Abstract
Multipotent mesenchymal stem cells (MSCs) are promising candidates for regenerative cell-based therapy. The mechanisms underlying MSC differentiation and other functions relevant to therapeutic avenues remain however a matter of debate. Recent reports imply a critical role for intercellular contacts in MSC differentiation. We studied MSC differentiation to vascular smooth muscle cells (VSMCs) in a coculture model using human primary MSCs and VSMCs. We observed that under these conditions, MSCs did not undergo the expected differentiation process. Instead, they revealed an increased proliferation rate. The upregulated MSC proliferation was initiated by direct contacts of MSCs with VSMCs; indirect coculture of both cell types in transwells was ineffective. Intercellular contacts affected cell growth in a unidirectional fashion, since VSMC proliferation was not changed. We observed formation of so-called tunneling nanotubes (TNTs) between MSCs and VSMCs that revealed an intercellular exchange of a fluorescent cell tracker dye. Disruption of TNTs using cytochalasin D or latrunculin B abolished increased proliferation of MSCs initiated by contacts with VSMCs. Using specific fluorescent markers, we identified exchange of mitochondria via TNTs. By generation of VSMCs with mitochondrial dysfunction, we show that mitochondrial transfer from VSMCs to MSCs was required to regulate MSC proliferation in coculture. Our data suggest that MSC interaction with other cell types does not necessarily result in the differentiation process, but rather may initiate a proliferative response. They further point to complex machinery of intercellular communications at the place of vascular injury and to an unrecognized role of mitochondria in these processes.
Introduction
Molecular and cellular mechanisms of arterial response to injury remain, despite extensive research, not well understood. Consequently, an integrated view of vascular injury-associated diseases that could be translated to effective treatment of these patients is still missing. Over the past decade, stem cell-based therapy has been attracting an increasing interest of biologists and clinicians as a new alternative therapeutic approach to repair injured tissues and restore their function. Mesenchymal stem cells (MSCs) have emerged as the most promising candidate for these cell-based therapeutic avenues. MSCs are adult stem cells localized in and mobilized from bone marrow (BM), retaining self-renewal capability and unique multilineage potential [1]. Beyond their ability to differentiate into multiple cell lineages, MSCs reveal immunosuppressive and anti-inflammatory activities contributing additionally by these ways to tissue repair [2]. MSCs can be easily isolated from BM and other tissues and expanded in vitro under standard cell culture conditions that enhance from translational perspective benefits of their potential use for therapeutic applications.
Most studies on MSC-based therapy address cancer, osteogenesis, chondrogenesis, adipogenesis, and cardiac repair [3]. Despite some contradictions in the results coming from these studies, they provide clear evidence for a high potential and safety of MSC-based therapy for these disorders. Participation and contribution of MSCs to vascular remodeling and repair after vascular injury are less well explored and understood. Although several in vitro and in vivo studies demonstrated the ability of MSCs to differentiate to endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) or VSMC-like cells and to engraft at the place of vascular injury, the underlying molecular and cellular events remain unresolved [4,5]. The lack of our knowledge on mechanisms controlling the MSC functional behavior upon response-to-vascular injury leads to limitations in the MSC use for related therapies.
Recent reports documented an important role of intercellular contacts and communications for MSC differentiation. Several groups have demonstrated that in coculture models, MSC differentiation to cardiomyocytes, osteocytes, and further lineages can be triggered via intercellular interplay [6–11]. In most, but not all of these studies, a direct intercellular contact was found to be mandatory to induce MSC differentiation. We have shown recently that human MSCs can differentiate to VSMC-like cells in vitro and engraft at the place of vascular injury in vivo [12]. We were interested to investigate whether and how MSCs may utilize intercellular communications with VSMCs for their differentiation to the VSMC phenotype. We report here that in a coculture model, MSCs did not undergo the expected differentiation process. Instead, they revealed an increased proliferation rate. The upregulated MSC proliferation was initiated by direct contacts of MSCs with VSMCs, formation of tunneling nanotubes (TNTs), and transfer of VSMC mitochondria to MSCs.
Materials and Methods
Cell culture and coculture of MSCs and VSMCs
Human bone marrow MSCs and primary human coronary artery VSMCs were obtained from Lonza (Lonza Walkersville, Inc.), and cultivated in the medium recommended by the suppliers, and used between passages 6 and 7. Monocultures and cocultures of MSCs and VSMCs were grown in a medium consisting of an MSC basal medium (MSCBM; Lonza Walkersville, Inc.), supplemented with 2% fetal bovine serum (Promocell GmbH), 4 mM l-glutamine (Lonza Walkersville, Inc.), and 0.01% penicillin/streptomycin (Biochrom AG). The medium was changed every 2 days, and cocultures were carried out for 4 days in 6-well plates with 0.1 million cells/well (MSC:VSMC in 1:1 ratio). To induce mitochondrial dysfunction by mitochondrial DNA (mtDNA) mutation and depletion, VSMCs were treated with 100 ng/mL ethidium bromide (EtBr). Cells became incapable of aerobic respiration and growth, unless the medium was additionally supplemented with 110 μg/mL sodium pyruvate and 50 μg/mL uridine to facilitate anaerobic glycolysis. After 40 days of culture in the presence of EtBr in the supplemented medium, mtDNA-depleted VSMCs were used for cocultures. The efficacy of mitochondrial dysfunction was confirmed by cell death in a medium lacking pyruvate/uridine.
Transwell cocultures were performed using 0.4-μm polyester membrane 24-mm inserts (Corning Life sciences) in a 6-well plate format. Potent actin polymerase inhibitors cytochalasin D and latrunculin B (Sigma-Aldrich) were used in coculture studies at 1 and 0.5 μM concentrations, respectively.
Endocytosis by transferrin- and dextran-uptake assay
VSMCs and MSCs (with or without cytochalasin D and latrunculin B) were grown in an MSCBM supplemented with 2% fetal bovine serum. About 5 μg/mL Alexa Fluor® 488-conjugated transferrin (Molecular Probes) was added to the medium and incubated on ice for 30 min. For internalization, washed thrice with ice-cold medium cells were incubated at 37°C for 1 h. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature (RT), and embedded in a ProLong® Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes). For the dextran-uptake assay, cells were incubated with Oregon Green® 488-conjugated dextran (70,000 MW; Molecular Probes) and incubated at 37°C for 1 h.
