Abstract
AIMS
The use of statins has been suggested to protect against atrial fibrillation (AF) in some clinical observational and experimental studies but has remained inadequately explored. This study was designed to examine whether statins can reduce the risk of AF.
METHODS
Meta-analysis of randomized, controlled trials with use of statins on incidence or recurrence of AF was performed.
RESULTS
Twenty studies with 23 577 patients were included in the analysis. Seven studies investigated the use of statins in patients with AF, 11 studies investigated the primary prevention of statins in patients without AF, and two studies investigated mixed populations of patients. The incidence or recurrence of AF occurred in 1543 patients. Overall, statin therapy was significantly associated with a decreased risk of AF compared with control (odds ratio 0.49, 95% confidence interval 0.37–0.65; P < 0.00001). A beneficial effect was found in the atorvastatin subgroup and the simvastatin subgroup, but not in the pravastatin subgroup or the rosuvastatin subgroup. The benefit of statin therapy appeared to be more pronounced in secondary prevention (odds ratio 0.34, 95% confidence interval 0.18–0.64; P < 0.0008) than in primary prevention (odds ratio 0.54, 95% confidence interval 0.40–0.74; P < 0.0001).
CONCLUSIONS
Statin therapy was significantly associated with a decreased risk of incidence or recurrence of AF. Heterogeneity was explained by differences in statin types, patient populations and surgery types. The benefit of statin therapy seemed more pronounced in secondary than in primary prevention.
Keywords: atrial fibrillation, meta-analysis, statins
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia, and AF is associated with relatively higher all-cause mortality in both men and women.
However, there are limited treatment options for AF.
Statins are hypothesized to have a benefit against arrhythmias in addition to well-established secondary prevention benefit for atherosclerotic coronary artery disease, yet the data are inconsistent
WHAT THIS STUDY ADDS
Statin therapy was significantly associated with a decreased risk of incidence or recurrence of AF.
The benefit of statin therapy seemed more markedly in secondary prevention than primary prevention.
These results provided some evidence for the benefit of statins beyond their lipid-lowering activity
Introduction
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia [1–3]. Individuals with AF have a greater long-term risk of ischaemic stroke and heart failure. Atrial fibrillation is also associated with relatively higher all-cause mortality in both men and women [4]. Secular trends show that the number of individuals with AF is increasing faster than previously projected, underscoring an urgent need for therapies that prevent AF [5].
There are, however, limited treatment options for this purpose. Traditional antiarrhythmic agents, such as amiodarone, sotalol, flecainide and propafenone, are associated with potentially serious adverse effects and, in some cases, increase overall mortality [4]. Safer and more efficacious agents are needed to prevent AF [5].
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are pleiotropic agents known to reduce inflammation, which are believed to play a key role in atrial remodelling [6, 7]. Statins are hypothesized to have a benefit against arrhythmias in addition to the well-established benefit of secondary prevention for atherosclerotic coronary artery disease, yet the data are inconsistent. Statins had been suggested to protect against AF in some clinical and experimental studies, but this remained inadequately explored. Specifically, observational studies provided evidence supporting a protective role of statins against AF [8].
However, previous randomized controlled trials, many of which were relatively small and perhaps underpowered to evaluate this end-point adequately, have demonstrated mixed results [9–28]. A meta-analysis by Fauchier et al. also provided evidence on antiarrhythmic effect of statins [8], and a number of randomized trials have been published since then. Thus, we aimed to conduct a meta-analysis to evaluate the effect of statin use on the end-point of incidence or recurrence of AF.
Methods
The CONSORT guidelines were followed in the reporting of the present meta-analysis [29].
Data collection
A PubChem Compound search of all names of HMG-CoA reductase inhibitors (statins) was carried out. The name of each drug was searched as a keyword combined with other relevant keywords, such as ‘HMG-CoA reductase inhibitors’, ‘statins’, ‘atrial fibrillation’ and ‘random’. We conducted a systematic literature search of Medline (up to 31 December 2010), Embase (up to 2010), the Cochrane Controlled Trials Register (up to 2010) and the ISI (web of science) database. We looked for randomized controlled trials that met all of the following specified criteria: (i) direct comparison between a statin and control treatment or placebo, regardless of the background therapy in either group; (ii) publication before 31 December 2010, written in English; and (iii) incidence or recurrence of AF as a specified event, although not necessarily a primary end-point. We extracted information on study design, sample characteristics, sample size, intervention strategies, outcome measures and other study characteristics from the included trials. We reviewed the methodological quality of randomized controlled trials by using a scoring system developed by Jadad et al. [30]. The number of events in each trial was extracted on the basis of the intention-to-treat approach. All the analyses on the end-point of AF were performed at the trial level, and none of the data of the individual studies were obtained from sponsoring institutions.
Statistical analysis
Analyses were conducted based on the intention-to-treat principle. We calculated the odds ratio (OR) for each study outcome to allow for pooling of similar outcomes. We calculated ORs and 95% confidence intervals (CIs) for incidence or recurrence of AF of each trial separately and for combinations of studies according to fixed-effect and random-effect models. We used a chi-squared test to assess heterogeneity. In the presence of statistical homogeneity, defined as a chi-squared test P value > 0.10, we analysed the data using fixed-effect models. Pooled ORs and 95% CIs for fixed-effect models were calculated on the basis of the Mantel–Haenszel method [31]. Otherwise, we used random-effect models [32]. The P value threshold for statistical significance was set at 0.05 for effect sizes.
Sensitivity analysis was performed by omitting studies on the basis of a quality assessment and checking the consistency of the overall effect estimate. Publication bias was evaluated using the funnel plot and Egger's regression method [33]. Statistical calculations were performed by using RevMan, version 5.0.25.0 (The Cochrane Collaboration, Oxford, UK) and STATA 10.0 (STATA Corp., College Station, TX, USA). No funding source had a role in the design of the study or in the decision to submit the manuscript for publication.
