Abstract
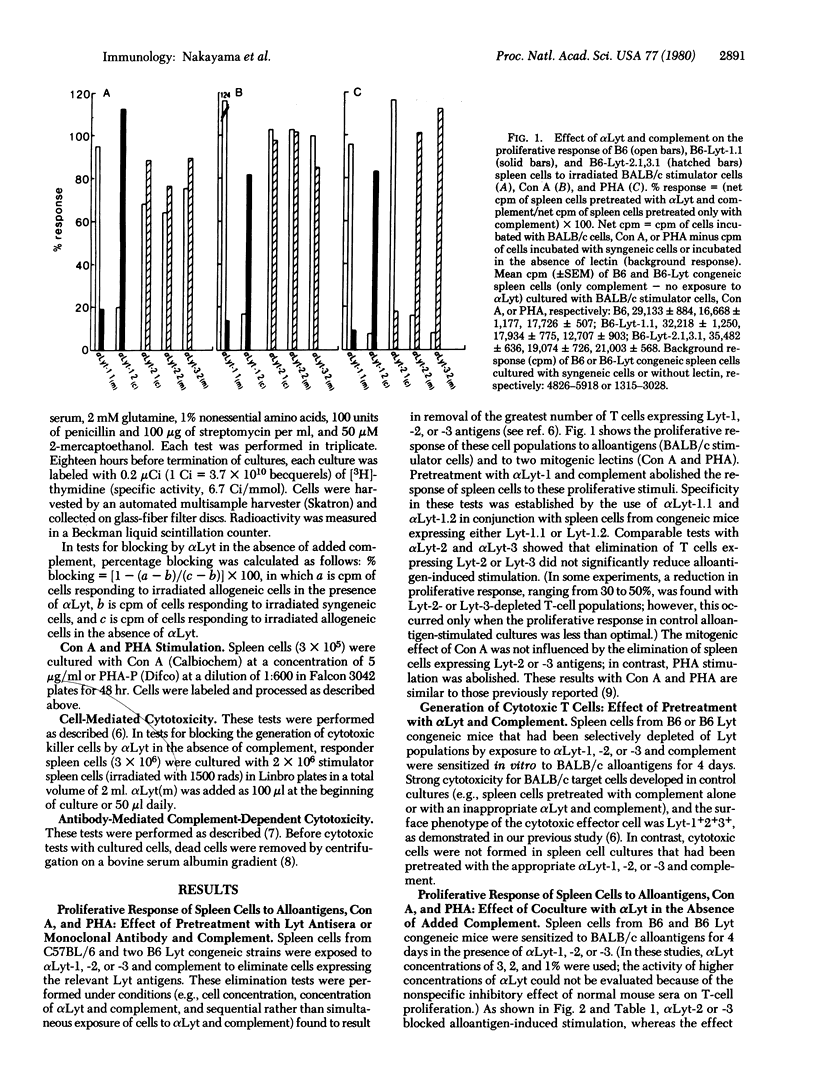
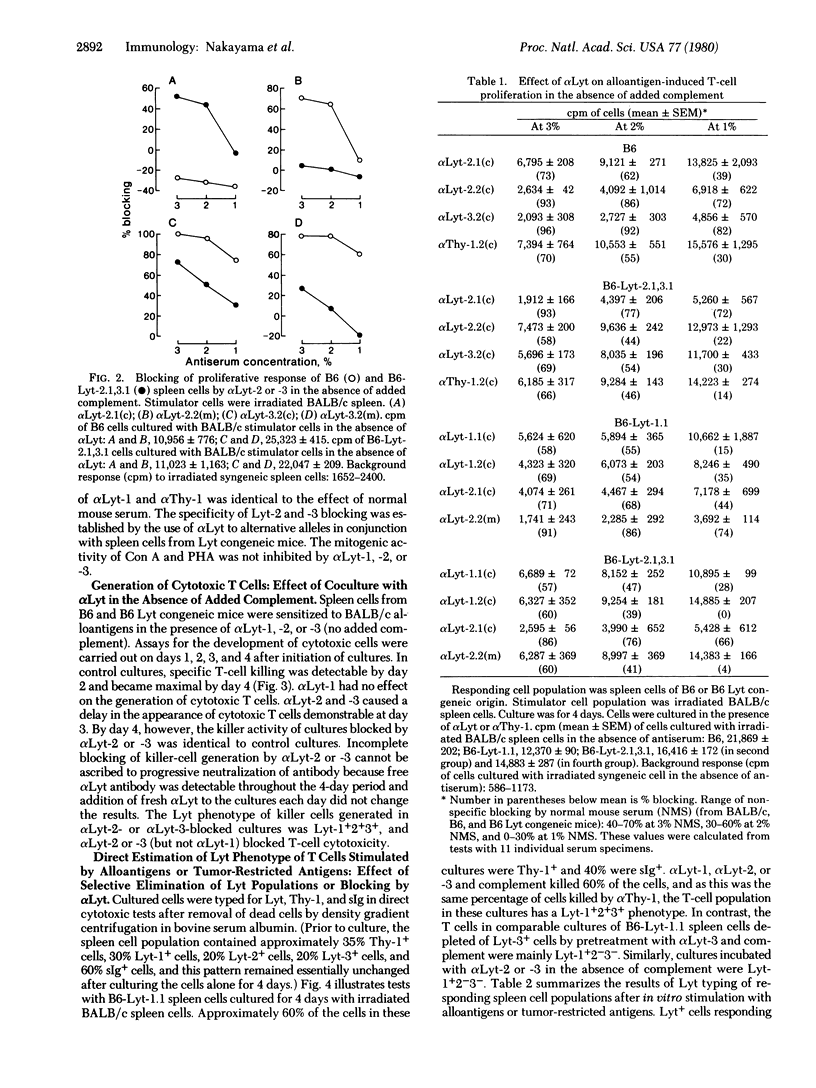
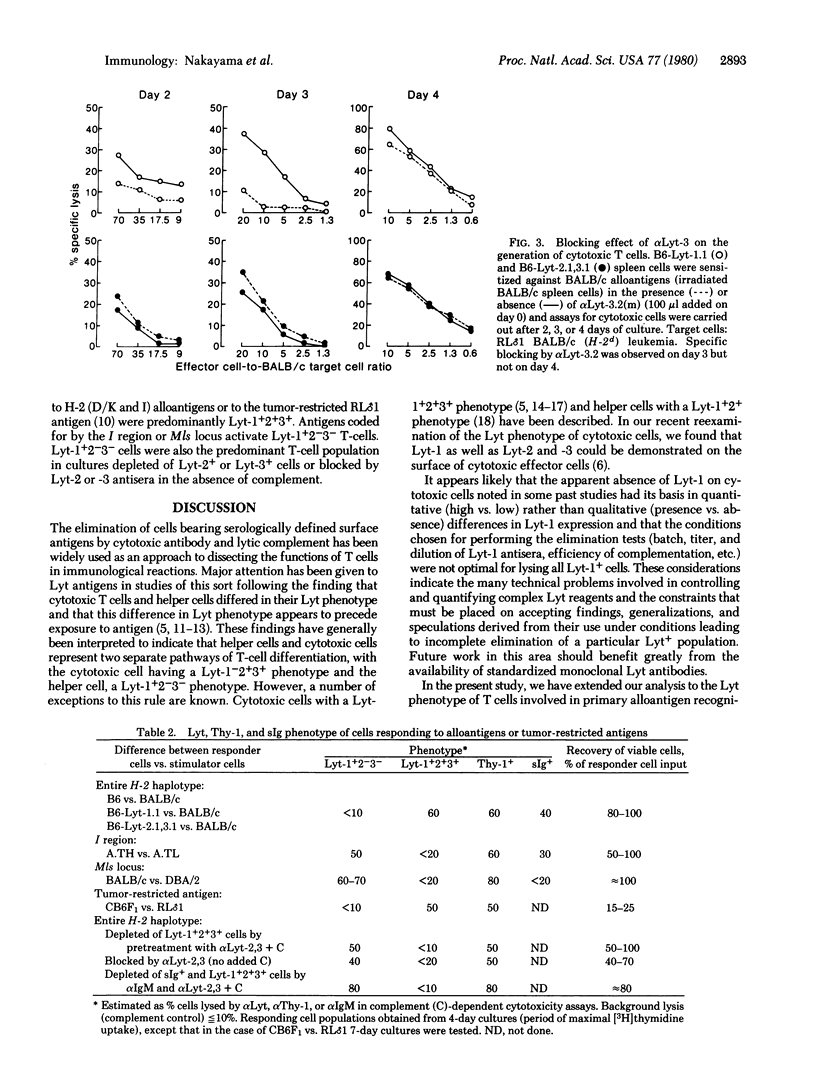
Cytotoxic T cells of the mouse express Lyt-1 as well as Lyt-2 and -3 on their surface, and T-cell cytotoxicity can be blocked by Lyt-2 and Lyt-3 (but not Lyt-1) antisera in the absence of added complement [Nakayama, E., Shiku, H., Stockert, E., Oettgen, H. F. & Old, L. J. (1979) Proc. Natl. Acad. Sci. USA 76, 1977-1981]. This analysis has now been extended to the study of the Lyt phenotype of T cells responding to alloantigens, concanavalin A (Con A), and phytohemagglutinin (PHA) and the effect of Lyt antibody on T-cell proliferation and the generation of H-2-specific killer T cells. H-2 (D/K and I), Con A, and PHA stimulation was abolished by