Abstract
Traumatic spinal cord injury (SCI) leads to oxidative stress, calcium mobilization, glutamate toxicity, the release of proinflammatory factors, and depletion of reduced glutathione (GSH) at the site of injury. Induction of the Keap1/Nrf2/ARE pathway can alleviate neurotoxicity by protecting against GSH depletion, oxidation, intracellular calcium overload, mitochondrial dysfunction, and excitotoxicity. Sulforaphane (SF), an isothiocyanate derived from broccoli, is a potent naturally-occurring inducer of the Keap1/Nrf2/ARE pathway, leading to upregulation of genes encoding cytoprotective proteins such as NAD(P)H: quinone oxidoreductase 1, and GSH-regulatory enzymes. Additionally, SF can attenuate inflammation by inhibiting the nuclear factor-κB (NF-κB) pathway, and the enzymatic activity of the proinflammatory cytokine macrophage inhibitory factor (MIF). Our study examined systemic administration of SF in a rat model of contusion SCI, in an effort to utilize its indirect antioxidant and anti-inflammatory properties to decrease secondary injury. Two doses of SF (10 or 50 mg/kg) were administered at 10 min and 72 h after contusion SCI. SF (50 mg/kg) treatment resulted in both acute and long-term beneficial effects, including upregulation of the phase 2 antioxidant response at the injury site, decreased mRNA levels of inflammatory cytokines (i.e., MMP-9) in the injured spinal cord, inactivation of urinary MIF tautomerase activity, enhanced hindlimb locomotor function, and an increased number of serotonergic axons caudal to the lesion site. These findings demonstrate that SF provides neuroprotective effects in the spinal cord after injury, and could be a candidate for therapy of SCI.
Key words: neuroprotection, spinal cord injury, sulforaphane
Introduction
Management of traumatic spinal cord injuries (SCI) is a major challenge with limited options. Immediate primary destruction of cells at the site of injury is followed by a cascade of secondary processes that include oxidative stress, inflammation, increased blood–spinal cord barrier permeability, ischemia of surrounding tissues, calcium overload, excitotoxicity, demyelination, glial scar formation, and axonal degeneration.1,2 Resident and infiltrating inflammatory cells also produce reactive oxygen species (ROS), release proinflammatory cytokines, and promote the activity of matrix metalloproteinases (MMPs), all of which can destroy the spinal cord tissue that survives the initial mechanical trauma.3,4
Preclinical studies have indicated that pharmacological approaches that minimize secondary damage improve both neurological and functional outcomes after SCI. In a groundbreaking study of a rat model of compression SCI, Kamencic and associates5 demonstrated that as early as 1 h after injury and extending to at least 48 h, the reduced glutathione (GSH) and glutathione reductase levels in the spinal cord were significantly lower, with a depletion gradient projecting both rostrally and caudally from the site of injury. Systemic treatment with a cysteine precursor (L-2-oxothiazolidine-4-carboxylate) increased levels of GSH and glutathione reductase in the spinal cord, and improved tissue sparing and recovery of hindlimb motor function after injury.5 These findings highlight the importance of restoring the levels and functional capacity of GSH, the principal endogenous cellular antioxidant. Many recent studies have shown that GSH levels are elaborately controlled in nervous tissue, and that glial cells are the principal protectors of neurons and provide the cysteine precursors for neuronal GSH synthesis.6–11
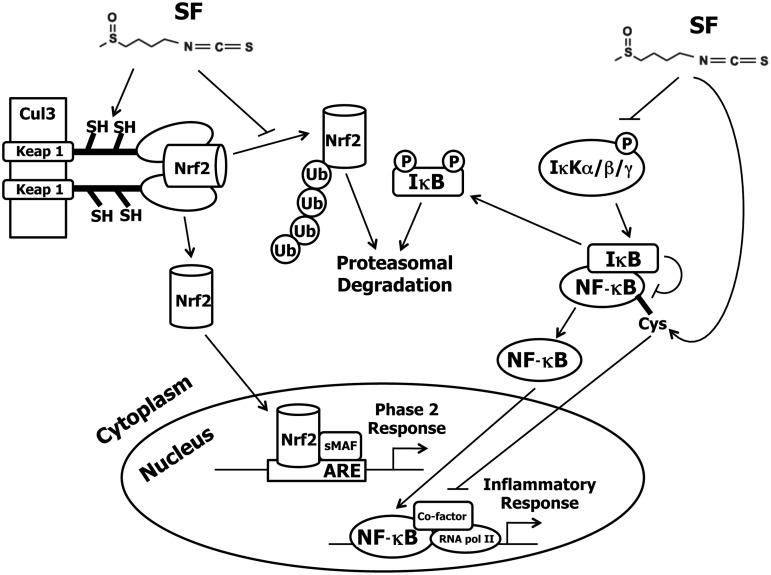
On the basis of these considerations we tested the hypothesis that the secondary damaging processes of SCI could be minimized by upregulating the intrinsic endogenous protective systems of neuronal tissue. The isothiocyanate (ITC) sulforaphane [SF; CH3S(O)(CH2)4NCS], which was isolated from broccoli, is a highly potent inducer of a network of cytoprotective phase 2 enzymes.12 SF has recently attracted widespread attention because it protects aerobic cells, including those of the nervous system, against oxidants, electrophiles, and inflammation.13–16 The chemoprotective effects of SF are mediated by upregulation of the Keap1/Nrf2/ARE pathway, and other anti-inflammatory mechanisms, such as inhibition of the nuclear factor-κB (NF-κB) pathway. The mechanisms of control of these regulatory pathways are described schematically in Figure 1. The cytoprotective genes code for enzymes that exert antioxidant, anti-inflammatory, and detoxification functions. They protect cells against the damage caused by reactive oxygen and nitrogen species, electrophiles, and other toxins, and modulate the redox balance of cells, especially with respect to GSH.
FIG. 1.
Transcriptional regulation of the cytoprotective phase 2 genes under control of the transcriptional factor nuclear factor-E2 p45-related factor 2 (Nrf2). Inducers such as sulforaphane (SF) modify specific reactive cysteine residues of the repressor of the Nrf2 transcription factor Kelch-like ECH-associated protein 1 (Keap1).50–54 Keap1 functions to sequester Nrf2 in the cytoplasm and to target Nrf2 for degradation via the 26S proteasome by association with the ubiquitin ligase Cullin 3-based E3 ligase (Cul3). Once Keap1 is modified by SF, Nrf2 is able to bypass the proteasomal degradation pathway and translocate to the nucleus, where it binds in conjunction with small Maf (sMaf) proteins to the antioxidant response elements (ARE) in the upstream regulatory regions of cytoprotective gene promoters. These genes encode proteins and enzymes that protect against oxidants, electrophiles, and radiation. The nuclear factor-κB (NF-κB) proinflammatory pathway can also be disrupted by SF through inhibition of IκB phosphorylation, thus blocking NF-κB translocation to the nucleus,55,56 or by directly interacting with cysteine residues in the DNA-binding region of the NF-κB transcription factor.57
The anti-inflammatory effects of SF are mediated at least in part through inhibition of the NF-κB pathway, resulting in decreased expression of many proinflammatory factors, such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α), and various interleukin cytokines (e.g., IL-1β and IL-6).17 This SF-mediated inhibition of NF-κB could play a role in promoting axon growth after injury, since transgenic inhibition of astroglial NF-κB has been shown not only to reduce inflammation in mice after contusion SCI, but also to reduce the chronic gliotic response, leading to an environment more conducive to axonal sparing and sprouting.18 SF also inactivates the tautomerase activity of the proinflammatory cytokine macrophage inhibitory factor (MIF), which may reduce the proinflammatory effects of MIF by blocking its interaction with the CD74 receptor of macrophages.19–23
In the present studies, we first established that systemic intraperitoneal (IP) administration of SF to rats resulted in adequate SF levels in the injured and normal spinal cord to upregulate cytoprotective genes. We then showed that SF (50 mg/kg) given at 10 min and 72 h after injury reduced the levels of inflammatory biomarkers in the urine and spinal cord after contusive SCI; in addition, this SF dose improved neurological recovery as assessed by both behavioral tests and by sparing or sprouting of serotonergic axons caudal to the injury site 5 weeks after injury. While our work was in progress, two sets of researchers reported that SF treatment of rodents subjected to SCI leads to improvements in functional and anatomical recovery.24,25 These studies are in full agreement with our findings that SF treatment after SCI improves hindlimb behavioral outcomes. Furthermore, increases in serotonergic axons caudal to the lesion site in injured SF-treated rats suggest a further protective role for SF administration after SCI.
