Abstract
In solid organ transplantation, mesenchymal stem cell (MSC) therapy is strongly emerging among other cell therapies due to the positive results obtained in vitro and in vivo as an immunomodulatory agent and their potential regenerative role. We aimed at testing whether a single dose of MSCs, injected at 11 weeks after kidney transplantation for the prevention of chronic mechanisms, enhanced regeneration and provided protection against the inflammatory and fibrotic processes that finally lead to the characteristic features of chronic allograft nephropathy (CAN). Either bone marrow mononuclear cells (BMCs) injection or no-therapy (NT) were used as control treatments. A rat kidney transplantation model of CAN with 2.5 h of cold ischemia was used, and functional, histological, and molecular parameters were assessed at 12 and 24 weeks after transplantation. MSC and BMC cell therapy preserves renal function at 24 weeks and abrogates proteinuria, which is typical of this model (NT24w: 68.9±26.5 mg/24 h, MSC24w: 16.6±2.3 mg/24 h, BMC24w: 24.1±5.3 mg/24 h, P<0.03). Only MSC-treated animals showed a reduction in interstitial fibrosis and tubular atrophy (NT24w: 2.3±0.29, MSC24w: 0.4±0.2, P<0.03), less T cells (NT: 39.6±9.5, MSC: 8.1±0.9, P<0.03) and macrophages (NT: 20.9±4.7, MSC: 5.9±1.7, P<0.05) infiltrating the parenchyma and lowered expression of inflammatory cytokines while increasing the expression of anti-inflammatory factors. MSCs appear to serve as a protection from injury development rather than regenerate the damaged tissue, as no differences were observed in Ki67 expression, and kidney injury molecule-1, Clusterin, NGAL, and hepatocyte growth factor expression were only up-regulated in nontreated animals. Considering the results, a single delayed MSC injection is effective for the long-term protection of kidney allografts.
Introduction
Chronic allograft nephropathy (CAN) is a multifactorial process that leads to late allograft dysfunction in renal transplantation. The joint association of nonalloreactive factors, as cold ischemia, and allogeneic factors significantly increases cellular infiltration at both early and late stages, aggravating the progression of CAN, which has been clearly shown in experimental models [1]. The main features of this entity include at end stages severe interstitial fibrosis and tubular atrophy (IFTA) with loss-of-renal function.
Cell therapies applied to solid organ transplantation have gained interest in the last years, and among them, mesenchymal stem cell (MSC) therapy has been strongly emerging. In addition to their potential role in therapies for renal repair, the immunomodulatory properties of MSCs offer promise as a novel cellular therapy for kidney transplantation.
To date, 3 groups have approached MSC cell therapy in the experimental kidney transplantation field but focusing only on the acute kidney injury [2–5]. The use of donor MSCs has been shown to induce tolerance and regulatory T-cell expansion by the induction of indoleamine 2,3-Dioxygenase (IDO) expression [2], while allogeneic MSCs enhanced functional recovery and attenuated histological damage from acute rejection by reducing cellular infiltrate in a 7 day follow-up in both syngeneic and allogeneic models of kidney transplantation [3,4]. Contrarily, Zhang et al. [5] did not observe a beneficial effect of treatment with MSCs (undetermined source) compared with cyclosporine A (CsA) monotherapy in a rat allogeneic kidney transplantation model.
MSCs treatment has also been tested in native chronic kidney disease models. In a 5/6 nephrectomy model, allogeneic MSCs injection decreased proteinuria and fibrosis [6–8] when intravenously injected or under the renal capsule, while the comparison between bone marrow mononuclear cells (BMCs) and MSCs injected in the renal parenchyma showed a better outcome with BMC cell therapy [9]. In a Col4A3 knock-out chronic model, syngeneic MSCs were able to reduce interstitial fibrosis [10], while allogeneic MSCs did not ameliorate the progression of the disease [11].
Moreover, acute kidney rejection can be effectively prevented in the early stages by using conventional drug-based immunosuppression, but unfortunately, there are no efficient immunosuppressive regimens that are able to guarantee long-term graft acceptance. Nowadays, the progressive loss of function secondary to IFTA is the major cause of graft loss.
In this work, we tested the effect of delayed therapy with an intravenous injection of a single dose of allogeneic BMCs or bone marrow-derived MSC on established CAN. We, specifically, assessed the potential immune mechanisms, the outcome of regeneration, and the protection against the inflammatory and fibrotic processes that finally lead to the development of late damage in a life-sustaining model of rat renal allotransplantation.
Materials and Methods
Rat kidney transplantation
For renal transplantation, inbred male Lewis rats (MHC haplotype: RT1I) (250 g body weight; Charles River) received a kidney from male Fischer-344 rats (MHC haplotype: RT1Iv1) (250 g body weight, Charles River) as previously described [12–14]. Briefly, kidneys were 2.5 h preserved in EuroCollins at 4°C. Recipient rats were bi-nephrectomized at the moment of transplantation. The animals received a single daily dose of 5 mg/kg CsA (Novartis) by oral gavage for 15 days. This model develops proteinuria and progressive renal damage [13]. All the procedures and housing conditions were in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals and Good Laboratory Practice.
Study design and follow-up
Rats were divided into 3 groups of treatment: NT: No-therapy group (n=11); BMC: BMCs injection (n=12); and MSC: MSCs injection (n=14). One rat was transplanted every day, and rats were included in 2 different badges of correlative transplantations (first badge: 21 animals, second badge: 15 animals). Before transplantation and then monthly until the end of the study, the rats were weighed and placed in metabolic cages for 24 h-urine and tail vein blood collection. Urine and serum creatinine (μM) were determined by Jaffe's reaction (Beckman Instruments), and proteinuria (Prot, mg/24 h) was determined by Ponceau's method (Bayer Diagnostics). Serum creatinine levels were assessed on days 1, 3, 5, and 7 after cell therapy. One week after cell therapy (12 weeks after transplantation), some rats were sacrificed (n=4 for NT and BMC and n=7 for MSC), and the grafted kidney was processed for histological and molecular studies. At the end of the study (24 weeks), the rest of the animals were sacrificed, and the grafted kidney was also processed.
