Abstract
Although neuroscience has made remarkable progress in understanding the involvement of prefrontal cortex in human memory, the necessity of dorsolateral prefrontal cortex (dlPFC) for key competencies of working memory remains largely unexplored. We therefore studied human brain lesion patients to determine whether dlPFC is necessary for working memory function, administering subtests of the Wechsler Memory Scale, the Wechsler Adult Intelligence Scale, and the N-Back Task to three participant groups: dlPFC lesions (n = 19), non-dlPFC lesions (n = 152), and no brain lesions (n = 54). DlPFC damage was associated with deficits in the manipulation of verbal and spatial knowledge, with left dlPFC necessary for manipulating information in working memory and right dlPFC critical for manipulating information in a broader range of reasoning contexts. Our findings elucidate the architecture of working memory, providing key neuropsychological evidence for the necessity of dlPFC in the manipulation of verbal and spatial knowledge.
Keywords: working memory, prefrontal cortex, dorsolateral prefrontal cortex, lesion data
INTRODUCTION
Working memory comprises a system for maintaining, monitoring and manipulating information in short-term memory, providing the interface between perception, long-term memory and action that enables goal-directed behavior (Baddeley, 1998; Baddeley and Petrides, 1996). Although cognitive neuroscience has made remarkable progress in understanding the involvement of the prefrontal cortex (PFC) in human memory, fundamental questions remain regarding the functional organization of the PFC with respect to working memory. One unresolved issue concerns whether subregions within the lateral PFC mediate functionally distinct processes or instead serve a common role in working memory. Anatomically, the lateral PFC consists of multiple subregions that differ in cytoarchitecture and connectivity (Petrides et al., 2012), raising the possibility that these subregions may guide goal-directed behavior through different mechanisms.
A seminal and longstanding debate in cognitive neuroscience has examined this issue, investigating alternative models for understanding the functional organization of the lateral PFC and its role in working memory. Domain-general models posit that the lateral PFC is functionally organized according to the type of working memory operations engaged, with the dorsolateral PFC (dlPFC) embodying computational mechanisms for monitoring and manipulating items in working memory (Owen et al., 1996; Duncan and Owen, 2000; Miller and Cohen, 2001; Koechlin et al., 2003; Petrides, 2000, 2005; Petrides et al., 2012). Monitoring operations are thought to support the active retention of information in working memory and computational mechanisms for manipulating items are recruited for updating (Petrides, 2000) or selecting between these representations (Rowe et al., 2000). In contrast, domain-specific models posit that the lateral PFC is functionally organized according to the domain of information processed. Advocates of this framework propose that dlPFC is functionally specialized to process visuospatial information in working memory, enabling mental representations of coordinates within the spatial domain (Awh et al., 1995; Butters and Pandya, 1969; Butters et al., 1971; Butters et al., 1972; Courtney et al., 1998; Courtney et al., 1996, 1997; Goldman-Rakic, 1995; Levy and Goldman-Rakic, 1999; Smith and Jonides, 1999).
The empirical case advanced in support of each model of dlPFC function has relied primarily upon (1) lesion studies in non-human primates demonstrating reliable deficits in working memory due to unilateral dlPFC lesions (Butters and Pandya, 1969; Butters et al., 1971; Butters et al., 1972; Jacobsen and Nissen, 1937; Levy and Goldman-Rakic, 1999) and (2) functional neuroimaging studies in humans reporting activity within the dlPFC for tests of working memory (for meta-analytic reviews, see (Owen et al., 2005; Wager et al., 2004; Wager and Smith, 2003). Two key findings from studies of non-human primates performing delayed response tasks suggest a crucial role for the dlPFC in working memory. First, experimental lesions of the principal sulcus in the dlPFC cause delay-dependent impairments, whereby forgetting increases with the length of the delay (Miller and Orbach, 1972; Bauer and Fuster, 1976; Funahashi et al., 1993). Second, neurophysiological unit recordings from the dlPFC often show persistent, sustained levels of neuronal firing during the retention interval of delayed response tasks (Funahashi et al., 1989; Fuster and Alexande.Ge, 1971; Kubota and Niki, 1971). This sustained activity is thought to provide a bridge between the stimulus cue (e.g., the location of a flash of light) and its contingent response (e.g., a saccade to the remembered location). Such data established a strong link implicating the dlPFC as a crucial node supporting working memory.
Conclusions drawn from these literatures, however, are characterized by the following well-known limitations. First, the precise localization of working memory functions cannot be directly transposed from monkeys to humans due to significant interspecies macroscopic anatomical differences (Petrides et al., 2012). Second, functional neuroimaging (fMRI) studies apply correlational methods and therefore cannot formally demonstrate whether dlPFC is necessary for working memory or instead serves an accessory role (Sarter et al., 1996). As a consequence, the precise localization of working memory function in humans and the contribution of dlPFC to the neural systems underlying working memory remain controversial.
In recent years, lesion studies in humans (Baldo and Dronkers, 2006a; D'Esposito and Postle, 1999; D'Esposito et al., 2006; Muller et al., 2002; Ptito et al., 1995; Tsuchida and Fellows, 2009; Volle et al., 2008) and repetitive transcranial magnetic stimulation (rTMS) experiments (Hamidi et al., 2009; Hamidi et al., 2008; Koch et al., 2005; Postle et al., 2006) have provided key evidence to inform the debate. Human lesion and rTMS research is able to overcome the methodological limitations of earlier non-human primate and functional neuroimaging studies by investigating the anatomical localization of working memory functions in the human brain (Rorden and Karnath, 2004) and evaluating the necessity of the dlPFC for specific components of working memory.
Findings from the contemporary literature, however, have been equivocal, with some investigators reporting specific patterns of working memory deficits (Baldo and Dronkers, 2006b; Mottaghy et al., 2002; Ptito et al., 1995; Tsuchida and Fellows, 2009; Volle et al., 2008) and others failing to observe reliable impairment (D'Esposito and Postle, 1999; D'Esposito et al., 2006; Hamidi et al., 2008; Koch et al., 2005; Muller et al., 2002). Difficulty interpreting the theoretical significance of these findings has resulted from (1) the often diffuse (rather than focal) lesions observed, (2) the lack of comparison subjects carefully matched for pre- and post-injury performance measures, and (3) the limited scope of working memory functions examined. The absence of such data represents a substantial gap in the understanding of both dlPFC function and the neural substrates of working memory. Here, we characterize key competencies of working memory function in a sample of patients with focal brain lesions involving dlPFC.
