Abstract
The gamma-aminobutyric acid (GABA) type A receptor (GABAAR) is responsible for most fast synaptic inhibition in the adult brain. The GABAAR protein is composed of multiple subunits that determine the distribution, properties, and dynamics of the receptor. Several studies have shown that the Janus kinase/signal transducer and activator of transcription (JaK/STAT) and early growth response 3 (Egr3) signaling pathways can alter GABAAR subunit expression after status epilepticus (SE). In this study we investigated changes in these pathways after experimental TBI in the rat using a lateral fluid percussion injury (FPI) model. Our results demonstrated changes in the expression of several GABAAR subunit levels after injury, including GABAAR α1 and α4 subunits. This change appears to be transcriptional, and there is an associated increase in the phosphorylation of STAT3, and an increase in the expression of Egr3 and inducible cAMP element repressor (ICER) after FPI. These findings suggest that the activation of the JaK/STAT and Egr3 pathways after TBI may regulate injury-related changes in GABAAR subunit expression.
Key words: Egr3 pathway, GABAA receptor, JaK/STAT pathway, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major public health problem worldwide, and has been called the signature injury of the U.S. conflicts in Iraq and Afghanistan. Each year, TBI is a leading cause of mortality and neurological morbidity.1,2 In addition, up to 50% of TBI survivors can go on to develop epilepsy,3,4 and some develop temporal lobe epilepsy (TLE).5,6 Preventive and therapeutic treatments for many of these patients have been unsuccessful due to a lack of knowledge of the cellular and molecular mechanisms by which brain trauma causes TLE. This lack of understanding limits how current medications are being implemented and designed.
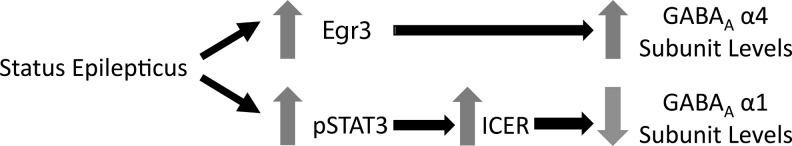
Trauma to the brain initiates a complex sequence of molecular responses involving a number of signaling pathways. One such pathway is the Janus kinase/signal transducer and activator of transcription (JaK/STAT) pathway, which has been shown to be activated after TBI,7–9 and following other brain insults such as stroke10,11 and status epilepticus (SE).12,13 Following SE, the JaK/STAT pathway has been shown to regulate gamma-aminobutyric acid (GABA) type A receptor (GABAAR) subunit α1 expression in the hippocampus by increasing the phosphorylation of STAT3.13 Phosphorylated STAT3 (pSTAT3) subsequently decreases transcription of the GABAAR α1 gene (Gabra1), by increasing the expression of the cyclic AMP response element-binding protein (CREB) family transcriptional inhibitor inducible cAMP element repressor (ICER).13 ICER binds to the Gabra1 promoter with phosphorylated CREB (pCREB) to inhibit the expression of Gabra1 (Fig. 1).13 The decreased levels of α1 subunit-containing GABAAR are thought to contribute to the increased hyperexcitability seen in the hippocampus of animals following SE, and to contribute to the subsequent development of TLE.14–17 Decreases in the GABAAR α1 subunit have been reported in injured hippocampus after TBI,18 but whether the JaK/STAT pathway regulates this decrease is not known. Other GABAAR subunits are also modulated after cerebral insults such as SE,14,15,19,20 and may also contribute to the alterations of neural excitability.
FIG. 1.
Overview of signaling pathways activated after status epilepticus (SE) that contribute to GABAAR subunit regulation (GABAAR, gamma-aminobutyric acid type A receptor; Egr3, early growth response 3; ICER, inducible cAMP element repressor; pSTAT3, phosphorylated STAT3).
Lateral fluid percussion injury (FPI) in the rat was used for our studies to determine if there were acute alterations in GABAAR subunit levels and the known signaling pathways that alter these receptor levels after other types of cerebral injury (such as SE). Lowenstein and associates,21 Toth and colleagues,22 and Gupta can co-workers,23 have shown that as early as 1 week after FPI there is enhanced excitability in the hippocampus, which indicates that TBI alters the balance of excitation and inhibition early post-injury. D'Ambrosio and associates,24 and Kharatishvili and colleagues,25 have shown that these animals can go on to develop epilepsy. The molecular mechanisms mediating early changes in excitability after TBI, as well as the contribution of early molecular and electrophysiological changes to later development of post-traumatic epilepsy (PTE), are not fully understood.
In this study we examined the levels of GABAAR subunits after lateral FPI in the rat, and found that the α1, α4, γ2, and δ subunit levels are decreased 1 week after injury, and that α2 and α5 subunit levels remain unchanged. We also found that two pathways known to regulate GABAAR subunit composition, the JaK/STAT and Egr3 pathways, are activated after FPI.
Methods
Establishment of dural access and fluid percussion injury
Adult male Sprague-Dawley rats (250–300 g) were anesthetized with 3–5% isoflurane via nose cone and placed in a stereotaxic head frame. After scalp incision and reflection, a 3-mm-diameter craniotomy was created centered at −3 mm from the bregma and 3.5 mm left of the sagittal suture. For support, a steel screw was placed in the right parietal bone opposite the craniotomy site. A female Luer-Lok hub was centered over the craniotomy site and bonded to the skull with cyanoacrylate adhesive. Dental acrylic was poured around the Luer hub and support screw. After the acrylic hardened, antibiotic ointment was placed around the injury cap and the animal was removed from the stereotaxic frame and returned to its cage to recover.