Cell preparation and injection
Male Sprague-Dawley green fluorescent protein (GFP) transgenic rats (GenOway) weighing between 250 and 300 g were used as a MSC and BMC source [15]. Briefly, the femurs and tibias were surgically removed and flushed under sterile conditions with phosphate buffer saline (PBS). Cells were passed through a 70 μm mesh and washed twice with PBS to obtain BMCs. For the MSCs isolation, 60×106 were cultured in 15 cm-diameter plates with DMEM (Gibco, Life Technologies) with 10% fetal bovine serum (FBS; Lonza). Between passages 3 and 5, the cells were harvested; depleted for CD45+ CD11b/c+ (autoMACS; Miltenyi Biotec); stained for CD90-Allophycocyanin (Miltenyi Biotec), CD73, MHC-I (ox-18-PE), and MHC-II (ox-6-PE) (Becton Dickinson); and analyzed by flow cytometry (FACSCanto II and Diva software for the analysis; Becton Dickinson). MSCs were proved to be multipotent with a conditioning medium: IMDM (PAA) with 20% FBS, 2 mM L-glut, 0.05 mM β-Mercaptoethanol, 10−9 Dexamethasone supplemented with 5 μg/mL Insuline for the adipogenic medium, and 50 μg/mL ascorbic acid 2-P and 10 mM β-Glycerophosphate for the osteogenic medium. A single-cell (MSC or BMC) suspension was prepared in PBS and maintained at 4°C until the moment of the injection. All animals received, through the tail vein, 0.5 mL of PBS alone (NT), 0.5×106 GFP+ cultured MSC (MSC), or 107 GFP+ freshly isolated BMC (BMC) at the 11th week after kidney transplantation. The timing chosen corresponds to the increasing proteinuria in this model, as previously described [12].
Histological studies
Transversal kidney graft, liver, and spleen slices (1–2 mm) were either fixed in buffered formalin and frozen in optimal cutting temperature compound or dehydrated and embedded in paraffin. For light microscopy, tissue sections (3–4 μm) were stained with hematoxylin-eosin, periodic acid-Schiff, and Masson's trichrom. A pathologist blinded to the treatment groups examined all sections. Glomerulosclerosis was expressed as a percentage of damaged glomeruli, while tubular atrophy, interstitial fibrosis, interstitial cell infiltration, and vasculopathy were graded following a semi-quantitative scale from 0 to 3 (0, no abnormalities; +1, abnormalities affecting <1/3 of the sample; +2, between 1/3 and 2/3; +3, >2/3 of the sample). IF/TA was assessed following the Banff criteria [16].
Immunohistochemistry
Representative tissue sections were immunoperoxidase stained for connective tissue growth factor (CTGF, rabbit polyclonal IgG; Santa Cruz Biotechnology, Inc.), alpha-smooth muscle actin (αSMA, MS-113, Neomarkers; LabVision Ltd.), CD3 (monoclonal mouse anti-rat, Serotec; Bionova cientifica), ED1 (Oxford Biomarketing), Ki67 (mouse anti-rat; BD Pharmingen), and rabbit anti-rat IDO [17] and immunofluorescence stained for GFP (ab5450; Abcam) in paraffin-embedded sections. The CTGF, αSMA, CD3, ED1, and Ki67 stained samples were revealed with diaminobenzidine (Sigma-Aldrich) and counterstained with hematoxylin. IDO immunostaining was performed as previously described [17]. GFP was directly observed under fluorescence light microscopy in kidney, liver, and spleen sections. Positive CTGF, αSMA, and IDO samples were semi-quantitatively scored from 0 to 3 in the tubules, the interstitial compartment, or the glomeruli. Positive CD3, ED1 infiltrating, and Ki67 tubular cells were quantitatively assessed at least in 20 power fields in a 400 magnification.
Quantification of circulating donor-specific antibodies
The presence of circulating donor-specific antibodies (DSAs) class-I and class-II was quantified on recipient serum samples that were incubated with donor spleen cells and measured by flow cytometry. Plasma samples were collected at the moment of sacrifice. Donor splenocytes were isolated from Fischer-344 or Sprague-Dawley rat spleens by Ficoll® density gradient and freshly used. Different controls were added: serum from nontransplanted Lewis rat as naïve; serum from a transplanted Lewis rat with high anti-MHC antibody titer as a positive control.
Briefly, 5×105 splenocytes were incubated with 25 μL of recipient serum for 30 min at room temperature, washed in PBS, incubated in the dark (30 min, 4°C) with a 1:25 mix of anti-CD3 (eBioscience) and anti-IgG Fc portion (Jackson Immuno Research), fixed with 1% paraformaldehyde, and analyzed by flow cytometry. A fluorescence increase of 15% with regard to the negative control was considered positive. Results were expressed as a percentage of positive cells with regard to the total number of CD3+ spleen cells.
Gene expression assays
Frozen kidney tissue was homogenized in trizol reagent. Total RNA was extracted and purified using PureLink RNA mini kit (Invitrogen, Life Technologies). Overall, 500 μg of total RNA was retrotranscribed with a high-capacity complementary DNA reverse transcription kit (Applied Biosystems). Negative controls for reverse transcriptions were carried out using distilled water. Gene expression of IL4 (Rn01456866_m1), IL6 (Rn00561420_m1), IL7 Receptor (Rn01402421_m1), IL10 (Rn00563409_m1), IL12p40 (Rn00575112_m1), IL15 (Rn00565548_m1), IL23α (Rn00590334_g1), tumor necrosis factor alpha (TNFα, Rn99999017_m1), Fibronectin1 (Rn00569575_m1), Ki67 (Rn01451448_g1), basic fibroblast growth factor (bFGF, Rn00570809_m1), hepatocyte growth factor (HGF, Rn00566673_m1), IDO (Rn00576778_m1), CXCR4 (Rn01483207_m1), CXCL12 (Rn00573260_m1), Clusterin (Rn00562081_m1), NGAL (Rn00590612_m1), and kidney injury molecule-1 (KIM-1, Rn00597703_m1) was performed by Taqman assay in a 7900HT real-time polymerase chain reaction (PCR) system (Applied Biosystems) by relative to 18S quantification using the CT method. PCR reactions and amplification were performed as previously described [12]. The gene expression of NT12w kidneys was used as reference values. Results were expressed as many folds of the unknown sample with regard to the reference value (arbitrary units).
Statistical analysis
Serum creatinine differences at any time point, DSA, gene expression, and plasma proteins were analyzed by analysis of variance and subsequent Scheffe's test. For a histological comparison of Banff classification, a Chi Square P value was calculated from the contingency table. A semi-quantitative histological evaluation was analyzed through the nonparametric Kruskal–Wallis test. Two-tailed P<0.05 was considered statistically significant. Data are presented as mean±SEM.
Results
MSCs show low immunogenicity
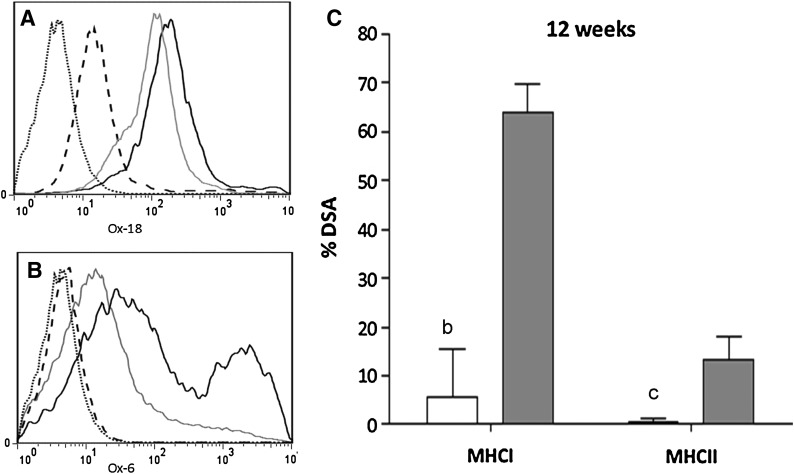
MSCs were isolated by plastic adherence, and the CD45−, CD11b/c−, and CD90+, CD73+ subpopulation was used for cell therapy. The multipotentiality of these cells was proved by their ability to differentiate into adipocytes or osteocytes (data not shown). They were selected for the therapy given their low immunogenicity, as they did not express MHC-II and had a very low expression of MHC-I (Fig. 1A, B). Contrarily, BMCs expressed MHC-II and, especially, MHC-I.
