Abstract
Aim of the study
Inflammatory cytokines have been implicated in the pathophysiology of post cardiac arrest syndrome, including myocardial dysfunction and hypotension, often leading to multi-organ system dysfunction and death. We hypothesized that administration of infliximab after return of spontaneous circulation (ROSC) would ameliorate hypotension and myocardial dysfunction and prolong survival.
Methods
Domestic swine were anesthetized and instrumented. Balloon occlusion of the LAD coronary artery just distal to the first septal perforator was performed and VF followed spontaneously in all animals. After 7 min,, chest compressions, defibrillation, and standard ACLS resuscitation was performed. Animals achieving ROSC (N=32) were randomized to receive infliximab (5 mg/kg, n=16) or vehicle (250 mL normal saline, n=16) immediately post-ROSC and survival and hemodynamics were monitored for 3 hours.
Results
There were no differences in prearrest hemodynamic variables, TNF-α levels, or resuscitation variables between groups. Both groups demonstrated a time dependent decline in mean arterial pressure (MAP) and stroke work (SW) post-ROSC with a nadir at 1 hour followed by recovery over hours 2 and 3. This decline was blunted in infliximab-treated swine (1-hour between group difference in MAP 21 mmHg, 95% CI 3–38 mmHg and SW 6.7 gm-m, 95% CI 0.4–13 at 1 hour). Left ventricular systolic dp/dt fell in the vehicle group (-437 mm Hg/sec, 95% CI -183 to -690) but did not in the infliximab group. Tau rose only in the vehicle group (44 msec, 95% CI 1–87). Short-term survival was higher in the infliximab group (Kaplan-Meier p = 0.022).
Conclusions
Blockade of TNF-α in the immediate post-ROSC period improved survival and hemodynamic parameters in this swine model of ischemic VF.
Keywords: cardiopulmonary resuscitation, ventricular fibrillation, post-resuscitation period, inflammatory response
INTRODUCTION
Emergency medical services (EMS) respond to approximately 300,000 out-of-hospital cardiac arrests (OOHCA) each year in the United States.1 A 5-fold variation in survival to hospital discharge following EMS-treated cardiac arrest was reported among the 10 municipalities reporting from the Resuscitation Outcomes Consortium.2 Several studies have demonstrated that post-cardiac arrest care is an important determinant of survival.3–6
Myocardial ischemia is a common cause of cardiac arrest, especially among patients with ventricular fibrillation (VF) as their initial arrest rhythm.7,8 Furthermore, ongoing ischemia likely contributes to post-cardiac arrest myocardial dysfunction in patients achieving return of spontaneous circulation (ROSC).9 Management of post-resuscitation myocardial dysfunction has focused on prevention and, more recently, on early intervention, particularly early percutaneous coronary intervention, following resuscitation.10.11 Optimal pharmacologic management has not been defined nor has a goal-directed approach been verified.9
An increase in blood proinflammatory cytokines have been reported following resuscitation and plasma levels of TNF-α have been shown to be inversely correlated with myocardial function.12,13 Using an electrical model of VF, we previously demonstrated that early administration of infliximab, an anti-TNF-alpha monoclonal antibody, following the return of spontaneous circulation (ROSC) in swine, ameliorates subsequent myocardial dysfunction.14 However, in this swine arrest model, post-arrest cardiac dysfunction is brief and moderate in severity.15 The purpose of this study was to determine if early blockade of TNF-alpha would prevent early post-cardiac arrest death and attenuate myocardial dysfunction following resuscitation from ischemically induced VF, a more clinically relevant arrest model with more profound post-resuscitation hemodynamic failure.
METHODS
This investigation was approved by the Animal Care and Utilization Review Committee of our institution and adheres to the American Physiological Society’s Guiding Principles in the Care and Use of Animals.
Male domestic swine (Yorkshire and Yorkshire/Hampshire crossbreed) three to four months of age (mean weight 38 ± 5 kg) were premedicated with IM ketamine (20 mg/kg) and xylazine (2 mg/kg). General anesthesia was induced with isoflurane via nose cone and, following endotracheal intubation, maintained with inhaled isoflurane (MAC 1.0–2.5%) and nitrous oxide in a 1 to 1 mixture with oxygen. End-tidal CO2 was continuously monitored via side-stream capnography and minute ventilation was adjusted to maintain end-tidal CO2 at 35–45 mm Hg. Standard lead II of the surface ECG was monitored continuously during instrumentation and throughout the study protocol.
