Abstract
Multipotent mesenchymal stromal cells (MSCs) from the human olfactory mucosa (OM) are cells that have been proposed as a niche for neural progenitors. OM-MSCs share phenotypic and functional properties with bone marrow (BM) MSCs, which constitute fundamental components of the hematopoietic niche. In this work, we investigated whether human OM-MSCs may promote the survival, proliferation, and differentiation of human hematopoietic stem cells (HSCs). For this purpose, human bone marrow cells (BMCs) were co-cultured with OM-MSCs in the absence of exogenous cytokines. At different intervals, nonadherent cells (NACs) were harvested from BMC/OM-MSC co-cultures, and examined for the expression of blood cell markers by flow cytometry. OM-MSCs supported the survival (cell viability >90%) and proliferation of BMCs, after 54 days of co-culture. At 20 days of co-culture, flow cytometric and microscopic analyses showed a high percentage (73%) of cells expressing the pan-leukocyte marker CD45, and the presence of cells of myeloid origin, including polymorphonuclear leukocytes, monocytes, basophils, eosinophils, erythroid cells, and megakaryocytes. Likewise, T (CD3), B (CD19), and NK (CD56/CD16) cells were detected in the NAC fraction. Colony-forming unit–granulocyte/macrophage (CFU-GM) progenitors and CD34+ cells were found, at 43 days of co-culture. Reverse transcriptase–polymerase chain reaction (RT-PCR) studies showed that OM-MSCs constitutively express early and late-acting hematopoietic cytokines (i.e., stem cell factor [SCF] and granulocyte- macrophage colony-stimulating factor [GM-CSF]). These results constitute the first evidence that OM-MSCs may provide an in vitro microenvironment for HSCs. The capacity of OM-MSCs to support the survival and differentiation of HSCs may be related with the capacity of OM-MSCs to produce hematopoietic cytokines.
Introduction
Hematopoietic stem cells (HSCs) grow and differentiate in the bone marrow (BM) microenvironment constituted by stromal cells, extracellular matrix proteins, and soluble extracellular matrix-bond growth factors [1]. BM stromal cells play a fundamental role in conditioning the microenvironment where self-renewal, proliferation, and differentiation of HSCs take place, by producing factors and expressing molecules that regulate hematopoiesis. In vitro expansion of HSCs is a rapidly developing area with an enormous potential for biomedical applications [2,3].
In vitro, it has been difficult to enhance the self-renewal and/or expansion of HSCs without stromal cells, even if all known exogenous growth factors and other materials are added to the cultures [4–6]. Despite this, several approaches have shown that human and mouse long-term hematopoiesis can be maintained by co-culturing HSCs with cell lines [7–13] or stromal cells, as feeder layers [14–19].
To date, multipotent mesenchymal stromal cells (MSCs) have shown the most promise cells for promoting in vitro hematopoiesis, as they support not only similar stromal and HSC interactions as those seen in the BM microenvironment [20,21], but also maintain the pluripotential characteristics of HSCs and the functionality of progenitor cells [22–24]. Although the most important source of MSCs is BM, these cells have been also isolated from various other sources [25–32]. Thus, MSCs from placenta, lung, and umbilical cord blood have been shown to support expansion of HSCs and hematopoietic progenitor cells [33,34].
MSCs from human olfactory mucosa (OM) have been recently isolated and characterized [35–38]. It has been reported that OM-MSCs have morphologic and phenotypic similarities with BM-MSCs [37]. Likewise, OM-MSCs have also the capacity to differentiate into ectoderm and mesoderm cell types [37,39]. These similarities have led us to investigate whether human OM-MSCs can be used as an in vitro microenvironment to support expansion and differentiation of human HSCs. In the present study, we show that OM-MSCs support in vitro hematopoiesis.
Materials and Methods
Reagents
Fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-conjugated mouse monoclonal antibodies anti-human CD90, CD73, CD166, CD49b, CD45, CD3, CD19, CD16, CD56, and CD34 were purchased from Becton Dickinson. Trizol was obtained from Sigma-Aldrich.
Isolation and culture of OM-MSCs
Human OM-MSCs were isolated from nasal mucosa biopsies obtained from patients undergoing nasal surgery under general anesthesia, as described before [40]. All patients gave their informed consent to participate in the study, and the study protocol was approved by the institutional review board of the participating institutions. OM-MSCs were obtained as previously described [35,38]. Briefly, biopsy specimens were dissected for explant cultures. Each explant was placed in 24-well plates with alpha-minimum essential medium (MEM)/Chang medium containing 20% fetal bovine serum (FBS) (Sigma). Two days after plating, cells began to migrate from explants as plastic-adherent cells. Two weeks later, adherent cells reached confluency and they were collected and seeded in 24-well plates with alpha-MEM/Chang medium containing 20% FBS.
Isolation and culture of human bone marrow cells
Human BM was obtained by aspiration from the posterior iliac crest of healthy adult volunteers. Informed consent was obtained for all BM collections, and the study was approved by the institutional bioethical committee. BM mononuclear cells were isolated after Ficoll-Paque (GE Healthcare Biosciences) density gradient, and cultured in alpha-MEM/Chang medium. After 24 h of culture, the nonadherent cell (NAC) fraction was collected and plated on a monolayer of OM-MSCs.
OM-MSC and bone marrow cell co-cultures
Bone marrow cells (BMCs) were seeded (15×104 cells per well) onto a confluent monolayer of OM-MSC cultures in 24-well plates with alpha-MEM/Chang medium containing 20% FBS. Half of the co-culture medium was collected and replaced each 5–7 days with fresh medium. The number and the viability of cells present in the NAC fraction of the BMC/OM-MSC co-cultures were determined by trypan blue staining. Aliquots of NACs from each well were examined for expression of HSCs, myeloid, and lymphoid cell markers by flow cytometry, and for morphology in cytospin and for morphology in cytospin preparations by May-Grünwald/Giemsa staining.