pretreating responding cell populations with Lyt-1 antiserum and complement. Pretreatment with Lyt-2 or -3 antiserum and complement did not decrease alloantigen or Con A stimulation but did abolish PHA stimulation. Cytotoxic cells were not generated in H-2 alloantigen-primed cultures pretreated with Lyt-1, -2, or -3 antiserum and complement. When responding cells were cultured with Lyt antiserum in the absence of added complement, Lyt-2 or -3 antiserum (but not Lyt-1 antiserum) blocked alloantigen-induced proliferation and delayed generation of killer cells. Under similar conditions, Con A and PHA stimulation was not blocked by Lyt-1,-2, or -3 antiserum. Evidence from these Lyt elimination and blocking tests and from direct Lyt phenotyping of responding cells leads to the following conclusions. Two populations of Lyt+ cells are involved: Lyt-1+2-3- and Lyt-1+2+3+. Current evidence does not favor the existence of Lyt-1-2+3+ cells but indicates that pre-killer and killer cells derive from the Lyt-1+2+3+ population and have a Lyt-1+2+3+ phenotype. H-2 (D/K and I) and PHA stimulation ordinarily activate the Lyt-1+2+3+ population, whereas Con A and I region or Mls locus antigens activate the Lyt-1+2-3- population. However, when Lyt-1+2+3+ cells are eliminated or blocked by Lyt-2 or -3 antiserum, H-2 alloantigen stimulation leads to proliferation of the Lyt-1+2-3- population. Blocking of H-2-induced proliferation by Lyt-2 or -3 antiserum adds further support to the possibility that molecules bearing Lyt-2 and -3 determinants are involved in T-cell recognition.