MATERIALS AND METHODS
Participant Data
We drew brain-injured participants from the Vietnam Head Injury Study (VHIS) registry, which includes American veterans who suffered brain damage from penetrating head injuries in the Vietnam War (n = 199), as well as neurologically healthy Vietnam veterans (n = 54). The VHIS has been organized in three phases. Phase 1 (1967–1970) was the initial enrollment; Phase 2 (1981–1984) included a cognitive evaluation; and Phase 3 (2003–2006) included a more comprehensive evaluation as well as CT brain imaging. Further details regarding the VHIS participants, including methods for visualizing and quantifying brain lesions, have previously been reported (Barbey et al., 2012; Barbey et al., 2011). Subjects were eligible for the present study if they participated in Phases 2 and 3 evaluations.
To preclude the possibility that impaired performance on working memory and executive function tests could be secondary to deficits in the production and/or comprehension of language, we excluded any participant who had significant impairment on a neuropsychological test of language comprehension and production (i.e., defined as performance at least two standard deviations below the mean of the neurologically healthy group on the Boston Naming Test). From the remaining brain-injured veterans we selected those with significant damage to dlPFC (Brodmann's area 9/46) in the left and/or right hemisphere(s) (dlPFC Lesion Group; Fig. 1; n = 19). The dlPFC is located on the lateral and dorsal part of the medial convexity of the frontal lobe and comprises BA 9 and 46 and a few transitional areas: 9-8, 9-45, 46-10, and 46-45 (for a detailed description of anatomical boundaries, see (Rajkowska and Goldman-Rakic, 1995b, a). In addition, we investigated a comparison group of brain-injured veterans whose damage did not involve dlPFC or the superior parietal lobe, a cortical region necessary for certain aspects of working memory (Non-dlPFC Lesion group; Supplemental Fig. 1; n = 152; (Koenigs et al., 2009). As Supplemental Figure 1 illustrates, the greatest area of lesion overlap within the non-dlPFC sample entailed the ventral portion of the medial prefrontal cortex (below the level of the genu of the corpus callosum) and medial portion of the orbital surface (approximately the medial one-third of the orbitofrontal cortex in each hemisphere) as well as the subjacent white matter. Neurologically healthy veterans served as an additional comparison group (No Lesion group; n = 54). Demographic and background cognitive function data for the three groups are presented in Table 1. No significant group differences were observed with respect to basic demographic variables (age, sex, years of education), pre- and post-combat measures of cognitive function, post-combat measures of verbal IQ and verbal comprehension, and total percent volume loss. All patient groups were therefore well matched with respect to (1) basic demographic variables, (2) pre- and post-combat measures of cognitive function and (3) lesion size.
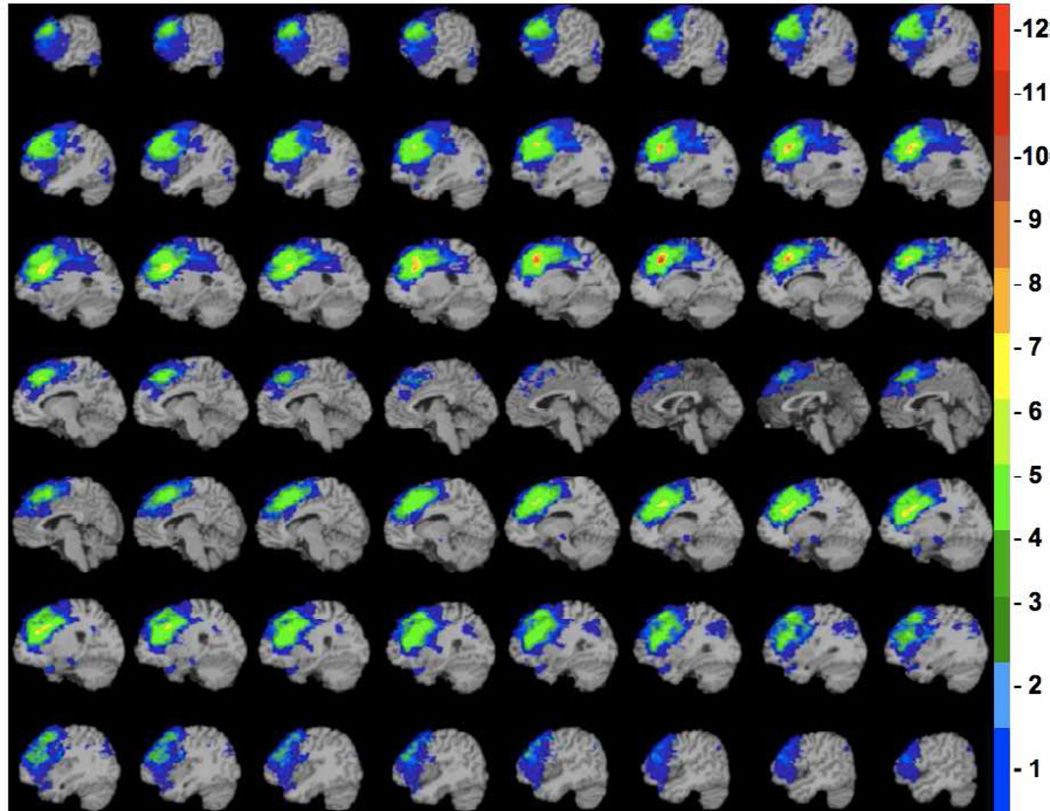
Figure 1.
Diagram of the lesion overlap map for the dorsolateral prefrontal patients. The color indicates the number of veterans in the left dorsolateral prefrontal group (n = 19) with damage to a given voxel. The greatest lesion overlap (red) occurred in the dorsolateral prefrontal cortex (BA 9). The depicted sagittal slices progress from the midline (top left) to lateral regions of the left hemisphere (bottom right).
Table 1.