Mitotracker Red and CM-DiI staining
Mitotracker Red (Molecular Probes) was used to visualize mitochondria. Cells were stained with 200 nM Mitotracker Red dissolved in an MSCBM for 10 min at 37°C. Excess of the dye was washed out with the medium. About 2 μmol/mL chloromethylbenzamido (CellTracker™ CM-DiI; Molecular Probes) was used to stain membranes of adherent MSCs in phosphate-buffered saline (PBS) for 5 min at 37°C and then for 15 min at 4°C. Excess of dye was washed out with the medium. Stained MSCs were detached and seeded for mono- and cocultures.
Immunofluorescent confocal microscopy
Prestained MSCs and VSMCs were seeded in mono- and cocultures on glass coverslips (20,000 cells/coverslip). At different time points (ranging from 2 to 24 h), cells were fixed with 4% PFA for 15 min at RT, and cells were embedded in a ProLong Gold antifade reagent with DAPI (Molecular Probes). The fluorescence cell images were captured using a Leica TCS-SP2 confocal microscope (Leica Microsystems). Images were taken with an oil-immersed 63×objective.
Western blotting
VSMC-specific cytoskeletal protein expression in mono- and cocultured MSCs and VSMCs was identified by western blotting. Cells were lyzed with a buffer containing 50 mM Tris–HCl, pH 7.4, 1% Triton X-100, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethylsulfonyl fluorid, 1 mg/mL aprotinin, 1 mg/mL leupeptin, 1 mM Na3VO4, and 1 mM NaF directly on culture dishes. Lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The protein was transferred onto a polyvinylidene fluoride membrane (Roche Diagnostics). Membranes were probed with appropriate antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz biotechnology). Chemiluminescent images were captured using VersaDoc-3000 (Bio-Rad Laboratories) and quantified using Quantity One software (Bio-Rad Laboratories).
Cell proliferation assays
xCELLigence-based assay
MSCs and VSMCs were seeded for mono- and cocultures in a 16-well plate (E-plate 16; Roche) (3,000 cells in 200 μL medium/well), following the xCELLigence Real Time Cell Analyzer (RTCA) DP instrument manual as provided by the manufacturer (Roche), and the experiment was allowed to run for 120 h. Average slopes for mono- and cocultures were calculated for at least 6 measurements from 2 replicate experiments±standard deviation.
Flow cytometry-based assay
The fluorescein-based dye 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) was used to track proliferation of MSCs and VSMCs in coculture as described [13]. MSCs were detached from the plate using 0.04% Trypsin/0.03% EDTA (PromoCell GmbH) and then incubated with pretitrated 10 μmol/mL CFSE in the MSCBM, supplemented with 2% fetal bovine serum for 10 min at 37°C. The cells were then washed twice with a 6-fold CFSE volume of the medium. Mono- and cocultures were cultured for 4 days and detached from culture plates with 0.04% Trypsin/0.03% EDTA and resuspended in 1% bovine serum albumin in PBS, and the flow cytometry analysis was performed using the FACS CANTO system (BD Biosciences) using acquisition software FACS DIVA (BD Biosciences). Cells were gated for stained MSCs or VSMCs according to forward scatter/side scatter and CFSE labeling. Data are presented as a mean fluorescence intensity, which was calculated using Summit software (Dako)±standard deviation of at least 3 independently performed experiments.
Statistical analysis
All the experiments were performed in triplicate. Data from all experiments are presented as mean±standard deviation. The Student t-test was used for statistical analysis.
Results
MSC coculture with VSMCs does not induce MSC differentiation
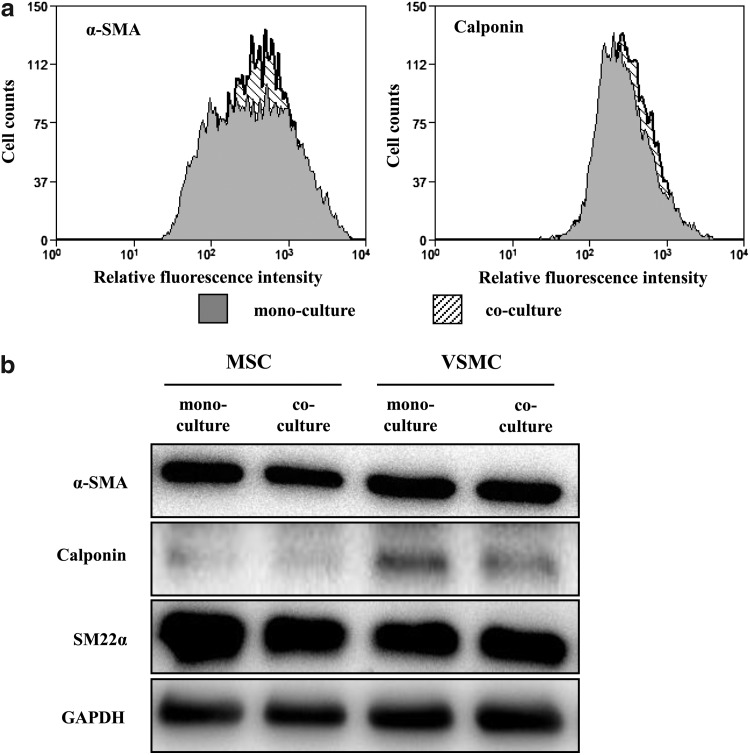
MSC differentiation triggered by coculture with other cell types has been demonstrated. To explore the role of intercellular communications for MSC differentiation to a VSMC-like phenotype, we used direct coculture of human primary MSCs and VSMCs. We have shown previously that MSCs we used in that model are able to undergo differentiation to VSMCs when stimulated with transforming growth factor (TGF)-β in monoculture [12]. The differentiation state of MSCs cocultured with VSMCs up to 7 days was monitored by expression of corresponding VSMC markers, such as smooth muscle α-actin, calponin, and SM22α proteins, in combination with cell sorting. Despite our expectations, we did not observe any differentiation of MSCs under coculture conditions, as conducted by flow cytometry and western blot analyses (Fig. 1a, b), though MSCs retained differentiation potential and underwent differentiation to VSMCs in response to TGF-β stimulation (data not shown).