FIG. 1.
MSCs are low-immunogenic cells. Levels of MHC-I (A) and MHC-II (B). MSCs (dashed line) express low levels of MHC-I and are negative for MHC-II, while BMCs (gray line) express high levels of MHC-I and low levels of MHC-II. As a positive control of MHC-I and MHC-II expression, we used Sprague-Dawley rat splenocytes (black line). We also quantified the percentage of DSA against third-party injected cells (MSCs or BMCs) 1 week after the injection (C), and observed no antibodies in MSC-injected animals (open bars) and positive MHC-I in BMC-injected animals (gray bars) (bP=0.005, cP=0.06). MSC, mesenchymal stem cell; BMCs, bone marrow mononuclear cells; DSA, donor specific antibody.
Moreover, rats treated with MSCs did not develop cell DSAs against Sprague-Dawley MHC class I or class II early post-transplantation, while rats receiving BMCs developed high levels of DSA against Sprague-Dawley MHC-I within the first week after a cell injection (Fig. 1C). As expected, NT rats did not present circulating antibodies against Sprague-Dawley (data not shown), indicating specificity of the circulating antibodies present in both cell treatment groups of animals.
MSCs prevent from renal dysfunction
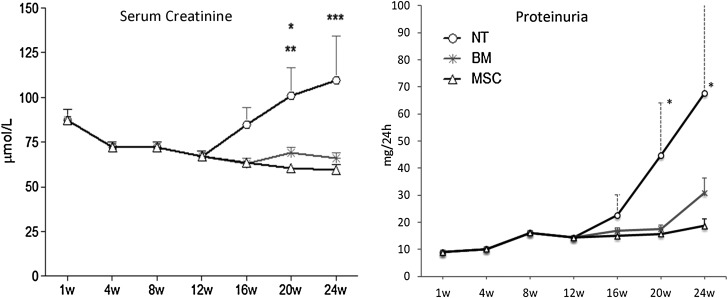
All the animals followed up to 24 weeks post-transplantation survived the study period. As shown in Fig. 2, the animals that were not treated developed progressive proteinuria and renal insufficiency from the 12th week onward. In marked contrast, the animals receiving either BMCs or MSCs therapy had no increase in proteinuria and also preserved normal serum creatinine levels.
FIG. 2.
MSCs and BMCs prevent from developing late allograft dysfunction. The animals treated with MSCs or BMCs did not increase the proteinuria levels, reaching significant differences at 20 and 24 weeks when compared with NT animals (P<0.05). In parallel, serum creatinine levels were nonpathologically maintained in both cell-injected groups (*P<0.05 NT vs. BMC, **P<0.001 vs. MSC, ***P<0.001 NT vs. MSC and BMC). NT, no-therapy.
One week after treatment, there was no difference in kidney function between PBS and MSC or BMC treatment.
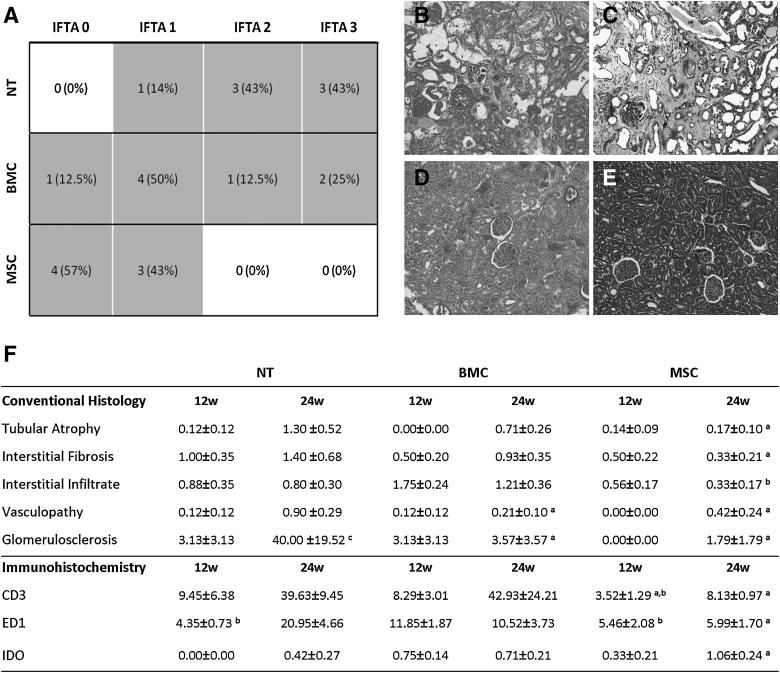
MSCs preserve renal histology along the follow-up
As expected, at 24 weeks, the conventional histology of NT grafts showed extensive tubular atrophy with widespread interstitial fibrosis and moderate diffuse interstitial infiltration (Fig. 3). Although those kidney grafts displayed a diverse degree of damage, most of them showed the highest IFTA values among the 3 groups of treatment (Mean value: 2.3, Fig. 3A). The MSC grafts displayed a normal histological picture with minimal tubular atrophy, interstitial fibrosis, and cellular infiltration (Fig. 3F). This resulted in very low IFTA scores in this group, contrary to NT (Mean value: 0.4). BMCs-injected animals presented the most heterogeneous histological damage distribution with IFTA scores ranging from 0 to 3 (Mean value: 1.6), significantly higher than the ones of the MSC group but not lower than the ones of the NT group.
FIG. 3.
Conventional histology. NT animals showed the worst outcome when pathological histology was analyzed (A). Not only IFTA degree in MSC animals was lower compared with NT animals, but also BMC animals show the worst histological preservation (P<0.003 vs. NT, P<0.05 vs. BMC). (B) and (C) are representative pictures of the NT kidney at 24 weeks of PAS and Masson staining, respectively. (D) and (E) show better-preserved and also less fibrotic MSC kidneys at 24 weeks with the same staining. In (F), we have specified the histological parameters evaluated by a pathologist at 12 and at 24 weeks and immunohistochemical-staining quantification of lymphocytic (CD3) and macrophagic (ED1) infiltrates (mean positive cells/field viewed±SEM) as well as semi-quantitative evaluation of IDO expression (score 0 to 3), (aP<0.05 vs. NT group at the same time point, bP<0.05 vs. BMC group at the same time point, cP<0.05 vs. same group at 12 weeks). IDO, indoleamine 2,3-dioxygenase; IFTA, interstitial fibrosis and tubular atrophy.