Keywords: cellular immunology, T-cell subsets, T-cell receptors
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Alter B. J. Alternative pathways of T lymphocyte activation. J Exp Med. 1978 Sep 1;148(3):829–834. doi: 10.1084/jem.148.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley P. C., Woody J., Dunkley M., Feldmann M., McKenzie I. Separation of suppressor and killer T cells by surgace phenotype. Nature. 1976 Aug 5;262(5568):495–497. doi: 10.1038/262495a0. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Miyazawa M., Aoki T., Old L. J. Ly-A and Ly-B: two systems of lymphocyte isoantigens in the mouse. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):175–193. doi: 10.1098/rspb.1968.0032. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Old L. J. The immunogenetics of differentiation in the mouse. Harvey Lect. 1978;71:23–53. [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. M., Taylor B. A., Cherry M. Evidence for close linkage of a mouse light chain marker with the Ly-2,3 locus. J Immunol. 1978 Oct;121(4):1585–1590. [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb P. D. Genetic correlation of a mouse light chain variable region marker with a thymocyte surface antigen. J Exp Med. 1974 Nov 1;140(5):1432–1437. doi: 10.1084/jem.140.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J. A., Beverley P. C., Kisielow P., Hoffmann M. K., Oettgen H. F. Ly antigens: markers of T cell function on mouse spleen cells. J Immunol. 1975 Dec;115(6):1555–1557. [PubMed] [Google Scholar]
- Itakura K., Hutton J. J., Boyse E. A., Old L. J. Genetic linkage relationships of loci specifying differentiation alloantigens in the mouse. Transplantation. 1972 Mar;13(3):239–243. doi: 10.1097/00007890-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Hirst J. A., Shiku H., Beverley P. C., Hoffman M. K., Boyse E. A., Oettgen H. F. Ly antigens as markers for functionally distinct subpopulations of thymus-derived lymphocytes of the mouse. Nature. 1975 Jan 17;253(5488):219–220. doi: 10.1038/253219a0. [DOI] [PubMed] [Google Scholar]
- Nakayama E., Shiku H., Stockert E., Oettgen H. F., Old L. J. Cytotoxic T cells: Lyt phenotype and blocking of killing activity by Lyt antisera. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1977–1981. doi: 10.1073/pnas.76.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama E., Shiku H., Takahashi T., Oettgen H. F., Old L. J. Definition of a unique cell surface antigen of mouse leukemia RL male 1 by cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3486–3490. doi: 10.1073/pnas.76.7.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y., Stockert E., O'Donnell P. V., Okubo S., Snyder H. W., Jr, Old L. J. G(RADA1): a new cell surface antigen of mouse leukemia defined by naturally occurring antibody and its relationship to murine leukemia virus. J Exp Med. 1978 Apr 1;147(4):1089–1105. doi: 10.1084/jem.147.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Boyse E. A. Current enigmas in cancer research. Harvey Lect. 1973;67:273–315. [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Shiku H., Kisielow P., Bean M. A., Takahashi T., Boyse E. A., Oettgen H. F., Old L. J. Expression of T-cell differentiation antigens on effector cells in cell-mediated cytotoxicity in vitro. Evidence for functional heterogeneity related to the surface phenotype of T cells. J Exp Med. 1975 Jan 1;141(1):227–241. doi: 10.1084/jem.141.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiku H., Takahashi T., Bean M. A., Old L. J., Oettgen H. F. Ly phenotype of cytotoxic T cells for syngeneic tumor. J Exp Med. 1976 Oct 1;144(4):1116–1120. doi: 10.1084/jem.144.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N., Sachs D. H. Mouse alloantibodies capable of blocking cytotoxic T-cell function. I. Relationship between the antigen reactive with blocking antibodies and the Lyt-2 locus. J Exp Med. 1979 Sep 19;150(3):432–444. doi: 10.1084/jem.150.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Panfili P. R. Helper cells activated by allogeneic H-2K or H-2D differences have a Ly phenotype distinct from those responsive to I differences. J Immunol. 1979 Feb;122(2):383–391. [PubMed] [Google Scholar]
- Wettstein P. J., Bailey D. W., Mobraaten L. E., Klein J., Frelinger J. A. T lymphocyte response to H-2 mutants: cytotoxic effectors are Ly-1+2+. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3455–3459. doi: 10.1073/pnas.76.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]


