Abstract
The specific molecular determinants that govern progenitor expansion and final compartment size in the myogenic lineage, either during gestation or during regenerative myogenesis, remain largely obscure. Recently, we retrieved d-asb11 from a zebrafish screen designed to identify gene products that are downregulated during embryogenesis upon terminal differentiation and identified it as a potential regulator of compartment size in the ectodermal lineage. A role in mesodermal derivatives remained, however, unexplored. Here we report pan-vertebrate expression of Asb11 in muscle compartments, where it highly specifically localizes to the Pax7+ muscle satellite cell compartment. Forced expression of d-asb11 impaired terminal differentiation and caused enhanced proliferation in the myogenic progenitor compartment both in in vivo and in vitro model systems. Conversely, introduction of a germline hypomorphic mutation in the zebrafish d-asb11 gene produced premature differentiation of the muscle progenitors and delayed regenerative responses in adult injured muscle. Thus, the expression of d-asb11 is necessary for muscle progenitor expansion, whereas its downregulation marks the onset of terminal differentiation. Hence, we provide evidence that d-asb11 is a principal regulator of embryonic as well as adult regenerative myogenesis.
Introduction
The establishment of the relative sizes of the various compartments in the vertebrate body is one of the most important and defining processes of developmental biology. During embryonic development, tissue-specific progenitor compartments must undergo massive cell expansion to generate enough volume to produce functionally differentiated tissues. The increase and diversification of vertebrate compartments played a crucial factor in the terms of evolution and enabled organisms to cope with different environmental conditions [1–3]. Further, aberrant compartment regulation is implicated in many serious pathologies (eg, cancer) [4]. The factors that determine and regulate cell proliferation and differentiation, thus, defining compartment size and function, remain poorly understood and elucidation of the underlying molecular mechanisms driving expansion of progenitor compartments represents an important scientific question.
Likewise, little is known with respect to biological events triggering muscle compartment expansion [5–7]. In vertebrates the skeletal muscle arises from an embryonic compartment called myotome, originating from a transient epithelial structure, the dermomyotome, which in turn is derived from somites. Somites are formed sequentially, as paired segments of the paraxial mesoderm on either side of the neural tube, from anterior to posterior, at regular time intervals. The somites are transient structures patterned by signals from the surrounding tissue into compartments that later differentiate into different types of tissues that will give rise to several trunk structures: sclerotome (precursor of the bones, cartilages, and tendons), myotome (precursor of the muscle), and dermatome (precursor of the dermis) [8–10].
The primary myotome is formed as the first differentiated muscle from the dermomyotome between E11.5 and E15.5 in the mouse. There, some myoblasts irreversibly exit the cell cycle, align with each other, and fuse, forming multinucleated myotubes. After primary myogenesis, secondary myoblasts in the dermomyotome use the primary myotome as a scaffold to attach to and fuse with each other, giving rise to secondary myotubes. A similar molecular process of myogenesis occurs postnatally, to recruit adult muscle precursors into new myofibers during skeletal muscle damage [11]. Enhanced knowledge of the molecular determinants in the formation of muscular tissue, both in embryos and adult organisms, will help to elucidate important processes involved in developmental biology and give a better understanding of degenerative diseases, such as muscular dystrophy, as well as the process of repair, reproduction, or replacement of lost or injured cells, possibly leading to new ways of treatment.
Earlier we reported on efforts to discover the elements involved in progenitor compartment size regulation by isolating genes that are specifically repressed during terminal differentiation and thus may be responsible for the proliferation and expansion of the stem cell compartment [12]. To this end, mRNA from zebrafish embryos treated with 0.5 μM all-trans retinoic acid (which terminates progenitor expansion and induces full terminal differentiation) was isolated and compared with untreated embryos. Differentially expressed mRNA fragments were tested using whole mount in situ hybridization (WISH) at different developmental stages. Finally, 1 fragment was singled out for detailed characterization based on its restricted spatiotemporal expression pattern during late gastrulation and early somitogenesis. The full-length sequence of the downregulated fragment revealed that the gene is homologous to the mammalian ankyrin repeat and suppressor of cytokine signaling (SOCS) box-containing protein 11 (Asb11). The amino acid sequence of zebrafish Asb11 has 293 residues, composed of a series of 6 ankyrin repeats at the N-terminus, and a C-terminal SOCS box domain [12].
The Asb family constitutes a (hemi)chordate-unique gene family whose members are characterized by variable numbers of N-terminal ankyrin repeats and a C-terminal SOCS box [13]. ASB proteins act as substrate receptor subunits of E3 ligases, enzymes that mediate ubiquitylation and degradation of target proteins [14–16] and ubiquitin ligases regulate a plethora of physiologically important processes [17,18]. Although the zebrafish Asb11 has the most functional homology with human ASB11, sequence analysis also showed high homology of zebrafish Asb11 with other mammalian members of the ASB family, most markedly with ASB9. Considering the fact that mouse and human Asb9 and Asb11 are also located close to each other on the genome (which seems to have arisen as the result of a recent gene duplication event), we proposed that these genes are both mammalian homologues of zebrafish asb11 [12]. Further, ASB proteins show very high pan-chordate conservation, Homo sapiens ASB11 and its orthologue in the urochordate Ciona intestinalis sharing 50% overall similarity on an amino acid basis, which points to very fundamental functions in chordate physiology.
Consistently, forced expression of Asb11 in the presumptive nervous system of zebrafish embryos maintained cell progenitor proliferation and increased the neuronal compartment size, whereas in the absence of Asb11 premature terminal differentiation was induced resulting in a reduced compartment size. The molecular mechanisms by which Asb11 sustains progenitor expansion likely involve Notch activation as we established that Asb11 is a positive regulator of canonical Notch signaling [12,19,20]. However, d-Asb11 function in embryogenesis and adult organisms has not been fully explored, and thus, it is possible that d-Asb11 is relevant for compartment definition outside the neuronal system, prompting more comprehensive analysis of its in vivo expression. Indeed, Asb11 was well capable of activating Notch signal transduction outside the neuronal system as heterologous expression of this gene activates Notch reporters in a variety of cell types [19].
In this context, muscle development may constitute an interesting target for Asb11 action as other ASB family members have been especially implicated in the regulation of this compartment [6,19]. ASB2β was first identified in muscle cells during embryogenesis and in adult tissue, and was shown to regulate muscle differentiation by targeting actin filamin B (FLNb) for proteasomal degradation. Inhibition of ASB2β blocked myoblast fusion and myotube formation, crucial processes in the later phase of muscle development [21]. Additionally, ASB15 has emerged as a regulator of protein synthesis and muscle growth [22], possibly mediated by the PI3K/Akt signal transduction pathway [23]. Besides, analysis of Asb11 transcripts showed that the expression of this gene in muscle tissue has a pan-vertebrate characteristic, presenting a particularly high expression in mammalian muscle (mouse and human). Hence, we decided to characterize the function of d-Asb11 during myogenesis. Further analysis showed that d-Asb11 expression is specifically restricted to Pax7+ compartment and may be important for progenitor maintenance. Downregulation of d-Asb11 activity, using a mutated form of d-Asb11, interfered with myotome formation during embryogenesis and adult muscle regeneration, whereas forced expression led to expansion of the muscle compartment both in vitro and in vivo. We conclude that d-Asb11 constitutes an important regulator of primary and regenerative myogenesis.
Materials and Methods
Fish and embryos
Embryos were obtained by natural matings, cultured in embryo medium, and staged according to methods previously described [24]. Zebrafish were kept at 27.5°C.
Cell culture
C2C12 mouse myoblasts were obtained from the ATCC and cultured in Dulbecco's modified Eagle's medium (Gibco) with 4.5 g/L glucose and L-glutamine and supplemented with penicillin (50 U/mL), streptomycin (50 μg/mL), and 10% fetal calf serum (FCS). Cells were grown in monolayers in a humidified atmosphere containing 5% CO2. The differentiation medium contained 2% horse serum instead of 10% FCS.
