Abstract
Background
Pulmonary hypertension (PH) is associated with a poor prognosis in idiopathic pulmonary fibrosis (IPF). Endothelin-1 (ET-1) and vascular endothelial growth factor (VEGF) are important in both fibrosis and vascular remodeling.
Objectives
We sought to determine the relationship between ET-1 and VEGF levels and hemodynamics in patients with IPF. We hypothesized that higher levels of ET-1 and VEGF would be associated with higher pulmonary artery pressures (PAP) and pulmonary vascular resistance (PVR) in patients with IPF.
Methods
We performed a cross-sectional analysis of 52 adults with IPF enrolled in a prospective cohort with available clinical data, platelet-free plasma, and hemodynamics. ET-1 and VEGF levels were measured via immunoassay. The associations of ET-1 and VEGF with PAP and PVR were examined using generalized additive models adjusted for age, gender, race/ethnicity, and forced vital capacity (% predicted).
Results
Sixteen of 52 (30.8%) had PH (mean PAP ≥ 25 mmHg). After multivariable adjustment, higher ET-1 levels were significantly associated with higher systolic (p = 0.01), diastolic (p = 0.02), and mean (p = 0.01) PAP and possibly higher PVR (p = 0.09). There were no significant associations between VEGF levels and hemodynamics.
Conclusions
Higher levels of ET-1 were associated with higher PAP and possibly higher PVR in participants with IPF. In a sub-group of patients, ET-1 may be a contributor to pulmonary vascular disease burden in IPF.
Keywords: idiopathic pulmonary fibrosis, endothelin-1, vascular endothelial growth factor, biomarkers, pulmonary hypertension
INTRODUCTION
Pulmonary hypertension (PH) is a common complication of idiopathic pulmonary fibrosis (IPF) that is associated with a high mortality rate when present [1–7]. While most individuals with IPF experience mild-to-moderate elevations in pulmonary artery pressures (PAP), the development (and severity) of PH is not associated with the severity of lung function impairment [2,4,5]. It has been postulated that PH in IPF is mediated in large part by hypoxic pulmonary vasoconstriction, but this is unlikely to be the sole determinant of pulmonary vascular disease, given the lack of a clear relationship between lung function and PH [2,4,5].
Endothelin-1 (ET-1) is a potent vasoconstrictor and promoter of pulmonary arterial smooth muscle cell growth with a pivotal role in pulmonary arterial hypertension (PAH) [8–10]. Elevated levels of ET-1 have been demonstrated in the peripheral and pulmonary circulations of patients with PAH and secondary PH [8,11–15]. In addition to its impact on the pulmonary endothelium, ET-1 has mitogenic effects that might contribute to the pathogenesis of pulmonary fibrosis [16–23]. Adults with interstitial lung disease (ILD) have been shown to have elevated circulating ET-1 levels, however few studies have examined the association of ET-1 with pulmonary vascular disease in IPF [24–26].
As with ET-1, vascular endothelial growth factor (VEGF) is an important mediator in the development of PAH and may play a role in the pathogenesis of IPF (and IPF with PH) [27–29]. In animal models of IPF, VEGF appears to modulate both fibrosis and endothelial function, although its effects are somewhat controversial [29–32]. VEGF levels may correlate with lung function in IPF patients, but to our knowledge the relationship between VEGF levels and hemodyamics has not been studied [26,33].
We examined the cross-sectional associations between plasma ET-1 and VEGF levels and hemodynamics, specifically PAP and pulmonary vascular resistance (PVR), in a cohort of patients with IPF. We hypothesized that higher levels of ET-1 and VEGF would be associated with higher PAP and PVR.