Flow cytometry analysis of MSCs and hematopoietic marker expression
To evaluate the expression of MSC markers, OM-MSCs were grown to 90% of confluency, and then the cells were harvested and analyzed by flow cytometry for the expression of the CD90, CD73, CD166, and CD49b markers. On the other hand, NACs were harvested from BMC/OM-MSC co-cultures and analyzed for the expression of HSC (CD34), pan-leukocyte (CD45), and lymphoid (CD3, CD19, CD16, and CD56) markers. Analysis of HSCs and blood cell markers was performed every 7 days. Simultaneous negative control staining reactions were performed by incubating the cells with the FITC- and PE-labeled IgG isotype. Data collection and analysis of the fluorescent intensities were made using an FACScalibur (Becton Dickinson). Ten thousand events were acquired and analyzed using the Cell Quest software program.
Clonogenic assays
Colony-forming unit–granulocyte/macrophage (CFU-GM) progenitors were assessed by methylcellulose colony-forming assay. For this purpose, NACs were seeded at 3×104 cells in Methocult GF H4034 (Stem Cell Technologies). Colonies were scored after 14 days of culture using standard morphological criteria. Single colonies were collected and spread on a glass slide using a cyto-centrifuge and stained with May-Grünwald/Giemsa to observe cell morphology.
Detection of hematopoietic cytokine transcripts in OM-MSCs by RT-PCR
Total RNA was extracted from monolayers of OM-MSCs using a TRIZOL reagent, following manufacturer's instructions. Reverse transcription was carried out using random hexamer oligonucleotides and 4 U AMV reverse transcriptase (Promega) for cDNA synthesis. Polymerase chain reaction (PCR) amplification of the cDNA was then performed using specific oligonucleotides (Table 1) for the detection of stem cell factor (SCF), granulocyte- macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-11, IL-7, and β-actin transcripts. PCR conditions were 1 min of denaturation at 94°C, annealing at 52°C for 1 min, and extension at 72°C for 1 min for 35 cycles. Analysis of the PCR products was performed by comparing them with the predicted PCR fragment size after ethidium bromide staining of the PCR products separated by electrophoresis in a 1.8% agarose gel.
Table 1.
Primer Sequences Specific for Hematopoietic Cytokines
| Primer | Sequences | Base pairs |
|---|---|---|
| IL-6 | 5′-GGTATACCTAGAGTACCTCCAGAA-3′ | 648 |
| 5′-AGTCTACTCCCTGCTGTCTGAATA-3′ | ||
| IL-7 | 5′-GAGTGTTCTAATGGTCAGCATC-3′ | 533 |
| 5′-GGTGCATTCAGTAACTTCTAGG-3′ | ||
| IL-11 | 5′-GTCATACATATCCACTTGAGGG-3′ | 574 |
| 5′-GTACTGTTGATCACAGGGTGACT-3′ | ||
| SCF | 5′-GAGACAGCCAAGTCTTACAAGG-3′ | 437 |
| 5′-ATGGTACATGCAGTCTGAGACAC-3′ | ||
| GM-CSF | 5′-CCTGAACCTGAGTAGAGACACT-3′ | 382 |
| 5′-CCATCCTGAGTTTCTAGCTCTT-3′ | ||
| β-Actin | 5′-TCCTGTGGCATCCACGAAACT-3′ | 314 |
| 5′-GAAGCATTTGCGGTGGACGAT-3′ |
IL, interleukin; SCF, stem cell factor; GM-CSF, granulocyte- macrophage colony-stimulating factor.
Results
Isolation and culture of human OM-MSCs
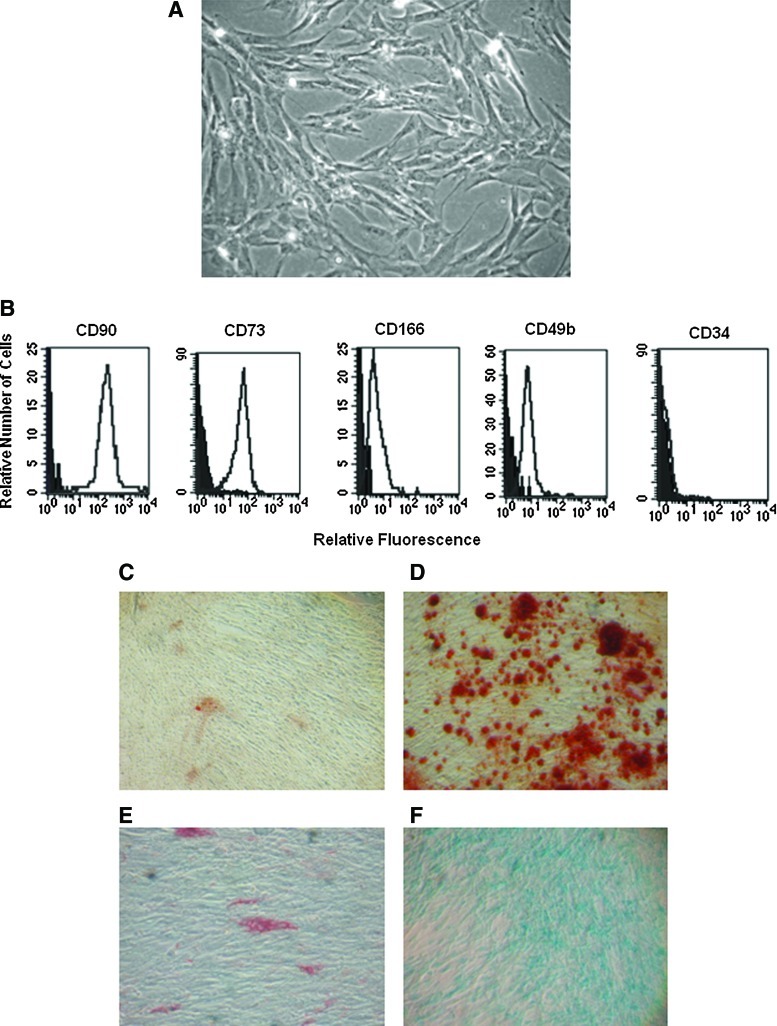
OM explants were placed in 24-well plates containing culture medium supplemented with 20% FBS. After 48 h of culture, adherent cells were observed migrating from OM explants (not shown). These cells were maintained in culture until they reach a confluent monolayer. Microscopic evaluation of cell cultures showed the presence of cells with elongated and fibroblastoid morphology (Fig. 1A). Flow cytometric analysis of these cells showed that they express the MSC markers CD90, CD73, CD166, and CD49b, but not CD34 (Fig. 1B). Differentiation assays showed the osteogenic, adipogenic, and chondrogenic capacity of these cells (Fig. 1C–F).