Plasmid construction
Plasmids, containing asb11 and related sequences, were constructed as described previously [12]. A partial cDNA fragment of asb11 in pBluescript was used as a template to generate a riboprobe for in situ hybridizations.
mRNA synthesis, mRNA, and cDNA microinjections
Capped mRNAs were synthesized using the mMESSAGE mMACHINE kit (Ambion). mRNAs were injected into 1-cell-stage embryos or in 1 cell of the 2-cell stage blastomere. cDNA and mRNA were injected by using a microinjector (World Precision Instruments). About 300 pg of Asb11 mRNA or 10 pg of cDNA was used unless otherwise indicated. Total volume of the injection was set at 1 nL.
RNA isolation and quantitative reverse transcriptase–polymerase chain reaction
Total RNA extraction and purification was performed by using standard Trizol and isopropanol precipitation. cDNA synthesis was performed using hexamer primers and MLV reverse transcriptase. Transcript levels were quantified by real-time polymerase chain reaction (PCR) using ABsolute QPCR SYBR Green Fluorescein Mix (Westburg) on an iCycler iQ Real-Time PCR detection system (Bio-Rad). Results are expressed as a relative ratio to the housekeeping gene actin.
Primers
Zebrafish: asb11-F: CTGCAAAGAGAGGTCACACG and asb11-R: TCCTTTTTGTCCCAGTGAGC. Mouse: Asb11-F: GTCAGAAGGCCTGGACCAT and Asb11-R: CTCATGGAG TGGGGATCG. Asb9-F: TCCTCTTCATGATGCTGCAA and Asb9-R: CACGTGATCTGCTGTGATGA.
In situ hybridization
mRNA WISH was carried out as previously described [25]. Embryos were fixed in 4% paraformaldehyde (PFA) overnight at 4°C and digoxigenin-tagged probes to myoD, muscle creatine kinase (mck), and myogenin were made with Roche labeling mix [26]. Images were obtained using a Zeiss Axioplan Stereomicroscope (Oberkochen) equipped with a Leica (Wetslar) digital camera.
Immunolabeling of zebrafish embryos
Embryos were fixed for antibody staining with 4% PFA and whole mount immunohistochemistry was performed according to Du et al. [27], using primary antibodies Pax7 1:20 (Developmental Studies Hybridoma Bank) and PH3 (Upstate Biotechnology; #06570) 1:1,000. Appropriate secondary antibodies were used at 1:200. Immunohistochemistry was analyzed at the level of yolk extension.
Immunolabeling of adult muscle
All stainings were initially performed as monostainings to assess the problem of bleed-through of the fluors. Subsequently, concentrations were titrated to minimize the problem (ie, if staining in 1 channel was markedly stronger as in another channel, causing bleed-through antibody, then concentrations were adjusted to reduce staining intensity). In addition, only double stainings were accepted for further analysis when in the same field clear monostained structures could also be recognized.
Short-term (7 days) and long-term (2 months) bromodeoxyuridine (BrdU) incorporation and labeling was performed by immersing and allowing the zebrafish to swim in BrdU (150 mg/L for 4 h per day) for 7 days. Fish were fixed at 2 months after the BrdU pulse. Adult muscle tissue was isolated and frozen in liquid nitrogen, postfixed in 4% PFA, cryosectioned in 10 μm, and dried overnight. Sections were postfixed in acetone, washed, and kept in 100% methanol overnight. Sections were permeabilized with 0.2% Triton X-100 and quenched with 100 mM glycine-NaOH (pH 10). Sections were washed in PBS-Tween-20 (PBS-T) and incubated in 2 N hydrochloric acid. To restore the pH, sections were washed in 0.1 M sodium borate. After washing in PBS-T, sections were blocked [2% goat serum, 1% bovine serum albumin (BSA), and 1% dimethyl sulfoxide (DMSO) in PBS-T] at room temperature for 1 h. BrdU antibody (Abcam; Ab6326) was incubated 1:200 in block overnight at 4°C. After washing in PBS-T, sections were blocked again for 1 h and anti-rat conjugated secondary antibody (Cy2/Jackson Immunoresearch; 1:200) was incubated for 2 h at room temperature (RT). After washing, sections were blocked (20% goat serum and 0.1% BSA in PBS) for 1 h. Asb11 antibody [12] was pre-incubated in fish powder and subsequently incubated in block (10% goat serum and 0.1% BSA) overnight at 4°C. After washing in PBS-T, sections were blocked for 1 h and incubated in anti-rabbit secondary antibody (Cy3/Jackson Immunoresearch) for 2 h at RT. After washing, sections were blocked (10% goat serum, 0.1% BSA, and 1% DMSO in PBST) for 1 h at RT and incubated in Pax7 antibody (Developmental Studies Hybridoma Bank) 1:20 in block overnight at 4°C. After washing in PBS-T, sections were blocked and incubated in anti-mouse secondary antibody (Rhodamine Red-X/Jakson Immunoresearch) for 2 h at RT. After immunolabeling, sections were counterstained in DAPI (Sigma) and analyzed by confocal microscopy as described earlier [28].
Tissue histology
For cryosections, adult muscle tissues were embedded in Jung Tissue Freezing Medium, and sectioned at 10 μm. For plastic sections, adult muscle tissues were dehydrated and rehydrated in an ethanol gradient. Adult muscle tissues were embedded in Technovit 8100 (Kulzer) and sectioned at 7 μm. Sections were then counterstained with Mayer's hematoxylin and eosin.
Zebrafish cell lysate for immunoblotting
Embryos were dechorionated at the appropriate stage and subsequently transferred to cell lysis buffer [50 mM HEPES (pH 7.5), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, and 1% Triton-X 100] at 4°C (2 μL/embryo), and subsequently lysed by pippeting up and down and stored at −20°C. Before loading, 2× loading buffer was added and the sample was boiled for 5 min. A total of 7 embryos was loaded.
Immunoblotting
Cell lysates were sonicated and then centrifuged at 1,200 g for 10 min at 4°C. The pellet was discarded and the protein concentration was measured with the BCA protein assay kit (Pierce chemical Co.). Lysates were diluted 1:2 in protein sample buffer [125 mM Tris/HCl (pH 6.8), 4% sodium dodecyl sulfate (SDS), 2% β-mercaptoethanol, 20% glycerol, and 1 mg bromophenol blue] and incubated at 95°C for 5 min. Twenty-five micrograms of protein per lane was loaded onto SDS–polyacrylamide gel electrophoresis and subsequently transferred onto PVDF membrane (Millipore) as described earlier [29]. The blots were blocked with 2% low-fat milk in Tris-buffered saline supplemented with 0.1% Tween-20 (TBST) for 1 h at room temperature and washed in TBST before overnight incubation at 4°C with primary antibody in 2% low-fat milk in TBST. Blots were then washed with TBST and incubated for 1 h at room temperature in 1:1,000 horse radish peroxidase conjugated secondary antibody in 2% low-fat milk in TBST. After a final wash with TBST, blots were incubated for 5 min in Lumilite plus (Boehringer-Mannheim) and then chemiluminescence detected using a Fuji LAS3000 illuminator (Fuji Film Medical Systems).
Luciferase reporter assay
C2C12 cells were transiently cotransfected with an MCK promoter-driven luciferase construct and one of the following constructs: myc-tagged Asb11 (MT-Asb11), HA-tagged SOCS box deficient Asb11 (HA-Asb11ΔSOCS), myc-tagged Asb9 (MT-Asb9), or myc-tag empty vector (MT). To correct for transfection efficiency or dilution effects, cells were transfected as explained previously but reporter vector was replaced with a cytomegalovirus (CMV) promoter-driven Renilla luciferase (Promega). Lipofectamine Plus (Invitrogen) method was used according to manufacturer's instruction. After 48, 72, or 96 h of incubation in differentiation medium, cells were lysed with passive lysis buffer as provided by Promega and luciferase activity was assayed according to the Dual-Glo-Luciferase Assay System (Promega) protocol on a Lumat Berthold LB 9501 Luminometer (Berthold Technologies).