Results
Twenty studies [9–28] with 23 577 patients were included in the analysis. Seven studies [10, 14, 18, 19, 22, 26, 28] investigated the use of statins in patients with a history of AF undergoing electrical cardioversion (EC; AF recurrence, n = 5) [10, 18, 22, 26, 28] or pharmacological care (n = 2) [14, 19], 11 studies [11–13, 15, 16, 20, 21, 23–25, 27] investigated the use of statins in primary prevention of AF in patients with cardiac surgery (postoperative AF, n = 8) [11–13, 16, 20, 21, 23, 24] or implantation of a pacemaker (postoperative AF, n = 1) [25] or pharmacological treatment (new-onset AF, n = 2) [15, 27], while the MIRACL study [17] and the ALLHAT study [9] included both AF patients and non-AF patients. MIRACL 1 and 2 indicated the effect of statin in subgroups of patients in the MIRACL study with or without a previous AF, respectively. The study of García-Fernández A et al. [34] also met the inclusion criteria, but the results of this trial are not provided in the article. Table 1 summarizes the characteristics of the 20 trials.
Table 1.
Trial design and baseline characteristics of the 20 trials included in the meta-analysis
| Study | n | Population | Treatment arms | Duration | Specified end-point | Quality scores |
|---|---|---|---|---|---|---|
| ALLHAT; Haywood et al. [9] | 8582 (4327:4255) | Hypertensive people >55 years of age with at least one additional CVD risk factor | Intervention: pravastatin | 4.8 years | New onset AF/AFL | 1 |
| Control: usual care | ||||||
| Almroth et al. [10] | 234 (118:116) | Persistent AF following EC | Intervention: atorvastatin 80 mg day−1 | 44 days | The number of patients in sinus rhythm at EC and day 30 after EC | 5 |
| Control: placebo | ||||||
| ARMYDA-3; Patti et al. [11] | 200 (101:99) | Patients undergoing cardiac surgery | Intervention: atorvastatin 40 mg day−1 | 37 days | The occurrence of postoperative AF | 5 |
| Control: placebo | ||||||
| Caorsi et al. [12] | 43 (21:22) | Patients undergoing cardiopulmonary bypass | Intervention: pravastatin 40 mg day−1 | 9 days | The occurrence of postoperative AF | 2 |
| Control: placebo | ||||||
| Chello et al. [13] | 40 (20:20) | Patients undergoing coronary bypass surgery | Intervention: atorvastatin 20 mg day−1 | 3 weeks | The occurrence of postoperative AF | 4 |
| Control: placebo | ||||||
| Dernellis et al. [14] | 80 (40:40) | Patients with proven PAF | Intervention: atorvastatin 20–40 mg day−1 | 6 months | The number of patients resolved of AF | 3 |
| Control: placebo | ||||||
| GISSI-HF; Maggioni et al. [15] | 3690 (1855:1835) | Chronic HF patients without AF | Intervention: rosuvastatin 10 mg day−1 | 3.7 years | New-onset AF | 5 |
| Control: placebo | ||||||
| Mannacio et al. [16] | 200 (100:100) | 200 patients undergoing coronary surgery | Intervention: rosuvastatin 20 mg day−1 | 1 week | The occurrence of postoperative AF | 4 |
| Control: placebo | ||||||
| MIRACL; Schwartz et al. [17] | 3087 (1539:1548) | Acute coronary syndrome | Intervention: atorvastatin 80 mg day−1 | 16 weeks | Incidence or recurrence of AF | 5 |
| Control: placebo | ||||||
| Ozaydin et al. [18] | 48 (24:24) | Persistent AF after EC | Intervention: atorvastatin 10 mg day−1 | 92 days | The reccurrence of AF | 2 |
| Control: control group | ||||||
| Qian et al. [19] | 99 (49:50) | Permanent AF patients after prosthetic mitral valve replacement | Intervention: combination pharmacological therapy with low-dose oral amiodarone (2 mg kg−1), captopril (0.25 mg kg−1) and simvastatin (0.3 mg kg−1) daily | 1 year | Efficacy of AF conversion | 2 |
| Control: digoxin and a CCB, diltiazem, alone or in combination to control heart ventricular rate, 12 months | ||||||
| Song et al. [20] | 124 (62:62) | Patients after off-pump CABG | Intervention: atorvastatin 20 mg day−1 for 3 days before the surgery and 30 days after surgery | 33 days | The occurrence of postoperative AF | 3 |
| Control: control group | ||||||
| Spadaccio et al. [21] | 50 (25:25) | Patients undergoing cardiopulmonary bypass surgery | Intervention: atorvastatin 20 mg day−1 | 3 weeks | The occurrence of postoperative AF | 5 |
| Control: placebo | ||||||
| SToP AF trial; Negi et al. [22] | 64 (33:31) | Patients with AF/AFL who underwent EC | Intervention: atorvastatin 80 mg day−1 | 12 months | The recurrence of AF | 4 |
| Control: placebo | ||||||
| Sun et al. [23] | 140 (71:69) | Patients undergoing elective off-pump CABG | Intervention: atorvastatin 20 mg day−1 | 37 days | The occurrence of postoperative AF | 5 |
| Control: placebo | ||||||
| Tamayo et al. [24] | 44 (22:22) | Patients undergoing CABG | Intervention: simvastatin 20 mg day−1 | 3 weeks | The occurrence of postoperative AF | 3 |
| Control: control group | ||||||
| Tsai et al. [25] | 106 (52:54) | Bradyarrhythmias and implantation of an atrial-based or dual-chamber pacemaker | Intervention: atorvastatin 20 mg day−1 | 1 year | The occurrence of AHE or AF episodes | 2 |
| Control: control group | ||||||
| Tveit et al. [26] | 114 (57:57) | AF patients undergoing EC | Intervention: pravastatin 40 mg day−1 | 9 weeks | The recurrence of AF | 3 |
| Control: standard therapy | ||||||
| WOSCOPS; Macfarlane & Norrie [27] | 6595 (3302:3293) | 6595 men with moderate hyperlipidaemia and no previous history of myocardial infarction | Intervention: pravastatin 40 mg day−1 | 4.9 years | The occurrence of AF | 3 |
| Control: placebo | ||||||
| Xia et al. [28] | 64 (32:32) | Persistent AF patients undergoing EC | Intervention: rosuvastatin 10 mg day−1 | 92 days | The recurrence of AF | 1 |
| Control: control group |
Abbreviations: AF, atrial fibrillation; AFL, atrial flutter; AHE,atrial high rate episodes; ALLHAT, antihypertensive and lipid-lowering treatment to prevent heart attack trial; ARMYDA-3, atorvastatin for reduction of myocardial dysrhythmia after cardiac surgery study; CABG, coronary artery bypass grafting; CCB, calcium channel blockers; CVD, cardiovascular disease; EC, electrical cardioversion; HF, heart failure; MIRACL, myocardial ischaemia reduction with aggressive cholesterol lowering study; PAF, paroxysmal atrial fibrillation; SToP AF trial, statin therapy for the prevention of atrial fibrillation trial; and WOSCOPS, West of Scotland Coronary Prevention Study.