Methods
Spinal cord injury
All procedures were approved by the Johns Hopkins Animal Care and Use Committee, and were consistent with federal law and National Institutes of Health (NIH) regulations. Johns Hopkins Medical Institutions are accredited by the American Association for Accreditation of Laboratory Animal Care. Female Sprague-Dawley rats (250–280 g; Charles River, Wilmington, MA) had ad libitum access to an inducer-free AIN-76A diet (Harlan Inc., Fredrick, MD) for 1 week prior to and during all experiments. The animals were anesthetized by IP injection of a mixture of ketamine HCl (60 mg/kg; Phoenix Pharmaceuticals, St. Joseph, MD) and dexmedetomidine (0.4 mg/kg; Pfizer Animal Health, New York, NY). A laminectomy was performed at the T9 vertebral level without disrupting the dura. A contusion injury of 200 kdyn was delivered using the Infinite Horizon impactor (Precision Systems and Instrumentation, Lexington, KY). Following injury, the dorsal musculature and skin were closed and the animals were revived from anesthesia using atipamezole (0.25 mg per animal IM; Pfizer). The animals recovered in a 30°C incubator for at least 2 h. Post-operative care for survival experiments included fluid supplementation (10 mL of lactated Ringer's solution SC; Hospira, Inc., Lake Forest, IL), and gentamicin (5 mg/kg IM; Quality Biological, Inc., Gaithersburg, MD), administered daily for 7 and 14 days, respectively. Manual bladder expression was performed twice daily until the micturition reflex returned (about 7 days).
Administration of sulforaphane
R,S-sulforaphane (LKT Laboratories, Inc., St. Paul, MN) was dissolved in corn oil (Sigma-Aldrich, St. Louis, MO). Two doses of SF, low dose (10 mg/kg) or high dose (50 mg/kg), in ∼250–280 μL corn oil were IP injected at 10 min and 72 h after injury. For short-term experiments, a single dose of SF (50 mg/kg) was administered IP 10 min after injury. Corn oil alone was used as the vehicle control.
Behavioral testing
Hindlimb locomotor score
Hindlimb motor function was assessed using the open-field Basso, Beattie, and Bresnahan (BBB)26 locomotor test on days 1, 4, and 7 following injury, and weekly thereafter. BBB subscores were calculated as previously described.27 The animals were evaluated by two experienced investigators who were blinded with respect to treatment group.
Horizontal ladder testing
Rats were assessed for their ability to cross a horizontal ladder with a fixed set of randomly spaced support bars at 3 and 5 weeks following injury. Four trials were video recorded for subsequent analysis over a 70-cm stretch. Foot-slip errors were designated as: minor (only the ankle joint fell below the plane of the ladder), or major (both the ankle and knee joint crossed the horizontal plane). Missteps were evaluated by a blinded examiner and calculated as the percentage of missteps per total number of attempted hindlimb steps.
CatWalk-assisted quantitative gait analysis
At 5 weeks after SCI, further gait analysis was performed using the Noldus CatWalk XT 9.0 system (Noldus Information Technology, Wageningen, The Netherlands).28 The data were segmented, including only the portion of the run that had the appropriate number of consecutive steps, speed, and speed variation. Runs were excluded from analysis if the rat stopped during the run, or if hindlimb footprints were not detected by the CatWalk software. Each animal had two to five runs which were analyzed based on these criteria. Three uninjured rats were subjected to the CatWalk test to establish a normal baseline for Sprague-Dawley female rats that were weight-matched to our study animals.
Cyclocondensation reaction and high-pressure liquid chromatography (HPLC) of rat tissue homogenates
At 4 h after injury, the animals (control, n=3; SF-treated [50 mg/kg], n=4) were anesthetized with the ketamine/dexmedetomidine mixture and euthanized by transcardial perfusion with cold Dulbecco PBS solution. Spinal cords (10 mm centered on the lesion epicenter), brains, and livers were harvested, frozen on dry ice, and stored at −80°C until use. Levels of SF metabolites (dithiocarbamates; DTCs) in the tissue homogenates were determined by a slight modification of the cyclocondensation reaction.29,30 For sample preparation, frozen tissues were homogenized in glass Dounce homogenizers with 0.25 M sucrose/10 mM Tris-HCl (pH 7.4). Sample homogenates (100- and 200-μL aliquots of each sample) were diluted to a volume of 0.5 mL with water. The reaction mixture (2.0 mL total volume) consisted of 0.5 mL of 150 mM K2HPO4 (pH 8.5), 1.0 mL of acetonitrile containing 1,2-benzenedithiol (15 mM final concentration; Sigma-Aldrich), and 0.5 mL of the sample to be analyzed. The mixture was incubated at 65°C for 2 h, and then cooled at 4°C for 1 h. The samples were centrifuged to remove precipitate, and 1 mL of the supernatant fraction was transferred to autosampler vials with caps containing Teflon/polypropylene septa. The HPLC analysis was conducted as previously described.29,30 Protein concentration of the tissue homogenates was determined by the bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL) and used to normalize the DTC levels.
mRNA isolation and real-time PCR analysis
At 4 h following injury, the animals (control, n=3; SF-treated, n=4) were anesthetized as described above and euthanized by transcardial perfusion with cold Dulbecco PBS solution. Spinal cords were quickly dissected and stored in RNAlater (Sigma-Aldrich) at 4°C for mRNA isolation and real-time PCR analysis. Total mRNA from spinal cords (10-mm sections centered on the epicenter of the lesion) was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA), and purified using the RNeasy Mini Kit (Qiagen, Valencia, CA) with DNase I digestion per the manufacturer's protocol. The iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA) was used to synthesize cDNA. Quantitative real-time PCR analysis was performed using the 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). All primers were optimized and a final primer concentration of 300 nM was used for all reactions. Primer sequences for gene amplification were as follows: NAD(P)H: quinone oxidoreductase 1 (NQO1): fwd 5′-tccagaaacgacatcacagg-3′, rev 5′-ttcagctacaatatccgggc-3′; heme oxygenase-1 (HO-1): fwd 5′-cagggtgacagaagaggctaagac-3′, rev 5′-tgaggacccatcgcaggag; interleukin-1β (IL-1β): fwd 5′-ctattcctaatgccttccccag-3′, rev 5′-cacggttttcttatggctctg-3′; matrix metalloproteinase-9 (MMP-9): fwd 5′-cttgaagtctcagaaggtgggatc-3′, rev 5′-cgccagaagtatttgtcatgg-3′; cyclophilin (endogenous control): fwd 5′-gcagacaaagttccaaagacag-3′, rev 5′-catttgccatggacaagatgcc-3′. The reactions were assembled using 100 ng of cDNA, 1×SYBR Green with Rox Master Mix (Bio-Rad Laboratories), forward and reverse primers, and DEPC-treated water. Relative mRNA expression for spinal cords was normalized to cyclophilin. Gene expression was calculated using the comparative 2−ΔΔCT method as previously described.31
Urine collection and urinary MIF tautomerase activity assay
Urine was manually collected from animals at various time points: after each animal was anesthetized (pre-surgery), immediately after contusion, and 3, 24, 72 (collected before treatment), 75, and 96 h after injury, for analysis of MIF enzymatic activity.19 Urine was stored at 4°C and assays were performed within 48 h of collection.