Demographic and background data
| Demographic Data | DLPFC | NonDLPFC | NO LESION | ANOVA F value |
ANOVA p value |
Significant between-group differences |
|---|---|---|---|---|---|---|
| Age | 58.68 (2.75) | 58.88 (3.20) | 59.52 (3.42) | 0.91 | 0.41 | none |
| Sex (% male) | 100 | 100 | 100 | n/a | n/a | none |
| Years of Education | 14.74 (2.83) | 14.93 (2.53) | 15.19 (2.47) | 0.3 | 0.74 | none |
| Pre-combat AFQT | 54.83 (27.75) | 62.40 (24.78) | 65.40 (22.91) | 1.09 | 0.34 | none |
| Post-combat AFQT | 59.61 (24.20) | 67.82 (22.81) | 72.34 (22.99) | 1.77 | 0.17 | none |
| Post-combat Verbal IQ | 102.84 (16.50) | 106.89 (12.38) | 109.87 (12.38) | 2.16 | 0.12 | none |
| Post-combat Verbal Comprehension | 105.00 (17.53) | 108.42 (13.60) | 109.66 (12.04) | 0.82 | 0.44 | none |
| Total Percent Volume Loss (cm3)* | 3.11 (1.98) | 2.70 (3.59) | n/a | 0.49 | 0.62 | none |
Data are presented as means with standard deviations in parentheses. “Age” refers to age at the time of Phase 3 evaluation. “Sex” refers to the percent of male veterans. “Years of Education” refers to the total number of years of education the veterans completed. “Pre-combat AFQT” refers to index scores on the Armed Forces Qualification Test, a battery of tests measuring basic cognitive function at the time of enlistment (pre-injury). “Post-combat AFQT” refers to index scores on the Armed Forces Qualification Test administered at Walter Reed Medical Center after injury. “Post-combat Verbal IQ” refers to the Phase 3 Verbal IQ Index score from the Wechsler Adult Intelligence Scale. “Post-combat Verbal Comprehension” refers to the Phase 3 Verbal Comprehension Index score from the Wechsler Adult Intelligence Scale. Significant between-group differences were determined with the Tukey’s honestly significant difference test.
An independent samples t-test was conducted (rather than an ANOVA) to determine significant between group differences for the DLPFC and NonDLPFC patient groups. The respective values represent the t score and the associated p value.
Lesion Analysis
We acquired computed tomography (CT) data during the Phase 3 testing period. Axial CT scans without contrast were acquired at the Bethesda Naval Hospital on a General Electric Medical Systems Light Speed Plus CT scanner in helical mode. We reconstructed the images with an in-plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm and a 1-mm slice interval. We determined lesion location and volume from CT images using the Analysis of Brain Lesion (ABLe) software (Makale et al., 2002; Solomon et al., 2007) contained in MEDx v3.44 (Medical Numerics) with enhancements to support the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). We applied the AAL atlas of the human brain to obtain neuroanatomical labels for locations in 3-dimensional space. For hypotheses about specific brain areas (dlPFC), we defined regions of interest (ROIs) in terms of AAL structures (Tzourio-Mazoyer et al., 2002) and Talairach coordinates. As part of this process, we spatially normalized the CT image of each subject’s brain to a CT template brain image in Montreal Neurological Institute (MNI) space (Collins et al., 1994). We determined the percentage of AAL structures the lesion entailed by analyzing the overlap of the spatially normalized lesion image with the AAL atlas image. We calculated lesion volume by manually tracing the lesion in all relevant slices of the CT image, and then summing the traced areas and multiplying by slice thickness. The tracing technique isolated areas of missing brain and regions affected by metallic artifacts and penetrating objects. A trained neuropsychiatrist carried out the manual tracing, which was then reviewed by an observer that was blind to the results of the neuropsychological testing.
Neuropsychological Tests
We administered subtests of the Wechsler Memory Scale, 3rd Edition (WMS III; Wechsler, 1997b), the Wechsler Adult Intelligence Scale, 3rd Edition (WAIS III; (Wechsler, 1997b), and an experimental test of working memory, the N-Back Task (Cohen et al., 1997) to investigate the necessity of dlPFC for specific (1) cognitive operations (maintenance, monitoring and manipulation) and (2) modalities of information (verbal and spatial) in working memory. Maintenance operations enable the temporary online retention of information in working memory and are measured by simple retention tasks (e.g., the Digit Span Forward Task). Monitoring refers to the process of deliberately attending to information in working memory and is measured by active retention tasks (the N-Back Task). Manipulating items in working memory refers to the rearrangement and transformation of representations for goal-directed behavior and is measured by tasks that draw upon executive control functions (e.g., the Letter-Number Sequencing Task). The reported neuropsychological data from the WMS III and WAIS III represent standardized scores based on published norms from Wechsler (Wechsler, 1997b). Data for the N-back Task represent the mean number of errors in each patient group.
Maintenance
We investigated the patient’s ability to maintain information in working memory, administering a verbal/auditory maintenance measure, WAIS III: Digit Span Forward Task, and a nonverbal/spatial maintenance measure, WMS III: Spatial Span Forward Task, which is equivalent to the Corsi Span Task (Kessels et al., 2008). In Digit Span Forward, the patient hears a sequence of digits and attempts to repeat the sequence in order (Wechsler, 1997b). In Spatial Span Forward, the patient watches the examiner tap a sequence of locations on a board and attempts to repeat the tapping sequence in order (Wechsler, 1997b). Together, these tasks provide an assessment of the simple retention of verbal/auditory and non-verbal/spatial representations in working memory.
Monitoring
To examine the patient’s ability to actively monitor information in working memory, we administered the zero-back condition of the N-Back Task (Cohen et al., 1997). In this condition, the patient receives a sequence of visually presented letters and indicates whether the letter on the current trial matches a target stimulus. The zero-back condition therefore represents a pure measure of monitoring operations, examining the patient’s ability to identify a target stimulus by actively monitoring incoming visual stimuli (Owen et al., 2005).
Cognitive Load and Processing Demands on Working Memory
We additionally administered the one-, two-, and three-back conditions of the N-Back Task to investigate the recruitment of dlPFC with increasing cognitive load and processing demands on working memory. These conditions support the parametric manipulation of cognitive load, measuring the patient’s ability to determine whether each letter in the series matches the stimulus that occurred either one-, two- or three-trials previously. Successful performance requires that the patient (1) monitor a series of incoming stimuli, (2) maintain activation of recently processed and potentially relevant items, (3) discard recently processed but irrelevant information, and (4) make comparisons between items in the series to identify a correct match. The one-, two- and three-back conditions of the N-Back Task therefore support an investigation of the dlPFC’s role in working memory with increasing cognitive load and processing demands.