All included trials were randomized and received Jadad scores of 1 (n = 2) [9, 28], 2 (n = 4) [12, 18, 19, 25], 3 (n = 5) [14, 20, 24, 26, 27], 4 (n = 3) [13, 16, 22] or 5 points (n = 6) [10, 11, 15, 17, 21, 23]. The 20 eligible trials included 11 836 patients randomized to statins and 11 741 patients randomized to a placebo or control regimen. The following statins were studied: atorvastatin (n = 11) [10, 11, 13, 14, 17, 18, 20–23, 25], rosuvastatin (n = 3) [15, 16, 28], pravastatin (n = 4) [9, 12, 26, 27] and simvastatin (n = 2) [19, 24]. Intervention doses for statins were variable. Comparisons were made with placebo (n = 10) [10, 11, 13–16, 21–23, 27] or usual care (n = 3) [9, 19, 26]; control groups were not exactly described in seven studies [12, 17, 18, 20, 24, 25, 28].
In most of the trials, AF was diagnosed through ECG and/or 24 h Holter at several specific time points after randomization. In addition, in four trials [15, 20, 23, 28] participants were also asked to undergo ECG and/or 24 h Holter at any time if they had symptoms of AF. In the study of Tsai et al. [25], AF was diagnosed by pacemaker. The other trials did not mention this as a specific requirement.
Statin vs. non-statin – meta-analysis
Combination of the results of the 20 trials [9–28] revealed that statin users had a significantly lower risk of AF compared with nonstatin users (OR 0.49; 95% CI 0.37–0.65). Heterogeneity was substantial as assessed by the I2 statistics (Q(d.f. = 19) = 61.05, P < 0.00001, I2 = 69%) (Figure 1).
Figure 1.
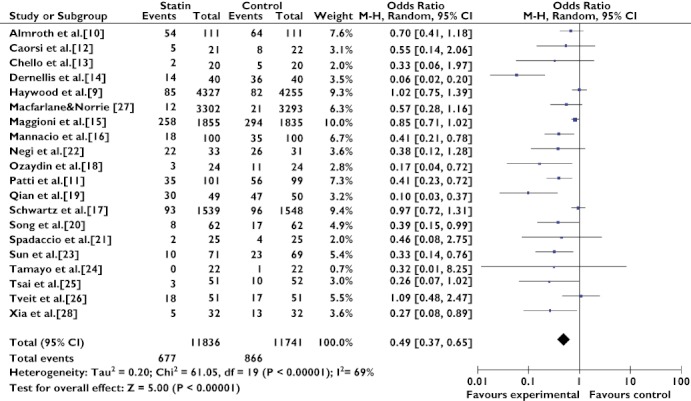
Effect of statin treatment on atrial fibrillation. Abbreviations: ALLHAT, antihypertensive and lipid-lowering treatment to prevent heart attack trial; ARMYDA-3, atorvastatin for reduction of myocardial dysrhythmia after cardiac surgery study; MIRACL, myocardial ischemia reduction with aggressive cholesterol lowering study; SToP AF trial, statin therapy for the prevention of atrial fibrillation trial; WOSCOPS, west of scotland coronary prevention study
Subgroup analysis
In order to address heterogeneity, two subgroup analyses were performed. In the subgroup analysis assessing the effect of different statins in the prevention of AF, atorvastatin (OR 0.38; 95% CI 0.24–0.61) and simvastatin (OR 0.12; 95% CI 0.04–0.40) had a preventive effect on AF occurrence, while pravastatin (OR 0.93; 95% CI 0.71–1.20) and rosuvastatin (OR 0.53; 95% CI 0.26–1.05) did not reduce the occurrence of AF. Heterogeneity remained substantial in the atorvastatin subgroup (Q(d.f. = 10) = 34.43, P = 0.0002, I2 = 71%) and in the rosuvastatin subgroup (Q(d.f. = 2) = 7.61, P = 0.02, I2 = 74%); studies in the provastatin subgroup (Q(d.f. = 3) = 2.95, P = 0.40, I2 = 0%) and the simvastatin subgroup (Q(d.f. = 1) = 0.41, P = 0.52, I2 = 0%) were very homogeneous (Figure 2).
Figure 2.