FIG. 1.
MSC coculture with VSMCs does not induce MSC differentiation. (a) Representative flow cytometric analysis shows no change of VSMC markers α-SMA and calponin in MSCs and VSMCs in mono- and cocultures; n=3. (b) Expression of VSMC markers in MSCs and VSMCs after cell sorting in mono- and cocultures was assessed by western blotting, with GAPDH as loading control; n=3. MSC, mesenchymal stem cells; VSMC, vascular smooth muscle cells; α-SMA, alpha-smooth muscle actin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
VSMCs induce upregulation of MSC proliferation
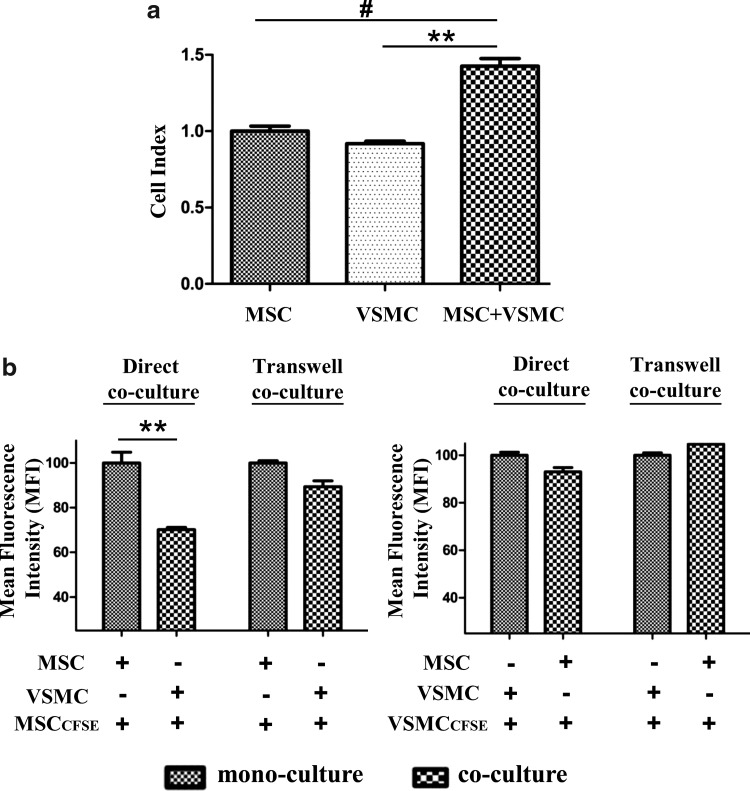
Examining MSC functional behavior upon coculture with VSMCs, we observed unexpectedly that cell growth in coculture was upregulated, as monitored using the xCELLigence system (Fig. 2a). This increase in cell proliferation was time dependent with a maximum at 3–4 days of coculture. This experimental approach did not allow, however, distinguishing between MSC and VSMC proliferation. Therefore, to determine the cell type responsible for the observed increase in the proliferation rate and to quantitatively analyze the extent of cell growth, we applied the proliferation assay based on usage of the CFSE cell proliferation dye [13]. MSCs or VSMCs were separately labeled with CFSE, used for coculture experiments, and analyzed by flow cytometry after 4 days of coculture. We performed first CSFE labeling of MSCs and found a time-dependent induction of MSC proliferation in coculture with VSMCs. This effect required direct contacts between both cell types, because MSC growth in transwells, which physically separated both cell types, was not affected (Fig. 2b, left panel). To examine whether the upregulated proliferation rate was bidirectional in coculture, we performed CFSE labeling of VSMCs, but not MSCs. In this case, however, we observed no change in VSMC proliferation in both direct and transwell cocultures compared to their proliferation in monoculture (Fig. 2b, right panel).
FIG. 2.
VSMCs induce increased MSC proliferation. (a) Real-time cell proliferation of mono- and cocultures was monitored by using the xCELLigence Real Time Cell Analyzer DP Instrument (Roche). Cell proliferation in coculture was significantly increased when compared to monocultures. Average slope for mono- and cocultures was calculated between 30 and 120 h for at least 6 measurements, each from 2 replicate experiments±standard deviation. #P<0.05; **P<0.012. (b) Graph showing MFI represents the cell proliferation in CFSE-labeled MSCs and VSMCs in direct and transwell cocultures after 4 days. Cell proliferation was indicated by decrease in MFI as a result of cell divisions. Reduced MFI was observed in CFSE-labeled MSCs in direct coculture with VSMCs when compared to monoculture and transwell cocultures (left panel). **P<0.012; n=4. However, no change in MFI was observed in VSMC proliferation (right panel) either in direct or transwell cocultures when compared to monocultures. MFI, mean fluorescent intensity; CFSE, 5,6-carboxyfluorescein diacetate succinimidyl ester.