METHODS AND MATERIALS
Study Design
Participants were prospectively evaluated at the New York Presbyterian/Columbia University Medical Center Interstitial Lung Disease (ILD) and Lung Transplantation Programs between June 2007 and December 2009. Patients meeting criteria for the diagnosis of IPF based on the 2000 American Thoracic Society/European Respiratory Society consensus statement were included [34]. Patients with clinical evidence of collagen vascular disease and lung disease due to environmental or drug exposure were excluded. Patients referred to the ILD and Lung Transplantation Programs undergo transthoracic echocardiogram as part of their routine work-up. Patients with a suggestion of PH (estimated systolic PAP > 40 mmHg or evidence of right ventricular dysfunction when an adequate tricuspid regurgitant jet is not seen) and those being considered for lung transplantation go on to have hemodynamics performed. Demographic and clinical data were collected by research staff. Additional physiologic data including pulmonary function testing and six-minute walk distance (6MWD) were performed according to standard clinical protocols [35,36]. The study was approved by the Columbia University Medical Center Institutional Review Board.
We included patients with available hemodynamics and banked plasma. We excluded those with hemodynamics and blood draw that were more than 6 months apart, and those without available echocardiography to confirm the absence of left ventricular systolic dysfunction (ejection fraction < 50%) and/or more than moderate aortic or mitral valve disease.
Hemodynamics
Right heart catheterizations were performed at the Cardiac Catheterization Laboratory of New York Presbyterian hospital using standard clinical procedures. All catheter tracings were reviewed by two primary investigators (CEV, DJL); patients were excluded if pulmonary capillary wedge pressure (PCWP) was determined to be ≥ 15 mmHg by either investigator.
Biomarkers
Platelet-free plasma by venipuncture for each participant was collected by a trained phlebotomist, processed, and stored at −80°C. Plasma ET-1 and VEGF were measured by quantitative sandwich enzyme immunoassays (QuantiGlo, R&D Systems, Inc, Minneapolis, MN) at the Laboratory for Clinical Biochemical Research, University of Vermont. Reported manufacturer intra-assay coefficients of variation (CV) are between 2.6–3.4% and 2.8–7.9% and inter-assay CV are between 4.6–8.9% and 4.2–8.8% for ET-1 and VEGF, respectively.
Statistical Analysis
Continuous variables were expressed as means and standard deviations. Categorical variables were expressed as percentages. Independent sample t tests were used to compare continuous variables and chi-square or Fisher’s exact tests were used to compare categorical variables in study participants and those excluded. As they are not normally distributed, ET-1 and VEGF were natural logarithmically transformed. Generalized additive modeling was used to assess the relationship between continuous ET-1 and VEGF levels and hemodynamics. Models were adjusted for age, gender, race/ethnicity, and forced vital capacity (FVC) (percent predicted). Statistical significance was defined as P < 0.05. Analyses were performed using STATA 10.0 (StataCorp, College Station, TX).
RESULTS
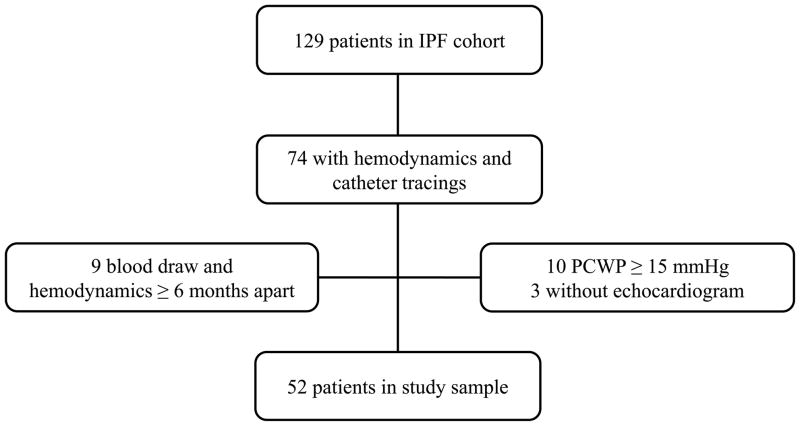
A total of 129 patients were enrolled in the IPF cohort during the study period (Figure 1). Of these, 74 (57%) had clinical data and right heart catheterization with available catheter tracings at the time of the study. We excluded 10 (14%) with PCWP ≥15 mmHg, 3 (4%) without available echocardiograms, and 9 (12%) patients whose blood draw and right heart catheterization were performed more than 6 months apart. A total of 52 participants were included in the study. Characteristics of the study sample and those excluded (N = 77) are shown in Table 1. Participants were more likely to be male, to have had systemic hypertension, to have received corticosteroids, and had more pronounced impairments in lung function and functional class than those excluded. Among participants, the mean age was 63 ± 6 years old, 83% were male, 94% were non-Hispanic white, and 61% were former smokers. None were receiving targeted PH therapy, such as endothelial receptor antagonists (ERAs) or phosphodiesterase-5 inhibitors. Mean FVC was 56 ± 15% predicted, mean 6MWD was 407 ± 126 m, and most participants were classified as New York Heart Association (NYHA) functional Class II or III. The average time between blood draw and hemodynamic assessment was 60 ± 49 days. Forty (77%) of the sample had study measurements performed less than 3 months apart.