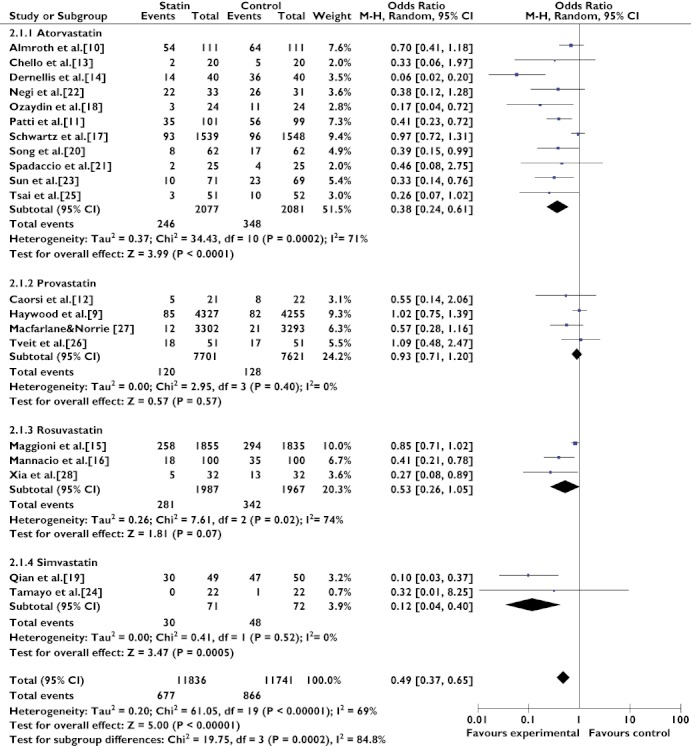
Effects of treatment with different statins on atrial fibrillation. Abbreviations: ALLHAT, antihypertensive and lipid-lowering treatment to prevent heart attack trial; ARMYDA-3, atorvastatin for reduction of myocardial dysrhythmia after cardiac surgery study; MIRACL, myocardial ischemia reduction with aggressive cholesterol lowering study; SToP AF trial, statin therapy for the prevention of atrial fibrillation trial; WOSCOPS, west of scotland coronary prevention study
In the subgroup analysis assessing the effect of statin therapy in different populations, statin therapy showed a greater preventive effect in the AF population (OR 0.34, 95% CI 0.18–0.64; P = 0.0008) than in the non-AF population (OR 0.54, 95% CI 0.40–0.74; P = 0.0001) and the mixed population (OR 1.02, 95% CI 0.75–1.39; P = 0.90; Figure 3). Heterogeneity remained substantial in the AF population subgroup (Q(d.f. = 7) = 26.90, P = 0.0003, I2 = 74%) and the non-AF population subgroup (Q(d.f. = 11) = 20.93, P = 0.03, I2 = 47%).
Figure 3.
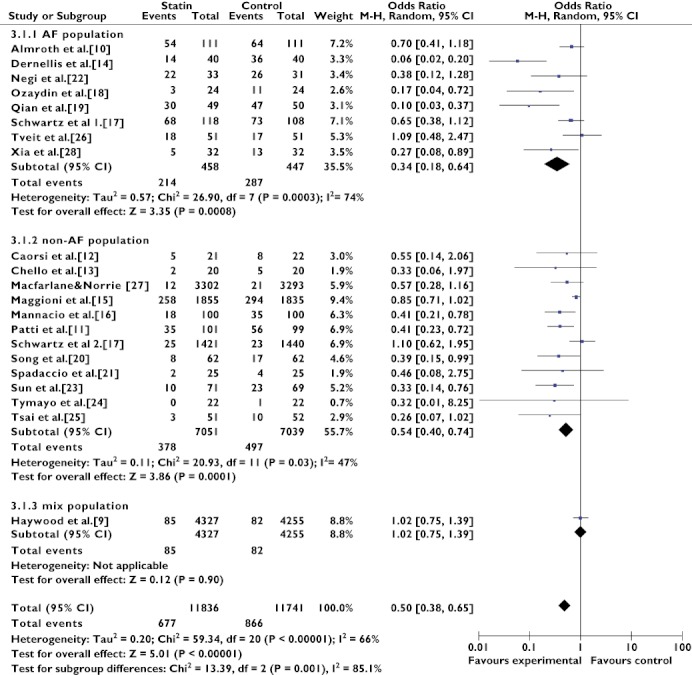
Primary and secondary prevention of atrial fibrillation with statins. Abbreviation: AF, atrial fibrillation; ALLHAT, antihypertensive and lipid-lowering treatment to prevent heart attack trial; ARMYDA-3, atorvastatin for reduction of myocardial dysrhythmia after cardiac surgery study; MIRACL, myocardial ischemia reduction with aggressive cholesterol lowering study; SToP AF trial, statin therapy for the prevention of atrial fibrillation trial; WOSCOPS, west of scotland coronary prevention study
Post hoc analysis
Heterogeneity was not addressed well through subgroup analyses; therefore, post hoc analyses were performed to elucidate the matter further.
We divided the seven trials [10, 14, 18, 19, 22, 26, 28] that assessed the preventive effect in AF populations into two subgroups on the basis of whether patients underwent EC. The MIRACL study [17], which was only published in abstract form, was removed from the analysis. Patients without EC (OR 0.08, 95% CI 0.03–0.19; P < 0.00001) had a significantly lower risk of recurrent AF compared with patients who underwent EC (OR 0.51, 95% CI 0.29–0.92; P = 0.03; Figure 4). The I2 statistics showed that both the EC group (Q(d.f. = 4) = 7.55, P = 0.11, I2 = 47%) and the without EC group (Q(d.f. = 1) = 0.33, P = 0.57, I2 = 0%) were very homogeneous.
We divided the 11 trials [11–13, 15, 16, 20, 21, 23–25, 27] that assessed the preventive effect in non-AF populations into two subgroups on the basis of whether patients underwent coronary surgery. The MIRACL study [17], which was only published in abstract form, was removed from the analysis. The benefits of statin therapy seemed to be more marked in patients with coronary surgery (OR 0.40, 95% CI 0.29–0.55; P < 0.00001) than in patients without coronary surgery (OR 0.81, 95% CI 0.68–0.96; P = 0.01; Figure 5). The I2 statistics showed that both the coronary surgery group (Q(d.f. = 7) = 0.52, P = 1.00, I2 = 0%) and the without coronary surgery group (Q(d.f. = 2) = 4.00, P = 0.14, I2 = 50%) were very homogeneous.
Figure 4.
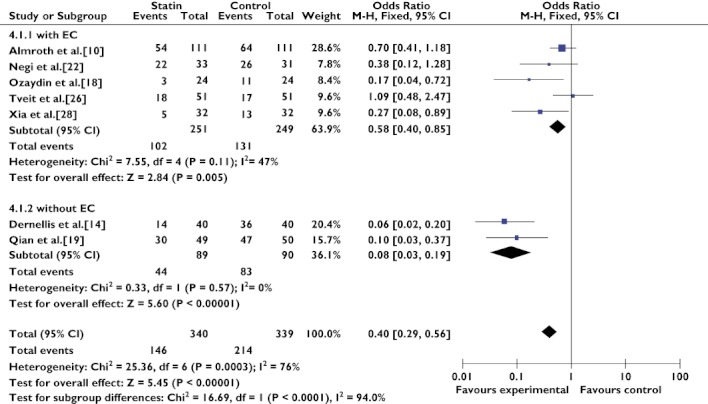
Secondary prevention of atrial fibrillation with statin therapy, either in the with EC group and in the without EC group. Abbreviations: EC, electrical cardioversion; SToP AF trial, statin therapy for the prevention of atrial fibrillation trial
Figure 5.