MSCs and VSMCs form TNT-like structures for intercellular contacts
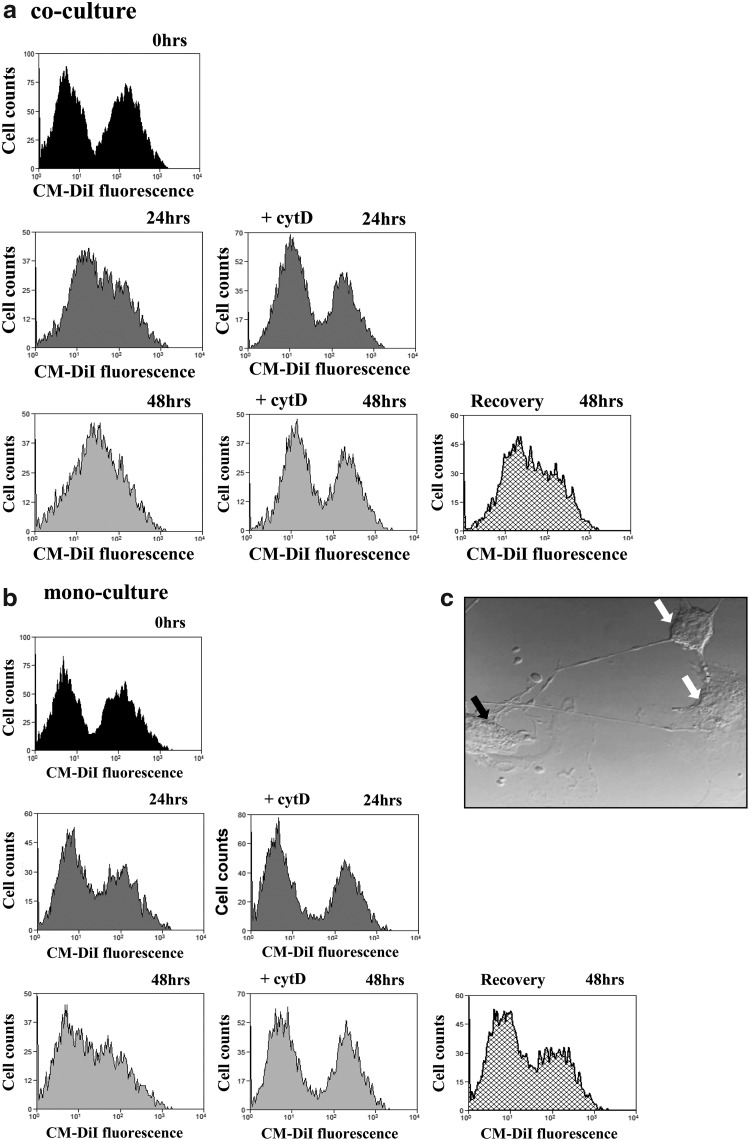
Since we found that direct intercellular contacts with VSMCs were required for increase in MSC proliferation, we next explored more carefully the mechanisms of MSC–VSMC direct communications. To examine the role of intercellular contacts in these processes, we analyzed intercellular transfer of membrane compartments through cell contacts by tracing of living cells using CellTracker (CM-DiI). MSCs were labeled with CM-DiI and used for coculture with unstained MSCs or VSMCs. Cells were analyzed by flow cytometry at different time points after coculture (Fig. 3a). Using coculture of labeled MSCs and nonstained VSMCs, we observed the appearance of a cell population with a transferred lipid probe after 24 h of coculture that further increased at 48 h. Although we observed a time-dependent CM-DiI transfer and formation of an additional cell population in MSC monoculture, where CM-DiI-loaded MSCs were cocultured with unlabeled MSCs, this effect was less expressed compared to the MSC and VSMC populations (Fig. 3b). Treatment of cocultured cells with cytochalasin D disrupting actin polymerization resulted in abrogation of intercellular transfer. Interestingly, if cytochalasin D was washed out after the treatment, cocultured cell populations were able to recover their ability for intercellular contacts (Fig. 3a, b, low-right panels).
FIG. 3.
MSCs and VSMCs form TNT-like structures for intercellular contacts. Flow cytometry of cocultured MSCs and VSMCs. MSCs were labeled with CellTracker™ (CM-DiI), whereas VSMCs were unlabeled; cytochalasin D (1 μM) was used to disrupt intercellular transfer. Flow cytometry analysis was performed after 2 h (a, b, top panels), 24 h without (a, b, middle left panels) or with (a, b, middle right panel) cytochalasin D, and 48 h without (a, b, bottom left panels) or with (a, b, bottom center panels) of mono- and coculture. Recovery of intercellular transfers was observed after cytochalasin D was washed out after the treatment (a, b, bottom right panels). (c) Representative phase-contrast images showing TNT formation between MSC-MSC and MSC-VSMC after 24 h of co-culture; MSCs (white arrow), VSMCs (black arrow). TNTs, tunneling nanotubes.
Together, these findings point to exchange of membranous structures between MSCs requiring formation of specific structures to realize this transfer. Increasing body of recent reports describes formation of ultrathin intercellular structures termed as TNTs connecting cells even over a long distance [14]. To explore nanotubular network formation, we used light microscopy and observed indeed formation of thin TNT-like structures between MSCs and VSMCs (Fig. 3c). TNT formation was spontaneous and time dependent, and TNTs were clearly visible 24 h after coculturing.