Perfusion and tissue processing
At 5 weeks after injury, the animals were deeply anesthetized with halothane and then euthanized by transcardial perfusion with 4% paraformaldehyde in Dulbecco PBS solution. Spinal cords were dissected and post-fixed 12–16 h in the same fixative, and then cryoprotected in 30% sucrose+0.01% sodium azide. The spinal cord was embedded in Shandon M-1 Embedding Matrix (Thermo Scientific), and serial 15-μm cryosections were cut longitudinally on a horizontal plane in 10-series, and thaw-mounted onto Super-Frost Plus slides (Thermo Scientific). Transverse 15-μm serial cryosections of spinal cord were collected 7 to 8 mm caudal to the injury site in 10-series.
Histology and immunohistochemistry
For analysis of the lesion area, longitudinal spinal cord sections were rehydrated and stained with eriochrome and cresyl violet, dehydrated, and cover-slipped with DPX mounting medium. For immunohistochemistry, longitudinal spinal cord sections were stained for reactive astrocytes, chondroitin sulfate proteoglycans (CSPGs), and activated microglia/macrophages. Transverse sections were stained for serotonergic neurons. CSPGs and reactive astrocytes were stained sequentially. Slides were treated with 5% goat serum in PBS, and incubated 2 h at 25°C, then overnight at 4°C with primary mouse monoclonal antibody against CSPGs (CS-56, 1:50; Sigma-Aldrich), followed by incubation with goat anti-mouse Cy3-conjugated secondary antibody (1:200; Jackson ImmunoResearch, West Grove, PA) at 25°C for 2 h. Slides were then washed and incubated with primary rabbit polyclonal antibody against glial fibrillary acidic protein (GFAP, 1:200; Dako, Carpinteria, CA) in 5% goat serum and 0.3% Triton X-100 in PBS for 2 h at 25°C, then overnight at 4°C, followed by incubation with a goat anti-rabbit Alexa 488-conjugated secondary antibody (1:200; Invitrogen) at 25°C for 2 h. Activated microglia/macrophages were stained using a primary antibody against CD68 (ED-1, 1:200; AbD Serotec, Raleigh, NC), followed by incubation with goat anti-mouse Cy3-conjugated secondary antibody (1:200; Jackson ImmunoResearch). Serotonergic neurons were stained using a primary antibody against serotonin (5-HT, 1:5000; ImmunoStar, Inc., Hudson, WI), followed by incubation with goat anti-rabbit Cy3-conjugated secondary antibody (1:200; Jackson ImmunoResearch). All slides were incubated with 4,6-diamino-2-phenylindole (DAPI; 1:10,000) in PBS for 5 min, washed, dried overnight, and cover-slipped with slide mounting medium.
Microscopic images were analyzed by observers blinded to treatment group. Spared tissue and lesion volume were analyzed using StereoInvestigator 8 Software (MicroBrightField, Inc., Williston, VT). A 6-mm length, centered on the lesion epicenter of each serial section, was evaluated by the Cavalieri estimator method, with a 150-μm-spaced grid, for analyzing the volume of white matter, grey matter, and penumbra. GFAP-, CS-56-, and ED-1-positive cells were quantified in a boxed region (4.7×2.2 mm) centered over the lesion epicenter in three serial spinal cord sections. 5-HT fibers were quantified in a boxed region (450×460 μm) over the ventral horns of five serial sections ∼7.5 mm caudal to the lesion. Images were taken at equal intensities and positive immunoreactivity was distinguished from background and quantified using NIS-Elements software (Nikon, Melville, NY).32 Relative pixel areas represented by GFAP-, CS-56-, and ED-1-positive cells were calculated as a percentage of the total area of the boxed region. Total 5-HT fiber areas were converted into relative pixel areas.
Statistical analysis
Stata 11.0 (Stata Corporation, College Station, TX) was used for statistical analysis. Statistical analyses were conducted using the non-paired Student's t-test, the Wilcoxon-Mann-Whitney U test, or one-way analysis of variance (ANOVA), followed by post-hoc Bonferroni recalculation of pairwise significance, where appropriate. Statistical analyses of the BBB scoring were performed using the general estimating equations (GEE) statistical model with an autoregressive correlation matrix for longitudinal data. This statistical model accounts for correlations between repeated subject measurements, and allows for robust estimation parameters even when the correlation matrix is not correctly specified. p Values less than 0.05 were considered statistically significant, whereas p values between 0.05 and 0.1 were considered a trend.
Results
Sulforaphane metabolites accumulate in rat spinal cord
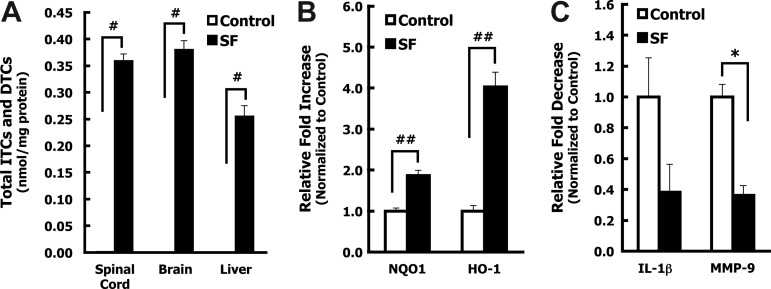
To evaluate the effects of the isothiocyanate SF on SCI we first established the levels of SF and its metabolites in the thoracic spinal cord after systemic administration in naïve and spinal cord-injured rats. Although tissue levels of the free isothiocyanates (ITCs) are usually very low, their GSH conjugates formed by GSH transferases predominate, and are successively hydrolyzed to dithiocarbamates (DTCs), and ultimately excreted as the N-acetylcysteine derivatives. Conjugation with GSH promotes accumulation of SF within cells. Interestingly, DTC metabolites upregulate the Nrf2 pathway in cell culture.33–35 The cyclocondensation reaction used here provides a simple HPLC-spectroscopic method for quantifying the sum of ITC and DTC in the spinal cord.29,30 We found that SF readily crossed the blood–brain barrier in naïve animals, reaching DTC levels of 0.74 and 0.87 nmol/mg protein in spinal cord and brain, respectively, and 1.2 nmol/mg protein in the liver 1 h after administration of SF (50 mg/kg IP). DTC levels fell by nearly 90% in all organs studied 16 h later. To ensure that the injury did not interfere with bioavailability, DTC levels were determined in the spinal cord, brain, and liver of rats 4 h after contusion SCI and SF administration. Levels of DTC (0.36, 0.38, and 0.25 nmol/mg protein, in spinal cord, brain, and liver, respectively) were within the range of those of uninjured animals, considering the estimated 1- to 2-h half-lives of SF metabolites, and the 3-h delay difference in observation time point (Fig. 2A).30,36 These studies support the view that there is no significant barrier to the entry and accumulation of SF metabolites in the injured rat spinal cord.
FIG. 2.