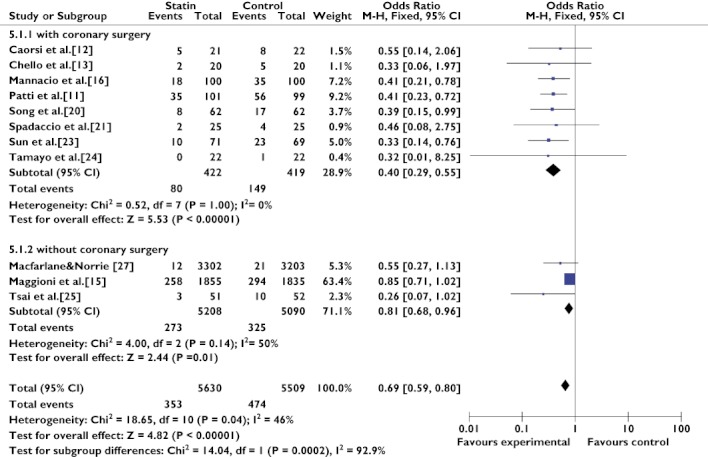
Primary prevention of atrial fibrillation with statins in patients with or without coronary surgery. Abbreviations: ARMYDA-3, atorvastatin for reduction of myocardial dysrhythmia after cardiac surgery study; WOSCOPS, west of scotland coronary prevention study
Sensitivity analysis and publication bias
Finally, results were similar when ORs were calculated after exclusion of the MIRACL study [17], which was only published in abstract form (OR 0.45, 95% CI 0.33–0.61; P < 0.00001, on the end-point of either incidence or recurrence of AF; Supporting Information Figure S1), or when studies with Jadad score <3 were removed from the analysis (OR 0.53, 95% CI 0.39–0.72; P < 0.0001; Supporting Information Figure S2).
Publication bias was evaluated using the funnel plot and Egger's regression test when the subgroup and post hoc analyses were performed. In the subgroup analysis assessing the effects in different populations (i.e. AF population, non-AF population and mixed population), publication bias was not evident in the AF population subgroup [t = −2.23, P = 0.076, 95% CI −3.00 to 0.21 (Egger's Test)] and, in post hoc analyses, publication bias was not evident in the subgroup of AF population with EC [t = −0.16, P = 0.883, 95% CI −4.16 to 3.71(Egger's Test)] and the non-AF population with coronary surgery [t = 0.12, P = 0.907, 95% CI −0.226 to 0.250 (Egger's test; Figure 6].
Figure 6.
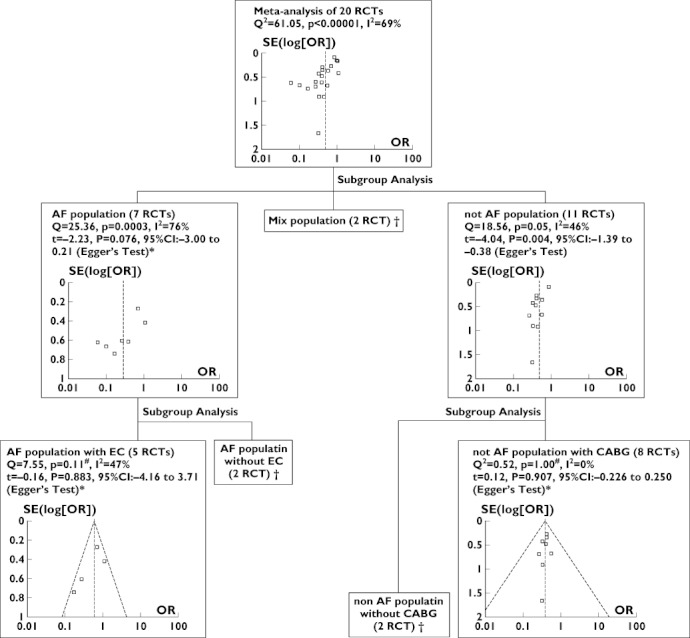
Evaluation of heterogeneity and publication bias. * Publication bias is not evident; # heterogeneity is not evident; and † the number of trials was too small to allow for evaluation of heterogeneity
Discussion
The results of our meta-analysis suggested that statin therapy reduced the occurrence or recurrence of AF. Statin therapy had significant effects on prevention of AF in the AF population or the non-AF population, but no significant effects in the mixed population. Statin therapy showed a greater preventive effect in the AF population than in the non-AF population and the mixed population.
Study design
Duration of follow-up in the 20 studies was variable because different types of AF have varying expected times for development or onset. In each study, patients were appropriately monitored on the basis of the type of AF. Recurrence of paroxysmal AF or AF after EC frequently occurred within the first month [4]. All of the patients with recurrent AF [10, 14, 18, 19, 22, 26, 28] included in our analysis had a follow-up period >1 month (44 days to 1 year). Postoperative AF patients [11–13, 16, 20, 21, 23, 24] were followed for at least 7 days and up to 37 days. As the risk of developing postoperative AF is greatest on postoperative days 2 and 3 and lower after day 10, these periods of follow-up were sufficient [35]. The MIRACL study [17] was the only one that did not show a clear reduction in AF with atorvastatin therapy, particularly in the subgroup of patients who had new-onset AF without coronary artery bypass grafting, with a relatively short follow-up of 16 weeks. This duration of follow-up (considering new-onset AF) might partly explain why a beneficial effect against AF was not observed in this study. However, the GISSI-HF study [15], with a relatively long follow-up of 3.7 years, showed a weak reduction in AF occurrence. It is very possible that the antiarrhythmic effect of statins in AF did not depend on the duration of follow-up.