Manipulation
We examined the patient’s ability to manipulate items in working memory, employing two measures of the rearrangement of verbal/auditory information, WMS III: Letter-Number Sequencing and WMS III: Digit Span Backward, and a measure of the manipulation of nonverbal/spatial representations, WMS III Spatial Span Backward. In Letter-Number Sequencing, the patient hears a sequence of alternating digits and letters, and attempts to rearrange the order of each item by repeating the digits in numerical order, followed by the letters in alphabetical order (Wechsler, 1997b). Digit Span Backward (Wechsler, 1997a) and Spatial Span Backward (Wechsler, 1997a) are the same as their forward counterparts, except that the subject attempts to repeat each sequence in reverse order. Together, these measures support an assessment of the manipulation and rearrangement of verbal and spatial representations in working memory.
Reasoning
To investigate whether the dlPFC is necessary for manipulating information in tasks that do not exclusively depend on working memory, we examined manipulation processes in a broader range of verbal and spatial reasoning contexts, administering neuropsychological tests of mental arithmetic, WAIS III: Arithmetic, and visuospatial reasoning, WAIS III: Matrix Reasoning. In Arithmetic the subject hears numerical problems in story format, performs mental arithmetic (i.e., without paper and pencil), and provides a verbal response (Wechsler, 1997b). In Matrix Reasoning, the patient receives pictures of geometric shapes and draws an analogical inference about the missing shape that completes the pattern (Wechsler, 1997b). This task is comparable to Raven’s Progressive Matrices (Raven, 2000). The inclusion of verbal and spatial reasoning tasks complements our analysis of these operations in working memory, supporting an assessment of the contribution of the dlPFC to cognitive operations for manipulating information in a broader range of contexts.
Statistical Analyses
We report two main analyses. First, we conducted a one-way ANOVA for each neuropsychological measure of working memory and executive function to examine the performance of dlPFC lesion patients (n = 19) with respect to non-dlPFC lesion patients (n = 152) and neurologically healthy participants (n = 54), followed by Tukey’s honestly significant difference (HSD) test to determine significant between-group differences. Second, we conducted a follow-up analysis to investigate the performance of a smaller sample of patients with focal dlPFC lesions, applying non-parametric statistics to test for group effects and for pairwise comparisons.
RESULTS
To summarize the results reported in Table 2, no significant group differences in the dlPFC patient sample were observed for neuropsychological tests of working memory maintenance (Digit Span Forward and Spatial Span Forward), monitoring (Zero-Back), or under conditions of increasing cognitive load and processing demands (One-, Two- and Three-Back). However, deficits were observed in the dlPFC patient group for a test of mental arithmetic requiring the manipulation of verbal information (Arithmetic) and approached significance for a working memory test also requiring the manipulation of verbal items (Letter-Number Sequencing; p < .05, uncorrected). This pattern of findings suggests that the dlPFC may be critical for the manipulation of verbal knowledge in mathematical reasoning and working memory. To substantiate this conclusion, however, it is necessary to examine several factors that are relevant to the interpretation of the observed results: (1) anatomical specificity of the lesions, (2) lesion laterality, and (3) specificity of the cognitive deficit.
Table 2.
Neuropsychological tests of working memory and reasoning.
| Cognitive Function | Cognitive Measure | DLPFC | NonDLPFC | NO LESION | ANOVA F value |
ANOVA p value |
Significant between-group differences |
|---|---|---|---|---|---|---|---|
| Maintenance | Digit Span Forward | 6.05 (1.22) | 6.33 (1.20) | 6.68 (1.22) | 2.47 | 0.09 | none |
| Maintenance | Spatial Span Forward | 9.16 (3.20) | 9.52 (3.27) | 10.22 (2.69) | 1.25 | 0.29 | none |
| Monitoring: No Cognitive Load | Zero-Back Errors | 1.71 (2.66) | 1.26 (1.73) | 1.08 (2.33) | 0.65 | 0.53 | none |
| Monitoring: Low Cognitive Load | One-Back Errors | 3.12 (2.98) | 2.98 (2.26) | 2.57 (2.48) | 0.66 | 0.52 | none |
| Monitoring: Medium Cognitive Load | Two-Back Errors | 4.76 (2.02) | 4.55 (2.48) | 3.85 (2.39) | 1.8 | 0.17 | none |
| Monitoring: High Cognitive Load | Three-Back Errors | 5.65 (2.98) | 5.95 (2.66) | 4.68 (2.40) | 4.39 | 0.01 | NonDLPFC > NO LESION** |
| Manipulation | Letter-Number Sequencing | 9.32 (3.25) | 10.08 (2.67) | 11.04 (2.66) | 3.63 | 0.03 | DLPFC < NO LESION* |
| Manipulation | Digit Span Backward | 4.53 (1.35) | 4.59 (1.34) | 4.96 (1.41) | 1.62 | 0.20 | none |
| Manipulation | Spatial Span Backward | 10.58 (3.37) | 11.23 (2.89) | 12.02 (3.12) | 2.09 | 0.13 | none |
| Reasoning | Arithmetic | 8.74 (3.12) | 10.49 (2.77) | 11.00 (2.25) | 4.78 | 0.01 | DLPFC < NonDLPFC* |
| Reasoning | Matrix Reasoning | 10.47 (3.22) | 11.52 (2.85) | 12.28 (2.94) | 2.94 | 0.06 | DLPFC < NO LESION** |
Means are presented with standard deviations in parentheses. Significant between-group differences were determined with the Tukey’s honestly significant difference test.
p < .05;
p < .01.