Imaging and quantifications
Fluorescent labeling was imaged using a Leica TCS SPE confocal microscope. BrdU+, Pax7+, and Asb11+ cells in the adult muscle tissue were counted at comparable positions at the dorsal part of the myotome. For statistical analysis, heteroscedastic 2-tailed Student's t-test was performed using Microsoft Excel.
Results
Specific expression of Asb11 in the Pax7+ muscle satellite cell
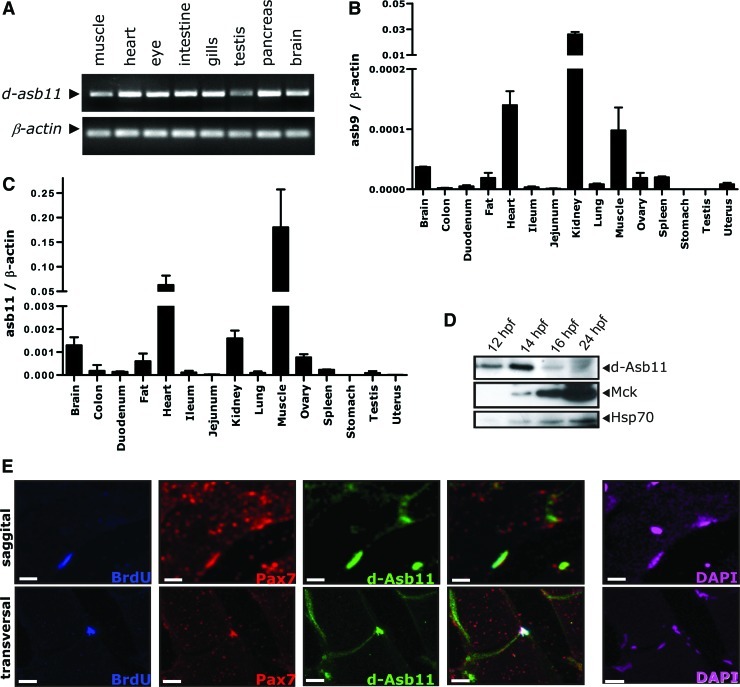
In an effort to determine possible extra-neuronal functions for Asb11, we explored the specific distribution of d-asb11 transcripts in adult zebrafish tissues by reverse transcription–PCR. d-asb11 cDNA from total mRNA extracted from muscle, heart, eye, intestine, gills, testis, pancreas, and brain was amplified. β-Actin was used as a control. d-asb11 was expressed in all the tissues we explored (Fig. 1A), indicating functionality outside the nervous system, and possibly in muscle. This notion is reinforced by experiments attempting a pan-vertebrate comparison of Asb11 expression.
FIG. 1.
Distribution of Asb11 expression in different tissues and colocalization with Pax7+ satellite cells. (A) RT–polymerase chain reaction analysis of zebrafish d-Asb11 (asb11) in mRNA samples isolated from muscle, heart, eye, intestine, gill, testis, pancreas, and brain of adult fish. Zebrafish β-actin was used as template control. (B, C) Various types of tissue from adult mice were analyzed for mRNA expression of murine Asb9 (Asb9) (B) and Asb11 (Asb11) (C). Results were normalized against β-actin expression. Asb9 is showing the highest expression in kidney, heart, and muscle. Asb11 shows the highest expression in muscle, heart, kidney, and brain. The bars indicate the standard error of the mean and were calculated from the results of 3 individual experiments. (D) Analysis of protein levels of Asb11 and muscle creatine kinase (MCK), a marker of muscle terminal differentiation, in different stages during embryogenesis. Hsp70 expression is used as input control. Expression of MCK and Asb11 showed inverse correlation during zebrafish embryogenesis. The images shown are representative for 3 independent experiments. (E) Adult zebrafish muscle was immunostained with bromodeoxyuridine (BrdU), Pax7, and d-Asb11 antibodies. Top panels show a representative staining of sagittal sections and bottom panels show a representative of transverse sections. d-Asb11 revealed a distinct expression pattern on the sarcolemma where it colocalizes with Pax7+ cells. The capacity of Pax7+/Asb11+ cells for long-term BrdU incorporation was tested and confirmed that this double-positive compartment truly represents slow-cycling satellite cells. 4′,6-diamidino-2-phenylindole staining was included to determine cell locations (white bars are 10 μm). Color images available online at www.liebertpub.com/scd
In mammals and birds 2 orthologues of d-asb11 are present, Asb9 and Asb11, which are highly homologous, both sharing ∼70% sequence similarity to d-asb11. Asb9 and Asb11 lie adjacent to each other on the X-chromosome, suggesting a sarcopterygiic-specific gene duplication event, not present in teleosts. We investigated specific-tissue expression of murine Asb11 and murine Asb9 and observed particularly prominent Asb11 expression in skeletal and cardiac muscles and less, but significant, expression in brain (Fig. 1B), whereas Asb9 was strongly expressed in testis and kidney (Fig. 1C). This is in agreement with the earlier established function of asb11 in regulating compartment size in the central nervous system of zebrafish embryos, but the expression of Asb9 in testis and kidney shows that high expression of asb11 orthologues in distinct mammalian tissues is a phenomenon observed in evolutionary highly divergent vertebrates and calls for an investigation to the role for d-Asb11 in zebrafish compartment expansion in these tissues.
To analyze the endogenous expression of d-Asb11 during embryogenesis, we isolated total cell lysates of zebrafish embryos at 12, 14, 16, and 24 hours postfertilization (hpf ) and compared protein levels of d-Asb11 and MCK, a marker for terminal muscle differentiation whose onset of expression coincides with the end of progenitor expansion [30,31]. Indeed, as expected, expression of MCK protein increases throughout somitogenesis (Fig. 1D). Consistently with a role of d-asb11 during progenitor expansion in muscle development, the expression of Asb11 is high during early myogenesis and diminishes when the final stages in muscle differentiation ensue, being approximately complementary to the expression of MCK.
Next, we analyzed the histological localization of d-asb11 and its relationship with progenitor cells. Cross sections of skeletal muscle tissue of zebrafish were costained with an antibody specific for d-Asb11 and the well-established muscle satellite marker Pax7 [32,33]. Asb11 staining displayed a distinct expression pattern on the sarcolemma where it colocalizes with a portion of the Pax7+ compartment (Fig. 1E). To confirm that these Pax7+/Asb11+ cells represent muscle progenitor cells, we tested their long-term BrdU label retention. The results, presented in Fig. 1E, confirmed that this double compartment truly represented slowly-cycling satellite cells. Thus, the muscle stem cell compartment in adult muscle is characterized by highly specific Asb11 expression in this compartment.
Forced expression of asb11 inhibits muscle cell differentiation and supports progenitor expansion in the C2C12 model system
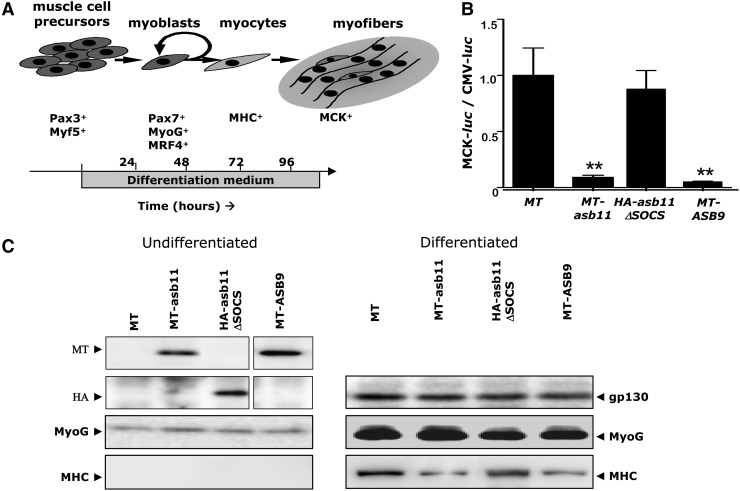
Heterologous expression of asb11 has been shown to support progenitor expansion while simultaneously inhibiting terminal differentiation in the PC12 and N-Tera2 in vitro model systems for neurogenesis [12]. Expression of Asb11 in the satellite cell compartment may suggest that Asb11 could also act in a similar manner in muscle formation. Mouse C2C12 myoblasts, upon application of differentiation medium, synchronously withdraw from the cell cycle, elongate, adhere, and finally fuse together to form myotubes exhibiting most mechanobiochemical adaptations associated with fully differentiated muscle [34] (Fig. 2A). Thus, this system represents a valuable model to study the effect of Asb11 on muscle cell differentiation.