FIG. 1.
Growth, expression analysis, and differentiation of OM-MSCs. MSCs were isolated from human OM and cultured until they reached confluency. Phase-contrast micrographs show cells with fibroblast-stromal morphology (A). Flow cytometry analysis of OM-MSCs shows the expression of CD90, CD73, CD166, CD49b, and CD34. Negative controls were stained with the respective isotype (black histograms) (B). OM-MSCs were collected and cultured in osteogenic, adipogenic, and chondrogenic media. After 14 days, cells were stained with Alizarin, oil red, and Alcian blue. Micrographs show the presence of mineralization in nucleus (Alizarin positive) (D), adipocytes (oil red staining) (E), and blue staining [Alcian blue positive, (F)], indicating the osteogenic, adipogenic, and chondrogenic capacity of OM-MSCs. As control, OM-MSCs were cultured in regular medium (C). OM, olfactory mucosa; MSCs, mesenchymal stromal cells. Color images available online at www.liebertonline.com/scd
OM-MSCs promote proliferation and survival of human BMCs
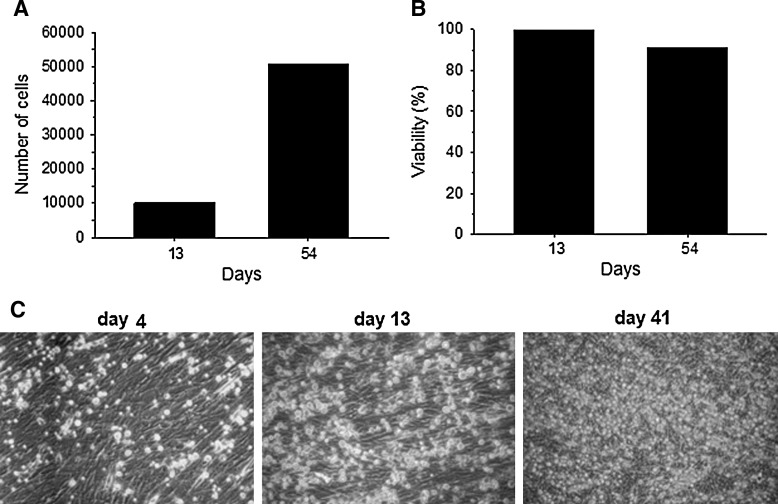
We evaluated the capacity of OM-MSCs to support the proliferation and survival of human BMCs in vitro, by seeding BMCs on confluent OM-MSC monolayers, in the absence of exogenous hematopoietic cytokines. Previously we showed by flow cytometry that these cells express the following markers: CD34, CD45, CD3, CD19, CD16, and CD56 (data not shown). At different intervals, and for almost 8 weeks, NACs were harvested from BMC/OM-MSC co-cultures. The highest number of NACs was collected after 54 days of co-cultures, as compared with day 13 (5-fold increase) (Fig. 2A). The viability of NACs harvested from BMC/OM-MSC co-cultures was always >90% (Fig. 2B). Microscopic evaluation of BMC/OM-MSC co-cultures showed the presence of rounded cells, which were increasing in number from day 4 of co-culture, on the OM-MSC monolayer (Fig. 2C).
FIG. 2.
OM-MSCs promote the proliferation and survival of human BMCs. NACs were collected from BMC/OM-MSC co-cultures and the cell number and viability were determined by trypan blue technique. Results show an increase in the number of NACs at 54 days with respect to 13 days of co-cultures (A). Cell viability was >80% at 54 days of co-culture (B). Microscopic evaluation shows proliferation of rounded cells on OM-MSC monolayer, at 13 and 41 days of BMC/OM-MSC co-cultures (C). Results are representative of at least 3 experiments, all with similar results. BMCs, bone marrow cells; NACs, nonadherent cells.
OM-MSCs promote myelopoiesis from human BMCs
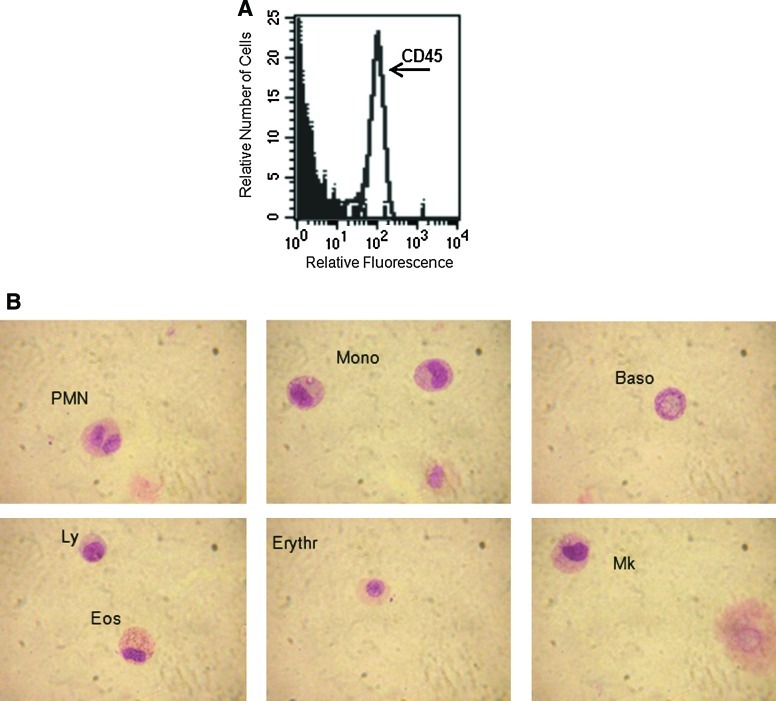
We were interested in evaluating the capacity of OM-MSCs to induce myeloid differentiation from BMCs. For this purpose, NACs were harvested from BMC/OM-MSC co-cultures and evaluated, using flow cytometry, for the expression of the leucocyte common antigen CD45. After 20 days, high percentage (73%) of cells expressing CD45 marker were detected in the NAC fraction (Fig. 3A). Cytospin and May-Grünwald/Giemsa staining of NACs showed the presence of cells of myeloid origin, including polymorphonuclear cells (neutrophils, basophils, and eosinophils), monocytes, erythroid cells, and megakaryocytes (Fig. 3B).