Even though the 3 groups showed similar initial IFTA at 12 weeks (data not shown), MSC treatment appeared to decrease the number of infiltrating cells within the first week after therapy compared with the other 2 groups, and interestingly, BMCs-injected animals slightly increased interstitial infiltrate mainly composed of ED1+ macrophages. It is worth mentioning that the MSC injection prevented the inflammatory infiltrate also at 24 weeks and maintained lower IFTA values until the end of the study, suggesting a preventive rather than corrective role of this cell therapy.
Although the model is not characterized by severe vasculopathy, both cellular treatments reduced vascular damage at 24 weeks. Notably, cellular therapy also halted the progression of glomerulosclerosis development (Fig. 3F).
MSCs modulate the immune-inflammatory response
Further immunohistochemical analysis revealed that a BMC injection immediately recruited increased numbers of ED1+ (macrophages) infiltrating cells with regard to NT- and MSC-treated animals at 12 weeks (Fig. 3F). At this time point, MSC therapy reduced the number of both ED1+ and CD3+ infiltrating cells not only with regard to the BMC group but also with regard to the NT group (Fig. 3F), and this observation persisted at 24 weeks. Contrarily, ED1+ and CD3+ infiltrating cells in NT animals increased at 24 weeks. BMC-injected animals maintained the number of infiltrating macrophages over time and highly increased the number of infiltrating T lymphocytes.
In accordance, MSC-treated animals showed a reduction in the parenchymal gene expression of inflammation-related genes along the follow-up, especially in IL6, IL23α, and IL7R (Table 1), although no differences were observed in IL15, IL12p40, or IL4 (data not shown). Furthermore, we also observed an immediate anti-inflammatory effect of MSC treatment through low gene expression of TNFα 1 week after cell therapy (NT12w: 1.00±0.00, BMC12w: 0.97±0.23, MSC12w: 0.49±0.10a,b; P<0.05 a: vs. NT, b: vs. BMC) along with a long-term effect on the up-regulation of the anti-inflammatory IL10 at 24 weeks (Table 1).
Table 1.
Gene Expression
| |
NT |
BM |
MSC |
|||
|---|---|---|---|---|---|---|
| Gene name | 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks |
| Inflammation | ||||||
| Il6 | 1.00±0.00 | 1.19±0.53 | 1.14±0.40 | 0.61±0.13a | 0.99±0.31 | 0.58±0.15a |
| Il7r | 1.00±0.00 | 1.02±0.26 | 0.83±0.18 | 0.79±0.17 | 0.98±0.21 | 0.58±0.14a |
| Il23a | 1.00±0.00 | 0.82±0.11 | 0.87±0.23 | 0.653±0.284 | 0.77±0.79 | 0.55±0.38a |
| Il10 | 1.00±0.00 | 0.93±0.38b | 0.95±0.26 | 0.68±0.21 | 0.51±0.13 | 1.03±0.37b |
| Fibrosis | ||||||
| bFGF | 1.00±0.00 | 1.14±0.22 | 0.66±0.22 | 0.51±0.21a | 0.41±0.06a | 0.59±0.14a |
| Fibronectin | 1.00±0.00 | 0.42±0.15c | 0.64±0.14 | 0.41±0.14 | 0.43±0.10a | 0.59±0.14a |
| Homing | ||||||
| Cxcl12 | 1.00±0.00 | 0.51±0.15c | 0.40±0.053a | 0.57±0.05 | 0.44±0.036a | 0.43±0.04 |
| Cxcr4 | 1.00±0.00 | 0.52±0.13c | 0.66±0.22a | 0.37±0.07 | 0.4±0.09a | 0.31±0.02 |
Immunomodulation of gene expression analysis of the grafts at 12 and at 24 weeks shows a modulation in the immune response. Results are presented as folds versus NT 12 weeks. We observe a decrease over time in the inflammatory cytokine levels in MSC-treated animals, along with an increase in the anti-inflammatory cytokine IL10. Fibrosis gene expression analysis (expressed as many folds over NT12 weeks) revealed an early effect of MSC therapy, decreasing the expression of profibrotic genes. At 24 weeks, we only observe differences at a gene level in the expression of basic fibroblast growth factor. On the other hand, at 12 weeks, the expression of homing genes CXCL12 and CXCR4 was down-regulated in both treated groups, and no differences were observed at 24 weeks (aP<0.05 vs. NT group at the same time point, bP<0.05 vs. BMC group at the same time point, cP<0.05 vs. same group at 12 weeks).
MSC, mesenchymal stem cell; BMC, bone marrow mononuclear cell; NT, no-therapy.
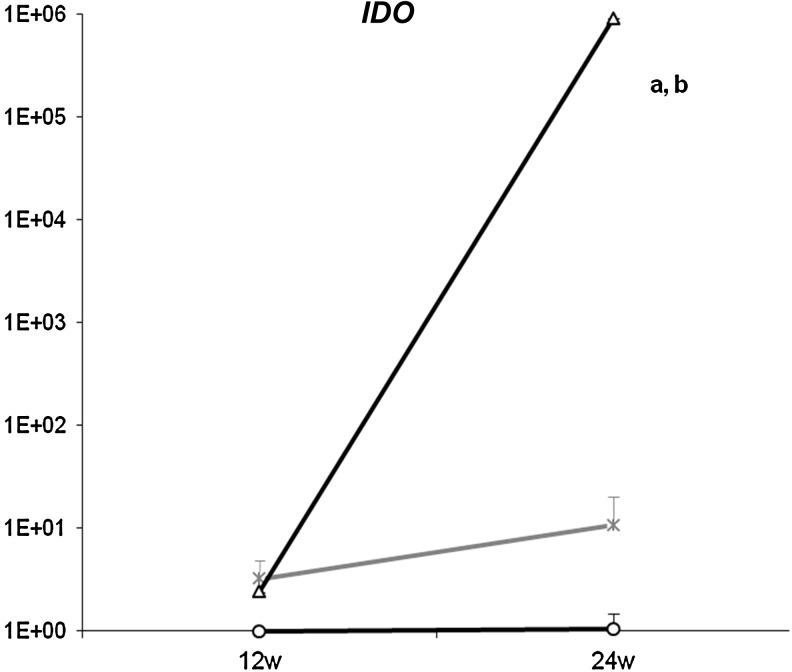
The immunomodulatory properties of MSC are supported by the IDO expression. An immunohistochemical analysis showed an increase of this protein in the MSC grafts at 24 weeks (Fig. 3F). The gene expression showed a logarithmic up-regulation a long time after the cell treatment (Fig. 4). In BMC-treated animals, IDO was 10-fold over-expressed with regard to NT at 24 weeks, but this was still far under the expression level reached by MSC treatment.
FIG. 4.