Figure 1. Study flow.
IPF=idiopathic pulmonary fibrosis; PCWP=pulmonary capillary wedge pressure
Table 1.
Study participants
|
|
|||
|---|---|---|---|
| Study Sample | Excluded | P value | |
|
| |||
| Number | 52 | 77 | |
| Age, years | 63 ± 6 | 65 ± 8 | 0.12 |
| Male gender, % | 83 | 69 | 0.08 |
| Race/ethnicity, %* | |||
| Non-Hispanic white | 94 | 90 | 0.65 |
| Non-Hispanic black | 2 | 3 | |
| Hispanic | 0 | 0 | |
| Non-Hispanic other | 4 | 7 | |
| Ever-smoker, % | 61 | 69 | 0.28 |
| Height, cm | 175 ± 10 | 169 ± 9 | 0.00 |
| Weight, kg | 86 ± 15 | 80 ± 15 | 0.03 |
| Body mass index, kg/m2 | 28 ± 4 | 28 ± 5 | 0.72 |
| Systemic hypertension, % | 41 | 33 | 0.04 |
| Corticosteroid use, % | 38 | 33 | 0.07 |
| PH therapy, % | |||
| Endothelin Receptor Antagonists | 0 | 1 | 1.00 |
| Phosphodiesterase-5 Inhibitors | 0 | 4 | 0.16 |
| FVC, % predicted | 56 ± 15 | 66 ± 20 | 0.01 |
| No.* | 50 | 63 | |
| DLCO, % predicted | 31 ± 10 | 36 ± 13 | 0.05 |
| No.* | 49 | 57 | |
| 6MWD, m | 407 ± 126 | 395 ± 142 | 0.57 |
| No.* | 47 | 36 | |
| NYHA Functional Class, % | 0.04 | ||
| I | 4 | 22 | |
| II | 56 | 36 | |
| III | 38 | 36 | |
| IV | 2 | 6 | |
| Hemodynamics | |||
| Right atrial pressure, mmHg | 3.1 ± 2.7 | ||
| Systolic PAP, mmHg | 38.4 ± 12.6 | ||
| Diastolic PAP mmHg | 13.3 ± 6.6 | ||
| Mean PAP, mmHg | 22.3 ± 8.5 | ||
| PCWP, mmHg | 7.3 ± 3.2 | ||
| PVR, dynes·s·cm−5 | 269.7 ± 195.8 | ||
| Cardic index, L/min/m2 | 2.6 ± 0.6 | ||
Data shown as mean ± standard deviation or %.