Under fluoroscopic guidance, high fidelity, micro-manometer tipped catheters (Millar Instruments, Houston, TX) were positioned in the ascending aorta and left ventricle (LV) via the femoral arteries and in the right atrium (RA) via a jugular vein. A thermistor-tipped catheter (Edwards Lifesciences, Irvine, CA) was positioned in a branch of the pulmonary artery for thermodilution cardiac output (CO) determinations. Commercially available, standard adhesive defibrillation electrode patches were applied to the left and right lateral aspects of the shaved thorax. Transthoracic impedance was measured using a tetrapolar constant current impedance measuring system (THRIM®, Morro Bay, CA). A small value non-inductive resistor (30) was then placed in series with the truncated exponential biphasic defibrillation waveform defibrillator (LifePak 12, Medtronic Emergency Response Systems, Redmond, WA).
Following instrumentation, heart rate, systolic and diastolic aortic and LV pressure, mean RA pressure, LV systolic (max) and diastolic (min) dP/dt, and CO were recorded and arterial blood was analyzed (I-Stat CG8+, I-Stat Corp, Princeton, NJ). Mean arterial pressure (MAP), stroke volume (SV), and LV stroke work were derived using standard formulae.
Using a 6 French HS-1 guiding catheter inserted via a carotid artery, a 4 mm × 20 mm angioplasty catheter (Abbott Vascular, Temecula CA) was positioned over a standard 0.014 coronary wire in the left anterior descending (LAD) coronary artery distal to the first septal perforator. The balloon was then inflated to 6–8 atms. All occlusions were performed by experienced interventionists. The time from guide insertion to LAD occlusion was typically <15 min and <20 ml of nonionic contrast was used. The site of coronary occlusion and confirmation of complete cessation of coronary flow distal to the balloon were confirmed with manual contrast injections. Balloon position was confirmed at necropsy.
Following the occurrence of ischemically induced spontaneous VF, animals were observed and untreated for 7 min. After 7 min, manual chest compressions were begun at a rate of approximately 100/min with force sufficient to depress the sternum 1.5 to 2.0 inches (determined visually) with the animal in the supine position. The occluding balloon remained inflated throughout resuscitative efforts. One minute after starting chest compressions, a transthoracic countershock at 200 J was given. For the purpose of these experiments, successful defibrillation was defined as termination of VF, regardless of the postshock cardiac rhythm or hemodynamic outcome, e.g., spontaneous QRS complexes with or without associated arterial pressure pulses, determined 5 sec after a defibrillation shock. If VF persisted, additional shocks in an escalating energy sequence (300, 360J) were administered. Chest compressions were performed between shocks and positive pressure ventilations (FiO2=1.00) were performed at a rate of 8 ventilations/min. If VF persisted after the initial three shocks, adrenaline, 1 mg, was administered and CPR continued for one to three minutes before additional shocks at 360 J were given. If VF was refractory or recurrent after the first three shocks, additional adrenaline and amiodarone, 150 mg, were administered and additional countershocks at 360 J were delivered every 2 minutes. Adrenaline was repeated every 3–5 minutes as needed for persistent or recurrent VF or if shocks resulted in pulseless electrical activity (PEA) or asystole. At the end of 30 minutes, animals remaining in VF, PEA, or asystole were considered resuscitation failures and efforts terminated.
For the purposes of these experiments, return of spontaneous circulation (ROSC) was defined as an arterial systolic blood pressure (SBP) of at least 60 mm Hg for > 2 minutes. Animals achieving ROSC immediately received either infliximab, an anti-TNF-alpha monoclonal antibody (5 mg/kg in 250 mL of normal saline, n = 16), or control (250 mL of NS, n =16), each to be infused through the RA catheter and administered over 30 minutes. Animals were randomized to groups via permuted block design and rescuers were blinded to treatment allocation. Animals were monitored hemodynamically throughout the post-ROSC period and dopamine was administered as an infusion if the SBP fell below 60 mm Hg for >10 minutes and was titrated to maintain an SBP > 90 mm Hg. Antiarrhythmic drugs were not administered after initiation of the control or infliximab occlusion. The angioplasty balloon remained inflated for the first 60 minutes of the post-ROSC period and was thereupon deflated and withdrawn. Death during the post-ROSC period and during treatment was defined as the occurrence of refractory hypotension with a systolic arterial pressure <50 mm Hg sustained for >10 min despite an additional dose of adrenaline and dopamine infusion at a rate >20 µg/kg/min or development of asystole. The majority of early deaths were the result of refractory PEA. In stable animals, hemodynamic and blood gas measurements were made at intervals for 3 hours. All animals were necropsied at the end of experiments. The site of balloon occlusion and the absence of coronary dissection were confirmed. Gross inspection of the intrathoracic and intraabdominal contents was also performed to determine if chest compression related organ injury was present.