FIG. 3.
OM-MSCs promote in vitro myelopoiesis from human BMCs. NACs were collected, at day 17, from BMC/OM-MSC co-cultures, and the cells were analyzed for their expression of the CD45 marker by flow cytometry (A). Cytospin from NACs and May-Grünwald/Giemsa staining were performed for morphology analysis showing the presence of polymorphonuclear (PMN), monocytes (mono), basophil (Baso), eosinophil (Eos), lymphocytes (Ly), erythroblast (Erythr), and megakaryocyte (Mk) (B). Results correspond to 1 experiment representative of 3 different assays, all with similar results. Color images available online at www.liebertonline.com/scd
OM-MSCs promote lymphopoiesis in vitro from human BMCs
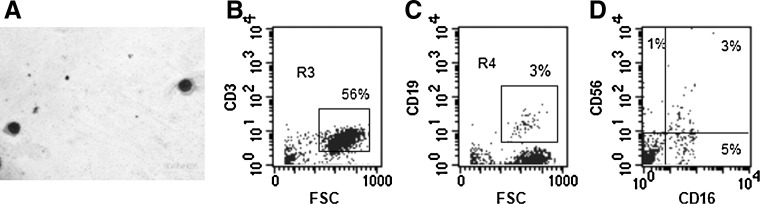
We evaluated the capacity of OM-MSCs to support lymphopoiesis from BMCs. Morphological evaluation of the NACs collected from BMC/OM-MSC co-cultures, after 20 days, showed the presence of lymphoid-like cells (Fig. 4A). Flow cytometric analysis showed the presence of a high percentage of CD3+ cells in the NAC fraction (Fig. 4B). Low percentages of cells expressing CD19+ and CD56+/CD16+ were also found in the NAC population (Fig. 4C, D, respectively).
FIG. 4.
OM-MSCs promote in vitro lymphopoiesis from human BMCs. NACs were collected from BMC/OM-MSC co-culture, at day 17, and the cells were analyzed for their morphology and surface expression markers. Microscopic evaluation shows the presence of lymphoid cells (A). Flow cytometry analysis shows the presence of cells expressing T [CD3, (B)], B [CD19, (C)], and NK [CD56/CD16, (D)] cell markers. Results correspond to 1 experiment representative of 3 different assays, all with similar results.
Detection of hematopoietic progenitors in the nonadhering fraction of human BMC/OM-MSC co-cultures
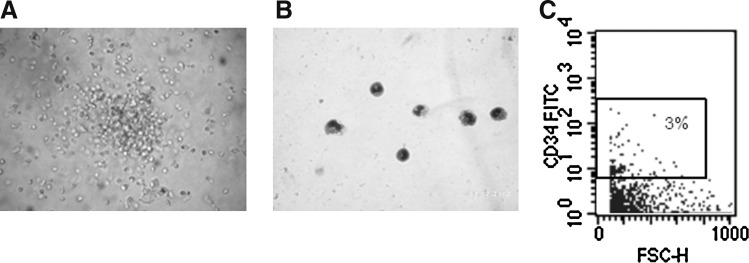
Because our results show that OM-MSCs promote hematopoiesis in vitro from BMCs in long-term cultures, we investigated the possible presence of hematopoietic progenitors in these cultures. For these purposes, NACs were collected from BMC/OM-MSC co-cultures, after 17 days, and evaluated in clonogenic assays for the presence of CFU-GM progenitors, using a MethoCult system. CFU-GM colonies were detected in the NAC fraction collected from BMC/OM-MSC co-cultures (Fig. 5A). Cytospin and May-Grünwald/Giemsa staining of desegregated CFU-GM colonies showed the presence of granulocytes and monocytes (Fig. 5B). The possibility that OM-MSCs support the survival of primitive HSCs in long-term cultures was investigated by evaluating the presence of CD34+ cells in the adherent fraction of the BMC/OM-MSC co-cultures. After 43 days of co-culture, CD34+ cells were detected in the adherent fraction of BMC/OM-MSC co-cultures (Fig. 5C). It is important to note that OM-MSCs do not express hematopoietic markers (CD34) (data not shown). Together, these findings indicate that OM-MSCs can support the survival of human primitive hematopoietic progenitor cells in vitro.
FIG. 5.
Detection of CFU-GM progenitors in BMC/OM-MSC co-cultures. NACs were collected from BMCs/OM-MSCs, after 17 days of co-culture, and colony-forming assays were performed. Microscopic evaluation shows the presence of a CFU-GM colony (A). Single CFU-GM colonies were taken from methylcellulose assays and subjected to cytospin, and May-Grünwald/Giemsa staining showing the presence of myeloid cells (B). Flow cytometry analysis of NACs shows the presence of cells expressing the CD34 marker (C). Results correspond to 1 experiment representative of 2 different assays, all with similar results. CFU-GM, colony-forming unit–granulocyte/macrophage.