MSCs and VSMCs utilize TNT for exchange of mitochondria
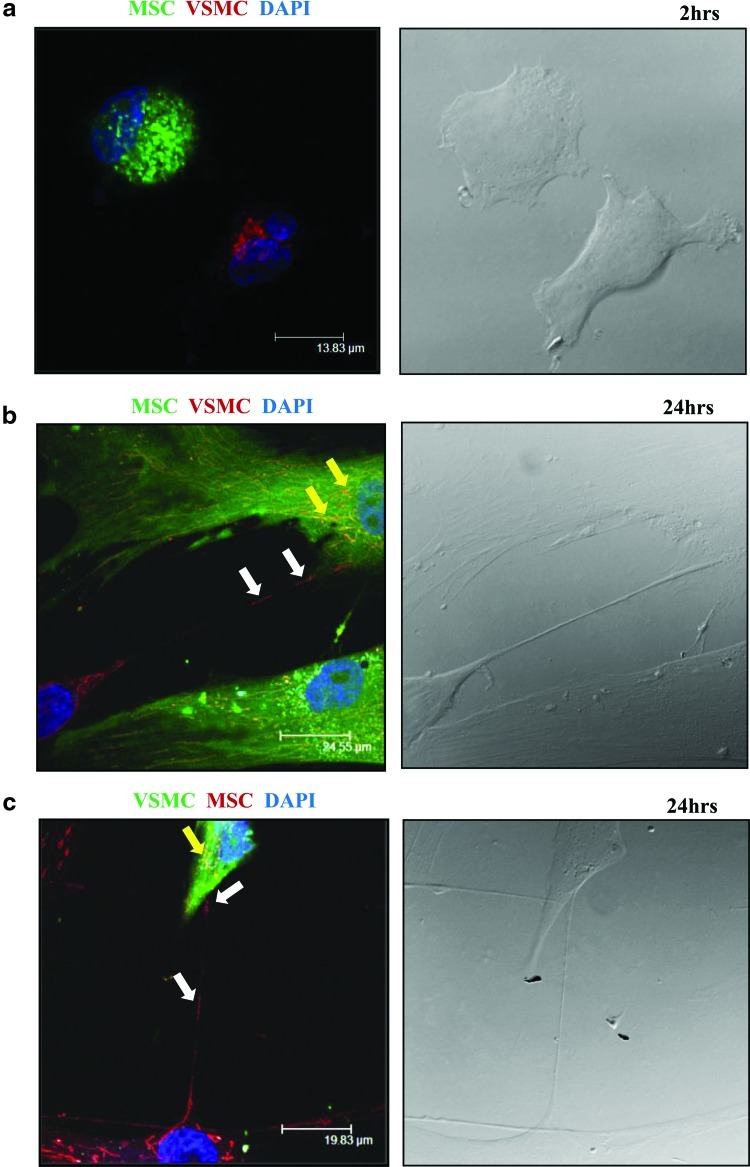
To analyze what kind of intracellular material or organelles may undergo transfer via TNTs, we first examined mitochondrial exchange, because recent reports imply an important role for mitochondria in stem cells [15]. MSCs or VSMCs were labeled separately with either the mitochondrial specific dye, MitoTracker Red, or CFSE and used for coculture experiments followed by confocal microscopy (Fig. 4). Although TNT formation began already after 2 h of coculture, mitochondrial transfer was not detected at this time point. After 24 h of co-culture, MitoTracker Red staining revealed functionally active mitochondria in lumen of TNTs connecting MSCs and VSMCs and mitochondrial transfer between cells. Transfer of mitochondria was bidirectional and did not reveal any preferential direction for their movement between MSCs and VSMCs.
FIG. 4.
Intercellular exchange of mitochondria between MSCs and VSMCs. MSCs or VSMCs were labeled with either MitoTracker Red (red) or CFSE (green). MitoTracker Red-labeled cells were cocultured for 2 and 24 h with CFSE-labeled cells. Fluorescence confocal microscopy revealed mitochondria in the lumen of the TNT formed between MSCs and VSMCs (left panels). Right panels show the corresponding phase-contrast images. (a) Initiation of TNT formation was observed between MSCs and VSMCs after 2 h of coculture. (b, c) Bidirectional exchange of mitochondria between MSCs and VSMCs after 24 h of coculture is shown (white arrows). Yellow arrows show double-positive cells with exchanged mitochondria; nuclear staining is shown in blue (DAPI). n=3. DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/scd
Transfer of mitochondria is required for MSC proliferation induced by VSMCs
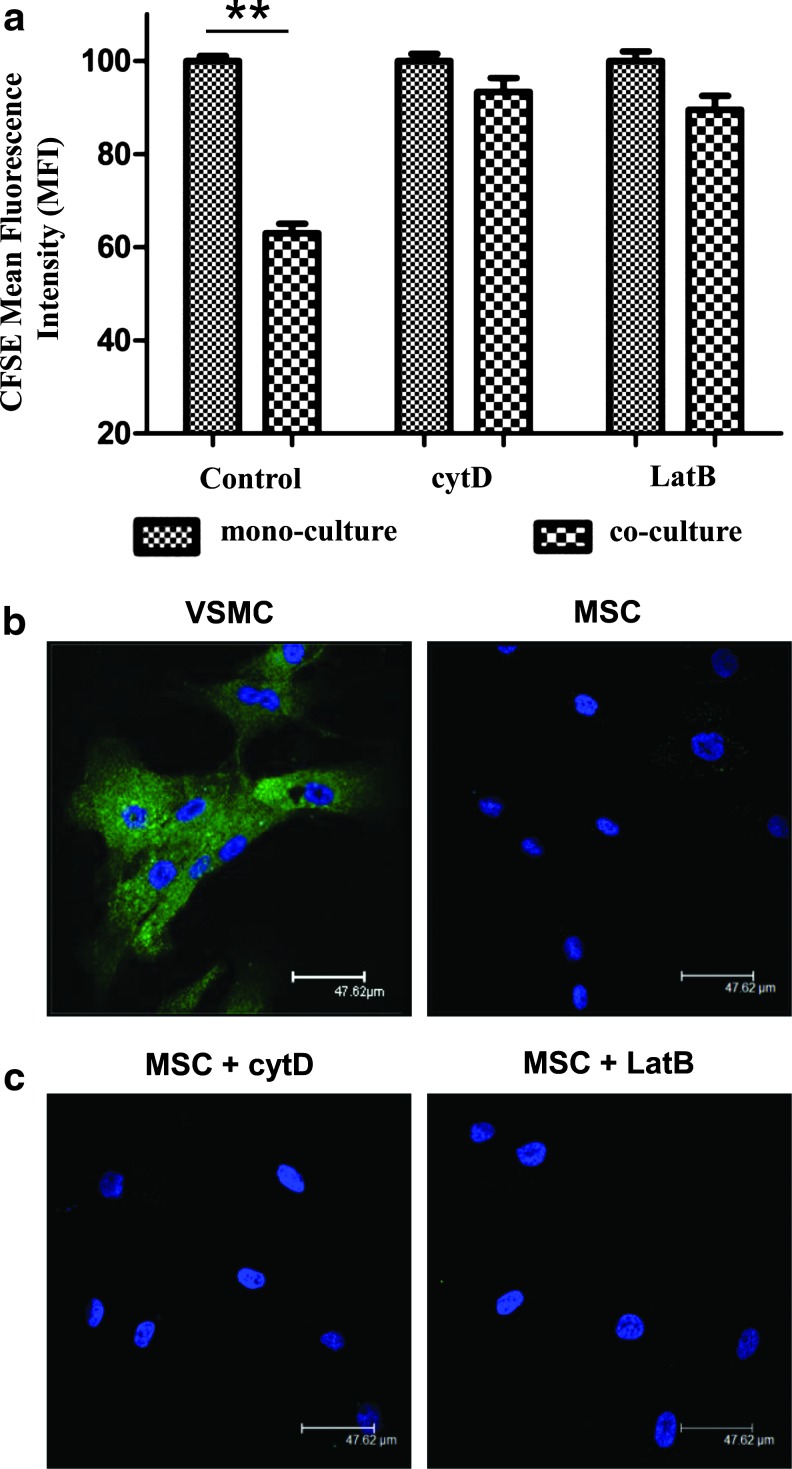
We asked whether the observed formation of the TNT network and transfer of mitochondria between MSCs and VSMCs might be utilized to trigger MSC proliferation observed in coculture with VSMCs. Therefore, cocultures of VSMCs with MSCs labeled with CFSE were subjected to treatment with cytochalasin D or latrunculin B. As expected and confirmed by light microscopy, this treatment resulted in substantial abrogation of TNT formation (data not shown). We observed that increase in proliferation of MSCs induced by VSMCs in control cocultures was completely abolished by both cytochalasin D and latrunculin B treatment (Fig. 5a). In control experiments, it was shown that neither cytochalasin D nor latrunculin B affected proliferation of monocultured MSCs (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd). To check whether cytochalasin D and latrunculin B treatment may elicit any side effects on MSC endocytosis, thus leading to changes in cell proliferation, we performed separately endocytosis assays using Alexa Fluor 488-conjugated transferring and Oregon Green 488-conjugated dextran uptake. In contrast to VSMCs, MSCs revealed a negligible potential for transferring endocytosis (Fig. 5b). Neither cytochalasin D nor latrunculin B had any effect on the very low endocytotic potential of MSCs (Fig. 5c). Similar results were observed for dextran uptake (data not shown).