Subset with available pulmonary function tests and 6MWD
Definition of abbreviations: FVC=forced vital capacity; DLCO=diffusion capacity of the lung for carbon monoxide; 6MWD=six-minute-walk distance; NYHA=New York Heart Association; PAP=pulmonary arterial pressures; PCWP=pulmonary capillary wedge pressure; PVR=pulmonary vascular resistance
The mean ET-1 level was 2.1 ± 0.6 pg/mL and the mean VEGF level was 54.9 ± 25.5 pg/mL. The mean right atrial pressure was 3.1 ± 2.7 mmHg, mean PAP was 22.3 ± 8.5 mmHg, PCWP was 7.3 ± 3.2 mmHg, PVR was 269.7 ± 195.8 dynes·s·cm−5, cardiac index (CI) was 2.6 ± 0.6 L/min/m2 (Table 1). Sixteen of 52 (30.8%) had PH (mean PAP ≥25 mmHg). Among those with PH, the mean ET-1 level was 2.3 ± 0.6 pg/mL and the mean VEGF level was 55.5 ± 25.9 pg/mL, as compared to 2.1 ± 0.6 pg/mL and 54.6 ± 25.6 pg/mL, respectively, in those without PH.
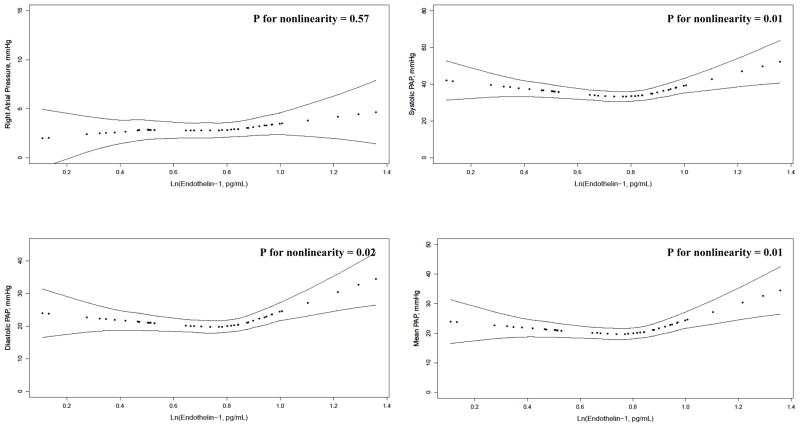
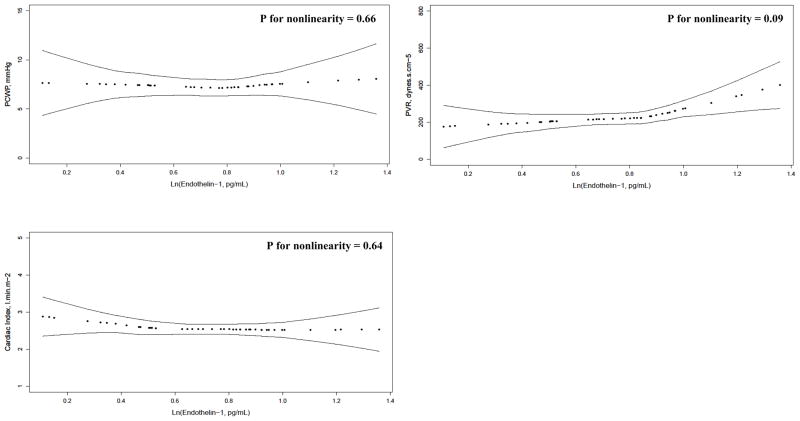
The continuous relationship between ET-1 levels and hemodynamics are depicted in Figure 2. After adjustment for age, gender, race/ethnicity, and percent predicted FVC, there was a blunted J-shaped relationship between ET-1 levels and systolic PAP (P for nonlinearity = 0.01), diastolic PAP (P for nonlinearity = 0.02), mean PAP (P for nonlinearity = 0.01), and possibly PVR (P for nonlinearity = 0.09). There was no association between ET-1 levels and RAP, PCWP, or CI. There were no significant associations between VEGF levels and hemodynamics (Figure E1). Results were unchanged when adjusted for diffusing capacity of the lung for carbon monoxide (DLCO) (data not shown). ET-1 and VEGF levels were not associated with FVC, DLCO, 6MWD, or NYHA functional class (data not shown).