Focal Dorsolateral Prefrontal Lesions
To strengthen the precision of our analysis, we examined the performance of a subset of patients in the dlPFC sample whose lesions were (1) confined to the frontal lobes, (2) lateralized, and (3) entailed damage within or adjacent to the peak area of dlPFC activation reported by a large-scale meta-analysis of fMRI studies on working memory (Wager and Smith, 2003). The results of this meta-analysis identified 86 peak activations reported by working memory studies within dlPFC, with a geometric center of activation in x = ± 40, y = 34, z = 29 (MNI coordinates; for further detail, see Wager and Smith, 2003). We assembled a left focal dlPFC sample (n = 7; Fig. 2) and a right focal dlPFC group (n = 9; Fig. 3) that each consisted of patients whose lesions were overlapping or adjacent to this peak activation site. In particular, 5 out of 7 left dlPFC patients (Fig. 4a) and 6 out of 9 right dlPFC patients (Fig. 4b) entailed damage to this region, supporting a more targeted assessment of the causal contribution of dlPFC to working memory. In addition, we further characterized the contribution of white matter pathways in the focal dlPFC samples, identifying that each patient group entailed damage within or adjacent to the (1) superior longitudinal fasciculus (primarily branch 1), (2) frontal aslant tract, and (3) fronto-striatal tracts (Thiebaut de Schotten et al., 2012; Mori et al., 2008).
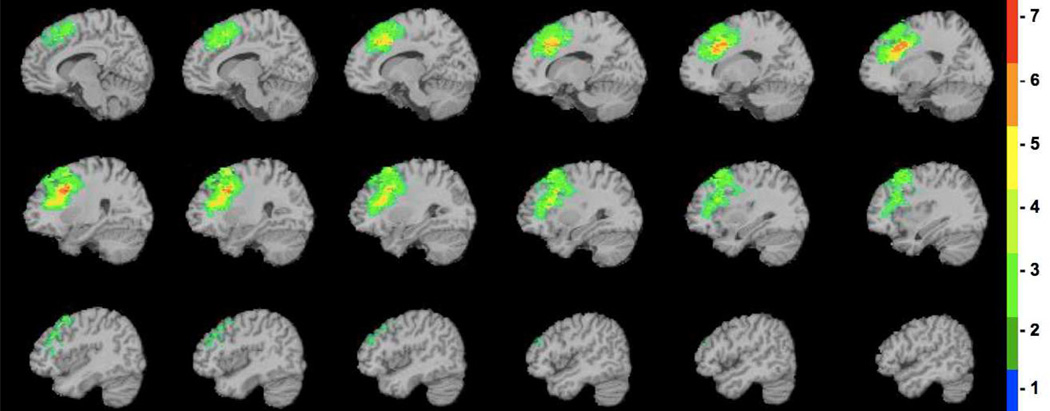
Figure 2.
Diagram of the lesion overlap map for the left dorsolateral prefrontal patients. The color indicates the number of veterans in the left dorsolateral prefrontal group (n = 7) with damage to a given voxel. The greatest lesion overlap (red) occurred in the dorsolateral prefrontal cortex (BA 9). The depicted sagittal slices progress from the midline (top left) to lateral regions of the left hemisphere (bottom right).
Figure 3.
Diagram of the lesion overlap map for the right dorsolateral prefrontal patients. The color indicates the number of veterans in the right dorsolateral prefrontal group (n = 9) with damage to a given voxel. The greatest lesion overlap (red) occurred in the dorsolateral prefrontal cortex (BA 9). The illustrated sagittal slices progress from the midline (top left) to lateral regions of the right hemisphere (bottom right).
Figure 4.
Lesion diagram of the (a) left and (b) right dorsolateral prefrontal patients illustrating the overlap with the peak activation reported in the Wager and Smith (2003) meta-analysis (in blue).
Comparison with Respect to Prefrontal Lesions
To further minimize differences between the dlPFC lesion patients and the brain-injured comparison group, we constructed a new comparison group consisting of patients with focal non-dlPFC lesions (Focal NonDLPFC patient group; Supplemental Fig. 2; n = 20). In contrast to the earlier non-dlPFC patient sample, this comparison group consisted of patients with lesions primarily confined to PFC rather than having lesions whose size and location was highly variable. As Supplemental Figure 2 illustrates, the focal non-dlPFC sample entailed lesions primarily within ventromedial PFC, representing the subset of patients from the earlier non-dlPFC sample with highly focal lesions. Demographic and background cognitive function data for each patient group are presented in Table 3. No significant group differences were observed with respect to basic demographic variables (age, sex, years of education), pre- and post-combat measures of cognitive function, post-combat measures of verbal IQ and verbal comprehension, and lesion size. In summary, the focal patient groups were well matched with respect to (1) basic demographic variables, (2) pre- and post-combat measures of cognitive function, (3) lesion size, and (4) lesion location (i.e., primarily confined to PFC).
Table 3.
Demographic and background data.
| Demographic Data |
L DLPFC | R DLPFC | Focal NonDLPFC |
NO LESION | Kruskal-Wallis χ2 |
Kruskal-Wallis p value |
Significant between-group differences |
|---|---|---|---|---|---|---|---|
| Age | 57.57 (1.13) | 60.00 (3.35) | 58.52 (2.29) | 59.52 (3.42) | 7.42 | 0.06 | none |
| Sex (% male) | 100 | 100 | 100 | 100 | n/a | n/a | none |
| Years of Education | 15.43 (2.99) | 14.22 (2.86) | 14.33 (2.99) | 15.19 (2.47) | 2.02 | 0.57 | none |
| Pre-combat AFQT | 53.43 (31.99) | 54.88 (28.61) | 54.20 (23.68) | 65.40 (22.91) | 3.70 | 0.29 | none |
| Post-combat AFQT | 57.50 (29.49) | 60.44 (25.89) | 61.69 (22.68) | 72.34 (22.99) | 5.75 | 0.12 | none |
| Post-combat Verbal IQ | 103.57 (20.69) | 99.89 (15.72) | 102.70 (11.91) | 109.87 (12.38) | 7.06 | 0.07 | none |
| Post-combat Verbal Comprehension | 107.57 (22.28) | 101.11 (15.88) | 102.30 (13.12) | 109.66 (12.04) | 6.48 | 0.09 | none |
| Total Percent Volume Loss (cm3) | 2.99 (1.42) | 2.55 (2.03) | 2.97 (1.90) | n/a | 1.19 | 0.55 | none |
Data are presented as means with standard deviations in parentheses. “Age” refers to age at the time of Phase 3 evaluation. “Sex” refers to the percent of male veterans. “Years of Education” refers to the total number of years of education the veterans completed. “Pre-combat AFQT” refers to index scores on the Armed Forces Qualification Test, a battery of tests measuring basic cognitive function at the time of enlistment (pre-injury). “Post-combat AFQT” refers to index scores on the Armed Forces Qualification Test administered at Walter Reed Medical Center after injury. “Post-combat Verbal IQ” refers to the Phase 3 Verbal IQ Index score from the Wechsler Adult Intelligence Scale. “Post-combat Verbal Comprehension” refers to the Phase 3 Verbal Comprehension Index score from the Wechsler Adult Intelligence Scale. There were no significant differences among groups for any measure. Non-parametric statistics were used to test for group effects and for the pairwise comparisons given the small number of participants in each sample. Significant between-group differences were determined with the Mann- Whitney U Test.