IDO gene expression. The gene expression of IDO in transplanted kidneys was analyzed by quantitative polymerase chain reaction. Results are normalized by the expression of housekeeping gene 18S and expressed as many folds of the NT at 12 weeks (y axis). MSC-treated animals (-o-) have increased the levels of IDO at 12 weeks that significantly increase at 24 weeks with regard to BMC treatment (-x-) and NT kidneys (-Δ-) (aP<0.05 vs. NT, bP<0.05 vs. BMC).
MSCs prevent from anti-donor-specific humoral responses
Without treatment, at 12 weeks, transplanted animals developed kidney DSAs against Fischer-344 MHC class I (DSA-I) but not against MHC class II (DSA-II, below 15%). Both DSA-I and DSA-II levels significantly increased over time in these NT animals.
MSC or BMC cell injections had a different effect in the development of DSA. The BMC animals presented DSA-I levels similar to NT rats and higher levels of DSA-II. In contrast, MSC treatment protected from DSA-I and DSA-II development at 12 weeks, and despite increasing over time, at 24 weeks, both DSA-I and DSA-II levels were still considerably reduced compared with NT animals (Table 2).
Table 2.
Mesenchymal Stem Cells Injection Reduces Kidney Donor-Specific Antibody Levels
| |
% F344 DSA-I |
||
|---|---|---|---|
| 12 weeks | 24 weeks | P | |
| NT | 54.3±19.9 | 86.1±2.284 | 0.043 |
| BMC | 65.8±13.5 | 46.8±15.5a | NS |
| MSC | 16.1±5.25b | 35.1±15.9a | NS |
| |
% F344 DSA-II |
||
|---|---|---|---|
| 12 weeks | 24 weeks | P | |
| NT | 8.87±4.9 | 31.6±5.2 | 0.03 |
| BMC | 22.4±6.6 | 17.1±6.1 | NS |
| MSC | 1.6±1.2b | 15.5±7.1c | NS |
The presence of circulating DSA class-I and class-II was quantified on recipient serum samples incubated with kidney donor (F344) spleen cells and measured by flow cytometry. A fluorescence increase of 15% with regard to the negative control was considered positive. Results were expressed as a percentage of positive cells with regard to the total number of CD3+ spleen cells. DSA titters were analyzed by analysis of variance followed by Scheffe's test. MSC treatment protects from developing DSAs from 1 week after the injection (12 weeks) and at 24 weeks. The BMCs injection has a different reaction, increasing the DSAs at 1 week after cell transplantation and maintaining them along with time.
P≤0.04 versus NT, bP=0.06 versus BMC; cP=0.06 versus NT.
DSAs, donor specific antibodies.
MSCs abrogate the onset of parenchymal fibrosis
At 12 weeks, no histological differences were observed in the conventional histology (Fig. 3F) or in the immunohistochemistry (IHC) of fibrosis markers (Fig. 5A) among the 3 groups. Nonetheless, the effect of MSC cell therapy was reflected at a gene level in the down-regulation of bFGF and fibronectin gene expression 1 week after the cell injection (Table 1). At 24 weeks, the expression of fibronectin was equivalent in the 3 groups of study, but bFGF gene expression was still significantly lower in MSC and also in BMC animals compared with NT (Table 1).
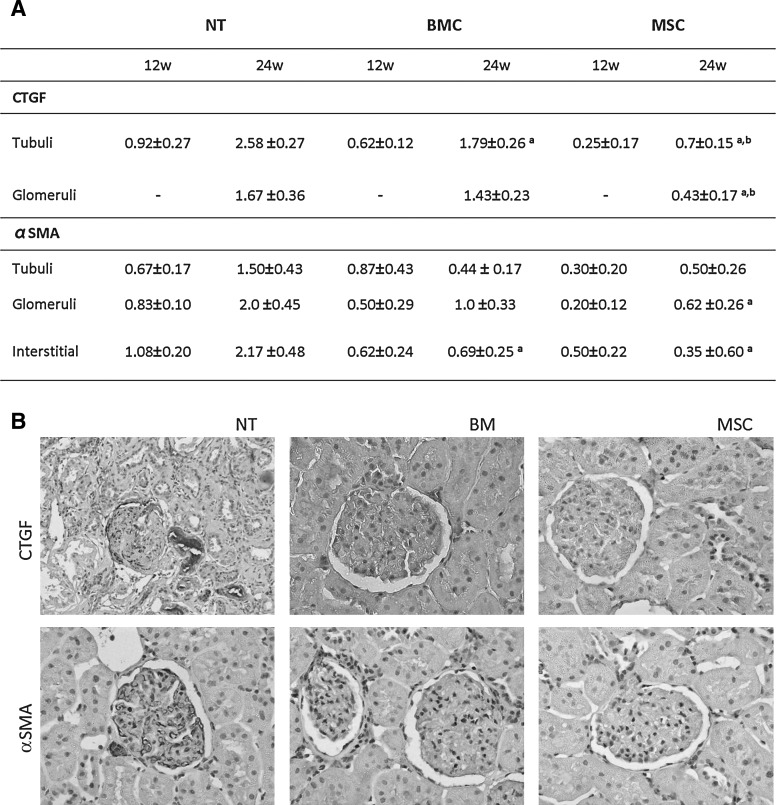
FIG. 5.
Parenchymal fibrosis. (A) shows the semi-quantitative analysis of CTGF and αSMA tissular expression (score 0 to 3). We observe lower αSMA parenchymal expression in MSC compared with NT at 24 weeks, and also lower CTGF tubular and glomerular expression when compared with both NT and BMC groups (aP<0.05 vs. NT group at the same time point, bP<0.05 vs. BMC group at the same time point). (B) depicts representative histological slides from all groups stained for αSMA and CTGF (400 magnification). CTGF, connective tissue growth factor. αSMA, alpha-smooth muscle actin.
At 24 weeks, a conventional histology evaluation showed a dramatic decrease in the glomerular and interstitial fibrosis in MSC-treated animals compared with NT (Fig. 3F). This effect is accompanied by a lower CTGF protein expression in both tubuli and glomeruli, in the MSC-treated kidneys. BMC treatment also reduced CTGF expression, only in the tubuli, although remaining significantly higher than MSC kidneys (Fig. 5). A similar pattern of reduced αSMA protein expression was observed in both glomeruli and interstitium, as shown in Fig. 5.
Cell homing is not enhanced by cell therapy
The expression of the cell homing genes (Cxcl12, Cxcr4) in the renal parenchyma was down-regulated at 12 weeks in both MSC and BMC groups, although at 24 weeks, these differences had already disappeared (Table 1).
On the other hand, the presence of injected MSC or BMC cells within renal, hepatic, and spleen parenchyma was assessed not only by direct GFP+ observation on frozen tissue but also by the enhancement of GFP by specific immunostaining in either frozen or paraffin-embedded tissues. We could not find GFP+ cells in any parenchyma either early after cell infusion at 12 weeks or lately at 24 weeks, in any of the treatment groups (data not shown).