Sulforaphane (SF) reaches the central nervous system (CNS) after IP administration in spinal cord-injured rats and alters spinal cord gene expression. Animals were subjected to contusion spinal cord injury (n=7). Within 10 min of injury, corn oil vehicle (n=3), or SF (50 mg/kg) in corn oil (n=4), was administered by IP injection. At 4 h after injury, the animals were euthanized and transcardially perfused with cold phosphate-buffered saline. (A) Brain and liver tissue and 10-mm segments of the spinal cord centered at the injury epicenter were used to detect SF metabolites (free isothiocyanates, ITCs, and dithiocarbamates, DTCs). ITC and DTC levels in the spinal cord, brain, and liver were analyzed by the cyclocondensation assay and normalized to protein levels. Measurable levels of ITCs and DTCs were detected in the spinal cord, brain, and liver at 4 h post-SCI in animals treated with SF, but not in corn oil-treated animals (#p<0.001). Values represent the mean±standard error of the mean (SEM), and p values were calculated by a non-paired Student's t-test. (B and C) The comparative 2−ΔΔCT method was used to compare mRNA levels in the lesion site of spinal cords isolated from animals 4 h after contusion and IP administration of corn oil (control, n=3) or SF (50 mg/kg, n=4). Cyclophilin was used as an endogenous control for all target genes, and the values are represented as the relative fold change in the mRNA levels of the SF-treated versus corn oil-treated animals. Real-time polymerase chain reaction (PCR) analysis indicated that SF treatment administered within 10 min of contusion increased the expression of the phase 2-related genes heme oxygenase-1 (HO-1, ##p<0.01), and NAD(P)H: quinone oxidoreductase 1 (NQO1, ##p<0.01, B), and decreased expression of the proinflammatory cytokine genes interleukin-1β (IL-1β, p=0.11) and matrix metalloproteinase-9 (MMP-9, *p<0.05, C), 4 h after injury and treatment. Error bars represent the SEM of the average 2−ΔΔCT values for each group. p Values were calculated using the non-paired Student's t-test.
Sulforaphane alters expression of mRNA levels of phase 2 genes and cytokines after SCI
Next, we determined if SF treatment resulted in changes in the expression of cytoprotective phase 2-related enzyme (HO-1 and NQO1), cytokine (IL-1β), and matrix metalloproteinase-9 (MMP-9) gene transcripts in spinal cord tissue harvested 4 h after contusion. Real-time PCR analysis of the spinal cord lesion site in SF-treated animals showed a significant fourfold increase in the mRNA levels of HO-1, and a 2-fold increase in NQO1 expression, over control animals 4 h after injury (Fig. 2B). SF treatment also caused a non-significant decrease in the mRNA levels of the cytokine IL-1β, and a significant ∼3-fold decrease in MMP-9 (Fig. 2C). These data demonstrate that SF treatment induces the protective phase 2 pathway in the spinal cord after injury, and may decrease important inflammatory stimuli within the injury epicenter shortly after contusion.
Sulforaphane decreases urinary macrophage inhibitory factor (MIF) tautomerase activity in spinal cord-injured rats
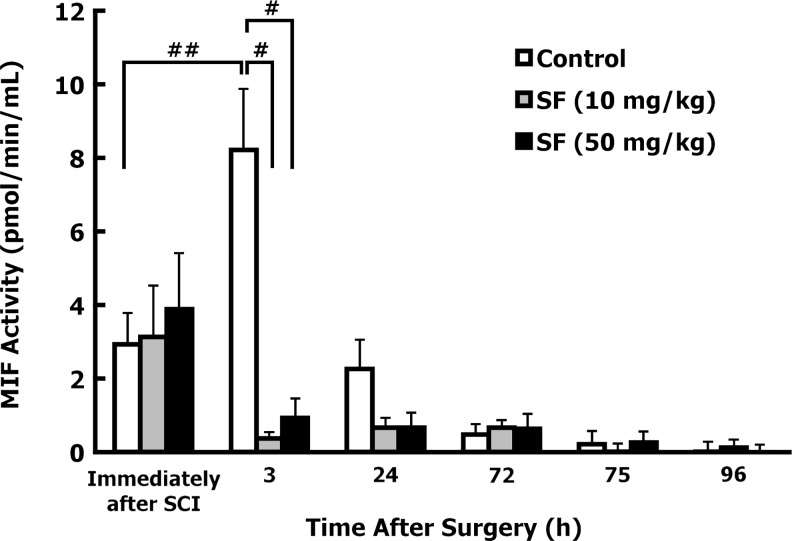
Preliminary studies were conducted to determine whether SCI increases urinary MIF tautomerase activity. MIF activity was measured immediately after the rats were anesthetized (pre-surgery), immediately after SCI, and 3 h after injury and treatment with corn oil (n=10), or SF (50 mg/kg; n=7). MIF activity in urine was significantly elevated in control animals at 3 h post-injury compared to pre-surgery levels (72% increase; p<0.05), or levels detected immediately after injury (88% increase; p<0.01). Administration of SF (50 mg/kg) profoundly decreased MIF activity by ∼90% at 3 h after SCI (p<0.01).
We examined the effects of both SF doses up to 96 h after injury. Urine was collected by manual bladder expression immediately after, and at 3, 24, 72, 75, and 96 h after injury. No differences were found in urine collection volumes (∼1.0 mL) at any time point during the observation period. Control animals showed similar trends for levels of MIF activity after SCI as seen in preliminary studies, with maximal activity at 3 h after injury, that decreased significantly 24 h later (Fig. 3). At 72, 75, and 96 h after injury, urinary MIF activity in control animals returned to pre-surgery levels. Sulforaphane treatment 10 min after injury (either 10 or 50 mg/kg) blocked the 3-h peak in MIF tautomerase activity observed in control-treated animals (p=0.0008 and 0.0012, respectively; Fig. 3).
FIG. 3.
Sulforaphane (SF) decreased urinary macrophage inhibitory factor (MIF) tautomerase activity in vivo after contusion. The total urinary MIF activity was determined by subtracting the residual activity in SF-treated (enzymatically-inactivated) urine samples from the total MIF activity, and then normalized to urine sample volume. The time point “Immediately after SCI” indicates urine collected immediately after spinal cord injury (SCI), but before any treatment was administered. MIF activity was analyzed in urine collected at various time points after injury, from animals treated with corn oil (control; n=11), low-dose SF (10 mg/kg SF; n=11), or high-dose SF (50 mg/kg; n=10), given at 10 min and 72 h after injury. MIF activity was found to significantly increase in the corn oil control animals 3 h after injury (##p<0.01 by non-paired Student's t-test). MIF tautomerase activity was significantly lower in both low-dose and high-dose SF-treated animals at 3 h after injury, compared to 3-h control levels (#p<0.001 and #p<0.001, respectively by one-way analysis of variance [ANOVA]). MIF activity returned to pre-surgery levels by 72 h after injury. Values represent the mean±standard error of the mean, and p values were calculated by a non-paired Student's t-test or one-way ANOVA.
Sulforaphane treatment 72 h after contusion elicits spontaneous movements of the hindlimbs and tail
All animals that were administered the second dose of SF (10 or 50 mg/kg IP) 72 h after contusion showed spontaneous ataxic movements of the hindlimbs and tail. These involuntary contractions began within 1 min and continued up to 10–15 min after SF injection, after which the hindlimbs and tail returned to their prior paralyzed state. The first dose of SF administered 10 min after injury was given while the animals were still under anesthesia and did not elicit any of these movements. This response was not observed in SCI animals treated only with corn oil. In addition, this reaction was not observed when uninjured rats were treated with the same doses of SF. The hindlimb and tail movements elicited by SF treatment 72 h after spinal cord injury were unexpected, and remain unexplained. The paralyzed hindlimbs and tail were the only appendages involved, indicating that this response only occurs caudally to the lesion.