Fifteen to 20 h after craniotomy and Luer hub implantation, the animals were anesthetized with isoflurane in an induction chamber. The animal was then removed from the chamber, immediately connected to the FPI apparatus, and received a 20-msec pulse of pressurized fluid (2.5–3.0 atm, moderate to severe impact) on the intact dural surface before awakening from anesthesia.26 Sham-injured animals underwent establishment of dural access and were anesthetized and connected to the FPI apparatus, but the injury pulse was not triggered. A total of 81 animals underwent surgery, of which 34 were sham-injured animals and 47 were FPI animals. Out of the animals that underwent FPI, 37 animals lived, thus we had a survival rate of 79%. The University of Colorado Institutional Animal Care and Use Committee approved all the procedures described here.
After the FPI the animals were returned to their cages and allowed to recover for 6 h, 24 h, 48 h, or 1 week after injury. At these time points the animals were deeply anesthetized with isoflurane in an induction chamber, decapitated, and their brains were rapidly removed. The hippocampi were dissected and flash frozen for storage in a freezer at −80°C.
Western blot
Western blot was performed with modifications of published protocols.17 Protein (25 μg) extracted from whole hippocampi was loaded into 8% SDS-polyacrylamide gels and run for 1.5 h at 115 V. The blots were then transferred to nitrocellulose membranes and blocked in 5% milk/trisphosphate-buffered saline with Tween-20 (TBS-T). The membranes were incubated with rabbit polyclonal antibodies raised against STAT3 phosphorylated at Tyr705 (anti-pSTAT3) (1:1000; Cell Signaling Technologies, Danvers, MA), STAT3 (1:2000; Cell Signaling Technologies), α1 (1:5000; Millipore), α2 (1:2000; Millipore), α4 (1:2000; Millipore), α5 (1:1000; Millipore), δ (1:1000; Millipore), or β actin (1:40,000; Sigma-Aldrich, St. Louis, MO) overnight at 4°C in 5% bovine serum albumin/TBS-T (for pSTAT3 and STAT3), or 1% milk/TBS-T (for GABAAR), then washed and incubated with anti-rabbit antibody (1:10,000) conjugated to horseradish peroxidase (HRP) for 1 h. Protein bands were detected with the use of chemiluminescent solution (Pierce Biotechnology, Rockford, IL), then the membranes were stripped and reprobed with rabbit polyclonal antibody raised against total STAT3 (anti-STAT3) (1:2000; Cell Signaling Technologies), or β-actin, and bands were quantified using Image J software (National Institutes of Health [NIH]), and expressed as percent change with respect to mean control values in the same run (defined as 100%).
RT-PCR
RNA was extracted from whole hippocampi with the use of a Trizol reagent protocol (Invitrogen, Carlsbad, CA). To synthesize complementary DNA (cDNA), 1 μg of RNA was separated and processed with the SuperScript II reverse transcription kit (Invitrogen), according to the manufacturer's instructions, and then diluted 1:4 for storage and subsequent RT-PCR. For RT-PCR reactions, each sample was run in triplicate and each 25-μL reaction contained 1.25 μL ICER, Egr3, GABAAR subunit α1, GABAAR subunit α4, or cyclophilin (ppia) Taqman gene expression primer/probe sets from Applied Biosystems (Foster City, CA), 12.5 μL of Taqman Master mix, and 10 μL of sample cDNA. RT-PCR was formed on the SDS-7500 PCR machine (Applied Biosystems). The RT-PCR runs consisted of 1 cycle of 50°C for 2 min, then 1 cycle of 95°C for 10 min, and 40 cycles of 95°C for 15 sec, and 60°C for 1 min. All values were normalized to cyclophilin expression in the same samples to control for loading variability, then expressed as fold change with respect to mean control values in the same run (defined as 1).
Statistical analysis
Statistical significance was set at p<0.05. All calculations were done using InStat software. Mean amounts of pSTAT3 and each GABAAR subunit protein assessed using Western blotting were compared between injured and sham-injured groups using the Student's t-test.
Mean RT-PCR expression of α1, α4, ICER, and Egr3 were compared between injured and sham-injured controls for the ipsilateral and contralateral brain regions (relative to FPI) using the Student's t-test, or the Mann-Whitney U test, as appropriate for non-parametric data.