FIG. 5.
VSMC-induced MSC proliferation requires TNT formation. (a) Graph showing MFI represents the cell proliferation in CFSE-labeled MSCs and VSMC cocultures. Increase in MSC proliferation was completely abolished by TNT disruption with cytochalasin D (1 μM) and latrunculin B (0.5 μM), when compared to the control coculture. **P<0.01; n=4. (b, c) Fluorescence confocal microscopy images represent the uptake of transferrin by endocytosis in VSMCs (b, left panel), whereas MSCs failed transferrin uptake without (b, right panel) or with CytD (c, left panel) or LatB (c, right panel). Color images available online at www.liebertpub.com/scd
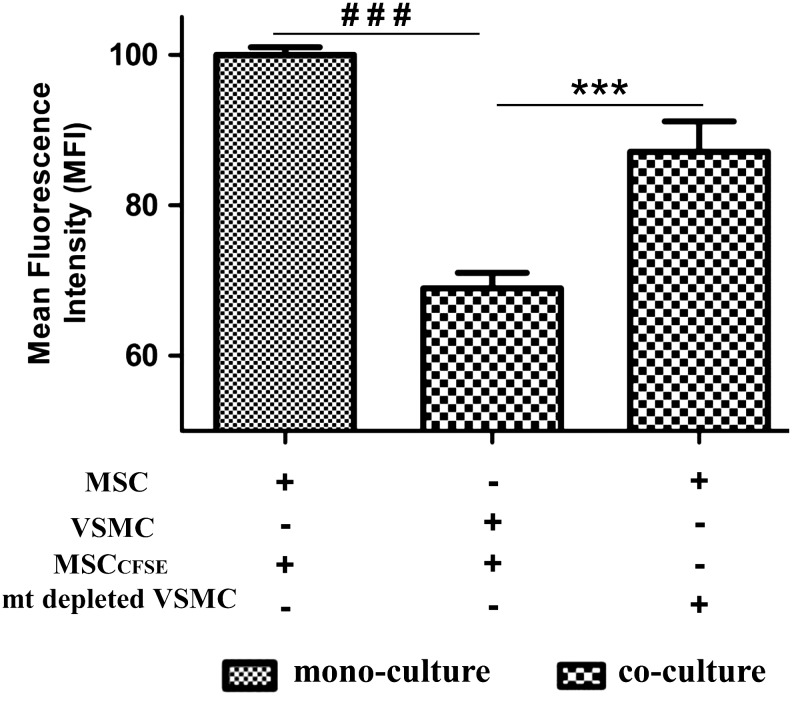
To provide direct evidence for the functional role of mitochondria in VSMC-induced MSC proliferation, we generated VSMCs with mitochondrial dysfunction using mtDNA depletion by long-term cell treatment with EtBr, which is known to destroy mtDNA, but not nuclear DNA. Cells became incapable of aerobic respiration and growth and could survive only in a permissive medium with pyruvate/uridine supplementation. After 40 days of culture in the presence of EtBr in a supplemented medium, VSMCs with mitochondrial dysfunction were used for cocultures followed by a proliferation assay. We found that whereas increased proliferation was observed for MSC cocultured with control VSMCs, this effect was abrogated in cocultures with VSMCs having mitochondrial dysfunction (Fig. 6). In control experiments, it was observed that VSMCs with dysfunctional mitochondria were able to form TNTs with MSCs frequent as normal VSMCs (data not shown). Together, results of these experiments provide direct evidence for requirement of TNT-mediated mitochondrial transfer from VSMCs to MSCs to induce MSC proliferation.
FIG. 6.
VSMC-induced MSC proliferation requires mitochondrial transfer from VSMCs to MSCs. Graph showing MFI represents the cell proliferation in CFSE-labeled MSCs and in cocultures with control VSMCs and with VSMCs having mitochondrial dysfunction. Cell proliferation was indicated by decrease in MFI as a result of cell divisions. Reduced MFI was observed in CFSE-labeled MSC cocultured with control VSMCs, but not in cocultures with mtDNA-depleted VSMCs. ***P<0.0002; ###P<0.0001, n=4.
Discussion
Four decades after the MSC discovery by Friedenstein et al. [16], these cells became a leading candidate for potential cell-based regenerative therapy. Despite increasing interest and extensive research, many aspects of MSC biology and applications yet remain a matter of debate. One of unresolved concerns is how the very low number of endogenous MSCs mobilized into circulation and engrafted at damaged tissue may provide the beneficial effect of MSCs in the long term. We report here that human MSCs may undergo upregulated proliferation at the place of vascular injury by interacting with resident vascular cells. We further show that formation of intercellular TNTs bridging MSCs and VSMCs and transfer of functional mitochondria from VSMCs to MSCs are decisive for this process.