Prior to LAD balloon inflation in all animals and at 15, 30, 60 min following restoration of circulation and at hourly intervals thereafter in surviving animals, arterial blood was sampled, placed in sterile, chilled (0° C), heparinized tubes, and centrifuged at 5000 rpm for 10 min. Plasma was immediately separated and stored at −80° C until analysis. TNF-α concentrations were determined by a quantitative sandwich ELISA using commercially available kits specific for this porcine cytokine (R&D Systems, Inc., Minneapolis, MN).
Data Analysis
Data were entered into an Excel Spreadsheet (v. 12.0, Microsoft Corp, Redmond, WA) and imported into SAS statistical software (v. 9.2, SAS Institute,Cary, NC) and SigmaStat 3.1 (Systat Software Inc, San Jose, CA) for analysis. Summary measures of normally distributed variables are reported as means and standard deviations (SD). Non-normally distributed variables are reported as medians and interquartile ranges (IQR). Means of normally distributed variables were compared using Student’s t-test. Mann-Whitney U test was performed to compare distributions of non-normally distributed variables. Kaplan-Meier survival plots were used to assess differences in short term survival between groups. Generalized linear mixed models were constructed to compare hemodynamic trajectories during the post-ROSC period between groups. The SAS procedure PROC MIXED was used for these analyses with time entered into the model as a random effect and the treatment assignment as a fixed effect with the assumption of an unstructured covariance matrix. All p values are two sided. When comparing post-ROSC values to controls values within treatment assignments, p values were adjusted using the simulate option in the LSMEANS statement.
RESULTS
Thirty-two animals achieved ROSC and were allocated to the infliximab group (n = 16) or to the control group (n = 16). Significant differences were not observed between with respect to prearrest hemodynamic variables (Table 1). Resuscitation variables between study groups were likewise not statistically different (Table 2).
Table 1.
Pre-arrest hemodynamic variables for the study groups
| Control (n = 16) | Infliximab (n = 16) | |
|---|---|---|
| Weight (kg) | 39 ± 5 | 40 ± 3 |
| Heart Rate (beats/min) | 99 ± 13 | 94 ± 14 |
| MAP (mm Hg) | 94 ± 9 | 90 ± 11 |
| LV EDP (mm Hg) | 8 ± 3 | 7 ± 3 |
| Stroke Work (gm-m) | 47 ± 12 | 48 ± 7 |
| Systolic dp/dt | 994 ± 130 | 1100 ± 202 |
| Tau (msec) | 37 ± 6 | 33 ± 4 |
| TNF-α (pg/ml) | 85 ± 77 | 99 ± 51 |
Values are mean (±SD).
Table 2.
Resuscitation variables for the study groups
| Transthoracic Impedance (Ω) |
Time to VF (sec) |
Time to ROSC (sec) |
Countershocks to 1st defib |
Adrenaline dose (mg) |
|
|---|---|---|---|---|---|
| Control | 71 ± 8 | 1489 ± 240 | 455 ± 228 | 3 ± 2 | 2 ± 1 |
| Infliximab | 69 ± 7 | 1535 ± 326 | 421 ± 229 | 4 ± 3 | 2 ± 1 |
Values are mean (±SD).