Expression of hematopoietic cytokines by OM-MSCs
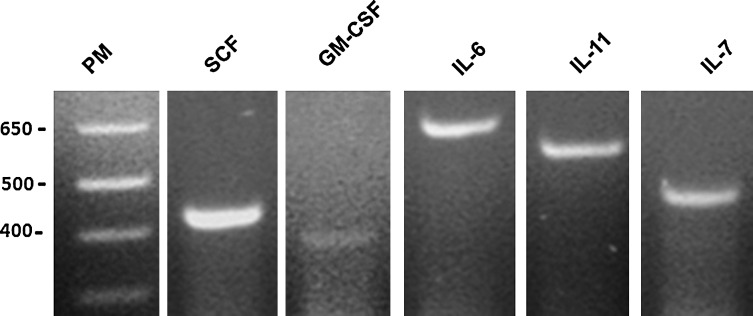
Based on results presented earlier that OM-MSCs support in vitro human hematopoiesis from BMCs, we investigated whether these cells express hematopoietic cytokines. OM-MSCs express mRNA messages for the early acting cytokine SCF, which exerts its effect on primitive hematopoietic progenitors (Fig. 6). They also express messages for GM-CSF, IL-6, and IL-11, which act on more committed hematopoietic progenitors (Fig. 6). Additionally, mRNA message was found for the lymphopoietic cytokine IL-7 (Fig. 6). Together, these results demonstrate that OM-MSCs constitutively express hematopoietic cytokines at the mRNA level.
FIG. 6.
OM-MSCs constitutively express hematopoietic cytokines. Reverse transcription and amplification were performed as described in the Materials and Methods section using specific primers for SCF, GM-CSF, IL-6, IL-11, and IL-7.
OM-MSCs maintain their mesenchymal phenotype after long-term co-culture
We evaluated the possibility that OM-MSCs change their phenotypical characteristics because of co-culturing with BMCs. Flow cytometric analysis of OM-MSCs showed that a high percentage of these cells maintain the expression of the MSC marker CD90 on their surface (Fig. 7), after long-term culture.
FIG. 7.
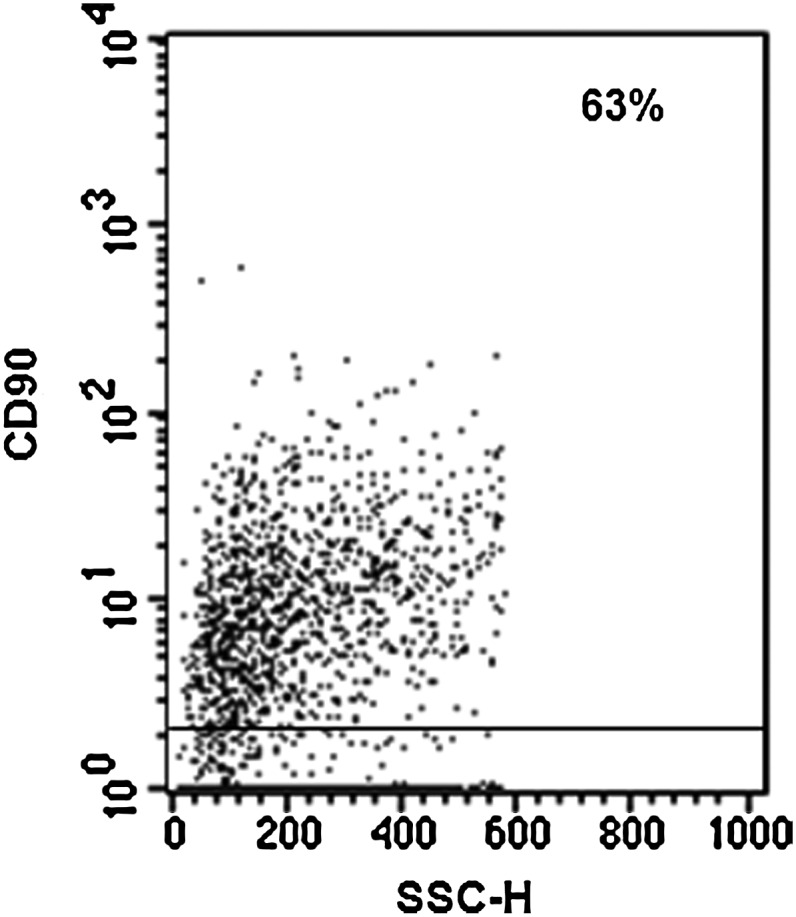
OM-MSCs maintain mesenchymal phenotype after long-term co-culture with BMCs. BMC/OM-MSC co-cultures were harvested after 54 days of co-culture, and the cells were analyzed for their expression of the CD90 MSC marker by flow cytometry. Negative controls were stained with the respective isotype (gray profile). Histogram analysis demonstrates that OM-MSCs maintain the CD90 expression on their surface. The results correspond to 1 experiment representative of 2 different assays, all with similar results.
Discussion
It is well known that BM stromal cells play an important role in the regulation of hematopoiesis [41–43]. There is evidence indicating that the ability of BM stromal cells to support hematopoiesis is due to their capacity to produce numerous hematopoietic cytokines and express adhesion molecules, both implicated in different aspects of hematopoiesis [1]. Numerous reports have shown the capacity of MSCs from BM to support the survival, proliferation, and differentiation of HSCs, in vitro. Although BM is the most well-known source for MSCs, there is evidence showing the presence of these cells in other tissues, and their capacity to maintain in vitro hematopoiesis [44–47]. Recently, MSCs from the human OM have been characterized [37], and it has been suggested that these cells might constitute a fundamental element of the neural stem cell niche in the human OM [36]. Because OM-MSCs share similar phenotypical and multipotential characteristics with MSCs from BM [39], in this work, we investigated the capacity of human OM-MSCs to support the survival, proliferation, and differentiation of human HSCs, in vitro. We show that OM-MSCs promote hematopoiesis from human BM hematopoietic cells.