Figure 2. Relationship between plasma ln(endothelin-1) and hemodynamics.
PAP=pulmonary artery pressures; PCWP=pulmonary capillary wedge pressure; PVR=pulmonary vascular resistance
DISCUSSION
We have shown that higher plasma ET-1 levels are associated with higher PAP and perhaps PVR in a cohort of patients with IPF after adjustment for demographics and lung function. This relationship is non-linear and J-shaped, with only a sub-group of patients demonstrating correlations between higher levels of ET-1 and higher PAPs. Conversely, there were no significant associations between VEGF levels and hemodynamics. To our knowledge, this is the largest observational study of these circulating biomarkers in IPF.
Several small studies have previously shown elevated levels of ET-1 in patients with ILD (but not necessarily IPF) and that ET-1 levels correlate with mean PAP [25,37–39]. Trakada and colleagues demonstrated that ET-1 concentrations were higher among those with ILD and concurrent PH compared to those without PH in 38 patients [38]. In IPF specifically, the relationship with hemodynamics has not been studied, but ET-1 has been shown to be elevated peripherally and to be overexpressed in the lung [24]. ET-1 appears to localize to small vessel endothelial cells but diffuse into surrounding tissue, suggesting that in certain individuals activation of the ET-1 pathway may result in uncontrolled fibrosis and perhaps widespread endothelial dysfunction via paracrine effects, resulting in IPF with PH [21,24,40,41]. Randomized clinical trials have studied the effect of ERAs on disease progression in IPF and IPF with PH (clinicaltrials.gov registry numbers NCT00879229 and NCT00768300) [42,43]. While the results of these trials have been somewhat disappointing, it is possible that a subgroup of patients with IPF (e.g., those with a particularly active endothelin pathway and therefore a large pulmonary vascular disease burden) may benefit from targeted PH therapy [44]. In fact, while most participants in our study clustered around mid-range ET-1 levels and had mild to modest elevations in PAP and PVR, a small group had strong linear associations between ET-1 and hemodynamics (as shown in Figure 2).
In PH and PAH, endothelial VEGF and VEGF receptor production is upregulated in response to vascular insult [27,28,45,46]. Evidence in humans is limited, but VEGF may also modulate angiogenesis and fibrosis in IPF [26,33]. While some studies have shown VEGF inhibition attenuates pulmonary fibrosis, areas of spared lung appear to have increased vascular density and VEGF upregulation [29–31]. Farkas et al. demonstrated reduced VEGF expression in fibrotic lung, an inverse correlation of VEGF levels with PAP, and improved endothelial apoptosis, vascular remodeling, and PH with VEGF augmentation, suggesting that decreased vascularity may underpin the development of PH in IPF [32]. While patients with IPF seem to have similar serum levels of VEGF compared with controls, in patients with severe disease there is significant correlation between VEGF levels and progression of disease [26,33]. These studies do not separately identify patients with concurrent PH, and our study suggests VEGF may not be a useful biomarker in IPF. The relationship between VEGF, angiogenesis/fibrosis and PH remains poorly described in humans.
Our study has several limitations. First, while the majority had blood draw and hemodynamics measured over a short interval (58% within 8 weeks and 39% within 4 weeks), ideally these would have been performed simultaneously and this may have introduced confounding. No patients were hospitalized between tests, implying relative clinical stability of the cohort. Second, patients were selected for hemodynamic evaluation based on screening echocardiography (or as part of lung transplantation evaluation) and therefore those included in the study tended to be sicker (e.g., had more impaired lung function) than those excluded, possibly resulting in bias. Third, as this is a cross-sectional study, no conclusions can be drawn about causality, i.e. whether elevated ET-1 precedes the development of PH complicating IPF or is simply a marker of pulmonary vascular disease burden. As is commonly seen in IPF, our study sample was predominantly white men, limiting the generalizability of these findings to women and individuals of other races. Last, because of the small number of patients with significant pulmonary vascular disease (31% with mean PAP ≥ 25 mmHg), we were underpowered to assess whether ET-1 may be useful to predict PH.