MSC therapy prevents injury
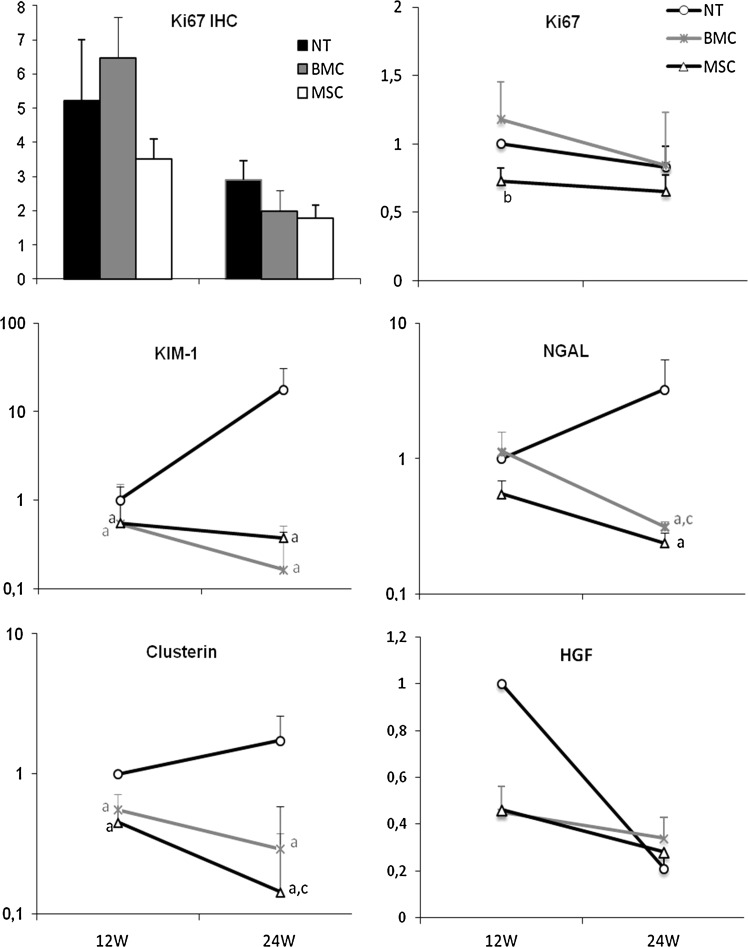
We stained tissue slides for Ki67 to check whether these protected tissues would show a higher number of positive proliferating tubular or glomerular cells. No differences were observed between the 3 groups of treatment neither at 12 nor at 24 weeks. A further analysis of the gene expression of Ki67 also showed no differences at 24 or at 12 weeks between any of the groups (Fig. 6).
FIG. 6.
Renal regeneration. Ki67 immunostaining of tubular epithelial cells (bars chart) was quantitatively assessed and expressed as mean of positive cells/per field viewed, and showed no differences between the groups at 12 or at 24 weeks. Gene expression of Ki67 shows slightly less expression at 12 weeks in the MSC group compared with the BMC group. Damage/proliferation markers as Kim-1, NGAL, and Clusterin show a dramatic increase in NT compared with MSC- and BMC-injected animals. HGF show the opposite pattern but again confirming no regeneration in the cell treatment group (aP<0.05 vs. NT group at the same time point, bP<0.05 vs. BMC group at the same time point, cP<0.05 vs. same group at 12 weeks). HGF, hepatocyte growth factor; KIM-1, kidney injury molecule-1.
When we checked for the expression of other genes involved in tubular damage and regeneration after injury such as Havcr1 (Kim-1), Lcn2 (NGAL), Clu (Clusterin), and HGF, the results were completely different. The expression of these genes was only up-regulated in the NT group both at 12 and/or at 24 weeks; meanwhile, both cell treatments abrogated the over-expression in time in these 3 genes. On the contrary, the dynamics of HGF was somewhat different, showing an initial increase in NT animals and a decrease over time in all groups.
Discussion
In this study, we observed for the first time the long-term beneficial effect of the MSC injection in a well-described CAN model. The rationale of using MSCs in this setting rose from the proposed immunomodulatory and remodelative properties of these cells [8,18]. In this model, these properties would help decrease the immune infiltrate, enhance renal parenchyma regeneration, and, therefore, counterbalance the fibrotic and inflammatory-driven chronic injury processes [1]. Our experience shows that an injection of MSCs or BMCs serves as protection from the development of proteinuria, glomerulosclerosis, and vasculopathy, typically observed in CAN, and also maintains stable function; albeit only MSC-injected animals showed decreased numbers of infiltrating cells, a fully preserved parenchyma structure, and were protected from developing graft fibrosis with a very homogeneous effect. However, we could not find any sign of kidney regeneration or homing of the injected cells in the graft.
Our main contribution to the success in the treatment of the CAN is the timing of the therapy, which leads to a complete prevention or protection of the graft 24 weeks after transplantation. A recent report from a clinical trial treating at the early stages after transplantation [19] showed the unexpected deleterious short-term effects of MSC therapy. We chose a later time after transplantation, as we and others [20] have shown a second deleterious inflammatory wave that leads to chronic tissue fibrosis. This type of approach has proved successful in our group with the use of HGF gene therapy [1].
An important point that needs to be addressed is the fact that we did not detect any of the injected MSCs 7 days after the therapy. As other authors have reported [21] those cells get trapped in the lungs within hours, and no cells or fluorescent signal is detected 3 days after the injection, suggesting that allogeneic MSCs die in the lungs early after the injection or are cleared from the circulation by immune cells. However, we know that the injected cells are low MHC class I expressers and MHC Class II negative before the injection, which theoretically makes them immune privileged to clearance by the adaptive immune system although more susceptible to the innate immune system. We cannot confirm whether there is a phenotypic change after an injection, as some authors have proved both MHC I and MHC II up-regulation after IFNγ stimulus in vitro [22]. However, the fact that the MSC-injected animals do not generate specific antibodies against the 3rd party cell donor, contrary to what happens in the BMC group, confirms that MSCs are not rejected by the recipient. Another option that should be contemplated is the possibility of MSCs being eliminated by CD8 T cells, as has been reported in vitro [23].
The use of BMCs as a therapeutic tool in solid organ transplantation had been already attempted in experimental [9] and clinical models [24] with very good results as a pro-tolerogenic agent. More recently, it has been suggested that whole BMCs would be more efficient in comparison to MSCs in the reduction of the progression of chronic kidney [9] and heart [25,26] diseases. We, therefore, injected whole BMC as a control group to MSC therapy. Since whole BMC includes MSCs, they represent a better control than sham injections.