FIG. 2.
Heterologous expression of d-Asb11 or h-Asb9 prevents terminal differentiation in the C2C12 model system of skeletal muscle differentiation. (A) Schematic illustration of the various stages of skeletal muscle differentiation as observed in the C2C12 model system and the associated expression of markers of muscle differentiation. (B) Following 96 h of exposure to differentiation medium, terminal differentiation of skeletal muscle, as assayed by luciferase activity driven by the MCK promoter, was inhibited by heterologous expression of either asb11 or h-Asb9. Transfection of a vector expressing only myc-tag (MT) or a HA-tag suppressor of cytokine signaling (SOCS) box deficient d-Asb11 (HA-d-Asb11ΔSOCS) showed no inhibitory effects. As a control for transfection efficiency, cytomegalovirus (CMV) promoter activity was used. Error bars show standard error of the mean (SEM) of 3 experiments. Statistical testing was done by comparing experimental groups to MT-only-transfected cells using a heteroscedastic paired Student's t-test. (C) C2C12 cells were transfected with a vector containing only the MT, MT-asb11, HA-asb11ΔSOCS, or MT-hAsb9 and allowed to differentiate for 72 h in the appropriate medium. Positive expression of the constructs was assessed and is shown on the left side of the panel. Effects of these transgenes on the expression of myogenin (MyoG; a myoblast marker) and myosin heavy chain (MHC; a marker of terminal muscle cell differentiation) were tested either in undifferentiated cells (left) or after 72 h under differentiation medium treatment (right). A representative image from 3 independent experiments is shown. **P<0.01.
Confirmation of the inhibitory effects of Asb11 on muscle cell differentiation was obtained from experiments in which C2C12 cells, upon differentiation medium, were transfected with different constructs in combination with MCK promoter-driven luciferase or a construct containing a CMV promoter-driven luciferase. Vectors containing either the HA-tag (HA) or a SOCS-box deficient version of asb11 (HA-Asb11ΔSOCS) were used as controls.
Differentiation induction of luciferase-mediated MCK promoter was significantly inhibited in the presence of MT-Asb11 or MT-Asb9 (orthologue of zebrafish asb11 in the human genome, together with Asb11), while no significant effect was shown by coexpression with the Asb11 lacking the SOCS box domain (Fig. 2B). Subsequently, we tested the effect of MT-Asb11 as well as MT-h-ASB9 on the expression of the early myogenic marker myogenin (MyoG) [35] and the terminal myocyte marker myosin heavy chain (MHC) [36] (Fig. 2C). As expected, both genes were substantially induced under differentiation conditions in control cells. After verification that the different Asb constructs were sufficiently expressed (Fig. 2C) we investigated the influence of such heterologous expression on in vitro muscle differentiation. Importantly, when cells were transfected with expressing MT-asb11 or MT-h-ASB9 constructs, differentiation no longer induced MHC expression (Fig. 2C). However, induction of MyoG was unaffected by expression of either MT-Asb11 or MT-ASB9. Together, these observations showed that forced expression of Asb11 prevents terminal myocyte differentiation but not myoblast differentiation of muscle precursors, at least in the C2C12 model system, consistent with a possible role of d-asb11 in maintaining progenitor proliferation in muscle.
Forced expression of Asb11 in vivo deregulates differentiation in the presumptive myotome and causes hyperplastic myotome formation
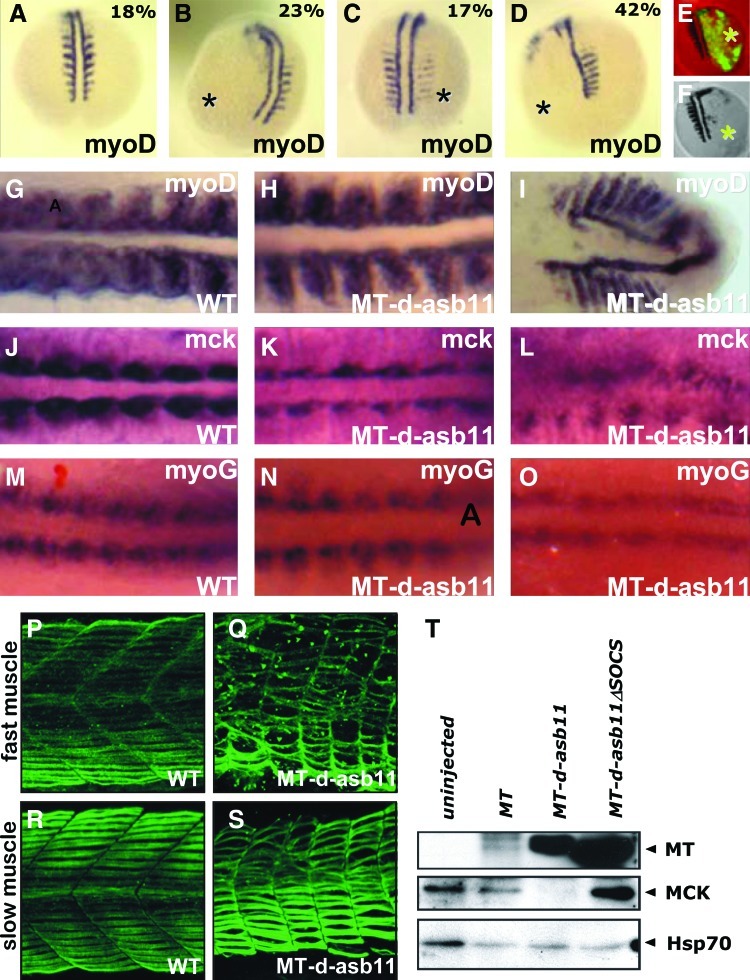
A prediction from the proposed role of d-asb11 as a factor inhibiting differentiation and maintaining proliferation in the Pax7+ muscle stem cell compartment would be that forced expression of asb11 is associated with impaired differentiation in zebrafish muscle. Thus, we injected MT-asb11 mRNA into 2-cell stage blastomeres, and investigated the expression pattern of myoD, a bHLH transcription factor that is expressed in adaxial cells and in the posterior region of each developing somite [31,37], by using WISH. An anti-Myc tag antibody was used to confirm expression of injected mRNA and to identify the half of the embryo derived from the injected blastomere. Compared with wild-type expression (Fig. 3A), forced expression of MT-asb11 resulted in loss of somitic myoD expression accompanied by variably reduced or absent expression in adaxial cells at 13–14 hpf in >80% of the injected embryos (n=82) (Fig. 3B–D). Further, the expression pattern of MT-d-Asb11 in the embryo coincided with exclusion of myoD expression (Fig. 3E–F). Additionally, when expressed in the whole embryo, Asb11 affected myoD in the entire myotome, showing a bended and/or displaced expression pattern at later stages (16 hpf ) (Fig. 3G–I). Hence, forced expression of d-Asb11 delays acquisition of myoD positivity in the presumptive axial myotome and deregulates important aspects of differentiation during zebrafish embryogenesis. As judged by mck expression—a marker for advanced muscle cell differentiation—d-asb11 misexpression inhibited terminal muscle differentiation (Fig. 3J–L), whereas myoG expression—a more early marker of muscle differentiation—was only marginally affected by such asb11 misexpression (Fig. 3O).
FIG. 3.