Our analysis ignored varying doses of statins and varying durations of therapy, as in most meta-analyses. As populations were different, it was inappropriate to compare the OR in each trial and to draw precise conclusions on dose effect. However, the benefit against AF did not seem to be clearly related to the statin dose. For example, ORs were not lower in the study by Almroth et al. [10], the MIRACL study [17] and the SToP AF study [22], in which a high dose of atorvastatin was used (80 mg day−1). Equivalence doses of the statins were calculated by Fauchier [36] according to the meta-analysis published by Law [37]. There was no significant correlation between OR and statin dose used. The effect of statins on atrial fibrillation was possibly not dose dependent [36]. The beneficial effect was also found in the studies performed with simvastatin, but not found in the studies performed with pravastatin and rosuvastatin. The benefits obtained against AF, at least at some extent, were different for the different statins.
Secondary prevention
Seven trials [10, 14, 18, 19, 22, 26, 28] were designed to evaluate the effects of statin therapy on AF as secondary prevention (AF population subgroup). The combined results of these seven trials revealed that statins had a significant preventive effect, but there was obvious heterogeneity between the results (Figure 3). Therefore, post hoc analyses were performed in order to address the heterogeneity and to identify some factors influencing the effect. Five studies [10, 18, 22, 26, 28] investigated patients who underwent EC, and they were homogeneous. We therefore divided the seven trials that assessed the preventive effect in AF populations into two subgroups on the basis of whether patients underwent EC. Statin therapy had a benefit for both the EC group and the without EC group (Figure 4).
During recent years, statins have emerged as one of the most effective treatments to reduce the burden of cardiovascular disease worldwide. Owing to their remarkably good safety profile and declining costs, there has been some interest in the potential use of statins as direct antiarrhythmic or anti-inflammatory drugs.
Previously conducted observational studies [38–40] have shown a benefit of statin therapy on recurrence rates of AF after EC, which is consistent with the overall conclusion of our meta-analysis. However, the meta-analysis of Bhardwaj et al. [41] disagreed with the results of our meta-analysis. The SToP AF trial [22] and the study of Xia et al. [28], which showed a significant preventive effect of statin therapy, were not included in the meta-analysis of Bhardwaj et al. A letter commenting by García-Fernández A et al. [34] showed different points on statin therapy, but the results of their trial were not provided in the comments.
Primary prevention
Eleven trials [11–13, 15, 16, 20, 21, 23–25, 27] were designed to evaluate the effects of statins on AF as primary prevention (non-AF population subgroup). The combined results of these 11 trials revealed that statins had significant preventive effects, but there was obvious heterogeneity within the results (Figure 3). Statin therapy showed a greater preventive effect in the AF subgroup than in the non-AF subgroup (Figure 3).
Therefore, post hoc analyses were performed in order to address the heterogeneity and to identify some factors influencing the effect. Eight studies [11–13, 16, 20, 21, 23, 24] investigated patients who underwent cardiac surgery, and they were homogeneous. We therefore divided the 11 trials into two subgroups on the basis of whether patients underwent cardiac surgery. Statin therapy was beneficial for both the cardiac surgery group and the without cardiac surgery group (Figure 5).
Atrial fibrillation is a common complication after cardiac surgery and has important implications [42]. Postoperative AF leads to prolonged hospital stays and increases hospitalization costs and utilization [42]. The ability of statins to reduce postoperative AF in patients undergoing cardiac surgery has been evaluated in two observational studies [43, 44]. Meta-analysis by Liakopoulos et al. [45, 46] and Takagi and Umemoto [47] also provided evidence that preoperative statin therapy was associated with a reduction in the incidence of AF after cardiac surgery.
Mechanisms of preventive effects of statins on AF
The mechanisms involved in the reduction of AF associated with statin therapy are unknown. One possible pathway involves inflammation, which has been recognized as an accomplice and a potential trigger for AF [48]. The capacity of statins to reduce inflammation and C-reactive protein levels is relatively well established. Seven studies [12–14, 16, 18, 22, 23] in our meta-analysis proved that statin treatment reduced biomarkers of inflammation, which might also explain a potentially beneficial effect of statins against AF.
Although inflammation has been proposed to have a pivotal role in postoperative AF, other systems are implicated in the pathophysiology of the development of AF. More recently, antioxidative actions have been hypothesized to prevent electrical remodelling [42]. This antioxidative effect was demonstrated with the use of ascorbate as an antioxidant administered before and after cardiac surgery, which was associated with a reduced incidence of postoperative AF [49]. Administration of statins significantly decreased generation of reactive oxygen species in vitro and in vivo[50].
Atrial remodelling of the extracellular matrix has been associated with the development and maintenance of AF. In the study of Marin et al. [43], statins prevented the development of AF by modulating extracellular remodelling. Statins modified extracellular components by regulating the expression of matrix metalloproteinases or their inhibitors.
Additionally, in the postoperative setting following cardiac surgery, it has been theorized that statins may downregulate the renin–angiotensin system and modulate autonomic nervous system-induced increases in sympathetic activity, which has been shown to promote atrial remodelling. Other potential mechanisms of action include stabilization of the atherosclerotic plaque, prevention of endothelial dysfunction and neurohormonal activation, and alteration of membrane fluidity and ion channel conductance [8, 36].
Statins have been shown to reduce the AF risk in the non-AF population as well as significantly to reduce the recurrence of AF in the non-AF population. However, statin therapy showed a greater preventive effect in the AF subgroup than in the non-AF subgroup and the mixed population subgroup (Figure 3). The reasons for this difference in different populations are unknown. Population characteristics were different in the AF subgroup and the non-AF subgroup. The recurrence rate of AF was 55.36% (501 of 905) in the AF population, while the occurrence rate of AF was 11.23% (842 of 7495) in the non-AF population. Most people in the AF group underwent EC, while some people in the non-AF group underwent coronary surgery. The mechanisms of atrial fibrillation after EC and coronary surgery were different. Combined medication of different population groups were not provided in the articles, and the effect of Combined medication could not be analysed. Further studies are needed to explore the reasons why statin therapy appeared to be more effective in secondary prevention than in primary prevention of AF.