Results
GABAAR subunit levels change after FPI
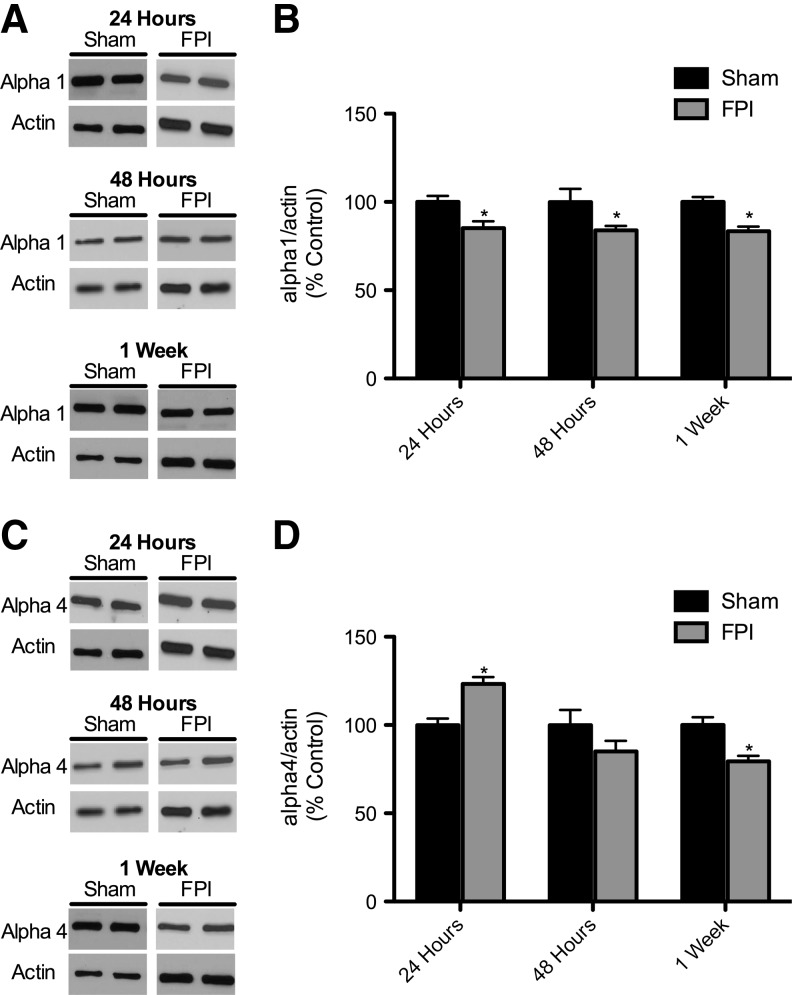
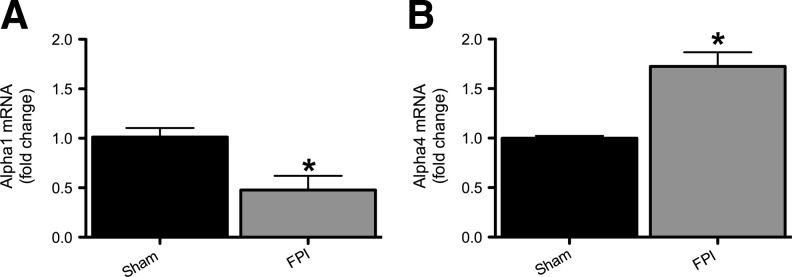
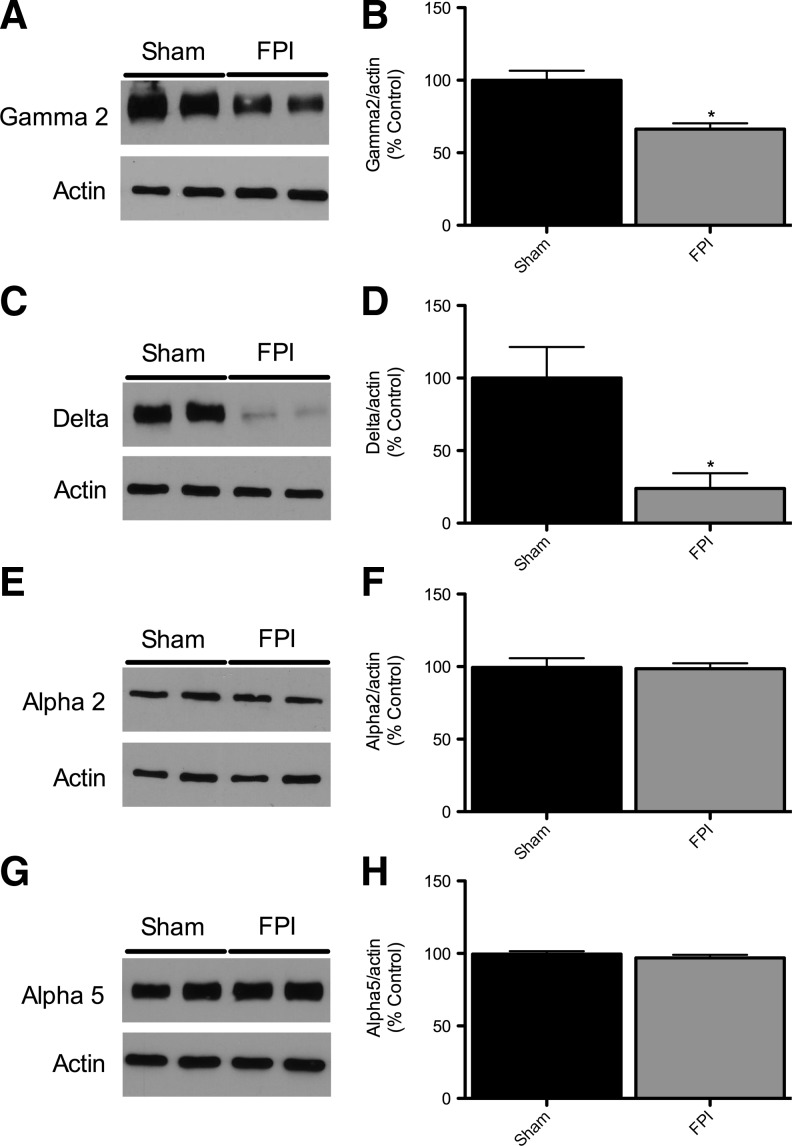
Figure 2 shows GABAAR subunit protein levels in hippocampi in sham-injured and FPI animals. Protein levels of the α1 subunit showed a significant decrease at 24 h, 48 h, and 1 week post-injury in the ipsilateral hippocampus (Fig. 2A and B). As shown in Figure 2C and D, α4 subunit levels were significantly increased at 24 h after injury, were not significantly different from controls at 48 h, and were significantly decreased compared to sham-injured controls at 1 week post-injury. There was no significant difference in α1 and α4 subunit levels at any time point in the hippocampus contralateral to the FPI compared to sham-injured controls (data not shown). To investigate whether the α1 and α4 subunit level changes at 24 h post-injury were due to transcriptional regulation we used quantitative RT-PCR on whole hippocampus samples 6 h after injury to determine the mRNA levels for these subunits. Figure 3 shows that in the ipsilateral hippocampus 6 h after FPI there was decreased expression of the α1 subunit (0.4-fold relative to controls), and an increase in the α4 subunit expression (1.7-fold relative to controls; Fig. 3A and B). There was no statistically significant difference in mRNA levels in the contralateral hippocampus compared to sham-injured controls (data not shown). Figure 4 shows that γ2 and δ subunit levels were significantly decreased 1 week after injury, but that the α2 and α5 subunits were unchanged compared to sham-injured controls. There was no significant difference in the γ2, δ, α2, or α5 subunit levels in the contralateral hippocampus compared to sham-injured controls (data not shown).