Sulforaphane treatment improves hindlimb locomotion
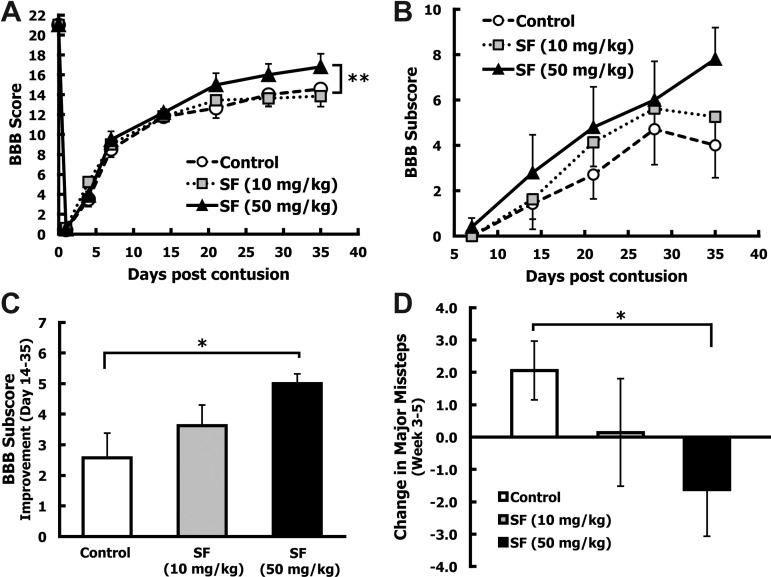
To evaluate whether SF treatment resulted in improved hindlimb locomotion, animals received a 200-kdyn contusion injury and were subjected to three behavioral assessments. In the BBB open-field locomotor test the extent of hindlimb movement was ranked on a 22-point scale (0, paraplegia to 21, normal). Five animals that achieved a score of 4 or higher at day 1 after injury were considered to have received inadequate initial injury and were removed from the study. Five animals in the high-dose SF group were removed from the study due to bladder calcification (n=1) or gastrointestinal complications (n=4). The final groups for hindlimb behavioral analysis were: control (n=7), low-dose SF (10 mg/kg; n=8), and high-dose SF (50 mg/kg; n=5). All groups achieved an average BBB score of ∼12 by 14 days after injury. The low-dose SF group did not show any improvement in hindlimb locomotion at any time point over the 35-day observation period, compared to the control animals (Fig. 4A). Starting 21 days after injury, the high-dose SF group had an average BBB score of 15.0±1.1, whereas the corn oil-treated animals averaged 12.6±1.0 (Fig. 4A). This improvement in BBB score was maintained between the high-dose SF and control animals for the rest of the 35-day study, with final average scores of 16.8±1.3 and 14.6±1.1, respectively (Fig. 4A). Although the higher SF treatment appeared to improve hindlimb function, the small final sample size (n=5) may have ultimately resulted in the lack of significance at the given time points. With use of the general estimating equations (GEE) statistical model with an autoregressive correlation matrix for longitudinal data, the high-dose SF group showed a trend (p=0.088), toward a higher BBB score compared to the control group when analyzing the BBB scores for days 21, 28, and 35.
FIG. 4.
Sulforaphane (SF) administered after spinal cord injury (SCI) improved the Basso, Beattie, and Bresnahan (BBB) score and subscore and horizontal ladder performance. After spinal cord contusion, animals received IP injections of corn oil (control; n=7), low-dose SF (10 mg/kg; n=8), or high-dose SF (50 mg/kg; n=5) at 10 min and 72 h after injury. (A) Hindlimb motor function was quantified using the BBB locomotor rating scale periodically for 35 days after injury. All groups displayed the same partial recovery (BBB score=∼12) during the first 14 days, then diverged over the last 3 weeks, with high-dose SF treatment resulting in a trend toward enhanced hindlimb function from days 21–35 post-injury (**p<0.1). p Values were calculated by general estimating equation (GEE) analysis by using an autoregressive matrix. (B) Subtle improvements in hindlimb motor function were quantified using the BBB subscoring method starting 7 days after injury. The high-dose SF (50 mg/kg) group showed improvements in BBB subscore compared to the control group, starting 14 days after injury and continuing to day 35. (C) Average improvement in BBB subscores between days 14 and 35 (mean±standard error of the mean [SEM]) are shown. The high-dose SF (50 mg/kg) treatment resulted in a significant improvement in BBB subscore over this time period compared to control animals (*p<0.05 by the Wilcoxon-Mann-Whitney U test). (D) At 3 and 5 weeks after injury, fine motor control was assessed using the horizontal ladder test. The total number of major hindlimb missteps was counted for each animal and expressed as the percent of total hindlimb steps attempted. The change in the number of major missteps between weeks 3 and 5 was calculated for each animal, and the group averages (mean±SEM) were established. The high-dose SF group showed a significant decrease in severe missteps between weeks 3 and 5 compared to the control group (*p<0.05 by the Wilcoxon-Mann-Whitney U test).
The BBB subscore allows subtle changes in toe clearance, paw rotation during stance and gait, trunk stability, and tail position, to be scored independently of overall coordination on a 13-point scale.27 The BBB subscores were analyzed starting at 7 days after injury. The high-dose SF group had higher BBB subscores compared to control animals starting 14 days after injury (Fig. 4B). Since BBB subscores appeared to diverge 14 days after injury, improvement in the BBB subscore was calculated for each animal from day 14 to day 35. Animals treated with the high-dose SF showed a statistically significant change in BBB subscore between days 14 and 35 compared to controls (p<0.05; Fig. 4C).
To assess sensorimotor function, animals were subjected to the horizontal ladder test at weeks 3 and 5 post-injury. Treatment with high-dose SF resulted in a non-significant decrease in the total number of missteps per total hindlimb steps attempted both at 3 (17.0% decrease) and 5 weeks (21.6% decrease) after injury compared to controls. When the change in major hindlimb missteps between weeks 3 and 5 was calculated for each group, a significant improvement in foot-fall errors was found for the high-dose SF group compared to the controls (p<0.05; Fig. 4D).
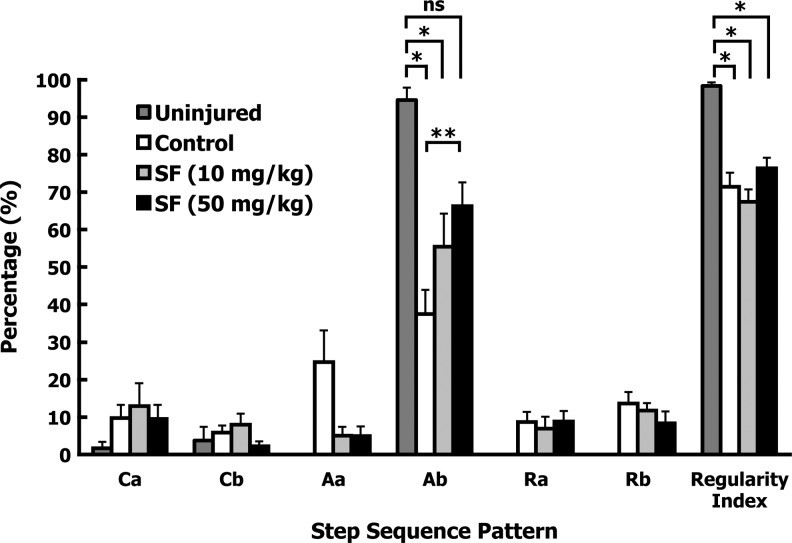
Finally, the CatWalk system was used to detect differences in gait between treatment groups at 5 weeks after injury. Analysis of the step sequence pattern determines the order in which frontlimbs and hindlimbs are placed or raised during locomotion.28 In quadrupeds, there are six different step sequence patterns (cruciate [Ca and Cb], alternate [Aa and Ab], and rotary [Ra and Rb]). The Ab alternate pattern accounts for over 90% of all step patterns. Rotary patterns are not normally used by uninjured animals.28 The normal Ab step sequence pattern was decreased significantly for both the control- and low-dose SF-treated groups, compared to a small subset of uninjured rats (n=3; p<0.05; Fig. 5). In contrast, there was no significant difference between the high-dose SF-treated and the uninjured groups (p=0.62; Fig. 5). There was an increase in Ab step sequence pattern for the high-dose SF group compared to the controls (p=0.081), indicating a trend toward improvement in an important gait parameter. All injured groups showed the same degree of increase in rotary step sequence patterns, and the corn oil treatment resulted in a non-significant increase in Aa pattern compared to both SF-treated groups (Fig. 5). The regularity index expresses the number of normal step sequence patterns relative to the total number of paw placements, and is an index of interlimb coordination.28 Spinal cord-injured animals showed significant decreases in regularity index compared to uninjured rats (p<0.05), regardless of treatment (Fig. 5). No differences in regularity index were observed with either SF treatment compared to the control-treated animals (Fig. 5). These findings indicate that although there was no difference in the number of missteps within the regular step sequence patterns between the groups, the high-dose SF-treated animals showed improvements in the normally favored Ab stepping pattern.