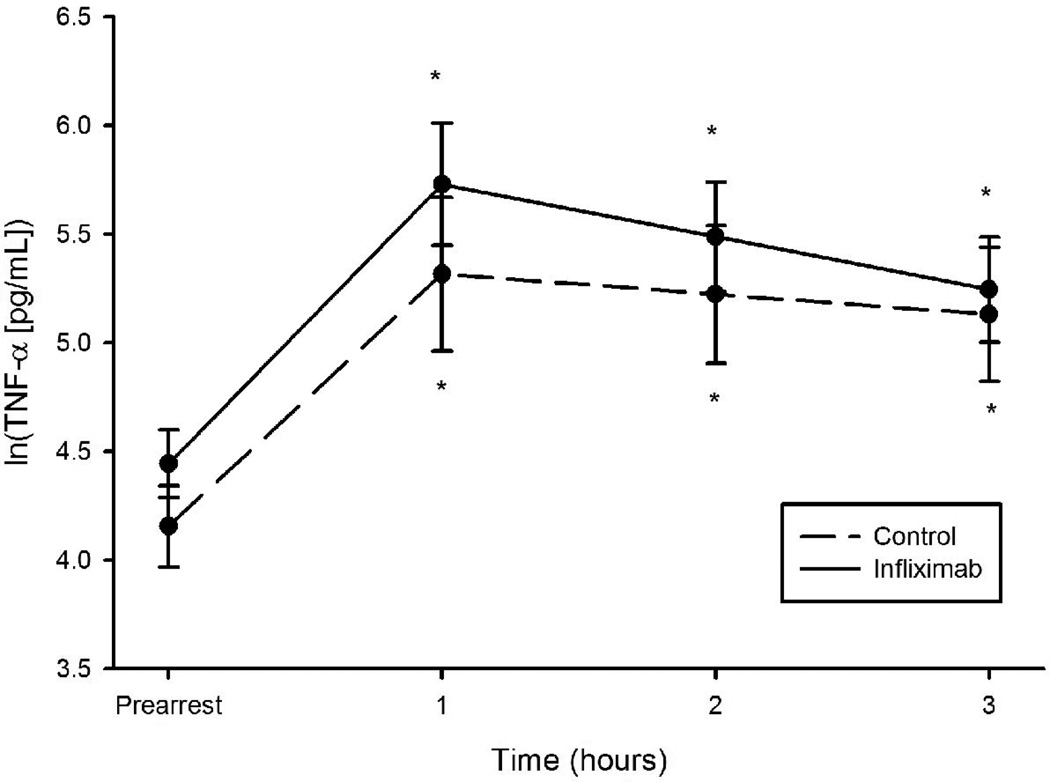
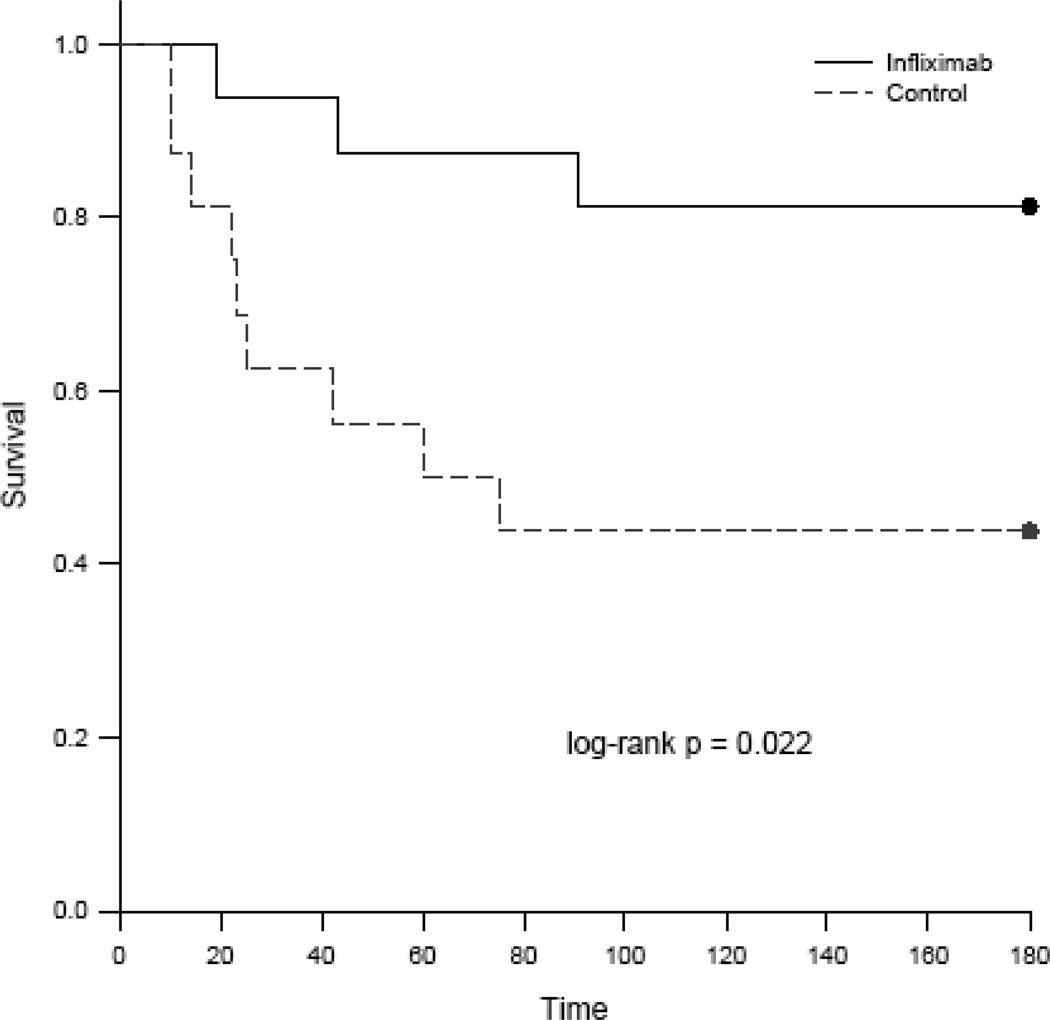
A significant increase in plasma TNF-α concentrations was observed in both groups within 30 min of ROSC and peaked at 1 hr (figure 1). Elevated TNF-α levels were sustained throughout the 3 hour post-resuscitation observation period and were not statistically different between the treatment groups. Survival was 13/16 (81.3%) in the infliximab group and 7/16 (43.8%) in the control group at 3 hours post-ROSC. There was early divergence of the survival plots with all deaths occurring within the first 100 minutes of the post arrest period (figure 2).