FIG. 2.
Temporal profile of GABAA α1 and α4 receptor subunits after fluid percussion injury (FPI). (A) Representative Western blots from whole hippocampi of rats 24 h, 48 h, and 1 week after FPI probed with anti-GABAAR α1 and β-actin antibodies. (B) Quantification of α1 blots showed a significant decrease at 24 h through 1 week after FPI injury relative to controls. α1 Levels were normalized to β-actin levels and expressed as percent change compared to sham-injured controls. (C) Representative Western blots from whole hippocampi of rats 24 h, 48 h, and 1 week after FPI probed with anti-GABAAR α4 and β-actin antibodies. (D) Quantification of α4 blots showed a significant increase at 24 h after FPI, but at 1 week there was a significant decrease relative to controls. α4 Levels were normalized to β-actin levels and expressed as percent change compared to sham animals (24 h: n=7 for sham, 6 for FPI; 48 h: n=6 for sham, 8 for FPI; 1 week: n=7 for sham, 7 for FPI; GABAAR, gamma-aminobutyric acid type A receptor).
FIG. 3.
α1 Expression is decreased and α4 expression is increased in injured hippocampi 6 h after fluid percussion injury (FPI). mRNA levels of α1 and α4 were quantified using RT-PCR analysis and represented as histograms showing the fold change of α1 (A) and α4 (B) 6 h after FPI and in sham-injured controls. α1 and α4 mRNA levels were normalized to cyclophilin mRNA levels in the same samples and expressed as fold change compared to sham animals (defined as 1; *p<0.05; n=4 for sham, 4 for FPI; RT-PCR, reverse transcriptase polymerase chain reaction).
FIG. 4.
GABAA receptor subunit γ2 is decreased, while α2 and α5 are unchanged, 1 week after fluid percussion injury (FPI). (A) Representative Western blots from whole hippocampi of rats 1 week after FPI probed with anti-GABAAR γ2 and β-actin antibodies. (B) Quantification of γ2 blots shows that the γ2 subunit is significantly decreased 1 week after FPI relative to sham-injured controls. (C) Representative Western blots from whole hippocampi of rats 1 week after FPI probed with anti-GABAAR δ and β-actin antibodies. (D) Quantification of δ blots shows that the δ subunit is significantly decreased 1 week after FPI relative to sham animals. (E) Representative Western blots from whole hippocampi of rats 1 week after FPI probed with anti-GABAAR α2 and β-actin antibodies. (F) Quantification of α2 blots shows that the α2 subunit is unchanged 1 week after FPI relative to sham animals. (G) Representative Western blots from whole hippocampi of rats 1 week after FPI probed with anti-GABAAR α5 and β-actin antibodies. (H) Quantification of α5 blots shows that the α5 subunit is unchanged 1 week after FPI. GABAAR subunit levels were normalized to β-actin levels and expressed as percent change compared to sham animals (*p<0.001; n=7 for sham and 7 for FPI; GABAAR, gamma-aminobutyric acid type A receptor).
FPI transiently activates the JaK/STAT pathway and affects downstream expression of ICER
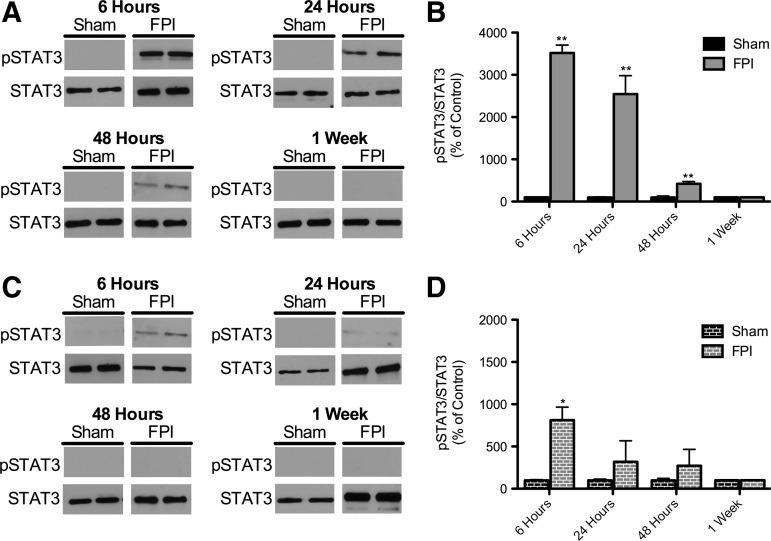
As shown in Figure 5A and B, there was a statistically significant increase in the levels of pSTAT3 in the ipsilateral hippocampus compared to sham-injured controls at 6 h after injury. This increase declines temporally and at one week there is no difference in the levels of pSTAT3 when compared to controls. Figure 5C and D shows that the contralateral hippocampus there was a statistically significant increase in the pSTAT3 levels 6 hours after injury but at 24 hours after FPI the levels were not statistically different from sham injured controls. This suggests that the pathway is transiently being activated after FPI.