Study limitations
Jadad scores were low in some studies, but results were similar after studies with Jadad score <3 were removed. It is noteworthy that the results of the MIRACL study with AF have not been published in a full-text article to date. However, results were similar when the MIRACL study was not included in our analysis.
We were unable to assess the impact of routine use of already proven medications (i.e. class Ic and III antiarrhythmics, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers [4]) on our conclusions because drug utilization data were not reported consistently.
We were only able to identify a handful of published randomized controlled trials and might have missed relevant unpublished information. If trials where information on AF had not previously been published were included, the results of our meta-analysis would be more persuasive.
Conclusions and implications for clinicians and future researchers
Statin therapy was significantly associated with a decreased risk of incidence or recurrence of AF. There was substantial heterogeneity among the trials included in this analysis, which was explained by differences in statin types, patient populations (AF population, non-AF population and mixed population), and whether EC or coronary surgery was done. The benefit of statin therapy seemed to be more marked in secondary prevention than in primary prevention. These results provided some evidence for the benefit of statins beyond their lipid-lowering activity. However, large-scale, prospective, randomized clinical trials are still needed to establish whether statins bring a similar benefit and are an appropriate therapeutic option in all subgroups of patients for the management of AF.
Competing Interests
There are no competing interests to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1
Effect of statins on the occurrence of AF in studies published in full text form
Figure S2
Effect of statins on the occurrence of AF in studies with Jadad score ≥3
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Moe GK, Abildskov JA. Atrial fibrillation as self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen PS, Athill CA, Wu TJ, Ikeda T, Ong JJ, Karagueuzian HS. Mechanisms of atrial fibrillation and flutter and implications for management. Am J Cardiol. 1999;84:125R–30R. doi: 10.1016/s0002-9149(99)00712-2. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of Atrial Fibrillation: report from a National Heart, Lung, and Blood Institute Workshop. Circulation. 2009;119:606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Heuzey Le JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S. ACC / AHA / ESC 2006 guidelines for the management of patients with AF. J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TSM. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 6.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for AF. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 7.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of AF: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–8. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Fauchier L, Pierre B, Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic Effect of Statin Therapy and Atrial Fibrillation: a Meta-Analysis of Randomized Controlled Trials. J Am Coll Cardiol. 2008;51:828–35. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 9.Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, Williard A. Atrial Fibrillation at Baseline and During Follow-Up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) J Am Coll Cardiol. 2009;54:2023–31. doi: 10.1016/j.jacc.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Almroth H, Hoglund N, Boman K, Englund A, Jensen S, Kjellman B, Tornvall P, Rosenqvist M. Atorvastatin and persistent atrial fibrillation following cardioversion: a randomized placebo-controlled multicentre study. Eur Heart J. 2009;30:827–33. doi: 10.1093/eurheartj/ehp006. [DOI] [PubMed] [Google Scholar]
- 11.Patti G, Chello M, Candura D, Pasceri V, Ambrosio AD, Covino E, Sciascio GD. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of Myocardial Dysrhythmia after cardiac surgery) study. Circulation. 2006;114:1455–61. doi: 10.1161/CIRCULATIONAHA.106.621763. [DOI] [PubMed] [Google Scholar]
- 12.Caorsi C, Pineda F, Munoz C. Pravastatin immunomodulates IL-6 and C-reactive protein, but not IL-1 and TNF-a, in cardio-pulmonary bypass. Eur Cytokine Netw. 2008;19:99–103. doi: 10.1684/ecn.2008.0124. [DOI] [PubMed] [Google Scholar]
- 13.Chello M, Patti G, Candura D, Mastrobuoni S, Sciascio GD, Agrò F, Carassiti M, Covino E. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med. 2006;34:660–7. doi: 10.1097/01.CCM.0000201407.89977.EA. [DOI] [PubMed] [Google Scholar]
- 14.Dernellis J, Panaretou M. Effect of C-reactive protein reduction on paroxysmal atrial fibrillation. Am Heart J. 2005;150:1064e7–e12. doi: 10.1016/j.ahj.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 15.Maggioni AP, Fabbri G, Lucci D, Marchioli R, Franzosi MG, Latini R, Nicolosi GL, Porcu M, Cosmi F, Stefanelli S, Tognoni G, Tavazzi L. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur Heart J. 2009;30:2327–36. doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 16.Mannacio VA, Iorio D, De Amicis V, Di Lello F, Musumeci F. Effect of rosuvastatin pretreatment on myocardial damage after coronary surgery: a randomized trial. J Thorac Cardiovasc Surg. 2008;136:1541–8. doi: 10.1016/j.jtcvs.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GG, Olsson AG, Chaitman B, Goldberger J, Szarek M, Sasiela WJ. Effect of intensive statin treatment on the occurrence of atrial fibrillation after acute coronary syndrome: an analysis of the MIRACL trial. Circulation. 2004;110(Suppl.):S740. [Google Scholar]
- 18.Ozaydin M, Varol E, Aslan SM, Kucuktepe Z, Dogan A, Ozturk M, Altinbas A. Effect of Atorvastatin on the Recurrence Rates of Atrial Fibrillation After Electrical Cardioversion. Am J Cardiol. 2006;97:1490–3. doi: 10.1016/j.amjcard.2005.11.082. [DOI] [PubMed] [Google Scholar]
- 19.Qian YJ, Xiao XJ, Yuan HS, Tang H, Shao HZ, Wei DM. Combination pharmacological cardioversion of permanent atrial fibrillation in post-prosthetic mitral valve replacement outpatients: a novel approach for the treatment of atrial fibrillation. J Int Med Res. 2008;36:537–43. doi: 10.1177/147323000803600319. [DOI] [PubMed] [Google Scholar]