FIG. 5.
Sulforaphane (SF) treatment after spinal cord injury improved normal step sequence pattern. Five weeks after injury, regularity index and step sequence patterns were assessed using the CatWalk system. The parameter averages were found for each animal, and group averages were calculated (mean±standard error of the mean). Gait parameters of a subset of uninjured animals (n=3) were analyzed in an identical fashion. All injured groups (i.e., control, low-dose, and high-dose SF) showed a decrease in regularity index compared to the uninjured animals (*p<0.05), and low-dose or high-dose SF treatment did not improve this parameter compared to control treatment. The Ab step sequence pattern was maintained in the high-dose SF group, to a point at which there was no significant difference with uninjured animals (nsp=0.62), and a trend towards improvement compared to control animals (**p<0.1 for SF). p Values were calculated by one-way analysis of variance.
Sulforaphane did not alter spared tissue volume or infiltration of activated microglia and macrophages into the lesion
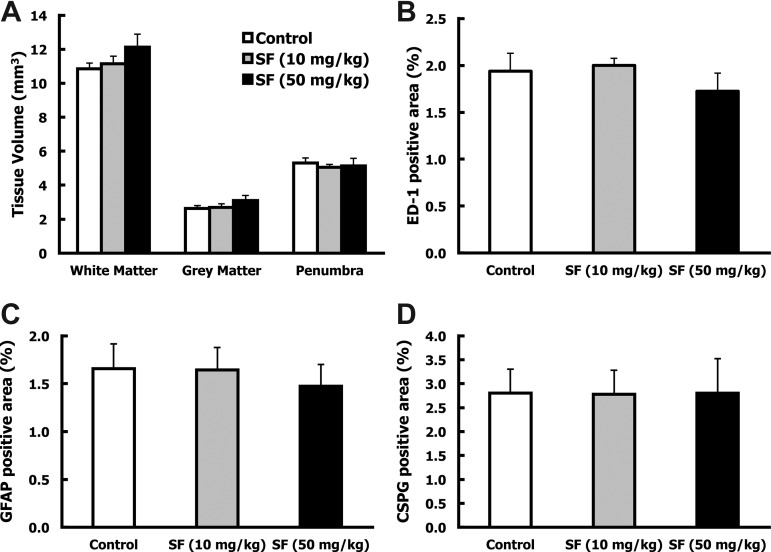
To determine whether SF provided long-lasting anti-inflammatory benefits, the volumes of the spinal cord lesions and the accumulation of activated microglia and macrophages (ED-1) within the lesions were examined 5 weeks after injury. Animals that were excluded from the behavioral analyses of the high-dose SF group because of bladder calcification and gastrointestinal illness (cecal impaction and enlarged colon), but survived to the termination of the study, were included in the histological examination of the spinal cord tissue (50 mg/kg SF; n=7). No differences were found between the control or either SF-treated group for the volume of spared white and grey matter, or in damaged tissue surrounding the cavity (penumbra) (Fig. 6A). Quantification of ED-1-positive activated microglia and macrophages within and surrounding the lesion site also showed no differences in infiltration of inflammatory cells between the control and the two SF-treated groups (Fig. 6B). Although the high-dose SF treatment improved functional outcomes, there were no differences in spared tissue and microglia and macrophage presence at the injury site 5 weeks after injury.
FIG. 6.
Sulforaphane (SF) did not alter spared tissue volume, activated macrophages and microglia, or glial scar around the injury site 5 weeks after spinal cord injury (SCI). Five weeks after SCI, injured spinal cords were removed for histological analysis. (A) Serial longitudinal spinal cord sections were evaluated for the volume of white matter, grey matter, and penumbra for each animal. Group averages were then calculated (mean±standard error of the mean [SEM[). No differences were found between treatment groups for spared or lesioned tissue. (B–D) Longitudinal sections containing the lesion epicenter were immunostained for activated macrophages/microglia (ED-1-positive), glial fibrillary acidic protein (GFAP-positive), and chondroitin sulfate proteoglycans (CSPGs; CS-56-positive) cells. Immunoreactivity was quantified within the lesion and surrounding area of three serial sections. The graphs represent the average percent of immunostained area within a defined region for each group (mean±SEM). No differences were detected between the control and SF-treated groups for (B) activated microglia and macrophages (ED-1), (C) reactive astrocytes (GFAP), or (D) inhibitory molecules (CSPGs).
Sulforaphane treatment did not affect glial scar formation 5 weeks after injury
Reactive astrocytes within the injury site express CSPGs, inhibitory molecules that hinder axon growth and regeneration. To determine whether SF administration affected formation of the glial scar, the lesion sites were examined for markers of reactive astrocytes (GFAP), and CSPGs (CS-56). Treatment with low-dose or high-dose SF had no effect on the glial scar at 5 weeks after injury (Fig. 6C and D). Assessment of earlier time points will be crucial to understanding the relationship between SF treatment and the gliotic response after SCI.
Sulforaphane treatment promotes sprouting or sparing of serotonergic fibers
Axons from serotonergic (5-HT) neurons descend from the raphe nucleus in the brainstem to all levels of the spinal cord and are important for hindlimb reflexes and locomotor function.37 To determine whether SF treatment affects the sparing or regeneration of these axons, 5-HT immunoreactivity in the ventral horns was examined in transverse sections ∼7.5 mm caudal to the lesion epicenter. Quantitative image analysis revealed a significant 57% increase in 5-HT-positive fibers in the ventral horns of the high-dose SF-treated animals compared to controls (p=0.03; Fig. 7A and B).
FIG. 7.
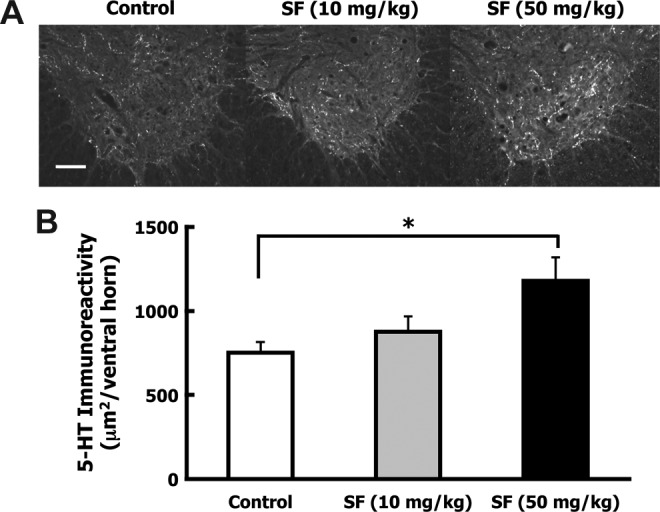
Sulforaphane (SF) treatment increased serotonergic axons caudal to the contusion injury site. After spinal cord injury (SCI), animals received IP injections of corn oil (control; n=7), low-dose SF (10 mg/kg; n=8), or high-dose SF (50 mg/kg; n=7) at 10 min and 72 h following injury. Five weeks after injury the animals were euthanized, perfusion-fixed, and the spinal cords were dissected. Transverse sections ∼7.5 mm caudal to the lesion were immunostained for serotonergic (5-HT) fibers and immunoreactivity was quantified in the ventral horns of five sections. (A) Representative ventral horn 5-HT immunostaining is shown for control, low-dose, and high-dose SF animals (scale bar=100 μm). (B) Bars indicate the average 5-HT pixel density area per ventral horn (mean±standard error of the mean). A significant 57% increase in 5-HT-stained area was detected in the ventral horns of the high-dose SF-treated group over the control group (*p<0.05 by one-way analysis of variance).