Forced expression of asb11 mRNA interferes with muscle differentiation in vivo. (A–D) Zebrafish embryos were injected at the 2-cell stage in 1 of the 2 blastomeres (marked with asterisk) with encoding full-length MT-d-asb11 mRNA (300 pg). The numbers indicate the percentages of injected embryos displaying the particular phenotype (total n=82). Panel A shows an uninjected wild-type embryo. (E, F) Immunolabeling of MT-Asb11 overlay with myoD in situ hybridization. (G–O) Embryos were injected at 1-cell stage with Asb11 mRNA and fixed at 16 hours postfertilization (hpf ). Whole mount in situ hybridization was performed using (G–I) myoD, (J–L) mck, and (M–O) myoG riboprobe. Expression was compared between uninjected (G, J, M) and injected embryos (H, I, K, L, N, O). (P–S) Abnormal axial muscle formation at 36 hpf in response to d-asb11 mRNA injections. Fast and slow muscle fibers were stained by immunolabeling as described earlier [33]. (T) Confirmation of d-asb11-forced expression effects on mck at the protein levels. Zygotes were injected MT-asb11 mRNA, MT only, or MT-asb11ΔSOCS-injected embryos, serving as controls. Total cell lysates were isolated at 16 hpf. The protein was analyzed using an anti-Mck antibody. Anti-MT antibody was used to verify induced expression of asb11 variants, and as a loading control an anti-Hsp70 antibody was used. A representative image from 3 independent experiments is shown. Color images available online at www.liebertpub.com/scd
In an effort to confirm these effects at the protein level, zygotes were injected with MT- asb11 and MT-Asb11ΔC mRNA. Uninjected embryos and embryos injected with MT only served as controls. Total cell lysates were isolated at 16 hpf and analyzed using an anti-MCK antibody, while an anti-MT antibody was used to verify induced asb11 variants (Fig. 3T). Indeed, forced expression of asb11 caused reduction of Mck, in agreement with its inhibitory function of cell differentiation.
Finally, to analyze the effects of forced d-Asb11 expression in slow and fast muscle fiber formation, we injected MT-Asb11 mRNA in zygotes that were then immunostained at 36 hpf. At this time point, aberrant expression of d-Asb11 caused gross abnormalities in both slow and fast muscles (Fig. 3P–S), accompanied by disruption of normal muscle architecture. Together, these results indicate an important role for d-asb11 in muscle progenitor expansion.
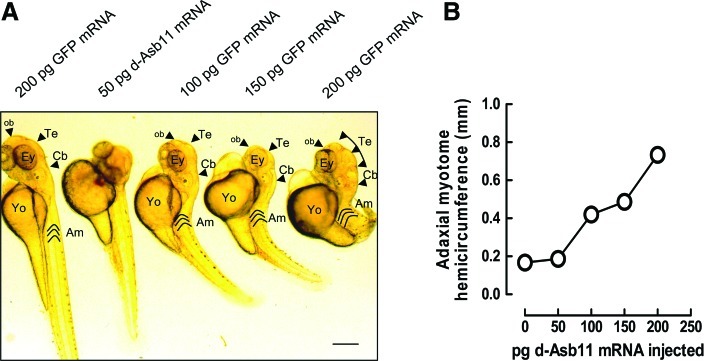
Earlier we have shown that in the presumptive neuronal system forced expression of d-Asb11 increases proliferation [12] concomitant with the delay in the acquisition of terminal differentiation markers. The in vitro experiments suggest that d-Asb11 can also maintain proliferation in the developing myotome. Thus, we assume that the misexpression of d-Asb11 will coincide with increased proliferation of the presumptive musculature. To analyze this possibility further, we injected different concentrations of d-Asb11 mRNA in zygotes, using GFP mRNA as a control. Misexpression of d-Asb11 mRNA in 3 independent experiments, each consisting of at least 60 asb-a mRNA-injected embryos, resulted among others in cyclopia, notochord defects, and tail phenotypes (23%), including no tail (ntl)–like phenotype and in a loss of 1 of the 2 pectoral fins (4%), whereas control mRNA never induced these effects, even when the control RNA was injected at twice the concentration of d-Asb11 mRNA. The notochord and tail phenotypes and the cyclopia are especially evident at higher d-Asb11 mRNA concentrations (Fig. 4A), which finally may cause severe dysmorphogenesis but also the myotome appears significantly enlarged (Fig. 4A). Morphometric analysis confirms this notion (Fig. 4B). Thus, it appears that the block in muscle differentiation provoked by d-Asb11 overexpression coincides with compartment expansion.
FIG. 4.
Forced expression of asb11 mRNA increases muscle compartment size. (A) Zebrafish zygotes were injected at the 2-cell stage with encoding full-length MT-d-asb11 mRNA at different concentrations or 200 pg of GFP-encoding mRNA as a control. Injection of increasing amounts of d-Asb11 mRNA (50, 100, 150, and 200 pg) in zygotes causes CNS and especially brain hypertrophy, which was described earlier at 48 h [12], but also the muscle compartment appears enlarged. Ob, olfactory bulb; Te, telencephalon; Cb, cerebellum; Am, adaxial myotomes; Ey, Eye; Yo, yolk sac; CNS, central nervous system. Bar indicates 100 μm. (B) Morphometric analysis of the adaxial myotome hemicircumference at equivalent position in the embryo is consistent with an increased muscle compartment size following d-Asb11 misexpression. Color images available online at www.liebertpub.com/scd
Fish homozygous for a hypomorphic Asb11 mutation are impaired in muscle progenitor expansion and myotome formation
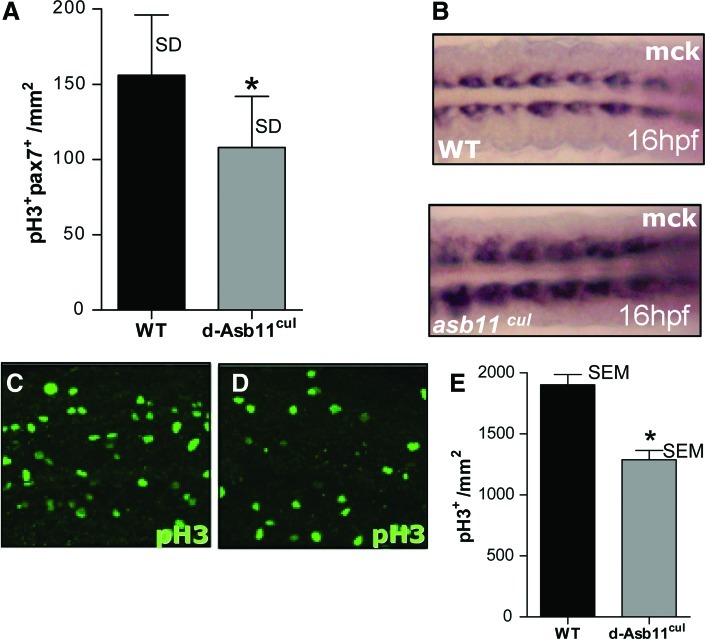
The expression of d-Asb11 in the muscle progenitor cell compartment and the inappropriate expansion of the myotome following its misexpression suggest that d-Asb11 is important for progenitor expansion. Thus, we decided to investigate the effect of moderate d-Asb11 deficiency during muscle development. To this end, we employed a mutant d-asb11 zebrafish germline (d-asb11cul) that lacks the cullin box, a SOCS box subdomain, resulting in a hypomorphic allele. We recently described the isolation of d-asb11cul mutants in detail and demonstrated that these are defective in Notch signaling and have impaired cell fate specification within the neurogenic regions of the embryos [20]. We did not analyze effects on muscle development, however. The d-asb11cul mutants survive until adulthood; however, they present a subtle shorter trunk and less well-defined somites. Interestingly, when the developing myotome was analyzed for the number of proliferating muscle progenitor cells, as defined by phospho-histone 3 (pH3) and Pax7 double staining, this number was significantly reduced in the d-asb11 mutants (Fig. 5A). Further, as judged by the expression of mck, muscle terminal differentiation in d-asb11cul embryos is temporally enhanced (Fig. 5B), whereas the cell proliferation in the myotome of these mutants is substantially reduced (Fig. 5C–E). Thus, we conclude that d-asb11 is required for maintaining myogenic proliferation in the stem cell compartment during embryogenesis.