FIG. 5.
Phosphorylated STAT3 (p STAT3) levels in injured hippocampus after fluid percussion injury (FPI). (A) Representative Western blots of protein homogenates from ipsilateral whole hippocampi (relative to FPI) of rats 6 h, 24 h, 48 h, and 1 week after FPI probed with pSTAT3 and STAT3 antibodies. (B) Quantification of pSTAT3 levels from FPI and sham-injured controls. (C) Representative Western blots of protein homogenates from contralateral whole hippocampi (relative to FPI) of rats 6 h, 24 h, 48 h, and 1 week after FPI probed with pSTAT3 and STAT3 antibodies. (D) Quantification of pSTAT3 levels from FPI and sham-injured controls. pSTAT3 levels were normalized to STAT3 levels and expressed as percent change compared to sham animals (*p<0.05, **p<0.001 for comparison of sham and FPI animals at the same time point; 6 h: n=5 for sham, 7 for FPI; 24 h: n=7 for sham, 6 for FPI; 48 h: n=6 for sham, 8 for FPI; 1 week: n=7 for sham, 7 for FPI; STAT3, signal transducer and activator of transcription 3).
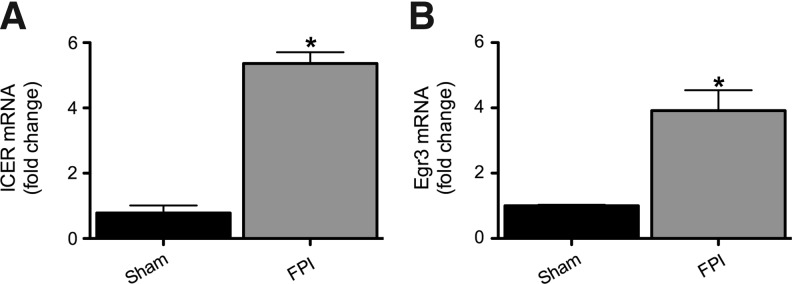
Figure 6A shows that ICER mRNA levels in the hippocampus ipsilateral to the injury were significantly increased 6 h after injury compared to sham-injured controls, although there was no difference between groups in the contralateral hippocampus (data not shown).
FIG. 6.
Inducible cAMP element repressor (ICER) and early growth response 3 (Egr3) expression are increased in injured hippocampi 6 h after fluid percussion injury (FPI). mRNA levels of ICER and Egr3 were quantified using RT-PCR analysis and represented as histograms showing the fold change of ICER (A) and Egr3 (B), 6 h after FPI in injured and sham-injured controls. ICER and Egr3 mRNA levels were normalized to cyclophilin mRNA levels in the same samples and expressed as fold change compared to shams (defined as 1; *p<0.05; n=4 for sham, 4 for FPI; RT-PCR, reverse transcriptase polymerase chain reaction).
FPI increases Egr3 levels
The Egr3 pathway has been shown to be a critical mediator of α4 upregulation after SE.27 Figure 6B shows that Egr3 mRNA levels in the hippocampus ipsilateral to injury were significantly increased 6 h after injury, compared to sham injured controls, although there was no difference between groups in the contralateral hippocampus (data not shown).
Discussion
In this study, we demonstrated that hippocampal injury after FPI alters the levels of protein and mRNA of several GABAAR subunits, activates the JaK/STAT pathway, and elevates Egr3 mRNA levels. The GABAAR α1 subunit level is decreased 24 h after injury, and remains lower than sham-injured controls for at least 1 week after FPI. The α4 subunit level is increased 24 h after injury, and then is lower than sham-injured controls by 1 week post-injury. We also found that mRNA expression of the α1 subunit was statistically significantly decreased, and that of the α4 subunit mRNA was statistically significantly increased, at 6 h after FPI, suggesting that the changes in the GABAAR subunit levels are at least in part due to transcriptional regulation.
Several other GABAAR subunits were analyzed to determine if their levels were also changed after injury. Our goal for looking at multiple GABAAR subunits was to determine if there was an overall decrease in all subunits at 1 week, or if there was evidence to suggest subunit-specific regulatory processes occurring after FPI. Further, in some transgenic mice with GABAAR subunit mutations, as well as after SE in the rat, it has been shown that changes in expression of one GABAAR subunit can be associated with unanticipated regulation of other subunits.14,28–31 While the γ2 and δ subunit levels were found to decrease at 1 week post-injury, the α2 and α5 subunit levels showed no significant difference between FPI animals and sham-injured controls at 1 week post-injury. These findings suggest that subunit changes are in part due to subunit-specific regulatory processes.