We have shown that higher ET-1 levels are associated with higher PAP and may be associated with higher PVR. Studies will be needed to evaluate ET-1 as a diagnostic screening tool for IPF with PH, and it remains to be seen whether subsets of individuals may benefit from targeted PH therapy.
Supplementary Material
PAP=pulmonary artery pressures; PCWP=pulmonary capillary wedge pressure; PVR=pulmonary vascular resistance
Acknowledgments
FUNDING SOURCES
American Heart Association 11FTF7400032, ASPIRE Pulmonary Vascular Disease Young Investigator Research Award WS1952812, NIH grants K23 HL086714 and KL2 RR024156, the Robert Wood Johnson Physician Faculty Scholars Program, and the Herbert and Florence Irving Scholar Award. This publication was made possible by grant number KL2 RR024156 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at the NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from the NIH Roadmap website.
Footnotes
Disclosures
CEV has received grant funding from Pfizer and has served on advisory boards for Actelion, Gilead, and United Therapeutics.
SMK has served on advisory boards for Bayer, Gilead, and Pfizer, has performed consulting work for Merck, Gilead, Novartis, has received grant funding from Actelion, Gilead, United Therapeutics, Lung Rx, Merck, and Bayer, and has received lecture fees from Gilead and Actelion.
DJL has served on a steering committee for a clinical trial of pirfenidone for IPF (Intermune, Inc.) and has served on advisory board for Gilead on the topics of IPF and PAH.
Contributor Information
Corey E. Ventetuolo, Email: corey_ventetuolo@brown.edu.
Steven M. Kawut, Email: kawut@mail.upenn.edu.
David J. Lederer, Email: dl427@columbia.edu.
References
- 1.Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30:715–721. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 2.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 3.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med. 2007;175:875–880. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 4.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 5.Nathan SD, Shlobin OA, Ahmad S, Urbanek S, Barnett SD. Pulmonary hypertension and pulmonary function testing in idiopathic pulmonary fibrosis. Chest. 2007;131:657–663. doi: 10.1378/chest.06-2485. [DOI] [PubMed] [Google Scholar]
- 6.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007;132:998–1006. doi: 10.1378/chest.06-3087. [DOI] [PubMed] [Google Scholar]
- 7.Pitsiou G, Papakosta D, Bouros D. Pulmonary hypertension in idiopathic pulmonary fibrosis: a review. Respiration. 2011;82:294–304. doi: 10.1159/000327918. [DOI] [PubMed] [Google Scholar]
- 8.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 9.Yoshibayashi M, Nishioka K, Nakao K, Saito Y, Matsumura M, Ueda T, Temma S, Shirakami G, Imura H, Mikawa H. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects. Evidence for increased production of endothelin in pulmonary circulation. Circulation. 1991;84:2280–2285. doi: 10.1161/01.cir.84.6.2280. [DOI] [PubMed] [Google Scholar]
- 10.Dupuis J, Stewart DJ, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation. 1996;94:1578–1584. doi: 10.1161/01.cir.94.7.1578. [DOI] [PubMed] [Google Scholar]
- 11.Montani D, Souza R, Binkert C, Fischli W, Simonneau G, Clozel M, Humbert M. Endothelin-1/endothelin-3 ratio: a potential prognostic factor of pulmonary arterial hypertension. Chest. 2007;131:101–108. doi: 10.1378/chest.06-0682. [DOI] [PubMed] [Google Scholar]