Figure 1.
Mean natural log transformed values for TNF-alpha levels between animals administered infliximab and control animals in a swine model of ischemic VF. * Adjusted p<0.05 for comparison with pre-arrest values.
Figure 2.
Kaplan-Meier survival plots for the comparison of short-term survival between study groups following return of spontaneous circulation in the ischemic VF model in swine.
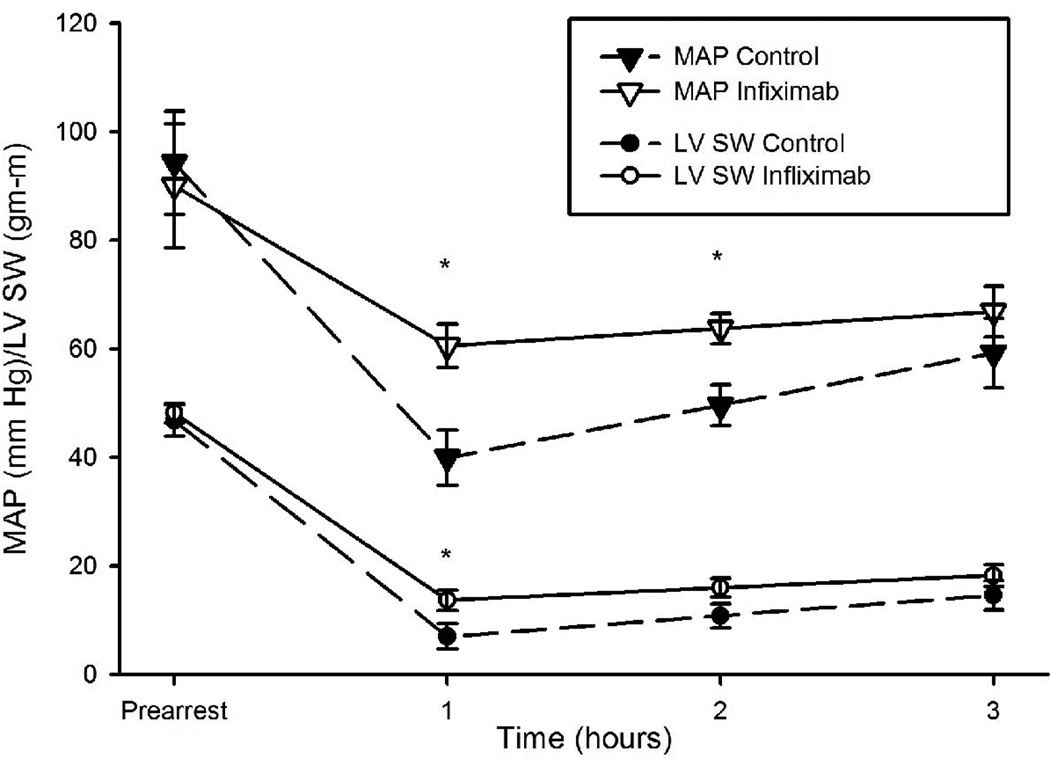
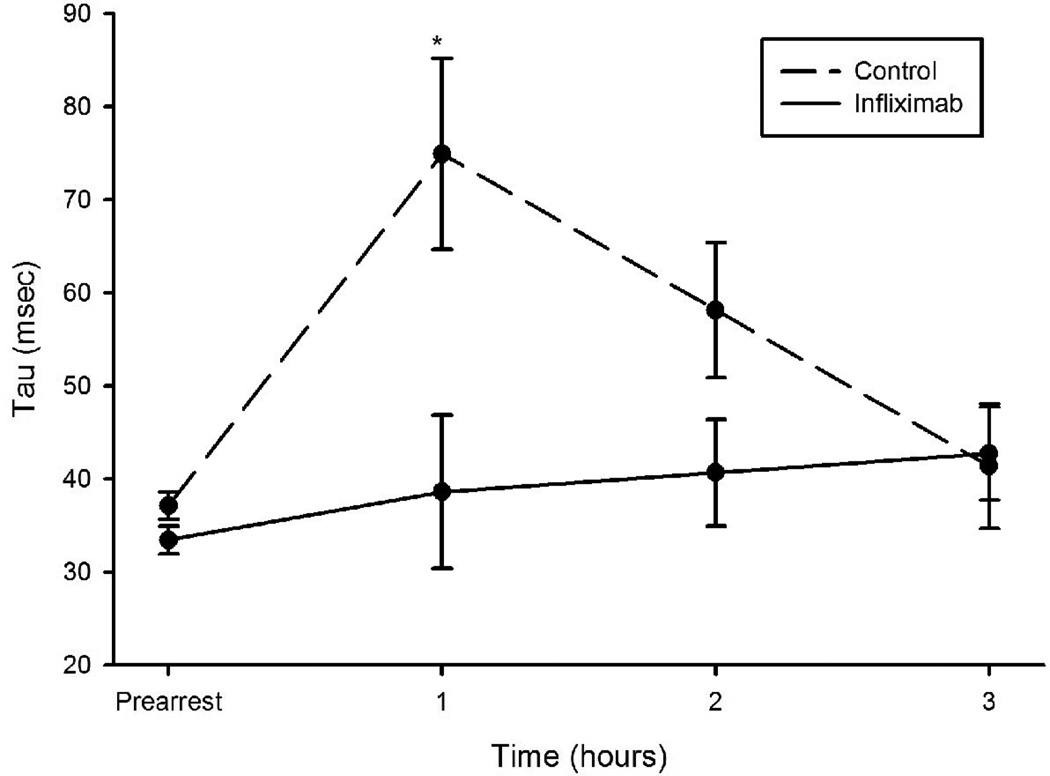
Both groups demonstrated a time dependent decline in mean arterial pressure (MAP) and stroke work (SW) post-ROSC with a nadir at 1 hour followed by recovery over hours 2 and 3 (figures 3 and 4). This decline was blunted in infliximab-treated swine (1-hour between group difference in MAP of 21 mmHg, 95% CI 3–38 and difference in SW of 6.7 gm-m, 95% CI 0.4–13 gm-m). The time derivative of LV pressure, dp/dt, fell in the control group over the first hour post-ROSC (-437 mm Hg/sec, 95% CI -183 to -690, p=0.0017) but did not in the infliximab group (between group difference at 1 hour -549 mm Hg/sec, 95% CI -1099 to 0.83, p=0.0503). Tau, the time constant for isovolumic relaxation, rose only in the control group over the first hour (average increase of 44 msec, 95% CI 1–87, p=0.04) with a higher value than the infliximab group by, on average, 36 msec (95% CI 9–64 msec, p=0.01) at 1 hour.
Figure 3.
Mean arterial pressure (MAP) and stroke work (SW) measured during the first three hours post-ROSC in animals administered infliximab or placebo. *p<0.05 for differences between groups.
Figure 4.
Tau measured during the first three hours post-ROSC in animals administered infliximab or placebo. *p<0.05 for differences between groups.
Following balloon deflation and reperfusion 1 hour after ROSC, there was a statistically significant increase in MAP and decrease in tau over the ensuing two hours in placebo treated animals. Similar changes were not observed in the infliximab treated group. Two hours after balloon deflation (3 hrs after ROSC) stroke work had significantly increased from nadir values in both groups.
DISCUSSION
We have demonstrated that, in a swine model of ischemic VF, immediate administration of a monoclonal antibody directed against TNF-α improves short-term survival in the early post cardiac arrest period. Consistent with our prior work in the electrical model of VF, infliximab also attenuates myocardial dysfunction and hypotension during this period. In this model, an abrupt and significant increase in TNF-α was observed within 30 min of resuscitation and plasma levels were persistently elevated during the 3 hr post-resuscitation observation period.