- 20.Song YB, On YK, Kim JH, Shin DH, Kim JS, Sung J, Lee SH, Kim WS, Lee YT. The effects of atorvastatin on the occurrence of postoperative atrial fibrillation after off-pump coronary artery bypass grafting surgery. Am Heart J. 2008;156:373e9–16. doi: 10.1016/j.ahj.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Spadaccio C, Pollari F, Casacalenda A, Alfano G, Genovese J, Covino E, Chello M. Atorvastatin increases the number of endothelial progenitor cells after cardiac surgery: a randomized control study. J Cardiovasc Pharmacol. 2010;55:30–8. doi: 10.1097/FJC.0b013e3181c37d4d. [DOI] [PubMed] [Google Scholar]
- 22.Negi S, Shukrullah I, Veladar E, Bloom HL, Jones DP, Dudley SC. Statin therapy for prevention of Atrial Fibrillation trial (STOP AF trial) Eur Heart J. 2010;31(Suppl. 1):885–6. doi: 10.1111/j.1540-8167.2010.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun YF, Mei YQ, Ji Q, Wang XS, Feng J, Cai JZ, Zhou YX, Xie SL. Effect of atorvastatin on postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. Chinese Med J. 2009;89:2988–91. [PubMed] [Google Scholar]
- 24.Tamayo E, Alvarez FJ, Alonso O, Bustamante R, Castrodeza J, Soria S, Lajo C. Effects of simvastatin on systemic inflammatory responses after cardiopulmonary bypass. J Cardiovasc Surg (Torino) 2009;50:687–94. [PubMed] [Google Scholar]
- 25.Tsai CT, Lai LP, Hwang JJ, Wang YC, Chiang FT, Lin JL. Atorvastatin prevents atrial fibrillation in patients with bradyarrhythmias and implantation of an atrial-based or dual-chamber pacemaker: a prospective randomized trial. Am Heart J. 2008;156:65–70. doi: 10.1016/j.ahj.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Tveit A, Grundtvig M, Gundersen T, Vanberg P, Semb AG, Holt E, Gullestad L. Analysis of pravastatin to prevent recurrence of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2004;93:780–2. doi: 10.1016/j.amjcard.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Macfarlane PW, Norrie J. The value of the electrocardiogram in risk assessment in primary prevention: experience from the West of Scotland Coronary Prevention Study. J Electrocardiol. 2007;40:101–9. doi: 10.1016/j.jelectrocard.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Xia W, Yin Z, Li J, Song Y, Qu X. Effects of rosuvastatin on asymmetric dimethylarginine levels and early atrial fibrillation recurrence after electrical cardioversion. Pacing Clin Electrophysiol. 2009;32:1562–6. doi: 10.1111/j.1540-8159.2009.02554.x. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869–97. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 31.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Fernández A, Marin F, Mainar L, Roldán V, Martínez JG. Effect of statins on preventing recurrence of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2006;98:1299–300. doi: 10.1016/j.amjcard.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–9. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 36.Fauchier L. Statins and atrial fibrillation – developments and advances. Eur Cardiol. 2008;4:93–5. [Google Scholar]
- 37.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423–7. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naji F, Suran D, Kanic V, Vokac D, Sabovic M. Statins and amiodarone improve freedom from recurrence of atrial fibrillation after successful cardioversion. Med Sci Monit. 2009;15:CR494–8. [PubMed] [Google Scholar]
- 39.Humphries KH, Lee M, Sheldon R, Ramanathan K, Dorian P, Green M, Kerr CR. Statin use and recurrence of atrial fibrillation after successful cardioversion. Am Heart J. 2007;154:908–13. doi: 10.1016/j.ahj.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Siu CW, Lau CP, Tse HF. Prevention of atrial fibrillation recurrence by statin therapy in patients with lone atrial fibrillation after successful cardioversion. Am J Cardiol. 2003;92:1343–5. doi: 10.1016/j.amjcard.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Bhardwaj A, Sood NA, Kluger J, Coleman CI. Lack of effect of statins on maintenance of normal sinus rhythm following electrical cardioversion of persistent atrial fibrillation. Int J Clin Pract. 2010;64:1116–20. doi: 10.1111/j.1742-1241.2010.02387.x. [DOI] [PubMed] [Google Scholar]
- 42.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Jr, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery.Current trends and impact on hospital resources. Circulation. 1996;94:390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 43.Marin F, Pascual DA, Roldan V, Arribas JM, Ahumada M, Tornel PL, Oliver C, Gómez-Plana J, Lip GY, Valdés M. Statins and postoperative risk of atrial fibrillation following coronary artery bypass grafting. Am J Cardiol. 2006;97:55–60. doi: 10.1016/j.amjcard.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 44.Dotani MI, Elnicki DM, Jain AC, Gibson CM. Effect of preoperative statin therapy and cardiac outcomes after coronary artery bypass grafting. Am J Cardiol. 2000;86:1128–30. doi: 10.1016/s0002-9149(00)01172-3. [DOI] [PubMed] [Google Scholar]
- 45.Liakopoulos OJ, Choi YH, Kuhn EW, Wittwer T, Borys M, Madershahian N, Wassme G, Wahlers T. Statins for prevention of atrial fibrillation after cardiac surgery: a systematic literature review. J Thorac Cardiovasc Surg. 2009;138:678–86. doi: 10.1016/j.jtcvs.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 46.Liakopoulos OJ, Choi YH, Haldenwang PL, Strauch J, Wittwer T, Dörge H, Stamm C, Wassmer G, Wahlers T. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30 000 patients. Eur Heart J. 2008;29:1548–59. doi: 10.1093/eurheartj/ehn198. [DOI] [PubMed] [Google Scholar]
- 47.Takagi H, Umemoto T. Effect of Preoperative Statin Therapy on Postoperative Atrial Fibrillation in Cardiac Surgery. Circ J. 2010;74:2788–9. doi: 10.1253/circj.cj-10-0707. [DOI] [PubMed] [Google Scholar]
- 48.Adabag AS, Nelson DB, Bloomfield HE. Effects of statin therapy on preventing atrial fibrillation in coronary disease and heart failure. Am Heart J. 2007;154:1140–5. doi: 10.1016/j.ahj.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 49.Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest. 2005;128:3421–7. doi: 10.1378/chest.128.5.3421. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe T, Yasunari K, Nakamura M. Antioxidative actions of statins: potential mechanisms for antiathersclerotic effects. Mini Rev Med Chem. 2006;6:505–8. doi: 10.2174/138955706776876249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