FIG. 5.
Fish harboring a hypomorphic asb11 allele show reduced size of the pax7+ progenitor compartment and precocious terminal differentiation. Isolation of the hypomorphic asb11 allele-containing asb11cul fish has been described before [20]. (A) At 24 hpf, the size of the expanding muscle progenitor compartment was determined by double labeling with anti-PH3 antibody (a marker for proliferating cells) and an anti-Pax7 antibody. Cells were counted at equivalent positions in the developing dorsal myotome. The asb11cul embryos have significantly lower numbers of proliferating muscle progenitor cells (n=3). The bars show standard deviation, the asterisk indicates P<0.05 (using a heteroscedastic paired Student's t-test). (B) Wild-type and mutant embryos were fixed at 16 hpf and tested for advanced muscle differentiation using mck riboprobes. (C–E) Proliferation in the presumptive myotome of wild-type (C) and d-asb11cul (D) embryos was visualized by anti-pH3 staining at 48 hpf, representative image (n=3). Quantification of total proliferation in zebrafish embryos using the number of PH3-positive cells as a surrogate measure (E). The results represent the average of 8 embryos. The bars depict SEM; *P<0.05 (using a heteroscedastic Student's t-test). Color images available online at www.liebertpub.com/scd
Expression of d-Asb11 in proliferating satellite cells is important for adult regenerative myogenesis
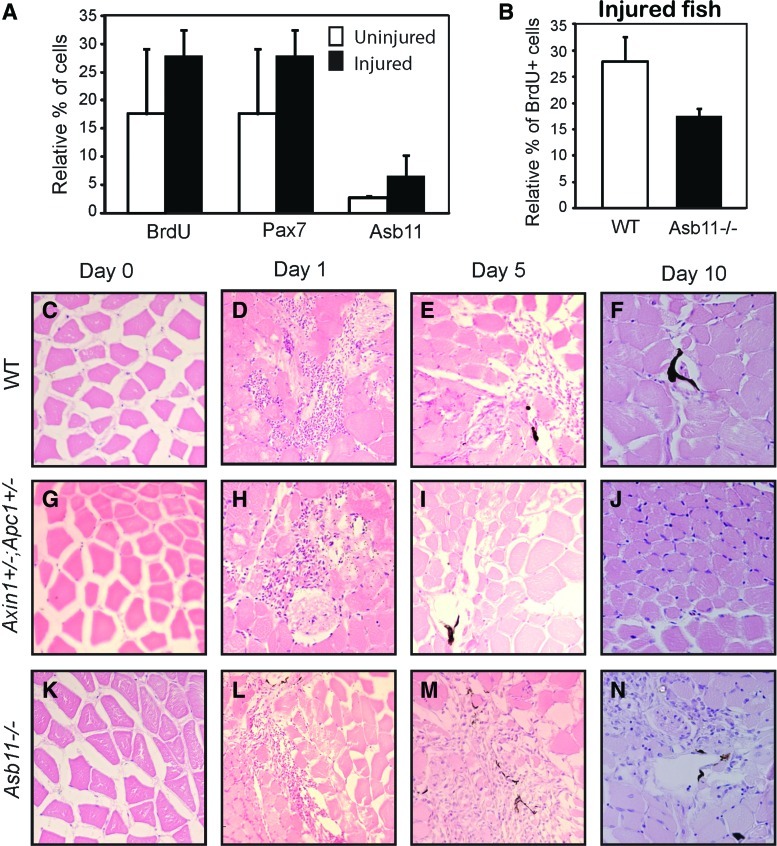
To determine whether regenerative myogenesis like primary embryogenesis is regulated by Asb11, we induced mechanical injury with a 30G needle on the adult zebrafish body musculature, dorsal to the anus, and investigated the number of BrdU+, Pax7+, and Asb11+ cells in lesion-induced versus uninjured wild-type zebrafish at 7 days postinjury. Although this procedure does not produce significant changes in the number of BrdU+ or Pax7+ cells, the double-positive d-Asb11+/Pax7+ compartment substantially increases in size following muscle injury, indicating that d-asb11 may be involved in response to trauma in this tissue (Fig. 6A). Subsequently, we compared the proliferation between injured d-asb11cul and wild-type zebrafish. Injured d-Asb11cul mutants showed a significant diminished numbers of BrdU+ cells (Fig. 6B), suggesting that regenerative proliferation is dependent on functional Asb11.
FIG. 6.
The hypomorphic asb11cul allele is associated with impaired regenerative responses following mechanical injury. Mechanical injury was induced using a needle to inflict trauma on the dorsal flank of the muscle in adult zebrafish. (A) Zebrafish were fixed and frozen 7 days postinjury. Adult muscle tissues were cryosectioned and immunostained with BrdU, Pax7, and d-Asb11. Quantification of the percentage of positively stained cells was performed. Error bars indicate the standard deviation calculated from data obtained from 8 different embryos. (B) Wild-type, axin−/apc−, and d-Asb11cul zebrafish were fixed and frozen 7 days postinjury. Adult muscle tissues were cryosectioned and immunostaining with BrdU antibody was performed. (C–N) Zebrafish were fixed at (C, G, K) 0, (D, H, L) 1, (E, I, M) 5, and (F, J, N) 10 days postinjury. Evans blue dye was used as a marker to identify the area of injury. The adult musculature was sectioned and counterstained with haematoxylin-eosin (HE). Color images available online at www.liebertpub.com/scd
Next, we performed a muscle regeneration comparative analysis using injured tissues of wild-type, d-Asb11cul, and axin1/apc1 mutants. axin1/apc1 double heterozygous mutants were used as positive controls as Wnt signaling is shown to positively regulate satellite cell proliferation on adult muscle fibers during wound-healing response in CD34+ cells [38] as well as cultured myofibers [39].
At day 0, muscle fiber morphology was comparable in wild-type, axin1/apc1, and d-Asb11cul zebrafish lines (Fig. 6C, G, K). At day 1 postinjury, there was a marked cellular invasion, and evident degeneration and necrosis of mature fibers of all tissues (Fig. 6D, H, L). At day 5 postinjury, axin1/apc1 mutants showed remarkable recovery and numerous small-diameter regenerating muscle fibers were observed (Fig. 6I), whereas wild type showed a slight recovery (Fig. 6E) and in d-Asb11cul the muscle fibers were still necrotic (Fig. 6M). At day 10 postinjury, axin1/apc1 showed a clear recovery and regeneration of the muscle fibers (Fig. 5J), while the aspect of wild-type fish appeared only moderately worse (Fig. 6F). In d-Asb11cul, however, little improvement could be noted with small regenerating fibers emerging (Fig. 6N). Altogether these results establish a crucial role of Asb11 in both embryonic and adult regenerative myogenesis.
Discussion
The determinants of the size of the muscle cell compartment remain poorly understood. During embryogenesis a group of Pax7+ stem cells arises and proliferates until the final compartment size is reached. In most precursor cells, a genomic program, responsible for terminal differentiation, is started resulting in functional muscle fiber, whereas a small subpopulation remains Pax7+ cells forming the satellite cell population from which regenerative myogenesis can start in response to injury. Our data presented here show that d-Asb11 is essential for maintaining muscle stem cell proliferation during zebrafish embryogenesis and is required for regenerative responses during injury as well. Importantly, we demonstrated that d-Asb11 is expressed beneath the basal lamina of adult zebrafish muscle fiber, and colocalized with a well-accepted muscle satellite cell specific marker Pax7. This, together with the coexpression of d-Asb11 with label-retaining BrdU slow-cycling cells suggested that the d-Asb11-positive cells are the muscle satellite cells themselves. Interestingly, there are significantly less Asb11+ cells compared with Pax7+ cells in the adult muscle fibers. It is tempting to speculate that the d-Asb11 cells are the primary stem cells, and thus, are activated and induced to proliferate in response to muscle damage/injury.