In this work, we provide evidence that OM-MSCs promote myeloid differentiation from BMCs. High percentage of cells (73%) expressing the leukocyte common antigen CD45 were detected in the NACs of the BMC/OM-MSC co-cultures. Cytological analysis showed the presence of cells belonging to the myeloid lineage with variable morphology, including neutrophils, monocytes, basophils, and eosinophils. These results support previous studies showing that BM-MSCs and other stromal cell lines promote hematopoietic differentiation to myeloid lineages [48–53]. However, most of these studies were performed using co-culture systems supplemented with exogenous cytokines [46,51,52,54,55]. In our study, OM-MSCs promoted myeloid differentiation in the absence of exogenous cytokines. It may be explained by the capacity of OM-MSCs to constitutively express hematopoietic cytokines that promote myeloid differentiation, such as GM-CSF. Importantly, the percentage of cells expressing the CD45 marker, in co-cultures with other stromal cell lines, were very low (0.1%–2% of CD45+ during 8–20 days of culture), as compared with those generated in co-cultures with OM-MSCs (73% of CD45 cells). Taken together, these results indicate that OM-MSCs promote myelopoietic differentiation from human BM hematopoietic cells, and provide the first evidence that OM-MSCs constitute a myelopoietic in vitro microenvironment.
Although previous reports have shown that hematopoietic differentiation promoted by stromal cells is mainly to the myeloid lineage [48,56], there is also evidence showing that MSCs from BM may also promote B and T-cell development [57]. Here, we provide evidence that OM-MSCs promote not only differentiation to myeloid lineages, but also lymphopoiesis from BMCs. We detected T, B, and NK cells in the NAC fraction of BMC/OM-MSC co-cultures. Similar results have been reported using co-cultures of murine ESCs with OP9 cell line, in which T, B, and NK cells were found [49,58]. However, these co-cultures were supplemented with lymphopoietic cytokines and lymphoid differentiation was mainly to generation of B-lineage cells. Our results showing that OM-MSCs promote lymphopoiesis from BMCs, in the absence of exogenous cytokines, may be explained by the capacity of these cells to constitutively express the lymphopoietic cytokine IL-7. Recent reports have also shown a restricted generation of B-lineage cells without using exogenous cytokines [59]. Other MSCs lines reported to promote hematopoiesis, in vitro, do not have the capacity to support lymphopoiesis in vitro [51]. Together, our results show for the first time that OM-MSCs constitute an in vitro lymphopoietic cell microenvironment.
Our results show that OM-MSCs express a wide array of hematopoietic cytokines, including the early acting cytokine SCF, which is known to maintain HSCs in quiescence or promote their self-renewal or differentiation [60,61]. Additionally, OM-MSCs express late-acting cytokines, such as GM-CSF, IL-6, IL-11, and IL-7, which are known to play an important role in various stages of myeloid and lymphoid differentiation [58,62]. The expression of other growth factors by OM-MSCs has been also previously reported [37,63].
In our co-culture system, we also found that OM-MSCs maintain their mesenchymal phenotype after long-term culture, expressing high levels of molecules characteristics of MSCs, such as CD90. This is very important because it has been reported that HSCs need to be re-plated into newly fresh feeder layers, to prevent transformation due to ageing of stroma.
In summary, our work provides evidence that OM-MSCs are capable to maintain in vitro hematopoiesis, in long-term cultures. The promoting effect of OM-MSCs is not limited to any specific hematopoietic lineage, because it involves myeloid and lymphoid cells, indicating that these cells may provide an excellent ex vivo microenvironment for hematopoietic progenitor expansion. The ability of OM-MSCs to support in vitro hematopoiesis could be related to the fact that OM-MSCs express a variety of hematopoietic cytokines.
Acknowledgments
This work was supported by IVIC and FONACIT grant G2005000405.
Author Disclosure Statement
The authors declare that they do not have any conflict of interest to disclose.
References
- 1.Dazzi F. Ramasamy R. Glennie S. Jones SP. Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Palsson BO. Paek SH. Schwartz RM. Palsson M. Lee GM. Silver S. Emerson SG. Expansion of human bone marrow progenitor cells in a high cell density continuous perfusion system. Bio/Technology. 1993;11:368–372. doi: 10.1038/nbt0393-368. [DOI] [PubMed] [Google Scholar]
- 3.Cabrita GJM. Ferreira BS. Silva CL. Goncalves R. Almeida-Porada G. Cabral JMS. Hematopoietic stem cells: from the bone to the bioreactor. Trends Biotechnol. 2003;21:233–240. doi: 10.1016/S0167-7799(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein ID. Andrews RG. Zsebo KM. Recombinant human stem cell factor enhances the formation of colonies by CD34+ and CD34+ lin- cells, and the generation of colony-formation cell progeny from CD34+ lin- cells cultured with interleukin-3, granulocyte colony-stimulating factor, or granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:2316–2321. [PubMed] [Google Scholar]
- 5.Lowry PA. Zsebo KM. Deacon DH. Eichman CE. Quesenberry PJ. Effects of rhSCF on multiple cytokine-responsive HPP-CFC generated from Sca-1+ Lin- murine hematopoietic progenitors. Exp Hematol. 1991;19:994–996. [PubMed] [Google Scholar]
- 6.Li CL. Johnson GR. Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic long-term repopulating cells. Blood. 1994;84:408–414. [PubMed] [Google Scholar]
- 7.Singer JW. Charbord P. Keating A. Nemunaitis J. Raugi G. Wight TN. Lopez JA. Roth GJ. Dow LW. Fialkow PJ. Simian virus 40-transformed adherent cells from human long-term marrow cultures: cloned cell lines produce cells with stromal and hematopoietic characteristics. Blood. 1987;70:464–474. [PubMed] [Google Scholar]