Specificity of Cognitive Deficit
To determine the effect of focal dlPFC lesions on components of working memory, we examined the performance of the left dlPFC (n = 7) and right dlPFC (n = 9) samples with respect to non-dlPFC lesion patients (n = 20) and neurologically healthy participants (n = 54). Because the assumptions underlying parametric statistics were not satisfied (e.g., homogeneity of variance, large sample size, and normality; Supplemental Table 1, Supplemental Figs. 3 – 24), nonparametric statistics were applied to test for group effects and for pairwise comparisons.
Working Memory
To summarize the results reported in Table 4 and Supplemental Figs. 3 – 22, focal lesions of the left or right dlPFC did not produce reliable deficits in working memory maintenance (Digit Span Forward and Spatial Span Forward), monitoring (Zero-Back), or under conditions of increasing cognitive load (One-, Two- and Three-Back). However, impairments in the left dlPFC patient group were observed for a test requiring the manipulation of verbal and auditory information in working memory (Letter-Number Sequencing) and approached significance for the test requiring the manipulation of non-verbal and spatial knowledge (Spatial Span Backward; p < .05, uncorrected). Additional analyses investigating the correlation between percent volume loss in dlPFC and performance on the administered tests of working memory revealed a converging pattern of findings (see Supplemental Table 2). In summary, our findings suggest that the left dlPFC is necessary for manipulating verbal/auditory and non-verbal/spatial information in working memory.
Table 4.
Neuropsychological tests of working memory and reasoning.
| Cognitive Function | Cognitive Measure |
L DLPFC | R DLPFC | Focal NonDLPFC |
NO LESION |
Kruskal- Wallis χ2 |
Kruskal- Wallisp value |
Significant betweengroup differences |
|---|---|---|---|---|---|---|---|---|
| Maintenance | Digit Span Forward | 6.00 (1.41) | 5.89 (1.05) | 6.40 (1.19) | 6.68 (1.22) | 4.83 | 0.19 | none |
| Maintenance | Spatial Span Forward | 8.57 (2.94) | 9.11 (3.89) | 9.89 (2.56) | 10.22 (2.7) | 3.05 | 0.39 | none |
| Monitoring: No Cognitive Load | Zero-Back Errors | 1.00 (0.71) | 2.56 (3.47) | 1.16 (1.89) | 1.08 (2.33) | 3.09 | 0.38 | none |
| Monitoring: Low Cognitive Load | One-Back Errors | 3.40 (2.97) | 3.67 (3.24) | 3.00 (2.75) | 2.57 (2.48) | 1.26 | 0.74 | none |
| Monitoring: Medium Cognitive Load | Two-Back Errors | 5.20 (1.92) | 4.89 (2.37) | 5.26 (2.81) | 3.85 (2.39) | 6.66 | 0.08 | Focal NonDLPFC > NO LESION* |
| Monitoring: High Cognitive Load | Three-Back Errors | 6.00 (2.74) | 5.67 (3.61) | 6.42 (2.65) | 4.68(2.40) | 5.27 | 0.15 | Focal NonDLPFC > NO LESION** |
| Manipulation | Letter-Number Sequencing | 8.14 (2.91) | 9.44 (3.68) | 9.95 (2.95) | 11.04 (2.66) | 7.73 | 0.05 | L DLPFC < NO LESION** |
| Manipulation | Digit Span Backward | 4.14 (1.22) | 4.67 (1.41) | 4.75 (1.21) | 4.96 (1.41) | 2.49 | 0.48 | none |
| Manipulation | Spatial Span Backward | 8.86 (3.81) | 11.33 (2.96) | 11.28 (2.49) | 12.02 (3.12) | 6.02 | 0.11 | L DLPFC < NO LESION* |
| L DLPFC < Focal NonDLPFC* | ||||||||
| Reasoning | Arithmetic | 8.71 (3.82) | 8.22 (3.11) | 10.40 (2.30) | 11.00 (2.25) | 8.70 | 0.03 | R DLPFC < NO LESION** |
| Reasoning | Matrix Reasoning | 10.57 (3.78) | 9.44 (2.79) | 11.89 (2.47) | 12.28 (2.94) | 7.94 | 0.04 | R DLPFC < NO LESION** |
| R DLPFC < Focal NonDLPFC* |
Means are presented with standard deviations in parentheses. Because the assumptions of parametric statistics were not satisfied (Supplemental Table 1, Supplemental Fig. 1 – 22), non-parametric statistics were used to test for group effects and for the pairwise comparisons. Significant between-group differences were determined with the Mann-Whitney U Test. Neuroscience evidence supporting the involvement of the dlPFC in working memory motivated the assessment of between-group differences when the Kruskal-Wallis test did not reach significance (see Introduction).
p < .05;
p < .01.
Reasoning
As Table 4 and Supplemental Figs. 3 – 24 illustrate, no reliable deficits were observed in the left dlPFC patient group for measures of mathematical (Arithmetic) or spatial reasoning (Matrix Reasoning). However, the right dlPFC patient group was significantly impaired for both neuropsychological tests of reasoning (for additional evidence, see Supplemental Table 2). This pattern of findings suggests that the right dlPFC is critical for manipulating information in the employed tests of arithmetic and spatial reasoning.
DISCUSSION
The aim of the current investigation was to examine the necessity of the dlPFC for key elements of working memory. Using a relatively large sample of patients with dorsolateral prefrontal damage (n = 19) and a wide-ranging assessment of cognitive function, we report several main findings. First, our results indicate that unilateral dlPFC is not necessary for working memory maintenance, monitoring, or for tasks that measure working memory performance under cognitive load. Second, our findings suggest that the dlPFC is important for manipulating representations in working memory (letter-number sequencing) and in reasoning (arithmetic; Table 2). Third, our results indicate that the left dlPFC is necessary for manipulating verbal and spatial knowledge in working memory (letter-number sequencing; spatial span backwards), while the right dlPFC is critical for the employed tests of verbal and spatial reasoning (arithmetic; matrix reasoning; Table 4).