The d-Asb11 gene has high homology to both mammalian Asb9 and Asb11; however, no obvious asb9 homologue is present in fish. As mammalian Asb9 and Asb11 lie adjacent on the same chromosome (X), it seems to represent the result of an evolutionary relatively recent genetic duplication event. In silico analysis revealed that 46.6% of the ancestral chordate genes appear in duplicate in one or more of the vertebrate lineages, with 34.5% having at least 1 duplication before the divergence of fish from tetrapods and 23.5% having at least 1 duplication afterward [40]. This suggests that zebrafish asb11 functions similarly to both mammalian Asb9 and Asb11, as it seems that d-Asb11, in zebrafish, bears a more varied number of functions, while in mammals the function of ASB proteins appears to be more specific. This hypothesis is supported by our results where zebrafish asb11 transcripts were present in all tissues analyzed, whereas mice showed more tissue-specific expression for both Asb9 (testis and kidney) and Asb11 (muscle and heart) transcripts, although there is no information of the function of these genes in male germ cell compartment, urogenital system, or muscle development. However, ASB1 [41] and ASB17 [42], which are also highly expressed in mice testis, have been implicated in mammalian spermatogenesis. Moreover, human and murine Asb9 were reported to interact with the creatine kinase isoforms, brain creatine kinase [14] and ubiquitous mitochondrial creatine kinase [43], targeting them to proteosomal degradation. In agreement with the notion that zebrafish use Asb proteins in multiple compartments, whereas in mammals ASB proteins function in specific compartments, zebrafish present a smaller number of Asb proteins compared to humans and mice. Clearly, more in-depth analysis of expression and function of different Asb proteins in different tissues is required; however, it is tempting to speculate on a function of d-Asb11 in zebrafish spermatogenesis and kidney development.
Earlier, we have demonstrated that d-Asb11 maintains the neuronal progenitor compartment, implying a critical role in the ectodermal compartment size. In zebrafish, this function does not seem to be restricted to this germ layer, as we now show that it is important for mesodermal lineage as well and hence d-Asb11 appears a regulator of vertebrate compartment size of more general importance. The effects of d-Asb11 on embryonic myogenesis are remarkably similar to its effects on embryonic neural precursors [12], suggesting that d-Asb11 functions in a similar way in regulating both the neuroectodermal and mesodermal cell fates. Whether d-Asb11 is important for compartment size in the endodermal lineage, however, is questionable. Interestingly, ASB9 expression has recently been linked to the maintenance of cell proliferation in colorectal cancer [44]. In this context it is interesting to note that we observe higher expression of the CysLT1 receptor in Asb9-expressing colon cancer cell lines (unpublished observations) and that expression of this receptor has been linked to compartment expansion in colon cancer [45]. Thus, further investigations on how endodermal progenitor expansion is regulated and whether they involve ASB-like proteins should be warranted.
Recently we showed that the functions of Asb11 in neurogenesis are mediated by its potential to enable Notch signaling activity [19]. Canonical Delta-Notch signaling plays a key role in satellite cell activation and muscle regeneration. There is a temporal switch between Notch and Wnt signaling, whereby Notch signaling has to be downregulated for myogenesis to proceed [46]. Consistently, our data showed that asb11 expression in muscle satellite cells is required to maintain the muscle precursor pool and efficient muscle regeneration. It is important to note, that although slower, muscle regeneration is still evident in d-asb11cul zebrafish.
In conclusion, based on the evolutionary conservation of asb11 with murine Asb9 and Asb11, it is tempting to hypothesize that the phenotypes we observed in the asb11 mutants could be linked to human muscular diseases, prompting an investigation into the role of Asb11 in muscle pathology.
Acknowledgments
The authors would like to thank S. Boj for the adult muscle tissue samples and Z.Y. Gong for the ck construct. M.A.S. da Silva and J.-M. Tee are paid by ALW grants No. 81702002 and No. 81502006, respectively, while S.H. Diks and P. van Tijn are supported by the TI Pharma grant T3-103, T1-215 and Internationale Stichting Alzheimer Onderzoek grant No. 07508, respectively.
Author Disclosure Statement
All authors do not have any conflict of interests.
References
- 1.Cheung AFP. Pollen AA. Tavare A. DeProto J. Molnar Z. Comparative aspects of cortical neurogenesis in vertebrates. J Anat. 2007;211:164–176. doi: 10.1111/j.1469-7580.2007.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence PA. Struhl G. Morphogens, compartments, and pattern: lessons from Drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- 3.Brand-Saberi B. Christ B. Evolution and development of distinct cell lineages derived from somites. Curr Top Dev Biol. 2000;48:1–42. doi: 10.1016/s0070-2153(08)60753-x. [DOI] [PubMed] [Google Scholar]
- 4.He XB. Marchionni L. Hansel DE. Yu W. Sood A. Yang J. Parmigiani G. Matsui W. Berman DM. Differentiation of a Highly Tumorigenic Basal Cell Compartment in Urothelial Carcinoma. Stem Cells. 2009;27:1487–1495. doi: 10.1002/stem.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahane N. Cinnamon Y. Kalcheim C. The origin and fate of pioneer myotomal cells in the avian embryo. Mech Dev. 1998;74:59–73. doi: 10.1016/s0925-4773(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 6.Tee JM. Peppelenbosch MP. Anchoring skeletal muscle development and disease: the role of ankyrin repeat domain containing proteins in muscle physiology. Crit Rev Biochem Mol Biol. 2010;45:318–330. doi: 10.3109/10409238.2010.488217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavanagh KD. Tabin CJ. A Developmental Model for the Evolution of Size Proportions in Fingers and Toes. Integr Comp Biol. 2010;50:E88. [Google Scholar]
- 8.Kahane N. Cinnamon Y. Kalcheim C. The cellular mechanism by which the dermomyotome contributes to the second wave of myotome development. Development. 1998;125:4259–4271. doi: 10.1242/dev.125.21.4259. [DOI] [PubMed] [Google Scholar]
- 9.Kalcheim C. Cinnamon Y. Kahane N. Myotome formation: a multistage process. Cell Tissue Res. 1999;296:161–173. doi: 10.1007/s004410051277. [DOI] [PubMed] [Google Scholar]
- 10.Kalcheim C. Kahane N. Cinnamon Y. Ben-Yair R. Mechanisms of lineage segregation in the avian dermomyotome. Anat Embryol. 2006;211:S31–S36. doi: 10.1007/s00429-006-0116-y. [DOI] [PubMed] [Google Scholar]
- 11.Brent AE. Tabin CJ. Developmental regulation of somite derivatives: muscle, cartilage and tendon. Curr Opin Genet Dev. 2002;12:548–557. doi: 10.1016/s0959-437x(02)00339-8. [DOI] [PubMed] [Google Scholar]
- 12.Diks SH. Bink RJ. van de Water S. Joore J. van Rooijen C. Verbeek FJ. den Hertog J. Peppelenbosch MP. Zivkovic D. The novel gene asb11: a regulator of the size of the neural progenitor compartment. J Cell Biol. 2006;174:581–592. doi: 10.1083/jcb.200601081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilton DJ. Richardson RT. Alexander WS. Viney EM. Willson TA. Sprigg NS. Starr R. Nicholson SE. Metcalf D. Nicola NA. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci U S A. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debrincat MA. Zhang JG. Willson TA. Silke J. Connolly LM. Simpson RJ. Alexander WS. Nicola NA. Kile BT. Hilton DJ. Ankyrin repeat and suppressors of cytokine signaling box protein Asb-9 targets creatine kinase B for degradation. J Biol Chem. 2007;282:4728–4737. doi: 10.1074/jbc.M609164200. [DOI] [PubMed] [Google Scholar]