- 8.Novotny JR. Duehrsen U. Welch K. Layton JE. Cebon JS. Boyd AW. Cloned stromal cell lines derived from human Whitlock/Witte-type long-term bone marrow cultures. Exp Hematol. 1990;18:775–784. [PubMed] [Google Scholar]
- 9.Cicuttini FM. Martin M. Salvaris E. Ashman L. Begley CG. Novotny J. Maher D. Boyd AW. Support of human cord blood progenitor cells on human stromal cell lines transformed by SV40 large T antigen under the influence of an inducible (metallothionein) promoter. Blood. 1992;80:102–112. [PubMed] [Google Scholar]
- 10.Roecklein BA. Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85:997–1005. [PubMed] [Google Scholar]
- 11.Loeuillet C. Bernard G. Remy-Martin J. Saas P. Herve P. Douay L. Chalmers D. Distinct hematopoietic support by two human stromal cell lines. Exp Hematol. 2001;29:736–745. doi: 10.1016/s0301-472x(01)00644-0. [DOI] [PubMed] [Google Scholar]
- 12.Garrido SM. Appelbaum FR. Willman CL. Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5) Exp Hematol. 2001;29:448–457. doi: 10.1016/s0301-472x(01)00612-9. [DOI] [PubMed] [Google Scholar]
- 13.Kawano Y. Kobune M. Yamaguchi M. Nakamura K. Ito Y. Sasaki K. Takahashi S. Nakamura T. Chiba H, et al. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a co-culture system with human telomerase catalytic subunit (hTERT)–transfected human stromal cells. Blood. 2003;101:532–540. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- 14.Kodama H. Sudo H. Koyama H. Kasa S. Yamamoto S. In vitro hemopoiesis within a microenvironment created by MC 3T3- G2/PA6 preadipocytes. J Cell Physiol. 1984;118:233–240. doi: 10.1002/jcp.1041180303. [DOI] [PubMed] [Google Scholar]
- 15.Itoh K. Tzuka H. Sakoda H. Konno M. Nagata K. Uchiyama T. Uchino H. Mori KJ. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–153. [PubMed] [Google Scholar]
- 16.Nishikawa M. Ozawa K. Tojo A. Yoshikubo T. Okano A. Tani K. Ikebuchi K. Nakauchi H. Asano S. Changes in hematopoiesis-supporting ability of C3H10T1/2 mouse embryo fibroblasts during differentiation. Blood. 1993;81:1184–1192. [PubMed] [Google Scholar]
- 17.Issaad C. Croisille L. Katz A. Vainchenker W. Coulombel L. A murine stromal cell line allows the proliferation of very primitive human CD34++/CD38− progenitor cells in long-term cultures and semisolid assays. Blood. 1993;81:2916–2924. [PubMed] [Google Scholar]
- 18.Temeles DS. McGrath HE. Kittler ELW. Shadduck RK. Kister VK. Crittenden RB. Turner BL. Quesenberry PJ. Cytokine expression from bone marrow derived macrophages. Exp Hematol. 1993;21:388–393. [PubMed] [Google Scholar]
- 19.Ye ZQ. Burkholder JK. Qiu P. Schultz JC. Shahidi NT. Yang NS. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci U S A. 1994;91:12140–12144. doi: 10.1073/pnas.91.25.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNiece I. Harrington J. Turney J. Kellner J. Shpall EJ. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–317. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 21.Robinson S. Niu T. de Lima M. Ng J. Yang H. McMannis J. Karandish S. Sadeghi T. Fu P, et al. Ex vivo expansion of umbilical cord blood. Cytotherapy. 2005;7:243–250. doi: 10.1080/14653240510027172. [DOI] [PubMed] [Google Scholar]
- 22.Xu MJ. Tsuji K. Ueda T. Mukouyama YS. Hara T. Yang FC. Ebihara Y. Matsuoka S. Manabe A, et al. Stimulation of mouse and human primitive hematopoiesis by murine embryonic aorta-gonad-mesonephros derived stromal cell lines. Blood. 1998;92:2032–2040. [PubMed] [Google Scholar]
- 23.Breems DA. Blokland EA. Siebel KE. Mayen AE. Engels LJ. Ploemacher RE. Stroma-contact prevents loss of hematopoietic stem cell quality during ex vivo expansion of CD34+ mobilized peripheral blood stem cells. Blood. 1998;91:111–117. [PubMed] [Google Scholar]
- 24.Bennaceur-Griscelli A. Tourino C. Izac B. Vainchenker W. Coulombel L. Murine stromal cells counteract the loss of long-term culture-initiating cell potential induced by cytokines in CD34(+)CD38(low/neg) human bone marrow cells. Blood. 1999;94:529–538. [PubMed] [Google Scholar]
- 25.Erices A. Conget P. Minguel JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 26.Campagnoli C. Roberts IA. Kumar S. Bennett PR. Bellantuono I. Fisk NM. Identification of mesenchymal stem/progenitor cells in human first trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 27.Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AL. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 28.In't Anker PS. Scherjon SA. Kleijburg-van der Keur C. de Groot-Swings GM. Claas FH. Fibbe WE. Kanhai HH. Isolation of mesenchymal stem cells of fetal and maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 29.Shih DT. Lee DC. Chen SC. Tsai RY. Huang CT. Tsai CC. Shen EY. Chiu WT. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005;23:1012–1020. doi: 10.1634/stemcells.2004-0125. [DOI] [PubMed] [Google Scholar]
- 30.Trubiani O. Di Primio R. Traini T. Pizzicannella J. Scarano A. Piattelli A. Caputi S. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol. 2005;18:213–221. doi: 10.1177/039463200501800204. [DOI] [PubMed] [Google Scholar]
- 31.Ame-Thomas P. Maby-El Hajjami H. Monvoisin C. Jean R. Monnier D. Caulet-Maugendre S. Guillaudeux T. Lamy T. Fest T. Tarte K. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109:693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 32.Krampera M. Sartoris S. Liotta F. Pasini A. Angeli R. Cosmi L. Andreini A. Mosna F. Bonetti B, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y. Li C. Jiang X. Zhang S. Wu Y. Liu B. Tang P. Mao N. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32:657–664. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Wang JF. Wang LJ. Wu YF. Xiang Y. Xie CG. Jia BB. Harrington J. McNiece IK. Mesenchymal stem/progenitor cells in human umbilical cord blood as support for ex vivo expansion of CD34(+) hematopoietic stem cells and for chondrogenic differentiation. Haematologica. 2004;89:837–844. [PubMed] [Google Scholar]