Discussion
SF readily crosses the blood–brain barrier and evokes acute changes in expression of Nrf2-dependent and inflammatory genes in the injured spinal cord. Urinary MIF activity was elevated after CNS injury, and this elevation in activity was blocked in SF-treated animals. These changes were associated with improvements in hindlimb function and increases in serotonergic axons caudal to the lesion, indicating a beneficial role of SF in recovery from SCI. Fortunately, our studies and those of others demonstrate protection when SF was administered soon after injury, and did not require treatment prior to injury.
Recent reports of the effectiveness of SF in mouse and rat models of SCI from two laboratories provide strong support for our findings.25,38 These independent studies were conducted in a mouse model of compression SCI, in which SF (5 mg/kg IP) was administered 1 h after injury,38 and in a rat model of contusion SCI, in which SF (5 mg/kg IP) was administered 15 min after injury and once daily for 3 days thereafter.25 Treatment with SF reduced NF-κB pathway activation and decreased levels of cytokine gene expression and protein levels of MMP-9, TNF-α, IL-6, and IL-1β in the spinal cord lesions at 12 and 24 h after SCI.25,38,39 We detected anti-inflammatory effects at earlier time points using a higher SF dose, with decreased mRNA levels of MMP-9 in the spinal cord seen at 4 h after injury. Our findings suggest that treatment with SF shortly after SCI elicits rapid anti-inflammatory effects.
Furthermore, we found an increase in MIF tautomerase activity 3 h after SCI, which is inactivated by SF treatment, and suggests that this may be a valuable quantitative biomarker for gauging the severity and rate of recovery from SCI that could substantially simplify assessment of the benefits of treatment. MIF is a proinflammatory cytokine that is upregulated early during episodes of acute inflammation, including SCI.40 MIF knockout mice show more rapid recovery of hindlimb function after SCI and have decreased neuronal cell death 6 weeks after injury, demonstrating that this cytokine plays a role in retarding recovery after SCI.40 Blocking MIF expression results in anti-inflammatory effects in vitro and in vivo.20,21 MIF also displays keto-enol tautomerase activity. Inactivation of its tautomerase activity may elicit anti-inflammatory effects in vivo by interfering with the binding of MIF to its receptor, CD74, on microglia and macrophages.20–23 Sulforaphane and other ITCs inactivate the tautomerase activity of MIF by reacting with a highly nucleophilic N-terminal proline residue in the active site of the enzyme.41 Recent studies have demonstrated MIF tautomerase activity in human urine, and that oral administration of SF-rich broccoli sprout extracts to volunteers almost completely abolished this activity.19 To our knowledge, urinary MIF tautomerase activity and the effects of SCI on urinary MIF activity have not previously been examined in rodents.
The anti-inflammatory activity of SF coincides with increased Nrf2-dependent gene expression (NQO1 and GST-α1) 12 h after SCI,38 and increased protein levels (Nrf2 and GCL) at 24 h following SCI.25 In our study SF treatment significantly increased expression of the quintessential Nrf2 markers NQO1 and HO-1 at 4 h after contusive SCI. The importance of the Nrf2 pathway in SF-mediated protection against SCI has been established in Nrf2 knockout mice,24,38 which showed increased edema and inflammation (IL-6 and IL-1β protein), and increased neuronal death in the injured spinal cord, and more severe hindlimb motor dysfunction with no improvements upon SF treatment.24,38 In wild-type mice, acute treatment with SF leads to protective effects, including decreased edema and fewer dying neurons in the spinal cord 48 h after injury, and improved hindlimb motor function during the first week after injury. Unfortunately, this study did not follow hindlimb functional recovery beyond 7 days after injury.38 We found a lasting improvement in hindlimb function in the open-field locomotor, horizontal ladder, and CatWalk tests. In agreement with our results, Wang and associates25 found that treatment with SF after contusion SCI leads to modest improvements in hindlimb coordination (i.e., regularity index) 7 weeks after injury. Our findings suggest that the use of a SF concentration up to 10 times higher than that used in previously published studies may provide protection that leads to beneficial behavioral changes. The study by Wang and colleagues25 demonstrated that treatment with SF after contusion SCI significantly reduces lesion volume (∼15%) 1 week after injury. Our results indicate that although short-term SF treatment after traumatic SCI does not affect lesion volume, inflammation, or glial scar, when assessed 5 weeks after injury, early stimulation of the Nrf2 pathway and inhibition of inflammation after injury can lead to beneficial outcomes after contusion SCI. Further investigation is needed to determine if long-term treatment with SF after SCI can chronically decrease lesion volume and inflammation.
In addition to an improved functional recovery, our high-dose SF-treated animals showed enriched serotonergic axonal density in the ventral horns caudal to the lesion. Previous studies have shown an association between enhancements of serotonergic fibers in the ventral horns of spinal cord-injured animals with improvements in hindlimb functional recovery.32,42–44 The ability of SF to protect neuronal cells against ROS and inflammation after injury has been shown in rodent models of ischemia-reperfusion45,46 and traumatic brain injury.47–49 Therefore, SF may protect serotonergic neurons, leading to sparing of these fibers caudal to the injury site. It has yet to be determined whether SF has the capability to enhance serotonergic axon sprouting after SCI.
The observation that SF increases serotonergic axons caudal to the spinal cord lesion provides novel insight into SF-mediated improvements in hindlimb behavior, that to our knowledge has not been documented previously. The finding that SF protected against SCI in three independent studies in a complex system in which a combination of behavioral and biochemical end-points were quantified, lends substantial credence to the conclusion that SF exerts protective effects in SCI. Our study has revealed interesting insights into the role of SF in recovery after traumatic SCI, and suggests that more extensive investigations are needed to determine the optimal dose and schedule of administration, and to define more clearly the mechanisms of action of SF in this injury model.
