brinton et al., 1991; Daling et al., 2005; Maden et al., 1993), phimosis (the inability of uncircumcised men to fully retract the foreskin) (Brinton et al., 1991; Daling et al., 2005; Hellberg et al., 1987; Maden et al., 1993; Madsen et al., 2008; Tsen et al., 2001), anogenital warts (Aynaud et al., 1994; Daling et al., 2005; Maden et al., 1993; Madsen et al., 2008), and HPV infection (Brinton et al., 1991; Maden et al., 1993). A very low incidence of penile cancer has been observed among Jewish populations that commonly practice neonatal circumcision (0.04 per 100,000) (Parkin & Bray, 2006). Circumcision most likely protects against penile cancer by reducing the risk of HPV acquisition (Gray et al., 2010), however, the timing of circumcision may infiuence the protective effect. Men circumcised after the neonatal period have a higher risk of penile cancer compared men who were circumcised at birth (Brinton et al., 1991; Daling et al., 2005). Delayed circumcision may not be protective because men who are circumcised later in life often undergo the procedure as treatment for phimosis or an existing chronic inflammatory condition. Other risk factors for penile cancer include current smoking (Daling et al., 2005; Hellberg et al., 1987; Maden et al., 1993; Tsen et al., 2001), early age at first sexual intercourse (Madsen et al., 2008), high lifetime number of female sexual partners (Daling et al., 2005; Maden et al., 1993; Madsen et al., 2008), lack of condom use (Madsen et al., 2008), chronic inflammatory conditions including balantitis and lichen sclerosus (Daling et al., 2005; Nasca et al., 1999), and treatment with ultraviolet photochemotherapy for psoriasis (Stern, 1990).
The role of HPV as a prognostic factor for penile cancer is not clear. In a study of 82 penile cancer cases, the rate of metastasis was lower among men with penile tumor tissue positive for HPV DNA compared to men with HPV negative tumors, but the difference was not statistically significant, likely due to limited power (Bezerra et al., 2001b; Lopes et al., 2002). The association between HPV status of penile tumors and survival is inconsistent, with one study reporting significantly higher survival rates among men with HPV positive tumors (Lont et al., 2006), but another study found survival rates did not differ between groups (Lopes et al., 2002). The biologic effect of why the presence of HPV DNA in penile tumors may be associated with better prognosis is not known and warrants further research.
Prevention of genital HPV infection and genital warts through vaccination
Several reports have demonstrated the efficacy of the quadrivalent HPV vaccine (HPV4) in preventing genital disease caused by HPV 6/11/16/18 in females including the precancerous lesions CIN 2 and 3 (FUTURE II Study Group, 2007; Brown et al., 2009; Garland et al., 2007; Wheeler et al., 2009). Male vaccination with the quadrivalent HPV vaccine has also been shown to significantly reduce HPV-associated anogenital infection and disease in men. In a recently completed international Phase III trial of the quadrivalent HPV vaccine Gardasil, prophylactic administration of HPV4 vaccine was efficacious in preventing HPV 6/11/16/18-related external genital lesions (EGL) in men aged 16–26 years (Giuliano et al., 2011b). Vaccine efficacy against HPV 6/11/16/18 related EGL in the intent-to-treat population was high (65.5% [95% CI: 45.8%, 78.6%]), as was efficacy against development of any EGL regardless of HPV type (60.2% [95% CI: 40.8%, 73.8%]). In the per protocol efficacy (PPE) population, the HPV4 vaccine reduced the incidence of HPV 6/11/16/18-related EGLs by 90.4% (95% CI: 69.2%, 98.1%). Efficacy against genital warts in the PPE population was 89.4% (95% CI: 65.5%, 97.9%). In addition, the HPV4 vaccine was efficacious against HPV 6/11/16/18-related persistent infection and any-time DNA detection. On the basis of these data the US FDA licensed Gardasil for use in males ages 9–26 for the prevention of genital warts in November 2009. The HPV4 vaccine was also recently approved for use in the prevention of anal cancer in men and women and the efficacy of the vaccine against oropharyngeal cancer is being evaluated. Altogether these results are encouraging and hold promise for an ultimate reduction in genital HPV infection and related lesions in men if the HPV4 vaccine is successfully disseminated to large segments of the young male population.
Footnotes
Conflict of Interest
None to report.