- 35.Féron F. Perry C. McGrath JJ. Mackay-Sim A. New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol Head Neck Surg. 1998;124:861–866. doi: 10.1001/archotol.124.8.861. [DOI] [PubMed] [Google Scholar]
- 36.Murrell W. Feron F. Wetzig A. Cameron N. Splatt K. Bellette B. Bianco J. Perry C. Lee G. Mackay-Sim A. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233:496–515. doi: 10.1002/dvdy.20360. [DOI] [PubMed] [Google Scholar]
- 37.Delorme B. Nivet E. Gaillard J. Häupl T. Ringe J. Devèze A. Magnan J. Sohier J. Khrestchatisky M, et al. The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. 2010;19:853–866. doi: 10.1089/scd.2009.0267. [DOI] [PubMed] [Google Scholar]
- 38.Girard SD. Devéze A. Nivet E. Gepner B. Roman FS. Féron F. Isolating nasal olfactory stem cells from rodents or humans. J Vis Exp 54e. 2011;2762:1–5. doi: 10.3791/2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calloni GW. Le Douarin NM. Dupin E. High frequency of cephalic neural crest cells shows coexistence of neurogenic, melanogenic, and osteogenic differentiation capacities. Proc Natl Acad Sci U S A. 2009;106:8947–8952. doi: 10.1073/pnas.0903780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winstead W. Marshall CT. Lu CL. Klueber KM. Roisen FJ. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am J Rhinol. 2005;19:83–90. [PubMed] [Google Scholar]
- 41.Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp Hematol. 1981;9:391–410. [PubMed] [Google Scholar]
- 42.Tavassoli M. Friedenstein A. Hemopoietic stromal microenvironment. Am J Hematol. 1983;15:195–203. doi: 10.1002/ajh.2830150211. [DOI] [PubMed] [Google Scholar]
- 43.Allen TD. Dexter TM. Simmons PJ. Marrow biology and stem cells. Immunol Ser. 1990;49:1–38. [PubMed] [Google Scholar]
- 44.Zuk PA. Zhu M. Ashjian P. De Ugarte DA. Huang JI. Mizuno H. Alfonso ZC. Fraser JK. Benhaim P. Hedrick NH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang CJ. Yen ML. Chen YC. Chien CC. Huang HI. Bai CH. Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 46.Battula VL. Treml S. Abele H. Buhring HJ. Prospective isolation and characterization of mesenchymal stem cells from human placenta using a frizzled-9-specific monoclonal antibody. Differentiation. 2007;76:326–336. doi: 10.1111/j.1432-0436.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 47.Nakao N. Nakayama T. Yahata T. Muguruma Y. Saito S. Miyata Y. Yamamoto K. Naoe T. Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: advantages over bone marrow-derived mesenchymal stem cells. Am J Pathol. 2010;177:547–554. doi: 10.2353/ajpath.2010.091042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardier JE. Barberá-Guillem E. Extramedullary hematopoiesis in the adult mouse liver is associated with specific hepatic sinusoidal endothelial cells. Hepatology. 1997;26:165–175. doi: 10.1002/hep.510260122. [DOI] [PubMed] [Google Scholar]
- 49.Nakano T. Kodama H. Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman DS. Hanson ET. Lewis RL. Auerbach R. Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X. Hisha H. Mizokami T. Cui W. Cui Y. Shi A. Song C. Okazaki S. Li Q, et al. Mouse mesenchymal stem cells can support human hematopoiesis both in vitro and in vivo: the crucial role of neural cell adhesion molecule. Haematologica. 2010;95:884–891. doi: 10.3324/haematol.2009.013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vodyanik MA. Bork JA. Thomson JA. Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 53.Qiu C. Hanson E. Olivier E. Inada M. Kaufman DS. Gupta S. Bouhassira EE. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33:1450–1458. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Sauvageau G. Iscove NN. Humphries RK. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23:7223–7232. doi: 10.1038/sj.onc.1207942. [DOI] [PubMed] [Google Scholar]
- 55.Robinson SN. Simmons PJ. Yang H. Alousi AM. Marcos de Lima J. Shpall EJ. Mesenchymal stem cells in ex vivo cord blood expansion. Best Pract Res Clin Haematol. 2011;24:83–92. doi: 10.1016/j.beha.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okubo T. Matsui N. Yanai N. Obinata M. Stroma-dependent maintenance of cytokine responsive hematopoietic progenitor cells derived from long-term bone marrow culture. Cell Struct Funct. 2000;25:133–139. doi: 10.1247/csf.25.133. [DOI] [PubMed] [Google Scholar]
- 57.Dejbakhsh-Jones S. Jerabek L. Weissman IL. Strober S. Extrathymic maturation of alpha beta T cells from hemopoietic stem cells. J Immunol. 1995;155:3338–3344. [PubMed] [Google Scholar]
- 58.Vegh P. Winckler J. Melchers F. Long-term “in vitro” proliferating mouse hematopoietic progenitor cell lines. Immunol Lett. 2010;130:32–35. doi: 10.1016/j.imlet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Wittig O. Paez-Cortez J. Cardier JE. Liver sinusoidal endothelial cells promote B lymphopoiesis from primitive hematopoietic cells. Stem Cells Dev. 2010;19:341–350. doi: 10.1089/scd.2009.0300. [DOI] [PubMed] [Google Scholar]
- 60.Schinköthe T. Bloch W. Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199–206. doi: 10.1089/scd.2007.0175. [DOI] [PubMed] [Google Scholar]
- 61.Hwang JH. Shim SS. Seok OS. Lee HY. Woo SK. Kim BH. Song HR. Lee JK. Park YK. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. Korean Med Sci. 2009;24:547–554. doi: 10.3346/jkms.2009.24.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majumdar MK. Thiede MA. Mosca JD. Moorman M. Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 63.Mackay-Sim A. Stem cells and their niche in the adult olfactory mucosa. Arch Ital Biol. 2010;148:47–58. [PubMed] [Google Scholar]