Gene expression gives us a more precise insight of the immune regulation while observing a decreased expression of inflammatory genes with a clear down-regulation over time, and an increased expression of anti-inflammatory and immunosuppressive genes. IDO appears to be a key factor in this regulation, as it is highly up-regulated in MSC-treated animals. IDO can block activation of T cells, which are particularly sensitive to loss of tryptophan [27]. Notably, IDO is needed to prevent T-cell-mediated rejection [28,29], and it has been shown to be responsible, at least in part, for the induction of kidney allograft tolerance through the generation of regulatory T cells in a mouse model of acute allograft rejection [2] and in a solid organ transplantation model, being also required for organ acceptance [30].
MSC cell therapy served as a protection not only from inflammatory cellular infiltrate but also from humoral responses. This is in tune with in vitro findings [31] showing how MSC suppress allo-specific antibody production by B cells. Interestingly, BMC therapy rapidly increases circulating alloantibodies specific against the cell donor along with an increase in circulating alloantibodies against the kidney donor.
Along with this cellular and humoral anti-inflammatory effect of the MSC early after treatment, we observe a gene modulation of pro-fibrotic genes. Shortly after the injection, MSCs show the ability to down-regulate bFGF and fibronectin, while without them, treatment would be increased. The down-regulation of fibronectin at the onset of the fibrotic process and the effective long-term down-regulation of bFGF are unequivocal signs of fibrosis inhibition or protection from damage by the treatment [32,33]. At 24 weeks, the anti-fibrotic effect may be seen in conventional histology and IHC with a significant decrease in CTGF and αSMA.
Interestingly, this effect was maintained along with time, although the cells were found neither at 24 weeks nor 1 week after the injection. CXCL12 expression has been widely shown to play a role in the mobilization and homing of CXCR4+ cells enhanced by tissue injury or DNA damage [34,35]. Since the expression of these 2 factors is only early enhanced in the non-MSC-treated groups, it gives strength to our idea of injury blockage by the cell therapy.
As a last step, we aimed at testing tissular regeneration. It remains controversial whether the differentiation of MSCs is produced, as some authors have localized injected MSC in chronic injury models and reported a regenerative effect [10], but they have not been able to observe kidney structures or cells derived from the injected MSCs. In our model, although we were expecting a pro-regenerative effect, we have observed neither the differentiation of injected MSCs in the renal parenchyma similar to that proposed by some authors nor the enhancement of regeneration. Since we have not detected the injected MSCs in the renal parenchyma or in liver or spleen, we cannot contribute to this subject.
Moreover, we observed a decreased expression of Kim-1, NGAL, clusterin, and HGF. The expression of these genes has been used as a biomarker of acute kidney damage and is also related to the regeneration to overcome injury [36–40]. Both cell treatments show no increase in the expression of these 4 genes in time along with a conserved parenchyma, indicating a prevention of the damage settling and again corroborating the idea of injury blockage by the MSC therapy.
In summary, we have observed a therapeutic effect of MSC attenuating the progression of CAN when this process is already in progress. This beneficial effect observed seems to be attributable to the immunomodulatory properties of MSCs, which rather than promoting tissue regeneration prevent the onset of the disease.
Acknowledgments
M.F. is a postdoctoral fellow funded by ERA-EDTA; E.R. is the recipient of a fellowship from IDIBELL. I.H-F. is a researcher from “Programa Estabilización Investigadores” financed by ISCIII and Dpt. Salut Generalitat Catalunya. This work was supported by grants from Instituto de Salud Carlos III/FIS (FIS06/0230, PS09/00107), REDinREN (FIS06/0016), and Fundación SENEFRO 2007. The authors thank N. Bolaños for immunohistochemical support and Centres Científics i Tecnològics - CCiTUB, UB-Bellvitge, for microscopy and flow cytometry technical support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Herrero-Fresneda I. Torras J. Franquesa M. Vidal A. Cruzado JM. Lloberas N. Fillat C. Grinyo JM. HGF gene therapy attenuates renal allograft scarring by preventing the profibrotic inflammatory-induced mechanisms. Kidney Int. 2006;70:265–274. doi: 10.1038/sj.ki.5001510. [DOI] [PubMed] [Google Scholar]
- 2.Ge W. Jiang J. Arp J. Liu W. Garcia B. Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 3.Zonta S. De Martino M. Bedino G. Piotti G. Rampino T. Gregorini M. Frassoni F. Dal Canton A. Dionigi P. Alessiani M. Which is the most suitable and effective route of administration for mesenchymal stem cell-based immunomodulation therapy in experimental kidney transplantation: endovenous or arterial? Transplant Proc. 2010;42:1336–1340. doi: 10.1016/j.transproceed.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 4.De Martino M. Zonta S. Rampino T. Gregorini M. Frassoni F. Piotti G. Bedino G. Cobianchi L. Dal Canton A. Dionigi P. Alessiani M. Mesenchymal stem cells infusion prevents acute cellular rejection in rat kidney transplantation. Transplant Proc. 2010;42:1331–1335. doi: 10.1016/j.transproceed.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W. Qin C. Zhou ZM. Mesenchymal stem cells modulate immune responses combined with cyclosporine in a rat renal transplantation model. Transplant Proc. 2007;39:3404–3408. doi: 10.1016/j.transproceed.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 6.Cavaglieri RC. Martini D. Sogayar MC. Noronha IL. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc. 2009;41:947–951. doi: 10.1016/j.transproceed.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 7.Choi S. Park M. Kim J. Hwang S. Park S. Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 8.Semedo P. Correa-Costa M. Antonio Cenedeze M. Maria Avancini Costa Malheiros D. Antonia dos Reis M. Shimizu MH. Seguro AC. Pacheco-Silva A. Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 9.Caldas HC. Fernandes IM. Gerbi F. Souza AC. Baptista MA. Ramalho HJ. Kawasaki-Oyama RS. Goloni-Bertollo EM. Pavarino-Bertelli EC. Braile DM. Abbud-Filho M. Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure. Transplant Proc. 2008;40:853–855. doi: 10.1016/j.transproceed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Ninichuk V. Gross O. Segerer S. Hoffmann R. Radomska E. Buchstaller A. Huss R. Akis N. Schlondorff D. Anders HJ. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70:121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 11.Prodromidi EI. Poulsom R. Jeffery R. Roufosse CA. Pollard PJ. Pusey CD. Cook HT. Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells. 2006;24:2448–2455. doi: 10.1634/stemcells.2006-0201. [DOI] [PubMed] [Google Scholar]