References
- Anic GM, Lee J, Stockwell H, et al. Incidence and HPV type distribution of genital warts in a multinational cohort of men: The HIM Study. JID. doi: 10.1093/infdis/jir652. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anic GM, Lee J, Stockwell H, et al. Risk factors for incident condyloma in a multinational cohort of men: The HIM Study. Sexually Transmitted Infections. doi: 10.1093/infdis/jir851. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin F, Pretet JL, Jacquard AC, et al. Human papillomavirus genotype distribution in external acuminata condylomata: a Large French National Study (EDiTH IV) Clin Infect Dis. 2008;47:610–615. doi: 10.1086/590560. [DOI] [PubMed] [Google Scholar]
- Aynaud O, Ionesco M, Barrasso R. Penile intraepithelial neoplasia. Specific clinical features correlate with histologic and virologic findings. Cancer. 1994;74:1762–1767. doi: 10.1002/1097-0142(19940915)74:6<1762::aid-cncr2820740619>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449–457. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- Baldwin SB, Wallace DR, Papenfuss MR, et al. Human papillomavirus infection in men attending a sexually transmitted disease clinic. J Infect Dis. 2003;187:1064–1070. doi: 10.1086/368220. [DOI] [PubMed] [Google Scholar]
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, Giuliano AR. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361–367. doi: 10.1016/j.urolonc.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Barrasso R. Latent and subclinical HPV external anogenital infection. Clin Dermatol. 1997;15:349–353. doi: 10.1016/s0738-081x(96)00161-7. [DOI] [PubMed] [Google Scholar]
- Bezerra AL, Lopes A, Landman G, Alencar GN, Torloni H, Villa LL. Clinicopathologic features and human papillomavirus DNA prevalence of warty and squamous cell carcinoma of the penis. Am J Surg Pathol. 2001a;25:673–678. doi: 10.1097/00000478-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Bezerra AL, Lopes A, Santiago GH, Ribeiro KC, Latorre MR, Villa LL. Human papillomavirus as a prognostic factor in carcinoma of the penis: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. Cancer. 2001b;91:2315–2321. [PubMed] [Google Scholar]
- Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer CJ. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141–150. doi: 10.1007/s00345-008-0302-z. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Cardis E. Cancer incidence correlations: genital, urinary and some tobacco-related cancers. Int J Cancer. 1990;46:178–184. doi: 10.1002/ijc.2910460206. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Li JY, Rong SD, et al. Risk factors for penile cancer: results from a case–control study in China. Int J Cancer. 1991;47:504–509. doi: 10.1002/ijc.2910470406. [DOI] [PubMed] [Google Scholar]
- Brown DR, Schroeder JM, Bryan JT, Stoler MH, Fife KH. Detection of multiple human papillomavirus types in Condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–3322. doi: 10.1128/jcm.37.10.3316-3322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S3/52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Carter JJ, Madeleine MM, Shera K, et al. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 2001;61:1934–1940. [PubMed] [Google Scholar]
- Cates W., Jr Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. American Social Health Association. Panel Sex Transm Dis. 1999;26:S2–S7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- Chan KW, Lam KY, Chan AC, Lau P, Srivastava G. Prevalence of human papillomavirus types 16 and 18 in penile carcinoma: a study of 41 cases using PCR. J Clin Pathol. 1994;47:823–826. doi: 10.1136/jcp.47.9.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PK, Luk AC, Luk TN, et al. Distribution of human papillomavirus types in anogenital warts of men. J Clin Virol. 2009;44:111–114. doi: 10.1016/j.jcv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Cupp MR, Malek RS, Goellner JR, Smith TF, Espy MJ. The detection of human papillomavirus deoxyribonucleic acid in intraepithelial, in situ, verrucous and invasive carcinoma of the penis. J Urol. 1995;154:1024–1029. [PubMed] [Google Scholar]
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606–616. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- Dinh TH, Sternberg M, Dunne EF, Markowitz LE. Genital warts among 18- to 59-year-olds in the United States, national health and nutrition examination survey, 1999–2004. Sex Transm Dis. 2008;35:357–360. doi: 10.1097/OLQ.0b013e3181632d61. [DOI] [PubMed] [Google Scholar]
- Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194:1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- Favorito LA, Nardi AC, Ronalsa M, Zequi SC, Sampaio FJ, Glina S. Epidemiologic study on penile cancer in Brazil. Int Braz J Urol. 2008;34:587–591. doi: 10.1590/s1677-55382008000500007. discussion 591–3. [DOI] [PubMed] [Google Scholar]
- Ferreux E, Lont AP, Horenblas S, et al. Evidence for at least three alternative mechanisms targeting the p16INK4A/cyclin D/Rb pathway in penile carcinoma, one of which is mediated by high-risk human papillomavirus. J Pathol. 2003;201:109–118. doi: 10.1002/path.1394. [DOI] [PubMed] [Google Scholar]
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–814. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008a;17:2036–2043. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AR, Lu B, Nielson CM, et al. Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J Infect Dis. 2008b;198:827–835. doi: 10.1086/591095. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Tortolero-Luna G, Ferrer E, et al. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008c;26 (Suppl 10):K17–K28. doi: 10.1016/j.vaccine.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM Study. Int J Cancer. 2009;124:1251–1257. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011a;377:932–940. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011b;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Serwadda D, Kong X, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1455–1462. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire L, Cubilla AL, Reuter VE, Haas GP, Lancaster WD. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst. 1995;87:1705–1709. doi: 10.1093/jnci/87.22.1705. [DOI] [PubMed] [Google Scholar]
- Heideman DA, Waterboer T, Pawlita M, et al. Human papillomavirus-16 is the predominant type etiologically involved in penile squamous cell carcinoma. J Clin Oncol. 2007;25:4550–4556. doi: 10.1200/JCO.2007.12.3182. [DOI] [PubMed] [Google Scholar]
- Hellberg D, Valentin J, Eklund T, Nilsson S. Penile cancer: is there an epidemiological role for smoking and sexual behaviour? Br Med J (Clin Res Ed) 1987;295:1306–1308. doi: 10.1136/bmj.295.6609.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez BY, Wilkens LR, Zhu X, et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J Infect Dis. 2008a;197:787–794. doi: 10.1086/528379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998–2003. Cancer. 2008b;113:2883–2891. doi: 10.1002/cncr.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin. 2009;25:2343–2351. doi: 10.1185/03007990903136378. [DOI] [PubMed] [Google Scholar]
- Hughes G, Catchpole M, Rogers PA, et al. Comparison of risk factors for four sexually transmitted infections: results from a study of attenders at three genitourinary medicine clinics in England. Sex Transm Infect. 2000;76:262–267. doi: 10.1136/sti.76.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human papillomaviruses. IARC Monogr Eval Carcinog Risk Hum. 2007:1–636. [PMC free article] [PubMed] [Google Scholar]
- Humbey O, Cairey-Remonnay S, Guerrini JS, et al. Detection of the human papillomavirus and analysis of the TP53 polymorphism of exon 4 at codon 72 in penile squamous cell carcinomas. Eur J Cancer. 2003;39:684–690. doi: 10.1016/s0959-8049(02)00835-3. [DOI] [PubMed] [Google Scholar]
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis. 2003;36:1397–1403. doi: 10.1086/375074. [DOI] [PubMed] [Google Scholar]
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. PharmacoEconomics. 2005;23:1107–1122. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- Iwasawa A, Kumamoto Y, Fujinaga K. Detection of human papillomavirus deoxyribonucleic acid in penile carcinoma by polymerase chain reaction and in situ hybridization. J Urol. 1993;149:59–63. doi: 10.1016/s0022-5347(17)35999-2. [DOI] [PubMed] [Google Scholar]
- Jeynes C, Chung MC, Challenor R. ‘Shame on you‘ — the psychosocial impact of genital warts. Int J STD AIDS. 2009;20:557–560. doi: 10.1258/ijsa.2008.008412. [DOI] [PubMed] [Google Scholar]
- Jin F, Prestage GP, Kippax SC, et al. Risk factors for genital and anal warts in a prospective cohort of HIV-negative homosexual men: the HIM Study. Sex Transm Dis. 2007;34:488–493. doi: 10.1097/01.olq.0000245960.52668.e5. [DOI] [PubMed] [Google Scholar]