Acknowledgments
We are grateful to Dr. W.D. Holtzclaw, Jr., K.L. Wade, and K.K. Stephenson (Johns Hopkins University) for technical assistance. We would like to thank D. Wendell (Hugo W. Moser Research Institute at Kennedy Krieger) for help with surgical procedures and behavioral testing. This work was supported in part by NIH grant NS057338 and the Lewis B. and Dorothy Cullman Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Baptiste D.C. Fehlings M.G. Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- 2.Hagg T. Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma. 2006;23:264–280. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- 3.Hall E.D. Springer J.E. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly D.J. Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamencic H. Griebel R.W. Lyon A.W. Paterson P.G. Juurlink B.H. Promoting glutathione synthesis after spinal cord trauma decreases secondary damage and promotes retention of function. FASEB J. 2001;15:243–250. doi: 10.1096/fj.00-0228com. [DOI] [PubMed] [Google Scholar]
- 6.Dringen R. Kussmaul L. Gutterer J.M. Hirrlinger J. Hamprecht B. The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. J. Neurochem. 1999a;72:2523–2530. doi: 10.1046/j.1471-4159.1999.0722523.x. [DOI] [PubMed] [Google Scholar]
- 7.Dringen R. Pfeiffer B. Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J. Neurosci. 1999b;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dringen R. Hirrlinger J. Glutathione pathways in the brain. Biol. Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 9.Kraft A.D. Johnson D.A. Johnson J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson J.A. Johnson D.A. Kraft A.D. Calkins M.J. Jakel R.J. Vargas M.R. Chen P.C. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann. NY Acad. Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escartin C. Won S.J. Malgorn C. Auregan G. Berman A.E. Chen P.C. Deglon N. Johnson J.A. Suh S.W. Swanson R.A. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J. Neurosci. 2011;31:7392–7401. doi: 10.1523/JNEUROSCI.6577-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y. Talalay P. Cho C.G. Posner G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese V. Cornelius C. Dinkova-Kostova A.T. Calabrese E.J. Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox. Signal. 2010;13:1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh N. Ghosh R. Mandal S.C. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free. Radic. Res. 2011;45:888–905. doi: 10.3109/10715762.2011.574290. [DOI] [PubMed] [Google Scholar]
- 15.Elbarbry F. Elrody N. Potential health benefits of sulforaphane: A review of the experimental, clinical and epidemiological evidences and underlying mechanisms. J. Med. Plants. Res. 2011;5:473–484. [Google Scholar]
- 16.Guerrero-Beltran C.E. Calderon-Oliver M. Pedraza-Chaverri J. Chirino Y.I. Protective effect of sulforaphane against oxidative stress: Recent advances. Exp. Toxicol. Pathol. 2012;64:503–508. doi: 10.1016/j.etp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Cheung K.L. Kong A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brambilla R. Hurtado A. Persaud T. Esham K. Pearse D.D. Oudega M. Bethea J.R. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J. Neurochem. 2009;110:765–778. doi: 10.1111/j.1471-4159.2009.06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy Z.R. Liu H. Holtzclaw W.D. Talalay P. Inactivation of tautomerase activity of macrophage migration inhibitory factor by sulforaphane: a potential biomarker for anti-inflammatory intervention. Cancer Epidemiol. Biomarkers. Prev. 2011;20:1516–1523. doi: 10.1158/1055-9965.EPI-11-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Abed Y. Dabideen D. Aljabari B. Valster A. Messmer D. Ochani M. Tanovic M. Ochani K. Bacher M. Nicoletti F. Metz C. Pavlov V.A. Miller E.J. Tracey K.J. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J. Biol. Chem. 2005;280:36541–36544. doi: 10.1074/jbc.C500243200. [DOI] [PubMed] [Google Scholar]
- 21.Garai J. Lorand T. Macrophage migration inhibitory factor (MIF) tautomerase inhibitors as potential novel anti-inflammatory agents: current developments. Curr. Med. Chem. 2009;16:1091–1114. doi: 10.2174/092986709787581842. [DOI] [PubMed] [Google Scholar]
- 22.Leng L. Metz C.N. Fang Y. Xu J. Donnelly S. Baugh J. Delohery T. Chen Y. Mitchell R.A. Bucala R. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F. Wu H. Xu S. Guo X. Yang J. Shen X. Macrophage migration inhibitory factor activates cyclooxygenase 2-prostaglandin E(2) in cultured spinal microglia. Neurosci. Res. 2011;71:210–218. doi: 10.1016/j.neures.2011.07.1821. [DOI] [PubMed] [Google Scholar]
- 24.Mao L. Wang H.D. Wang X.L. Tian L. Xu J.Y. Disruption of Nrf2 exacerbated the damage after spinal cord injury in mice. J. Trauma Acute Care. Surg. 2012;72:189–198. doi: 10.1097/TA.0b013e31821bf541. [DOI] [PubMed] [Google Scholar]
- 25.Wang X. de Rivero Vaccari J.P. Wang H. Diaz P. German R. Marcillo A.E. Keane R.W. Activation of the Nuclear Factor E2-Related Factor 2/Antioxidant Response Element pathway is neuroprotective after spinal cord injury. J. Neurotrauma. 2012;29:936–945. doi: 10.1089/neu.2011.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 27.Basso D.M. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J. Neurotrauma. 2004;21:395–404. doi: 10.1089/089771504323004548. [DOI] [PubMed] [Google Scholar]
- 28.Hamers F.P. Koopmans G.C. Joosten E.A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y. Wade K.L. Prestera T. Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal. Biochem. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 30.Ye L. Dinkova-Kostova A.T. Wade K.L. Zhang Y. Shapiro T.A. Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 31.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Mountney A. Zahner M.R. Lorenzini I. Oudega M. Schramm L.P. Schnaar R.L. Sialidase enhances recovery from spinal cord contusion injury. Proc. Natl. Acad. Sci. USA. 2010;107:11561–11566. doi: 10.1073/pnas.1006683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y. Kolm R.H. Mannervik B. Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem. Biophys. Res. Commun. 1995;206:748–755. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- 35.Hwang E.S. Jeffery E.H. Induction of quinone reductase by sulforaphane and sulforaphane N-acetylcysteine conjugate in murine hepatoma cells. J. Med. Food. 2005;8:198–203. doi: 10.1089/jmf.2005.8.198. [DOI] [PubMed] [Google Scholar]
- 36.Cornblatt B.S. Ye L. Dinkova-Kostova A.T. Erb M. Fahey J.W. Singh N.K. Chen M.S. Stierer T. Garrett-Mayer E. Argani P. Davidson N.E. Talalay P. Kensler T.W. Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt B.J. Jordan L.M. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 38.Mao L. Wang H. Wang X. Liao H. Zhao X. Transcription factor Nrf2 protects the spinal cord from inflammation produced by spinal cord injury. J. Surg. Res. 2011;170:e105–e115. doi: 10.1016/j.jss.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 39.Mao L. Wang H.D. Wang X.L. Qiao L. Yin H.X. Sulforaphane attenuates matrix metalloproteinase-9 expression following spinal cord injury in mice. Ann. Clin. Lab. Sci. 2010;40:354–360. [PubMed] [Google Scholar]
- 40.Nishio Y. Koda M. Hashimoto M. Kamada T. Koshizuka S. Yoshinaga K. Onodera S. Nishihira J. Okawa A. Yamazaki M. Deletion of macrophage migration inhibitory factor attenuates neuronal death and promotes functional recovery after compression-induced spinal cord injury in mice. Acta. Neuropathol. 2009;117:321–328. doi: 10.1007/s00401-008-0476-x. [DOI] [PubMed] [Google Scholar]
- 41.Brown K.K. Blaikie F.H. Smith R.A. Tyndall J.D. Lue H. Bernhagen J. Winterbourn C.C. Hampton M.B. Direct modification of the proinflammatory cytokine macrophage migration inhibitory factor by dietary isothiocyanates. J. Biol. Chem. 2009;284:32425–32433. doi: 10.1074/jbc.M109.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bregman B.S. Kunkel-Bagden E. Schnell L. Dai H.N. Gao D. Schwab M.E. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita T. Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- 44.Bradbury E.J. Moon L.D. Popat R.J. King V.R. Bennett G.S. Patel P.N. Fawcett J.W. McMahon S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 45.Ping Z. Liu W. Kang Z. Cai J. Wang Q. Cheng N. Wang S. Wang S. Zhang J.H. Sun X. Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 2010;1343:178–185. doi: 10.1016/j.brainres.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J. Kobori N. Aronowski J. Dash P.K. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 47.Hong Y. Yan W. Chen S. Sun C.R. Zhang J.M. The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta. Pharmacol. Sin. 2010;31:1421–1430. doi: 10.1038/aps.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dash P.K. Zhao J. Orsi S.A. Zhang M. Moore A.N. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci. Lett. 2009;460:103–107. doi: 10.1016/j.neulet.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao J. Moore A.N. Redell J.B. Dash P.K. Enhancing expression of Nrf2-driven genes protects the blood-brain barrier after brain injury. J. Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinkova-Kostova A.T. Holtzclaw W.D. Cole R.N. Itoh K. Wakabayashi N. Katoh Y. Yamamoto M. Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang D.D. Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eggler A.L. Liu G. Pezzuto J.M. van Breemen R.B. Mesecar A.D. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. Sci. USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong F. Freeman M.L. Liebler D.C. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 2005;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 54.Hu C. Eggler A.L. Mesecar A.D. van Breemen R.B. Modification of keap1 cysteine residues by sulforaphane. Chem. Res. Toxicol. 2011;24:515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeong W.S. Kim I.W. Hu R. Kong A.N. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm. Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 56.Xu C. Huang M.T. Shen G. Yuan X. Lin W. Khor T.O. Conney A.H. Kong A.N. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 57.Heiss E. Herhaus C. Klimo K. Bartsch H. Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]