Our findings are therefore consistent with domain-general models of working memory, which posit that the dlPFC embodies specific computational mechanisms for monitoring and manipulating cognitive representations (Owen et al., 1996; Duncan and Owen, 2000; Miller and Cohen, 2001; Koechlin et al., 2003; Petrides, 2000, 2005).
A key contribution of our lesion study is to elucidate the nature of these mechanisms, demonstrating that the dlPFC is necessary for manipulating verbal and spatial knowledge. Functional neuroimaging evidence indicates that the dlPFC is selectively engaged in a wide range of working memory operations, with increased activation in this region observed (1) at the beginning of delayed-response trials in which the amount of to-be-remembered information approaches or exceeds short-term memory capacity, (2) during the subsequent delay interval when no information is accessible to the subject (Courtney et al., 1997; Zarahn et al., 1999), (3) for manipulating information during the delay period (D'Esposito and Postle, 1999; Postle et al., 1999; Rypma and D'Esposito, 1999), and (4) upon presentation of the probe stimulus, when a subject is required to select an appropriate response. These findings highlight the temporal dynamics of dlPFC function in working memory and suggest that this region is involved in several encoding- and response-related operations, as well as mnemonic and non-mnemonic processes that are engaged when manipulating information. The results of our lesion study demonstrate that although the dlPFC is associated with multiple cognitive operations, it is computationally necessary for the specific process of manipulating verbal and spatial knowledge.
The observed lateralization within the dlPFC further suggests that the left dlPFC supports manipulating representations in working memory and the right dlPFC supports the manipulation of information in a broader range of reasoning contexts. In both cases, the dlPFC implements specific processes for manipulating cognitive representations (in the verbal and spatial domain) and therefore supports a domain-general model of the functional organization of the dlPFC. This pattern of findings is consistent with the proposal that the left dlPFC supports cognitive processes that are temporally bounded within working memory (letter-number sequencing; spatial span backwards), whereas the right dlPFC supports cognitive processes that extend beyond the scope of working memory and enable goal-directed behavior and adaptive decision making (arithmetic; matrix reasoning; Barbey et al., 2009).
When evaluating the theoretical contributions of this study, it is important to emphasize the type of inferences that can be drawn from lesion data. While physiological studies of the nervous system are based on correlational methods (e.g., single- and multi-unit electrophysiology, EEG, MEG, measures of glucose metabolism and the BOLD response), lesion data support inferences about the necessity of a brain region for a given cognitive function. Interpretation of neuropsychological data, however, is subject to a different set of limitations. Lesion localization and the interruption of fibers of passage by a brain injury are often difficult to assess in human studies, and the damaged region may contribute in a non-specific way to the normal functioning of a distal region that is itself the true neural substrate of the function in question. It is important to emphasize that the dlPFC lesion patients under investigation here had damage within or adjacent to the (1) superior longitudinal fasciculus (primarily branch 1), (2) frontal aslant tract, and (3) fronto-striatal tracts (see (Thiebaut de Schotten et al., 2012; Mori et al., 2008). Lesions within these white matter fiber tracts damage neural circuitry by disconnecting dlPFC and medial parietal cortex (superior longitudinal fasciculus), ventrolateral PFC and bilateral medial premotor (frontal aslant tract), and dlPFC and the dorsal striatum (fronto-striatal tracts). As a consequence, the observed pattern of working memory deficits reflect damage not only to the dlPFC but also to a crossroad of tracts that allow communication between several brain regions that have been implicated in working memory (for a meta-analytic review, see Owen et al., 2005).
Accumulating neuroscience evidence indicates that working memory and other higher cognitive processes centrally depend on white matter fiber tracts that synthesize information across a broadly distributed neural system. Recent voxel-based lesion-symptom mapping studies have sharpened our understanding of the role of white matter fiber tracts in binding the dlPFC and parietal cortex into an integrated system subserving working memory and general intelligence (Barbey et al., 2012; Glascher et al., 2010; Glascher et al., 2009; Chiang et al., 2009; Rudrauf et al., 2008). Barbey et al. (2012) showed that the neural architecture of general intelligence and working memory is remarkably circumscribed, concentrated within the core of white matter fiber tracts that connect dlPFC with the inferior parietal cortex and that terminate in the superior parietal lobe. The observed reliance upon white matter fiber tracts suggests that working memory and other high-level cognitive processes are supported by the interregional communication among many brain areas, emphasizing the central role of the dlPFC and parietal cortex (Jung and Haier, 2007).