- 12.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 13.Carratu P, Scoditti C, Maniscalco M, Seccia TM, Di Gioia G, Gadaleta F, Cardone RA, Dragonieri S, Pierucci P, Spanevello A, Resta O. Exhaled and arterial levels of endothelin-1 are increased and correlate with pulmonary systolic pressure in COPD with pulmonary hypertension. BMC Pulm Med. 2008;8:20. doi: 10.1186/1471-2466-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiramoto Y, Shioyama W, Higuchi K, Arita Y, Kuroda T, Sakata Y, Nakaoka Y, Fujio Y, Yamauchi-Takihara K. Clinical significance of plasma endothelin-1 level after bosentan administration in pulmonary arterial hypertension. J Cardiol. 2009;53:374–380. doi: 10.1016/j.jjcc.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Hiramoto Y, Shioyama W, Kuroda T, Masaki M, Sugiyama S, Okamoto K, Hirota H, Fujio Y, Hori M, Yamauchi-Takihara K. Effect of bosentan on plasma endothelin-1 concentration in patients with pulmonary arterial hypertension. Circ J. 2007;71:367–369. doi: 10.1253/circj.71.367. [DOI] [PubMed] [Google Scholar]
- 16.Jain R, Shaul PW, Borok Z, Willis BC. Endothelin-1 induces alveolar epithelial-mesenchymal transition through endothelin type A receptor-mediated production of TGF-β1. Am J Respir Cell Mol Biol. 2007;37:38–47. doi: 10.1165/rcmb.2006-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giaid A, Michel RP, Stewart DJ, Sheppard M, Hamid Q, Corrin B. Expression of endothelin-1 in lungs of patients with crypogenic fibrosing alveolitis. Lancet. 1993;341:1550–1554. doi: 10.1016/0140-6736(93)90694-c. [DOI] [PubMed] [Google Scholar]
- 18.Saleh D, Furukawa K, Tsao M, Maghazachi A, Corrin B, Yanagisawa M, Barnes P, Giaid A. Elevated expression of endothelin-1 and endothelin-converting enzyme-1 in idiopathic pulmonary fibrosis: possible involvement of proinflammatory cytokines. Am J Respir Cell Mol Biol. 1997;16:187–193. doi: 10.1165/ajrcmb.16.2.9032126. [DOI] [PubMed] [Google Scholar]
- 19.Hocher B, Schwarz A, Fagan KA, Thone-Reineke C, El-Hag K, Kusserow H, Elitok S, Bauer C, Neumayer H-H, Rodman DM, Theuring F. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol. 2000;23:19–26. doi: 10.1165/ajrcmb.23.1.4030. [DOI] [PubMed] [Google Scholar]
- 20.Park S-H, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156:600–608. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 21.Ross B, D’Orleans-Juste P, Giaid A. Potential role of endothelin-1 in pulmonary fibrosis: from the bench to the clinic. Am J Respir Cell Mol Biol. 2010;42:16–20. doi: 10.1165/rcmb.2009-0175TR. [DOI] [PubMed] [Google Scholar]
- 22.Shi-wen X, Kennedy L, Renzoni EA, Bou-Gharios G, Bois RMd, Black CM, Denton CP, Abraham DJ, Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheumat. 2007;56:4189–4194. doi: 10.1002/art.23134. [DOI] [PubMed] [Google Scholar]
- 23.Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, Dashwood MR, du Bois RM, Denton CP, Black CM, Abraham DJ, Leask A. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol. 2006;26:5518–5527. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uguccioni M, Pulsatelli L, Grigolo B, Facchini A, Fasano L, Cinti C, Fabbri M, Gasbarrini G, Meliconi R. Endothelin-1 in idiopathic pulmonary fibrosis. J Clin Pathol. 1995;48:330–334. doi: 10.1136/jcp.48.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trakada G, Spiropoulos K. Arterial endothelin-1 in interstitial lung disease patients with pulmonary hypertension. Monaldi Arch Chest Dis. 2001;56:379–383. [PubMed] [Google Scholar]
- 26.Simler NR, Brenchley PE, Horrocks AW, Greaves SM, Hasleton PS, Egan JJ. Angiogenic cytokines in patients with idiopathic interstitial pneumonia. Thorax. 2004;59:581–585. doi: 10.1136/thx.2003.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuder R, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool C, Bishop A, Geraci M, Semenza G. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 28.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers: evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol. 1999;155:411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M, Sasano H, Kondo T, Nukiwa T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004;169:1203–1208. doi: 10.1164/rccm.200308-1111OC. [DOI] [PubMed] [Google Scholar]