The vast majority of patients resuscitated from out-of-hospital cardiac arrest die during subsequent hospitalization, with in-hospital mortality rates exceeding 70% in most reports.16,17 The ultimate cause of death is generally related to central nervous system, myocardial, or multiorgan system failure. The components of the post-resuscitation syndrome have been enumerated and include post-cardiac arrest brain injury, post-resuscitation myocardial dysfunction, a systemic ischemia/reperfusion response, and persistent precipitating pathology.9
Post-resuscitation myocardial dysfunction has been observed in the laboratory and in the clinical population. It is likely multifactorial in origin and contributing factors have been described and include ischemic injury with “stunning”18, electrical injury due to countershocks if ventricular fibrillation is encountered during resuscitation19, and drug administration, particularly epinephrine20, during resuscitation efforts. Myocardial dysfunction is likely to be more pronounced if acute coronary occlusion caused the cardiac arrest and evolves to myocardial infarction and necrosis. There is also evidence that a systemic inflammatory response follows resuscitation and is characterized by an increase in circulating inflammatory cytokines.13,13,21 Cytokines, particularly TNF-α, are known to cause myocardial depression by altering calcium homeostasis in myocytes.22
In this study, administration of infliximab immediately after resuscitation from ischemically induced VF significantly improved short-term (3 hour) survival and improved both systolic and diastolic myocardial function (stroke work, arterial pressure, and isovolumic relaxation). The hemodynamic differences between groups were similar to, but not as pronounced as, differences observed in an electrically induced VF swine model. Less dramatic improvement in cardiac function when models and therapy are compared is likely the result of differences in the time to ROSC, the magnitude of the TNF-α inflammatory response, and persistent ischemia after ROSC due to continued coronary occlusion for 60 min. This duration of occlusion was selected to approximate an achievable door-to-balloon time in the clinical cardiac arrest and resuscitation scenario. Persistent occlusion of the LAD after resuscitation represents a persistent precipitating pathology, one of the key components of the post-resuscitation syndrome not replicated in the electrically-induced VF model.
This study has several limitations. We used only male swine because previous work has demonstrated that the post-resuscitation inflammatory response in males is more robust than that observed in female swine of similar age.21 The anesthetics used in this study may affected pre-arrest and post-resuscitation cardiac function and possibly the innate inflammatory response.23 However, the anesthetic regimen was the same for the two treatment groups. Although time to restoration of spontaneous circulation and number of countershocks were not statistically different between the control and infliximab group, small differences may have been physiologically significant. We evaluated only one dose of infliximab in the study. However, this dose (5 mg/kg) was previously shown to be effective in an electrically-induced VF swine cardiac arrest model.14 We did not evaluate the potential role that other cytokines in the genesis of post-resuscitation myocardial dysfunction nor the effect of other cytokine inhibitors. IL-1β is an inflammatory cytokine with myocardial depressant properties and may have contributed to the observed changes in myocardial function after resuscitation.24 Lastly, although consistency of the site of coronary occlusion in the proximal LAD was confirmed angiographically and at autopsy, differences in infarct size may have existed between animals. However, the swine heart has predominately an end-artery coronary anatomy with minimal innate collateralization.25 Thus, the area at risk is likely to have been consistent across animals. The study model incorporating a 3 hour post-resuscitation observation period following ischemia as well as reperfusion does not lend itself to infract sizing with biomarkers or imaging.26,27
The findings of this investigation lend additional support to the potential role that TNF-α may play in the cardiac dysfunction that typifies the post-resuscitation syndrome. TNF-α blockade has now been shown to attenuate the decline in myocardial function following resuscitation in two different swine models of cardiac arrest. The occlusion induced, ischemically mediated model of VF more closely replicates the clinical scenario of cardiac arrest and is associated with a more profound ischemic insult to the myocardium and systemic inflammatory response. Infliximab and other cytokine blockers or immune mediators may be useful adjuncts in the management of post-resuscitation cardiogenic shock.
Acknowledgments
Funding Source: Funded, in part, by a grant from the NIH NHLBI R01 HL076671.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented, in part, at the Annual Meeting of the American Heart Association, Orlando, FL, November, 2011.