In a recent study using FPI in the rat, a reduction of approximately 55% was found in GABAAR α1 subunit levels compared to sham-injured controls, with no change seen in the α2 and α5 subunits in the ipsilateral hippocampus 24 h and 1 week post-injury, results similar to ours.18 These authors did not examine the α4 subunit or any of the signaling pathways that regulate GABAAR subunits. Another study using the FPI model in the rat showed that after experimental TBI there is a progressive loss of phasic GABAAR-mediated inhibition, which is suggestive of alterations in the GABAAR. This study, however, only showed GABAAR subunit mRNA expression changes in α4 (a ∼ 52% increase in expression compared to controls), and β1 (a ∼ 27% increase in expression compared to controls).32 The difference in the expression levels seen between that study and ours could be attributed to when the samples were collected, which was 6 h after injury in the current study, while the Pavlov group32 measured GABAAR mRNA levels 4 months after injury.32
Studies utilizing another injury model, controlled cortical impact (CCI) in the rat, showed a reduction in γ2 subunit levels ∼5–9 months after injury, similar to those found in the current study, but showed no change in α1 and α4 and an increase in δ.33 These differences may be attributed to the difference in injury methods (CCI versus FPI), the time point of collecting the samples, or may be because the Kharlamov group33 only analyzed the contralateral hippocampus, a brain region not known to be extensively affected in the CCI model. Electrophysiological studies done using the same injury model found that there is an increase in the GABAAR-mediated tonic currents, a loss of diazepam potentiation, and an increase in furosemide inhibition of synaptic GABAARs in dentate granule cells (DGCs), ipsilateral to the injury site 90 days after injury.34 These physiological changes are consistent with a decrease in α1-containing receptors, and an increase in α4-containing receptors in DGCs, similar to that seen in rats with epilepsy following SE.14 In addition, the γ2 and δ subunit levels have been shown to influence tonic and phasic synaptic inhibition and GABAAR location.20,35–44 Alterations of all of these levels, which we also saw after FPI, may result in altered GABAergic signaling, and could be a contributing factor to the development of TLE after head trauma.
Previous studies have shown that the JaK/STAT pathway mediates a decrease in α1 subunit levels after SE.13,14 SE results in phosphorylation of STAT3, which upregulates expression of ICER, and subsequently binds to the Gabra1 promoter and decreases transcription of Gabra1 in the hippocampus of rats.13 Multiple types of injuries to the hippocampus can lead to the development of TLE (including SE, ischemia, and head trauma). Interestingly, JaK/STAT pathway activation has been shown to occur in each of these types of injury models.7,8,9,10,11,13
In the current study we found that pSTAT3 levels were significantly increased in the injured hippocampus 6 h after FPI, and that there is a subsequent decline in pSTAT3 levels back to baseline levels by 1 week after injury. Our data are consistent with data from Oliva and associates,7 suggesting that the JaK/STAT pathway is transiently activated in the ipsilateral hippocampus after cerebral injury.7 Oliva and colleagues7 also showed pSTAT3 co-localizing, mostly with glial fibrillary acidic protein (GFAP)-positive cells, and that pSTAT3 was transcriptionally regulating known STAT3-regulatory genes such as nitric oxide synthase 2.7 However, they did not look for alterations in GABAAR genes, subunit levels, or ICER or Egr3 expression. Zhao and associates performed Western blot analysis and immunohistochemistry, which showed that in the injured ipsilateral parietal cortex, pSTAT3 signal was enhanced at acute time points, and that pSTAT3 was co-localized with both NeuN- and GFAP-positive cells after TBI.9 It remains to be determined if the activation of the JaK/STAT pathway after TBI directly alters the expression of GABAAR subunits as has been shown after SE, and is a contributing factor in the development of TLE after TBI.
Last, we looked at the Egr3 mRNA levels 6 h after FPI because the α4 subunit was increased 24 h after injury, and previous studies in our lab have shown that the Egr3 pathway can regulate transcription of the α4 subunit.27,45,46 We have shown that Egr3 mRNA levels were increased in the ipsilateral hippocampus after TBI, suggesting that this pathway may be regulating the increase seen in α4 subunit levels 24 h after injury. The mechanism mediating the reduction in α4 subunit levels 1 week post-injury remains uncertain. Future studies will be required to elucidate these mechanisms, as well as to understand the functional role of Egr3 changes after FPI.46
Our study does have several limitations. First, the whole hippocampus was used to determine GABAAR subunit levels, as well as for the determination of pSTAT3 levels and expression of ICER and Egr3. Thus we are unable to determine where in the hippocampus these changes were occurring and in which cell type. Second, β-actin was used for normalization in this study to control for differences in protein loading, and β-actin levels can be affected by cell death and other regulatory processes. However, normalization of subunit densitometry to total protein loaded in each well produced results similar to those found with normalization to β-actin, and there was no difference in the expression of β-actin (normalized to total protein loaded) in the FPI samples compared to sham-injured controls (data not shown). Thus we could find no evidence that intergroup differences in β-actin levels were impacting our results. Further, we could find no studies in the literature suggesting that β-actin is actively regulated after FPI. Third, our data suggests an association, but not necessarily a causal relationship, between activation of the JaK/STAT and Egr3 pathways and GABAAR alterations. A causal relationship has been shown more definitively in TLE after SE,13,27,45,46 suggesting that a causal relationship between the two processes in TLE after TBI is plausible, if yet unproven. Fourth, we have only reported a set of molecular changes after TBI, but do not examine potential functional consequences of these changes. Results of previous studies examining inhibitory function utilizing similar injury models, however, are consistent with changes that we show.32,34 Lastly, while the current findings demonstrate changes in the GABAAR after TBI, they do not establish that these changes contribute to the later development of epilepsy. Establishing this connection will require future studies in which GABAAR changes after TBI are prevented or reversed, and showing that subsequent epilepsy outcome is altered.