- 30.Hamada N, Kuwano K, Yamada M, Hagimoto N, Hiasa K, Egashira K, Nakashima N, Maeyama T, Yoshimi M, Nakanishi Y. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol. 2005;175:1224–1231. doi: 10.4049/jimmunol.175.2.1224. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary NI, Roth GJ, Hilberg F, Muller-Quernheim J, Prasse A, Zissel G, Schnapp A, Park JE. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29:976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- 32.Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest. 2009;119:1298–1311. doi: 10.1172/JCI36136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ando M, Miyazaki E, Ito T, Hiroshige S, Nureki S, Ueno T, Takenaka R, Fukami T, Kumamoto T. Significance of serum vascular endothelial growth factor level in patients with idiopathic pulmonary fibrosis. Lung. 2010;188:247–252. doi: 10.1007/s00408-009-9223-x. [DOI] [PubMed] [Google Scholar]
- 34.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 35.ATS Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 36.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson D, MacIntyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 37.Yamakami T, Taguchi O, Gabazza E, Yoshida M, Kobayashi T, Kobayashi H, Yasui H, Ibata H, Adachi Y. Arterial endothelin-1 level in pulmonary emphysema and interstitial lung disease. Relation with pulmonary hypertension during exercise. Eur Respir J. 1997;10:2055–2060. doi: 10.1183/09031936.97.10092055. [DOI] [PubMed] [Google Scholar]
- 38.Trakada G, Nikolaou E, Pouli A, Tsiamita M, Spiropoulos K. Endothelin-1 levels in interstitial lung disease patients during sleep. Sleep Breath. 2003;7:111–118. doi: 10.1007/s11325-003-0111-y. [DOI] [PubMed] [Google Scholar]
- 39.Maeder MT, Brutsche MH, Arenja N, Socrates T, Reiter M, Meissner J, Staub D, Morgenthaler NG, Bergmann A, Mueller C. Biomarkers and peak oxygen uptake in patients with chronic lung disease. Respiration. 2010;80:543–552. doi: 10.1159/000319038. [DOI] [PubMed] [Google Scholar]
- 40.Kim KK, Chapman HA. Endothelin-1 as initiator of epithelial-mesenchymal transition: potential new role for endothelin-1 during pulmonary fibrosis. Am J Respir Cell Mol Biol. 2007;37:1–2. doi: 10.1165/rcmb.2007-0001ED. [DOI] [PubMed] [Google Scholar]
- 41.Haynes WG, Webb DJ. The endothelin family of peptides: local hormones with diverse roles in health and disease? Clin Sci (Lond) 1993;84(5):485–500. doi: 10.1042/cs0840485. [DOI] [PubMed] [Google Scholar]
- 42.King J, Talmadge E, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, Behr J. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 43.King TE, Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stahler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 44.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier J-F, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ on behalf of the ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian J, Smith A, Nechtman J, Podolsky R, Aggarwal S, Snead C, Kumar S, Elgaish M, Oishi P, Goerlach A, Fratz S, Hess J, Catravas JD, Verin AD, Fineman JR, She J-X, Black SM. Effect of PPARγ inhibition on pulmonary endothelial cell gene expression: gene profiling in pulmonary hypertension. Physiol Genomics. 2009;40:48–60. doi: 10.1152/physiolgenomics.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho YJ, Han JY, Lee SG, Jeon BT, Choi WS, Hwang YS, Roh GS, Lee JD. Temporal changes of angiopoietins and Tie2 expression in rat lungs after monocrotaline-induced pulmonary hypertension. Comp Med. 2009;59:350–356. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PAP=pulmonary artery pressures; PCWP=pulmonary capillary wedge pressure; PVR=pulmonary vascular resistance