- 15.Kohroki J. Nishiyama T. Nakamura T. Masuho Y. ASB proteins interact with Cullin5 and Rbx2 to form E3 ubiquitin ligase complexes. Febs Lett. 2005;579:6796–6802. doi: 10.1016/j.febslet.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Guardavaccaro D. Pagano M. Oncogenic aberrations of cullin-dependent ubiquitin ligases. Oncogene. 2004;23:2037–2049. doi: 10.1038/sj.onc.1207413. [DOI] [PubMed] [Google Scholar]
- 17.Wullaert A. Heyninck K. Janssens S. Beyaert R. Ubiquitin: tool and target for intracellular NF-kappa B inhibitors. Trends Immunol. 2006;27:533–540. doi: 10.1016/j.it.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Scheffner M. Staub O. HECT E3s and human disease. BMC Biochem 8 Suppl. 2007;1:S6. doi: 10.1186/1471-2091-8-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diks SH. da Silva MAS. Hillebrands JL. Bink RJ. Versteeg HH. van Rooijen C. Brouwers A. Chitnis AB. Peppelenbosch MP. Zivkovic D. d-Asb11 is an essential mediator of canonical Delta-Notch signalling. Nat Cell Biol. 2008;10:1190–1198. doi: 10.1038/ncb1779. [DOI] [PubMed] [Google Scholar]
- 20.da Silva MAS. Tee JM. Paridaen J. Brouwers A. Runtuwene V. Zivkovic D. Diks SH. Guardavaccaro D. Peppelenbosch MP. Essential role for the d-Asb11 cul5 Box domain for proper notch signaling and neural cell fate decisions in vivo. Plos One. 2010;5:e14023. doi: 10.1371/journal.pone.0014023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bello NF. Lamsoul I. Heuze ML. Metais A. Moreaux G. Calderwood DA. Duprez D. Moog-Lutz C. Lutz PG. The E3 ubiquitin ligase specificity subunit ASB2 beta is a novel regulator of muscle differentiation that targets filamin B to proteasomal degradation. Cell Death Differ. 2009;16:921–932. doi: 10.1038/cdd.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDaneld TG. Hannon K. Moody DE. Ankyrin repeat and SOCS box protein 15 regulates protein synthesis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1672–R1682. doi: 10.1152/ajpregu.00239.2005. [DOI] [PubMed] [Google Scholar]
- 23.McDaneld TG. Spurlock DM. Ankyrin repeat and suppressor of cytokine signaling (SOCS) box-containing protein (ASB) 15 alters differentiation of mouse C2C12 myoblasts and phosphorylation of mitogen-activated protein kinase and Akt. J Anim Sci. 2008;86:2897–2902. doi: 10.2527/jas.2008-1076. [DOI] [PubMed] [Google Scholar]
- 24.Peppelenbosch MP. Tertoolen LGJ. deLaat SW. Zivkovic D. Tonic Responses to Epidermal Growth-Factor in Zebrafish Cells. Exp Cell Res. 1995;218:183–188. doi: 10.1006/excr.1995.1146. [DOI] [PubMed] [Google Scholar]
- 25.Jowett T. Double in situ hybridization techniques in zebrafish. Methods. 2001;23:345–358. doi: 10.1006/meth.2000.1147. [DOI] [PubMed] [Google Scholar]
- 26.Xu YF. He JY. Wang XK. Lim TM. Gong ZY. Asynchronous activation of 10 muscle-specific protein (MSP) genes during zebrafish somitogenesis. Dev Dyn. 2000;219:201–215. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1043>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Du SJ. Devoto SH. Westerfield M. Moon RT. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-beta gene families. J Cell Biol. 1997;139:145–156. doi: 10.1083/jcb.139.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peppelenbosch M. Boone E. Jones GE. van Deventer SJH. Haegeman G. Fiers W. Grooten J. Ridley AJ. Multiple signal transduction pathways regulate TNF-induced actin reorganization in macrophages: inhibition of Cdc42-mediated filopodium formation by TNF. J Immunol. 1999;162:837–845. [PubMed] [Google Scholar]
- 29.Versteeg HH. Sorensen BB. Slofstra SH. Van den Brande JHM. Stam JC. Henegouwen PMPV. Richel DJ. Petersen LC. Peppelenbosch MP. VIIa/tissue factor interaction results in a tissue factor cytoplasmic domain-independent activation of protein synthesis, p70, and p90 S6 kinase phosphorylation. J Biol Chem. 2002;277:27065–27072. doi: 10.1074/jbc.M110325200. [DOI] [PubMed] [Google Scholar]
- 30.Chamberlain JS. Jaynes JB. Auschka SD. Regulation of Creatine-Kinase Induction in Differentiating Mouse Myoblasts. Mol Cell Biol. 1985;5:484–492. doi: 10.1128/mcb.5.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lassar AB. Buskin JN. Lockshon D. Davis RL. Apone S. Hauschka SD. Weintraub H. Myod is a sequence-specific DNA-binding protein requiring a region of Myc homology to bind to the muscle creatine-kinase enhancer. Cell. 1989;58:823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- 32.Hawke TJ. Geary DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 33.Hood DA. Hawke TJ. Skeletal muscle stem cells: a symposium—introduction. Appl Physiol Nutr Metabol. 2006;31:771–772. doi: 10.1139/h06-052. [DOI] [PubMed] [Google Scholar]
- 34.Lawson MA. Purslow PP. Differentiation of myoblasts in serum-free media: effects of modified media are cell line-specific. Cells Tissues Organs. 2000;167:130–137. doi: 10.1159/000016776. [DOI] [PubMed] [Google Scholar]
- 35.Ji ZX. Du C. Wu GS. Li SY. An GS. Yang YX. Jia R. Jia HT. Ni JH. Synergistic up-regulation of muscle LIM protein expression in C2C12 and NIH3T3 cells by myogenin and MEF2C. Mol Genet Genomics. 2009;281:1–10. doi: 10.1007/s00438-008-0393-7. [DOI] [PubMed] [Google Scholar]
- 36.Wang L. Guo F. Wei S. Zhao R. Divergent effects of GLP-1 analogs exendin-4 and exendin-9 on the expression of myosin heavy chain isoforms in C2C12 myotubes. Peptides. 2011;32:1313–1319. doi: 10.1016/j.peptides.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C R Biol. 2007;330:530–533. doi: 10.1016/j.crvi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Polesskaya A. Seale P. Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45(+) adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 39.Otto A. Schmidt C. Luke G. Allen S. Valasek P. Muntoni F. Lawrence-Watt D. Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]
- 40.Dehal P. Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:1700–1708. doi: 10.1371/journal.pbio.0030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kile BT. Metcalf D. Mifsud S. DiRago L. Nicola NA. Hilton DJ. Alexander WS. Functional analysis of Asb-1 using genetic modification in mice. Mol Cell Biol. 2001;21:6189–6197. doi: 10.1128/MCB.21.18.6189-6197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo JH. Saiyin H. Wei YH. Chen S. Chen L. Bi G. Ma LJ. Zhou GJ. Huang CQ. Yu L. Dai L. Expression of testis specific ankyrin repeat and SOCS box-containing 17 gene. Arch Androl. 2004;50:155–161. doi: 10.1080/01485010490425485. [DOI] [PubMed] [Google Scholar]
- 43.Kwon S. Kim D. Rhee JW. Park JA. Kim DW. Kim DS. Lee Y. Kwon HJ. ASB9 interacts with ubiquitous mitochondrial creatine kinase and inhibits mitochondrial function. BMC Biol. 2010;8:23. doi: 10.1186/1741-7007-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tokuoka M. Miyoshi N. Hitora T. Mimori K. Tanaka F. Shibata K. Ishii H. Sekimoto M. Doki Y. Mori M. Clinical significance of ASB9 in human colorectal cancer. Int J Oncol. 2010;37:1105–1111. doi: 10.3892/ijo_00000762. [DOI] [PubMed] [Google Scholar]
- 45.Massoumi R. Sjolander A. The role of leukotriene receptor signaling in inflammation and cancer. Sci World J. 2007;7:1413–1421. doi: 10.1100/tsw.2007.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brack AS. Conboy IM. Conboy MJ. Shen J. Rando TA. A temporal switch from Notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]