Conflict of Interest: The authors have no conflicts of interest.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics� 2011 Update. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr B, Goyal M, Band R, et al. A national analysis of the relationship between hospital factors and post-cardiac arrest mortality. Intens Care Med. 2009;35:505–511. doi: 10.1007/s00134-008-1335-x. [DOI] [PubMed] [Google Scholar]
- 4.Liu JM, Yang Q, Pirrallo RG, Klein JP, Aufderheide TP. Hospital variability of out-of-hospital cardiac arrest survival. Prehospital Emergency Care. 2008;12:339–346. doi: 10.1080/10903120802101330. [DOI] [PubMed] [Google Scholar]
- 5.Cronier P, Vignon P, Bouferrache K, et al. Impact of routine percutaneous coronary intervention after out-of-hospital cardiac arrest due to ventricular fibrillation. Critical Care. 2011;15:R122. doi: 10.1186/cc10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stub D, Hengel C, Chan W, et al. Usefulness of cooling and coronary catheterization to improve survival in out-of-hospital cardiac arrest. Am J Cardiol. 2011;107:522–527. doi: 10.1016/j.amjcard.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Spaulding CM, Joly L, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 8.Gorjup V, Radsel P, Kocjancic ST, Erzen D, Noc M. Acute ST-elevation myocardial infarction after successful cardiopulmonary resuscitation. Resuscitation. 2007;72:379–385. doi: 10.1016/j.resuscitation.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Neumar RW, Nolan JP, Adrie C, et al. Post cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 10.Gazmuri RJ, Ayoub IM, Kolarova J. Myocardial protection during resuscitation from cardiac arrest. Curr Opin Crit Care. 2003;9:199–204. doi: 10.1097/00075198-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Zia A, Kern KB. Management of postcardiac arrest myocardial dysfunction. Curr Opin Crit Care. 2011;17:241–246. doi: 10.1097/MCC.0b013e3283447759. [DOI] [PubMed] [Google Scholar]
- 12.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 13.Niemann JT, Garner D, Rosborough JP, Lewis RJ. Tumor necrosis factor- alpha is associated with early post-resuscitation myocardial dysfunction. Crit Care Med. 2004;32:1753–1758. doi: 10.1097/01.ccm.0000132899.15242.d3. [DOI] [PubMed] [Google Scholar]
- 14.Niemann JT, Youngquist S, Rosborough JP, Shah AP, Phan QT, Filler SG. Infliximab attenuates early myocardial dysfunction after resuscitation in a swine cardiac arrest model. Crit Care Med. 2010;38:1162–1167. doi: 10.1097/CCM.0b013e3181d44324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemann JT, Rosborough JP, Youngquist S, Thomas J, Lewis RJ. Is all ventricular fibrillation the same? A comparison of ischemically induced with electrically induced ventricular fibrillation in a porcine cardiac arrest and resuscitation model. Crit Care Med. 2007;35:1356–1361. doi: 10.1097/01.CCM.0000261882.47616.7D. [DOI] [PubMed] [Google Scholar]
- 16.Steill IG, Wells GA, Field B, et al. Ontario Prehospital Advanced Life Support Study Group: Advanced cardiac life support in the out-of-hospital cardiac arrest. N Engl J Med. 2004;351:647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 17.Nolan JP, Laver SR, Welch CA, Harrison DA, Gupta V, Rowan K. Outcome following admission to UK intensive care units after cardiac arrest: A secondary analysis of the ICNARC Case Mix Programme Database. Anaesthesia. 2007;62:1207–1216. doi: 10.1111/j.1365-2044.2007.05232.x. [DOI] [PubMed] [Google Scholar]
- 18.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: An example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–240. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 19.Walcott GP, Killingsworth CR, Ideker RE. Do clinically relevant transthoracic defibrillation energies cause myocardial damage and dysfunction? Resuscitation. 2003;59:59–70. doi: 10.1016/s0300-9572(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 20.Tang W, Weil MH, Sun S, Noc M, Yang L, Gazmuri RJ. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92:3089–3093. doi: 10.1161/01.cir.92.10.3089. [DOI] [PubMed] [Google Scholar]
- 21.Niemann JT, Rosborough JP, Youngquist S. Is the tumor necrosis factor- alpha response following resuscitation gender dependent in the swine model? Resuscitation. 2008;77:258–263. doi: 10.1016/j.resuscitation.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Menyar AA. Cytokines and myocardial dysfunction: State of the Art. J Card Fail. 2008;14:61–74. doi: 10.1016/j.cardfail.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson G, Hall GM. Effects of anesthesia on the inflammatory response to injury. Curr Opin in Anesth. 2011;24:370–374. doi: 10.1097/ACO.0b013e328348729e. [DOI] [PubMed] [Google Scholar]
- 24.Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15:392–397. doi: 10.1097/MCC.0b013e3283307a4e. [DOI] [PubMed] [Google Scholar]
- 25.Heusch G, Skyschally A, Schylz R. The in-situ pig heart with regional ischemia/reperfusion—Ready for translation. J Mol Cell Cardiol. 2011;50:951–963. doi: 10.1016/j.yjmcc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons RJ, Valeti US, Araoz PA, Jaffee AS. The quantification of infarct size. J Am Coll Cardiol. 2004;44:1533–1542. doi: 10.1016/j.jacc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 27.Botker HE, Kaltoft AK, Pedersen SF, Kin WY. Measuring myocardial salvage. Cardiovasc Res. doi: 10.1093/cvr/cvs081. epub ahead of print. [DOI] [PubMed] [Google Scholar]