An increasing body of recent findings suggests importance of heterotypic intercellular crosstalk in the regulation of MSC functional behavior [3]. Several studies using coculture models, including the three-dimensional spheroid coculture system [11], revealed that the MSCs' differentiation capacity is significantly determined by their microenvironment. It was demonstrated that intercellular interactions of MSCs with cardiomyocytes [7] and osteoclasts [9] induced MSCs to acquire the phenotypical characteristics of the interacting cell type. A couple of studies documented similar behavior of MSCs upon coculture with vascular cells, such as ECs and smooth muscle cells [6,11]. When indirect coculture or a conditioned medium was used, MSC differentiation was less expressed or completely abolished, thus pointing to direct intercellular contact as an obligatory event in MSC reprogramming.
We have shown recently that MSCs may undergo differentiation into VSMCs upon optimized conditions and revealed some of the underlying molecular mechanisms [12]. In this study, we were originally interested to investigate these mechanisms upon MSC interplay with VSMCs under the premise that MSCs would acquire the VSMC phenotype. Contrary to the findings of others, we could not document MSC differentiation to VSMCs upon their coculture up to 7 days. MSCs did not become positive for VSMC marker proteins, as examined by various techniques established by us to monitor MSC-VSMC transdifferentiation. Instead, we observed increased proliferation of MSCs mediated by direct intercellular interactions with VSMCs. A conditioned medium or indirect coculture in transwells was ineffective to stimulate cell growth. Interestingly, the proliferation process was asymmetric and directed to MSCs; VSMC growth was not affected. We do not believe that there is a contradiction between our data and previous observations. One possible explanation why others did not observe the same effect may be the specificity of the cell origin. Thus, Wang et al. [6] used rat, but not human MSCs and VSMCs for co-culture; moreover, VSMCs were isolated from aorta, whereas in our study, human coronary artery cells were used. Differences in further experimental conditions may also have an outcome on MSC functional behavior. In support of our data, Sinclair and Burg [9] recently found a positive effect of osteoclasts on the proliferation of human MSCs. Further reports provide evidence that functional effects upon cell-to-cell contacts can be directed, in opposite to our observations, from MSCs to the interacting cell type. In particular, the antiapoptotic effect of MSCs on cardiomyocytes [17] and acute B lymphoblastic leukemia cells [18] was shown. Collectively, these data suggest a central role for intercellular crosstalk in regulation of MSC functional modulations. The revealed ability of VSMCs to induce MSC proliferation implies that this mechanism might be utilized at the place of vascular injury as a coordinated strategy to amplify the amount of engrafted MSCs followed by their reprogramming and differentiation.
We found that disruption of TNT-like structures bridging MSCs and VSMCs by cytochalasin D or latrunculin B abrogated VSMC-directed MSC growth pointing to critical role for these structures in intercellular communications and cell growth control. MSC ability to form highly dynamic membranous structures to establish intercellular connections, even over long distances, has been described in early report of Wuchter et al. [19]. These structures are now referred to as TNTs and have been recognized as a novel mechanism for intercellular signaling via fast exchange of the cytoplasmic material, organelles, viral and bacterial pathogens, and other components in vitro and in vivo [20,21]. Recent studies have demonstrated that TNTs mediated interplay of cardiomyocytes [8] and renal tubular cells [22] with MSCs, resulting in MSC differentiation. Other reports revealed a selective multiplicative effect of TNTs between endothelial progenitor cells and ECs, providing reconstitution of the lysosomal pool, and improved viability and function of stressed endothelia [23].
We provide evidence for mitochondrial transfer between MSCs and VSMCs via TNTs. We show further that transfer of functional mitochondria from VSMCs to MSCs was required to stimulate MSC proliferation in cocultures. VSMCs with mitochondrial dysfunction after mtDNA depletion were not able to affect MSC growth. Mitochondria have long been recognized as cellular energy centers providing necessary redox control and maintaining cell survival. An increasing body of evidence implies novel roles for mitochondria, in particular in stem cells, beyond this traditional view [15,24]. Involvement of mitochondria in regulation of stem cells growth has not been reported so far. Several recent studies demonstrated the importance of the bioenergetic function for differentiation potential and pluripotency of stem cells. In particular, MSC osteogenic differentiation was shown to correlate with increased mitochondrial biogenesis [25]. It was demonstrated that mitochondria play a key role in cardiac mesoangioblast differentiation, suggesting that treatments increasing cellular mitochondrial content may be beneficial for cardiac stem cell therapy [26]. In hematopoietic progenitor cells, mitochondrial defects affected the differentiation process [27]. One further recent report documented reprogramming of adult cardiomyocytes back to a progenitor-like state by mitochondrial transfer from and partial cell fusion with MSCs [28]. In our coculture conditions, cell fusion of MSCs with VSMCs was a rare event and not the principle mechanism of intercellular communications. Although we observed a very low endocytotic potential of MSCs, it cannot be excluded, however, that further organelles or/and macromolecules might be transferred between VSMCs and MSCs and participate in the regeneration process. We used coculture conditions supporting TNT formation and minimizing other cellular contacts. However, it would be interesting to examine whether further mechanisms of mitochondrial transfer beyond TNTs might exist. We observed that mitochondrial exchange was bidirectional. Although VSMCs did not reveal any change in the proliferation rate, it remains to be investigated whether delivery of MSC mitochondria to VSMCs may affect other functions of these cells. Thus, it has been suggested that delivery of stem cell mitochondria to cardiomyocytes may prolong their survival [28].
Altogether, our findings highlight the complex nature of MSC communications with resident vascular cells and point to multiple functional outcomes of these interactions that should be considered by potential MSC-based therapy aiming at vascular diseases.