- 12.Herrero-Fresneda I. Torras J. Cruzado JM. Condom E. Vidal A. Riera M. Lloberas N. Alsina J. Grinyo JM. Do alloreactivity and prolonged cold ischemia cause different elementary lesions in chronic allograft nephropathy? Am J Pathol. 2003;162:127–137. doi: 10.1016/S0002-9440(10)63804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrero-Fresneda I. Torras J. Vidal A. Lloberas N. Cruzado JM. Grinyo JM. Reduction of postischemic immune inflammatory response: an effective strategy for attenuating chronic allograft nephropathy. Transplantation. 2005;79:165–173. doi: 10.1097/01.tp.0000147198.88801.57. [DOI] [PubMed] [Google Scholar]
- 14.Herrero-Fresheda Z. Experimental kidney trasplant. www.renal.transplantation.com www.renal.transplantation.com
- 15.Remy S. Tesson L. Usal C. Menoret S. Bonnamain V. Nerriere-Daguin V. Rossignol J. Boyer C. Nguyen TH, et al. New lines of GFP transgenic rats relevant for regenerative medicine and gene therapy. Transgenic Res. 2010;19:745–763. doi: 10.1007/s11248-009-9352-2. [DOI] [PubMed] [Google Scholar]
- 16.Sis B. Mengel M. Haas M. Colvin RB. Halloran PF. Racusen LC. Solez K. Baldwin WM., 3rd Bracamonte ER, et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 17.Hill M. Pereira V. Chauveau C. Zagani R. Remy S. Tesson L. Mazal D. Ubillos L. Brion R, et al. Heme oxygenase-1 inhibits rat and human breast cancer cell proliferation: mutual cross inhibition with indoleamine 2,3-dioxygenase. FASEB J. 2005;19:1957–1968. doi: 10.1096/fj.05-3875com. [DOI] [PubMed] [Google Scholar]
- 18.Casiraghi F. Noris M. Remuzzi G. Immunomodulatory effects of mesenchymal stromal cells in solid organ transplantation. Curr Opin Organ Transplant. 2010;15:731–737. doi: 10.1097/MOT.0b013e328340172c. [DOI] [PubMed] [Google Scholar]
- 19.Perico N. Casiraghi F. Introna M. Gotti E. Todeschini M. Cavinato RA. Capelli C. Rambaldi A. Cassis P, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6:412–422. doi: 10.2215/CJN.04950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond JR. Tilney NL. Frye J. Ding G. McElroy J. Pesek-Diamond I. Yang H. Progressive albuminuria and glomerulosclerosis in a rat model of chronic renal allograft rejection. Transplantation. 1992;54:710–716. doi: 10.1097/00007890-199210000-00028. [DOI] [PubMed] [Google Scholar]
- 21.Yang X. Balakrishnan I. Torok-Storb B. Pillai MM. Marrow Stromal Cell Infusion Rescues Hematopoiesis in Lethally Irradiated Mice despite Rapid Clearance after Infusion. Adv Hematol. 2012;2012:142530. doi: 10.1155/2012/142530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crop MJ. Baan CC. Korevaar SS. Ijzermans JN. Pescatori M. Stubbs AP. van Ijcken WF. Dahlke MH. Eggenhofer E. Weimar W. Hoogduijn MJ. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin Exp Immunol. 2010;162:474–486. doi: 10.1111/j.1365-2249.2010.04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crop MJ. Korevaar SS. de Kuiper R. Ijzermans JN. van Besouw NM. Baan CC. Weimar W. Hoogduijn MJ. Human mesenchymal stem cells are susceptible to lysis by CD8+ T-cells and NK cells. Cell Transplant. 2011 doi: 10.3727/096368910X564076. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Mathew JM. Garcia-Morales RO. Carreno M. Jin Y. Fuller L. Blomberg B. Cirocco R. Burke GW. Ciancio G, et al. Immune responses and their regulation by donor bone marrow cells in clinical organ transplantation. Transpl Immunol. 2003;11:307–321. doi: 10.1016/S0966-3274(03)00056-X. [DOI] [PubMed] [Google Scholar]
- 25.Mazo M. Gavira JJ. Abizanda G. Moreno C. Ecay M. Soriano M. Aranda P. Collantes M. Alegria E, et al. Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant. 2010;19:313–328. doi: 10.3727/096368909X480323. [DOI] [PubMed] [Google Scholar]
- 26.Ghanem A. Ziomka A. Krausgrill B. Schenk K. Troatz C. Miszalski-Jamka T. Nickenig G. Tiemann K. Muller-Ehmsen J. Functional impact of targeted closed-chest transplantation of bone marrow cells in rats with acute myocardial ischemia/reperfusion injury. Cell Transplant. 2009;18:1289–1297. doi: 10.3727/096368909X12483162197286. [DOI] [PubMed] [Google Scholar]
- 27.Muller AJ. DuHadaway JB. Donover PS. Sutanto-Ward E. Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 28.DelaRosa O. Lombardo E. Beraza A. Mancheno-Corvo P. Ramirez C. Menta R. Rico L. Camarillo E. Garcia L, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 29.English K. Barry FP. Field-Corbett CP. Mahon BP. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110:91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Popp FC. Eggenhofer E. Renner P. Slowik P. Lang SA. Kaspar H. Geissler EK. Piso P. Schlitt HJ. Dahlke MH. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55–60. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Comoli P. Ginevri F. Maccario R. Avanzini MA. Marconi M. Groff A. Cometa A. Cioni M. Porretti L, et al. Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant. 2008;23:1196–1202. doi: 10.1093/ndt/gfm740. [DOI] [PubMed] [Google Scholar]
- 32.Vieira JM., Jr. Mantovani E. Rodrigues LT. Delle H. Noronha IL. Fujihara CK. Zatz R. Simvastatin attenuates renal inflammation, tubular transdifferentiation and interstitial fibrosis in rats with unilateral ureteral obstruction. Nephrol Dial Transplant. 2005;20:1582–1591. doi: 10.1093/ndt/gfh859. [DOI] [PubMed] [Google Scholar]
- 33.Liu N. Tolbert E. Pang M. Ponnusamy M. Yan H. Zhuang S. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol. 2011;22:1064–1075. doi: 10.1681/ASN.2010090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Togel F. Isaac J. Hu Z. Weiss K. Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 35.Ponomaryov T. Peled A. Petit I. Taichman RS. Habler L. Sandbank J. Arenzana-Seisdedos F. Magerus A. Caruz A, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra J. Ma Q. Prada A. Mitsnefes M. Zahedi K. Yang J. Barasch J. Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 37.Gobe GC. Buttyan R. Wyburn KR. Etheridge MR. Smith PJ. Clusterin expression and apoptosis in tissue remodeling associated with renal regeneration. Kidney Int. 1995;47:411–420. doi: 10.1038/ki.1995.54. [DOI] [PubMed] [Google Scholar]
- 38.Vinuesa E. Sola A. Jung M. Alfaro V. Hotter G. Lipocalin-2-induced renal regeneration depends on cytokines. Am J Physiol Renal Physiol. 2008;295:F1554–F1562. doi: 10.1152/ajprenal.90250.2008. [DOI] [PubMed] [Google Scholar]
- 39.Homsi E. Janino P. Biswas SK. Mizuno S. Nakamura T. Lopes de Faria JB. Attenuation of glycerol-induced acute kidney injury by previous partial hepatectomy: role of hepatocyte growth factor/c-met axis in tubular protection. Nephron Exp Nephrol. 2007;107:e95–e106. doi: 10.1159/000109828. [DOI] [PubMed] [Google Scholar]
- 40.Bailly V. Zhang Z. Meier W. Cate R. Sanicola M. Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]