- Kjaer SK, Munk C, Winther JF, Jorgensen HO, Meijer CJ, van den Brule AJ. Acquisition and persistence of human papillomavirus infection in younger men: a prospective follow-up study among Danish soldiers. Cancer Epidemiol Biomarkers Prev. 2005;14:1528–1533. doi: 10.1158/1055-9965.EPI-04-0754. [DOI] [PubMed] [Google Scholar]
- Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31:748–752. doi: 10.1097/01.olq.0000145851.76025.ad. [DOI] [PubMed] [Google Scholar]
- Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- Kreimer AR, Alberg AJ, Viscidi R, Gillison ML. Gender differences in sexual biomarkers and behaviors associated with human papillomavirus-16, -18, and -33 seroprevalence. Sex Transm Dis. 2004;31:247–256. doi: 10.1097/01.olq.0000118425.49522.2c. [DOI] [PubMed] [Google Scholar]
- Krustrup D, Jensen HL, van den Brule AJ, Frisch M. Histological characteristics of human papilloma-virus-positive and -negative invasive and in situ squamous cell tumours of the penis. Int J Exp Pathol. 2009;90:182–189. doi: 10.1111/j.1365-2613.2008.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey CJ. Therapy for genital human papillomavirus-related disease. J Clin Virol. 2005;32 (Suppl 1):S82–S90. doi: 10.1016/j.jcv.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Lajous M, Mueller N, Cruz-Valdez A, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–1716. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- Levi JE, Rahal P, Sarkis AS, Villa L. Human papillomavirus DNA and p53 status in penile carcinomas. Int J Cancer. 1998;76:779–783. doi: 10.1002/(sici)1097-0215(19980610)76:6<779::aid-ijc1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lont AP, Kroon BK, Horenblas S, et al. Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int J Cancer. 2006;119:1078–1081. doi: 10.1002/ijc.21961. [DOI] [PubMed] [Google Scholar]
- Lopes A, Bezerra AL, Pinto CA, Serrano SV, de Mell OC, Villa LL. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol. 2002;168:81–86. [PubMed] [Google Scholar]
- Lu B, Wu Y, Nielson CM, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: a prospective study. J Infect Dis. 2009;199:362–371. doi: 10.1086/596050. [DOI] [PubMed] [Google Scholar]
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19–24. doi: 10.1093/jnci/85.1.19. [DOI] [PubMed] [Google Scholar]
- Madsen BS, van den Brule AJ, Jensen HL, Wohlfahrt J, Frisch M. Risk factors for squamous cell carcinoma of the penis—population-based case–control study in Denmark. Cancer Epidemiol Biomarkers Prev. 2008;17:2683–2691. doi: 10.1158/1055-9965.EPI-08-0456. [DOI] [PubMed] [Google Scholar]
- Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis. 2009;200:1059–1067. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- McCance DJ, Kalache A, Ashdown K, et al. Human papillomavirus types 16 and 18 in carcinomas of the penis from Brazil. Int J Cancer. 1986;37:55–59. doi: 10.1002/ijc.2910370110. [DOI] [PubMed] [Google Scholar]
- Miralles-Guri C, Bruni L, Cubilla AL, Castellsague X, Bosch FX, de Sanjose S. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62:870–878. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911–914. doi: 10.1016/s0190-9622(99)70245-8. [DOI] [PubMed] [Google Scholar]
- Nielson CM, Flores R, Harris RB, et al. Human papillomavirus prevalence and type distribution in male anogenital sites and semen. Cancer Epidemiol Biomarkers Prev. 2007a;16:1107–1114. doi: 10.1158/1055-9965.EPI-06-0997. [DOI] [PubMed] [Google Scholar]
- Nielson CM, Harris RB, Dunne EF, et al. Risk factors for anogenital human papillomavirus infection in men. J Infect Dis. 2007b;196:1137–1145. doi: 10.1086/521632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson CM, Harris RB, Flores R, et al. Multiple-type human papillomavirus infection in male anogenital sites: prevalence and associated factors. Cancer Epidemiol Biomarkers Prev. 2009a;18:1077–1083. doi: 10.1158/1055-9965.EPI-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson CM, Schiaffino MK, Dunne EF, Salemi JL, Giuliano AR. Associations between male anogenital human papillomavirus infection and circumcision by anatomic site sampled and lifetime number of female sex partners. J Infect Dis. 2009b;199:7–13. doi: 10.1086/595567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriel JD. Natural history of genital warts. Br J Vener Dis. 1971;47:1–13. doi: 10.1136/sti.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S3/11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–1136. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- Pascual A, Pariente M, Godinez JM, et al. High prevalence of human papillomavirus 16 in penile carcinoma. Histol Histopathol. 2007;22:177–183. doi: 10.14670/HH-22.177. [DOI] [PubMed] [Google Scholar]