Supplementary Material
Acknowledgments
This work was supported by the grant DU 344/7-1 from the Deutsche Forschungsgemeinschaft and by the Ph.D. program Molecular Medicine of the Hannover Medical School. We express our grateful acknowledgments to Yulia Kiyan, Natalia Tkachuk, Sergey Tkachuk, Manoj B. Menon, Anurag Kumar Singh, Libin Abraham, and Pooja Mishra for their constant support throughout the work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ohishi M. Schipani E. Bone marrow mesenchymal stem cells. J Cell Biochem. 2009;109:277–282. doi: 10.1002/jcb.22399. [DOI] [PubMed] [Google Scholar]
- 2.Lee J. Fang W. Krasnodembskaya A. Howard J. Matthay M. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AR. Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang NF. Li S. Mesenchymal stem cells for vascular regeneration. Regen Med. 2008;3:877–892. doi: 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abedin M. Tintut Y. Demer LL. Mesenchymal stem cells and the artery wall. Circ Res. 2004;95:671–676. doi: 10.1161/01.RES.0000143421.27684.12. [DOI] [PubMed] [Google Scholar]
- 6.Wang T. Xu Z. Jiang W. Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. Int J Cardiol. 2006;109:74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 7.Rangappa S. Entwistle J. Wechsler A. Kresh J. Cardiomyocyte-mediated contact programs human mesenchymal stem cells to express cardiogenic phenotype. J Thorac Cardiovasc Surg. 2003;126:124–132. doi: 10.1016/s0022-5223(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 8.Plotnikov E. Khryapenkova T. Vasileva A. Marey M. Galkina S. Isaev N. Sheval E. Polyakov V. Sukhikh G. Zorov D. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med. 2008;12:1622–1631. doi: 10.1111/j.1582-4934.2007.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinclair S. Burg K. Effect of osteoclast co-culture on the differentiation of human mesenchymal stem cells grown on bone graft granules. J Biomater Sci Polym Ed. 2011;22:789–808. doi: 10.1163/092050610X496260. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre A. Planell J. Engel E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem Biophys Res Commun. 2010;400:284–291. doi: 10.1016/j.bbrc.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 11.Saleh F. Whyte M. Genever P. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater. 2011;22:242–257. doi: 10.22203/ecm.v022a19. [DOI] [PubMed] [Google Scholar]
- 12.Vallabhaneni K. Tkachuk S. Kiyan Y. Shushakova N. Haller H. Dumler I. Eden G. Urokinase receptor mediates mobilization, migration, and differentiation of mesenchymal stem cells. Cardiovasc Res. 2010;90:113–121. doi: 10.1093/cvr/cvq362. [DOI] [PubMed] [Google Scholar]
- 13.Sukkar M. Stanley A. Blake A. Hodgkin P. Johnson P. Armour C. Hughes J. ‘Proliferative’ and ‘synthetic’ airway smooth muscle cells are overlapping populations. Immunol Cell Biol. 2006;82:471–478. doi: 10.1111/j.0818-9641.2004.01275.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerdes H. Carvalho R. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470–475. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Chen C. Hsu S. Wei Y. Mitochondrial bioenergetic function and metabolic plasticity in stem cell differentiation and cellular reprogramming. Biochim Biophys Acta. 2012;1820:571–576. doi: 10.1016/j.bbagen.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Friedenstein A. Chailakhja R. Lalykina K. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 17.Cselenyák A. Pankotai E. Horváth E. Kiss L. Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29–40. doi: 10.1186/1471-2121-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nwabo Kamdje A. Mosna F. Bifari F. Lisi V. Bassi G. Malpeli G. Ricciardi M. Perbellini O. Scupoli M. Pizzolo G. Krampera M. Notch-3 and notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood. 2011;118:380–389. doi: 10.1182/blood-2010-12-326694. [DOI] [PubMed] [Google Scholar]
- 19.Wuchter P. Boda-Heggemann J. Straub B. Grund C. Kuhn C. Krause U. Seckinger A. Peitsch W. Spring H. Ho A. Franke W. Processus and recessus adhaerentes: giant adherens cell junction systems connect and attract human mesenchymal stem cells. Cell Tissue Res. 2007;328:499–514. doi: 10.1007/s00441-007-0379-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y. Tunneling-nanotube: a new way of cell-cell communication. Commun Integr Biol. 2011;4:324–325. doi: 10.4161/cib.4.3.14855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno H. Hase K. Kimura S. M-Sec: emerging secrets of tunneling nanotube formation. Commun Integr Biol. 2010;3:231–233. doi: 10.4161/cib.3.3.11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotnikov E. Khryapenkova T. Galkina S. Sukhikh G. Zorov D. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res. 2010;316:2447–2455. doi: 10.1016/j.yexcr.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Yasuda K. Khandare A. Burianovskyy L. Maruyama S. Zhang F. Nasjletti A. Goligorsky M. Tunneling nanotubes mediate rescue of prematurely senescent endothelial cells by endothelial progenitors: exchange of lysosomal pool. Aging (Albany NY) 2011;3:597–608. doi: 10.18632/aging.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkerson D. Sankar U. Mitochondria: a sulfhydryl oxidase and fission GTPase connect mitochondrial dynamics with pluripotency in embryonic stem cells. Int J Biochem Cell Biol. 2011;43:1252–1256. doi: 10.1016/j.biocel.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Chen C. Shih Y. Kuo T. Lee O. Wei Y. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 26.San Martin N. Cervera A. Cordova C. Covarello D. McCreath K. Galvez B. Mitochondria determine the differentiation potential of cardiac mesoangioblasts. Stem Cells. 2011;29:1064–1074. doi: 10.1002/stem.654. [DOI] [PubMed] [Google Scholar]
- 27.Inoue S. Noda S. Kashima K. Nakada K. Hayashi J. Miyoshi H. Mitochondrial respiration defects modulate differentiation but not proliferation of hematopoietic stem and progenitor cells. FEBS Lett. 2010;584:3402–3409. doi: 10.1016/j.febslet.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Acquistapace A. Bru T. Lesault P. Figeac F. Coudert A. le Coz O. Christov C. Baudin X. Auber F. Yiou R. Dubois-Randé J. Rodriguez A. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812–824. doi: 10.1002/stem.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.