We emphasize, in closing, that understanding the neural architecture of working memory will ultimately require knowledge of the entire network of brain regions that participate, the contribution made by each component, and the role of white matter fiber tracks that communicate and synthesize information between them. The results of the present investigation contribute to this emerging research program by elucidating the involvement of the dlPFC, demonstrating that this region supports the manipulation of verbal and spatial representations in working memory. Although activation within the dlPFC is associated with a broad range of cognitive operations, our study indicates that this region is a central component of the neural systems underlying the manipulation of verbal and spatial knowledge.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, V. Raymont, K. Reding, and G. Tasick for their invaluable help with the testing of participants and organization of this study. This work was supported by funding from the U.S. National Institute of Neurological Disorders and Stroke intramural research program and a project grant from the United States Army Medical Research and Material Command administered by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: a 30-year post-injury follow-up study, grant number DAMD17-01-1-0675).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Awh E, Smith EE, Jonides J. Human rehearsal processes and the frontal lobes: PET evidence. Annals of the New York Academy of Sciences. 1995;769:97–117. doi: 10.1111/j.1749-6632.1995.tb38134.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Recent developments in working memory. Current Opinion in Neurobiology. 1998;8(2):234–238. doi: 10.1016/s0959-4388(98)80145-1. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex - Discussion. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1996;351(1346):1461–1462. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006a;20(5):529–538. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006b;20(5):529–538. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain. 2012;135:1154–1164. doi: 10.1093/brain/aws021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J. Orbitofrontal Contributions to Human Working Memory. Cerebral Cortex. 2011;21(4):789–795. doi: 10.1093/cercor/bhq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. An evolutionarily adaptive neural architecture for social reasoning. Trends in Neurosciences. 2009;32(12):603–610. doi: 10.1016/j.tins.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. Journal of Comparative & Physiological Psychology. 1976;90(3):293–302. doi: 10.1037/h0087996. [DOI] [PubMed] [Google Scholar]
- Butters N, Pandya D. Retention of delayed-alternation: effect of selective lesions of sulcus principalis. Science. 1969;165(3899):1271–1273. doi: 10.1126/science.165.3899.1271. [DOI] [PubMed] [Google Scholar]
- Butters N, Pandya D, Sanders K, Dye P. Behavioral deficits in monkeys after selective lesions within the middle third of sulcus principalis. Journal of Comparative & Physiological Psychology. 1971;76(1):8–14. doi: 10.1037/h0031037. [DOI] [PubMed] [Google Scholar]
- Butters N, Pandya D, Stein D, Rosen J. A search for the spatial engram within the frontal lobes of monkeys. Acta Neurobiologiae Experimentalis (Warsaw) 1972;32(2):305–329. [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM. Genetics of brain fiber architecture and intellectual performance. Journal of Neuroscience. 2009;29(7):2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18(2):192–205. [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279(5355):1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral Cortex. 1996;6(1):39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386(6625):608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Cooney JW, Gazzaley A, Gibbs SE, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and FMRI data. Journal of the International Neuropsychological Society. 2006;12(2):248–260. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37(11):1303–1315. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. Journal of Neurophysiology. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic "scotomas". Journal of Neuroscience. 1993;13(4):1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Alexande.Ge Neuron Activity Related to Short-Term Memory. Science. 1971;173(3997):652. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Glascher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R. Distributed neural system for general intelligence revealed by lesion mapping. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Grabowski T, Damasio H, Adolphs R. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61(5):681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive Transcranial Magnetic Stimulation Affects behavior by Biasing Endogenous Cortical Oscillations. Frontiers in Integrative Neuroscience. 2009;3:14. doi: 10.3389/neuro.07.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR. Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain Research. 2008;1230:202–210. doi: 10.1016/j.brainres.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen CF, Nissen HW. Studies of cerebral function in primates IV. The effects of frontal lobe lesions on the delayed alternation habit in monkeys. Journal of Comparative Psychology. 1937;23(1):101–112. [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30(2):135–154. doi: 10.1017/S0140525X07001185. discussion 154–187. [DOI] [PubMed] [Google Scholar]
- Kessels RP, van den Berg E, Ruis C, Brands AM. The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment. 2008;15(4):426–434. doi: 10.1177/1073191108315611. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Carlesimo GA, Turriziani P, Caltagirone C. rTMS evidence of different delay and decision processes in a fronto-parietal neuronal network activated during spatial working memory. Neuroimage. 2005;24(1):34–39. doi: 10.1016/j.neuroimage.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior Parietal Cortex Is Critical for the Manipulation of Information in Working Memory. Journal of Neuroscience. 2009;29(47):14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. Journal of Neurophysiology. 1971;34(3):337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Association of storage and processing functions in the dorsolateral prefrontal cortex of the nonhuman primate. Journal of Neuroscience. 1999;19(12):5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behavior Research Methods Instruments & Computers. 2002;34(1):6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller MH, Orbach J. Retention of spatial alternation following frontal lobe resections in stump-tailed macaques. Neuropsychologia. 1972;10(3):291–298. doi: 10.1016/0028-3932(72)90020-6. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM, Doring T, Muller-Gartner HW, Topper R, Krause BJ. Bilateral parieto-frontal network for verbal working memory: an interference approach using repetitive transcranial magnetic stimulation (rTMS) European Journal of Neuroscience. 2002;16(8):1627–1632. doi: 10.1046/j.1460-9568.2002.02209.x. [DOI] [PubMed] [Google Scholar]
- Muller NG, Machado L, Knight RT. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. Journal of Cognitive Neuroscience. 2002;14(5):673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cerebral Cortex. 1996;6(1):31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. Journal of Neuroscience. 2000;20(19):7496–7503. doi: 10.1523/JNEUROSCI.20-19-07496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society London B Biological Sciences. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48(1):46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D'Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(22):12959–12964. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, Alexander A, Tononi G. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. Journal of Cognitive Neuroscience. 2006;18(10):1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- Ptito A, Crane J, Leonard G, Amsel R, Caramanos Z. Visual-spatial localization by patients with frontal-lobe lesions invading or sparing area 46. Neuroreport. 1995;6(13):1781–1784. doi: 10.1097/00001756-199509000-00018. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cerebral Cortex. 1995a;5(4):307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cerebral Cortex. 1995b;5(4):323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Raven J. The Raven's progressive matrices: change and stability over culture and time. Cognitive Psychology. 2000;41(1):1–48. doi: 10.1006/cogp.1999.0735. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5(10):813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288(5471):1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Grabowski TJ. Disconnection's renaissance takes shape: Formal incorporation in grouplevel lesion studies. Cortex. 2008;44(8):1084–1096. doi: 10.1016/j.cortex.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(11):6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Berntson GG, Cacioppo JT. Brain imaging and cognitive neuroscience. Toward strong inference in attributing function to structure. American Psychologist. 1996;51(1):13–21. doi: 10.1037//0003-066x.51.1.13. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe) Computer Methods and Programs in Biomedicine. 2007;86(3):245–254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Tsuchida A, Fellows LK. Lesion evidence that two distinct regions within prefrontal cortex are critical for nback performance in humans. Journal of Cognitive Neuroscience. 2009;21(12):2263–2275. doi: 10.1162/jocn.2008.21172. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volle E, Kinkingnehun S, Pochon JB, Mondon K, Thiebaut de Schotten M, Seassau M, Duffau H, Samson Y, Dubois B, Levy R. The functional architecture of the left posterior and lateral prefrontal cortex in humans. Cerebral Cortex. 2008;18(10):2460–2469. doi: 10.1093/cercor/bhn010. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler adult intelligence test administration and scoring manual. San Antonio, Texas: The Psychology Corporation; 1997b. [Google Scholar]
- Zarahn E, Aguirre GK, D'Esposito M. Temporal isolation of the neural correlates of spatial mnemonic processing with fMRI. Brain Res Cogn Brain Res. 1999;7(3):255–268. doi: 10.1016/s0926-6410(98)00029-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.