- Picconi MA, Eijan AM, Distefano AL, et al. Human papillomavirus (HPV) DNA in penile carcinomas in Argentina: analysis of primary tumors and lymph nodes. J Med Virol. 2000;61:65–69. doi: 10.1002/(sici)1096-9071(200005)61:1<65::aid-jmv10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rubin MA, Kleter B, Zhou M, et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159:1211–1218. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar EL, Mercado E, Calzada L. Human papillomavirus hpv-16 DNA as an epitheliotropic virus that induces hyperproliferation in squamous penile tissue. Arch Androl. 2005;51:327–334. doi: 10.1080/014850190923396. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Miles BJ, Plieth DH, Crissman JD. Detection of human papillomavirus in squamous neoplasm of the penis. J Urol. 1992;147:389–392. doi: 10.1016/s0022-5347(17)37245-2. [DOI] [PubMed] [Google Scholar]
- Scheurer ME, Tortolero-Luna G, Adler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15:727–746. doi: 10.1111/j.1525-1438.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- Stern RS. Genital tumors among men with psoriasis exposed to psoralens and ultraviolet A radiation (PUVA) and ultraviolet B radiation. The Photochemotherapy Follow-up Study. N Engl J Med. 1990;322:1093–1097. doi: 10.1056/NEJM199004193221601. [DOI] [PubMed] [Google Scholar]
- Svare EI, Kjaer SK, Nonnenmacher B, et al. Seroreactivity to human papillomavirus type 16 virus-like particles is lower in high-risk men than in high-risk women. J Infect Dis. 1997;176:876–883. doi: 10.1086/516505. [DOI] [PubMed] [Google Scholar]
- Tsen HF, Morgenstern H, Mack T, Peters RK. Risk factors for penile cancer: results of a population-based case–control study in Los Angeles County (United States) Cancer Causes Control. 2001;12:267–277. doi: 10.1023/a:1011266405062. [DOI] [PubMed] [Google Scholar]
- Vaccarella S, Lazcano-Ponce E, Castro-Garduno JA, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–1939. doi: 10.1002/ijc.21992. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Habel LA, Sherman KJ, McKnight B, Stergachis A, Daling JR. Risk factors for incident and recurrent condylomata acuminata among men. A population-based study. Sex Transm Dis. 1998;25:278–284. doi: 10.1097/00007435-199807000-00002. [DOI] [PubMed] [Google Scholar]
- Van Doornum GJ, Prins M, Juffermans LH, et al. Regional distribution and incidence of human papillomavirus infections among heterosexual men and women with multiple sexual partners: a prospective study. Genitourin Med. 1994;70:240–246. doi: 10.1136/sti.70.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doornum GJ, Korse CM, Buning-Kager JC, et al. Reactivity to human papillomavirus type 16 L1 virus-like particles in sera from patients with genital cancer and patients with carcinomas at five different extragenital sites. Br J Cancer. 2003;88:1095–1100. doi: 10.1038/sj.bjc.6600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepapeliere P, Barrasso R, Meijer CJ, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192:2099–2107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189:677–685. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- Wen LM, Estcourt CS, Simpson JM, Mindel A. Risk factors for the acquisition of genital warts: are condoms protective? Sex Transm Infect. 1999;75:312–316. doi: 10.1136/sti.75.5.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199:936–944. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- Wieland U, Jurk S, Weissenborn S, Krieg T, Pfister H, Ritzkowsky A. Erythroplasia of queyrat: coinfection with cutaneous carcinogenic human papillomavirus type 8 and genital papillomaviruses in a carcinoma in situ. J Invest Dermatol. 2000;115:396–401. doi: 10.1046/j.1523-1747.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- Wikstrom A, Popescu C, Forslund O. Asymptomatic penile HPV infection: a prospective study. Int J STD AIDS. 2000;11:80–84. doi: 10.1177/095646240001100203. [DOI] [PubMed] [Google Scholar]
- Wiley DJ, Douglas J, Beutner K, et al. External genital warts: diagnosis, treatment, and prevention. Clin Infect Dis. 2002;35:S210–S224. doi: 10.1086/342109. [DOI] [PubMed] [Google Scholar]