Conclusions
The current findings demonstrate that levels of the α1, α4, γ2, and δ GABAAR subunits are decreased in injured hippocampus at 1 week after experimental TBI, and that α2 and α5 subunit levels remain unchanged, suggesting that there is subunit-specific regulation of the GABAAR following TBI. We also found that mRNA expression of the α1 subunit was decreased, and that the α4 subunit mRNA was increased, suggesting that the changes in these GABAAR subunits are at least in part due to transcriptional regulation. Lastly, we found that two pathways known to regulate GABAAR subunit composition in other models of cerebral injury, the JaK/STAT and Egr3 pathways, are activated after FPI. Based on evidence from these other models, we can speculate that the activation of these pathways may mediate altered levels of GABAAR subunits, and may contribute to the early hippocampal hyperexcitability previously reported after TBI, and may lead to the development of later epilepsy. Future studies are needed to determine if there is a causal relationship between JaK/STAT activation, Egr3 elevations, and changes in GABAAR subunit composition, as well as to determine the contribution of these injury-related changes to the occurrence of experimental post-traumatic epilepsy.
Acknowledgments
The funding for this research was provided by Department of Defense (DoD) award number W81XWH-11-1-0501, and by NIH/NCRR Colorado CTSI Grant no. TL1 RR025778. The contents are the authors' sole responsibility and do not necessarily represent official DoD or NIH views.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Sosin D.M. Sniezek J.E. Thurman D.J. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10:47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- 2.Thurman D.J. Alverson C. Dunn K.A. Guerrero J. Sniezek J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Salazar A.M. Jabbari B. Vance S.C. Grafman J. Amin D. Dillon J.D. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- 4.Marcikic M. Melada A. Kovacevic R. Management of war penetrating craniocerebral injuries during the war in Croatia. Injury. 1998;29:613–618. doi: 10.1016/s0020-1383(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Arrastia R. Agostini M.A. Frol A.B. Mickey B. Fleckenstein J. Bigio E. Van Ness P.C. Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch. Neurol. 2000;57:1611–1616. doi: 10.1001/archneur.57.11.1611. [DOI] [PubMed] [Google Scholar]
- 6.Hudak A.M. Trivedi K. Harper C.R. Booker K. Caesar R.R. Agostini M. Van Ness P.C. Diaz-Arrastia R. Evaluation of seizure-like episodes in survivors of moderate and severe traumatic brain injury. J. Head Trauma Rehabil. 2004;19:290–295. doi: 10.1097/00001199-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Oliva A.A., Jr. Kang Y. Sanchez-Molano J. Furones C. Atkins C.M. STAT3 signaling after traumatic brain injury. J. Neurochem. 2011;120:710–720. doi: 10.1111/j.1471-4159.2011.07610.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J. Li G. Zhang Y. Su X. Hang C. The potential role of JAK2/STAT3 pathway on the anti-apoptotic effect of recombinant human erythropoietin (rhEPO) after experimental traumatic brain injury of rats. Cytokine. 2011a;56:343–350. doi: 10.1016/j.cyto.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J.B. Zhang Y. Li G.Z. Su X.F. Hang C.H. Activation of JAK2/STAT pathway in cerebral cortex after experimental traumatic brain injury of rats. Neurosci. Lett. 2011b;498:147–152. doi: 10.1016/j.neulet.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Planas A.M. Soriano M.A. Berruezo M. Justicia C. Estrada A. Pitarch S. Ferrer I. Induction of Stat3, a signal transducer and transcription factor, in reactive microglia following transient focal cerebral ischaemia. Eur. J. Neurosci. 1996;8:2612–2618. doi: 10.1111/j.1460-9568.1996.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S. Tanaka K. Nogawa S. Dembo T. Kosakai A. Fukuuchi Y. Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp. Neurol. 2001;170:63–71. doi: 10.1006/exnr.2001.7701. [DOI] [PubMed] [Google Scholar]
- 12.Choi J.S. Kim S.Y. Park H.J. Cha J.H. Choi Y.S. Kang J.E. Chung J.W. Chun M.H. Lee M.Y. Upregulation of gp130 and differential activation of STAT and p42/44 MAPK in the rat hippocampus following kainic acid-induced seizures. Brain Res. Mol. Brain Res. 2003;119:10–18. doi: 10.1016/j.molbrainres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Lund I.V. Hu Y. Raol Y.H. Benham R.S. Faris R. Russek S.J. Brooks-Kayal A.R. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci. Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks-Kayal A.R. Shumate M.D. Jin H. Rikhter T.Y. Coulter D.A. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 15.Peng Z. Huang C.S. Stell B.M. Mody I. Houser C.R. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J. Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang G. Raol Y.H. Hsu F.C. Coulter D.A. Brooks-Kayal A.R. Effects of status epilepticus on hippocampal GABAA receptors are age-dependent. Neuroscience. 2004;125:299–303. doi: 10.1016/j.neuroscience.2004.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raol Y.H. Lund I.V. Bandyopadhyay S. Zhang G. Roberts D.S. Wolfe J.H. Russek S.J. Brooks-Kayal A.R. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J. Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson C.J. Meyer R.C. Hamm R.J. Traumatic brain injury and the effects of diazepam, diltiazem, and MK-801 on GABA-A receptor subunit expression in rat hippocampus. J. Biomed. Sci. 2010;17:38. doi: 10.1186/1423-0127-17-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun C. Mtchedlishvili Z. Erisir A. Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the alpha4 subunit of GABA(A) receptors in an animal model of epilepsy. J. Neurosci. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N. Wei W. Mody I. Houser C.R. Altered localization of GABA(A) receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J. Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowenstein D.H. Thomas M.J. Smith D.H. McIntosh T.K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toth Z. Hollrigel G.S. Gorcs T. Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J. Neurosci. 1997;17:8106–8117. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta A. Elgammal F.S. Proddutur A. Shah S. Santhakumar V. Decrease in tonic inhibition contributes to increase in dentate semilunar granule cell excitability after brain injury. J. Neurosci. 2012;32:2523–2537. doi: 10.1523/JNEUROSCI.4141-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Ambrosio R. Fairbanks J.P. Fender J.S. Born D.E. Doyle D.L. Miller J.W. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharatishvili I. Nissinen J.P. McIntosh T.K. Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Frey L.C. Hellier J. Unkart C. Lepkin A. Howard A. Hasebroock K. Serkova N. Liang L. Patel M. Soltesz I. Staley K. A novel apparatus for lateral fluid percussion injury in the rat. J. Neurosci. Methods. 2009;177:267–272. doi: 10.1016/j.jneumeth.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts D.S. Hu Y. Lund I.V. Brooks-Kayal A.R. Russek S.J. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J. Biol. Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- 28.Sur C. Wafford K.A. Reynolds D.S. Hadingham K.L. Bromidge F. Macaulay A. Collinson N. O'Meara G. Howell O. Newman R. Myers J. Atack J.R. Dawson G.R. McKernan R.M. Whiting P.J. Rosahl T.W. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J. Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kralic J.E. Sidler C. Parpan F. Homanics G.E. Morrow A.L. Fritschy J.M. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J. Comp. Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- 30.Schneider Gasser E.M. Duveau V. Prenosil G.A. Fritschy J.M. Reorganization of GABAergic circuits maintains GABAA receptor-mediated transmission onto CA1 interneurons in alpha1-subunit-null mice. Eur. J. Neurosci. 2007;25:3287–3304. doi: 10.1111/j.1460-9568.2007.05558.x. [DOI] [PubMed] [Google Scholar]
- 31.Liang J. Suryanarayanan A. Chandra D. Homanics G.E. Olsen R.W. Spigelman I. Functional consequences of GABAA receptor alpha 4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcoholism Clin. Exper. Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 32.Pavlov I. Huusko N. Drexel M. Kirchmair E. Sperk G. Pitkanen A. Walker M.C. Progressive loss of phasic, but not tonic, GABAA receptor-mediated inhibition in dentate granule cells in a model of post-traumatic epilepsy in rats. Neuroscience. 2011;194:208–219. doi: 10.1016/j.neuroscience.2011.07.074. [DOI] [PubMed] [Google Scholar]
- 33.Kharlamov E.A. Lepsveridze E. Meparishvili M. Solomonia R.O. Lu B. Miller E.R. Kelly K.M. Mtchedlishvili Z. Alterations of GABA(A) and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 2011;95:20–34. doi: 10.1016/j.eplepsyres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Mtchedlishvili Z. Lepsveridze E. Xu H. Kharlamov E.A. Lu B. Kelly K.M. Increase of GABAA receptor-mediated tonic inhibition in dentate granule cells after traumatic brain injury. Neurobiol. Dis. 2010;38:464–475. doi: 10.1016/j.nbd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Somogyi P. Fritschy J.M. Benke D. Roberts J.D. Sieghart W. The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 36.Nusser Z. Sieghart W. Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sur C. Farrar S.J. Kerby J. Whiting P.J. Atack J.R. McKernan R.M. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acidA receptor in rat thalamus. Mol. Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- 38.Sassoe-Pognetto M. Panzanelli P. Sieghart W. Fritschy J.M. Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites. J. Comp. Neurol. 2000;420:481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Wei W. Zhang N. Peng Z. Houser C.R. Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semyanov A. Walker M.C. Kullmann D.M. Silver R.A. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Sun C. Sieghart W. Kapur J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia F. Pignataro L. Schofield C.M. Yue M. Harrison N.L. Goldstein P.A. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J. Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 43.Liang J. Zhang N. Cagetti E. Houser C.R. Olsen R.W. Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J. Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodkin H.P. Joshi S. Mtchedlishvili Z. Brar J. Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J. Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts D.S. Raol Y.H. Bandyopadhyay S. Lund I.V. Budreck E.C. Passini M.A. Wolfe J.H. Brooks-Kayal A.R. Russek S.J. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. Proc. Natl. Acad. Sci. USA. 2005;102:11894–11899. doi: 10.1073/pnas.0501434102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gangisetty O. Reddy D.S. Neurosteroid withdrawal regulates GABA-A receptor alpha4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience. 2010;170:865–880. doi: 10.1016/j.neuroscience.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]