Abstract
AIMS
To compare the O- (CYP2D6 mediated) and N- (CYP3A4 mediated) demethylation metabolism of tramadol between methadone and buprenorphine maintained CYP2D6 extensive metabolizer subjects.
METHODS
Nine methadone and seven buprenorphine maintained subjects received a single 100 mg dose of tramadol hydrochloride. Blood was collected at 4 h and assayed for tramadol, methadone, buprenorphine and norbuprenorphine (where appropriate) and all urine over 4 h was assayed for tramadol and its M1 and M2 metabolites.
RESULTS
The urinary metabolic ratio [median (range)] for O-demethylation (M1) was significantly lower (P= 0.0002, probability score 1.0) in the subjects taking methadone [0.071 (0.012–0.103)] compared with those taking buprenorphine [0.192 (0.108–0.392)], but there was no significant difference (P= 0.21, probability score 0.69) in N-demethylation (M2). The percentage of dose [median (range)] recovered as M1 was significantly lower in subjects taking methadone compared with buprenorphine (0.069 (0.044–0.093) and 0.126 (0.069–0.187), respectively, P= 0.04, probability score 0.19), M2 was significantly higher in subjects taking methadone compared with buprenorphine (0.048 (0.033–0.085) and 0.033 (0.014–0.049), respectively, P= 0.04, probability score 0.81). Tramadol was similar (0.901 (0.635–1.30) and 0.685 (0.347–1.04), respectively, P= 0.35, probability score 0.65).
CONCLUSIONS
Methadone inhibited the CYP2D6-mediated metabolism of tramadol to M1. Hence, as the degree of opioid analgesia is largely dependent on M1 formation, methadone maintenance patients may not receive adequate analgesia from oral tramadol.
Keywords: buprenorphine, CYP2D6 inhibition, in vivo, methadone, tramadol metabolism
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Management of pain in opioid dependent individuals is problematic due to numerous issues including cross-tolerance to opioids. Hence there is a need to find alternative analgesics to classical opioids and tramadol is potentially one such alternative.
Methadone inhibits CYP2D6 in vivo and in vitro.
We aimed to investigate the effect of methadone on the pathways of tramadol metabolism: O-demethylation (CYP2D6) to the opioid-active metabolite M1 and N-demethylation (CYP3A4) to M2 in subjects maintained on methadone or buprenorphine as a control.
WHAT THIS STUDY ADDS
Compared with subjects on buprenorphine, methadone reduced the clearance of tramadol to active O-desmethyl-tramadol (M1) but had no effect on N-desmethyltramadol (M2) formation.
Similar to other analgesics whose active metabolites are formed by CYP2D6 such as codeine, reduced formation of O-desmethyltramadol (M1) is likely to result in reduced analgesia for subjects maintained on methadone. Hence alternative analgesics whose metabolism is independent of CYP2D6 should be utilized in this patient population.
Introduction
Opioid abuse and dependence as a result of illicit or licit opioid use is of significant public health concern worldwide [1]. Maintenance therapies using substitution opioids, such as methadone and buprenorphine, are the most cost-effective treatments for opioid dependence [2] and are sanctioned by the World Health Organization to prevent opioid withdrawal. However, a high prevalence of pain has been reported among the opioid maintenance population [3, 4], and this population has been shown to be hyperalgesic to cold pressor-induced pain, most probably as a result of continued opioid exposure [4, 5]. Consequently, management of both acute and chronic pain in these patients can be problematic, with issues such as cross-tolerance to additional opioids, for example morphine, other than their maintenance opioid [6] and altered prescribing practices of opioids due to history of opioid abuse [7]. Therefore, there is a need to find suitable analgesic alternatives to the ‘typical’ opioids for the treatment of pain in these patients.
Tramadol, a synthetic mixed µ opioid receptor agonist [8], may be such an alternative. It is administered orally as the racemate and undergoes O-demethylation by cytochrome P450 2D6 (CYP2D6) to O-desmethyltramadol (M1, 5–15% oral dose), and N-demethylation by CYP3A4 to N-desmethyltramadol (M2, 4–31% oral dose) [9–12]. In comparison with tramadol, the M1 and M2 metabolites are more (444-fold) and less (0.2-fold) potent µ opioid receptor agonists, respectively [8], while tramadol itself also possesses serotonin and norepinephrine re-uptake blocking activity [13, 14]. Consequently, the opioid analgesic effect of tramadol is mediated almost exclusively by the M1 metabolite. Indeed, deficiency in CYP2D6 activity has been demonstrated to alter not only the pharmacokinetics of tramadol and M1, but also the clinical analgesic and pharmacodynamic responses [15–19].
With regard to tramadol use in opioid maintenance patients, there is potential for methadone to inhibit the formation of M1 due to its competitive inhibition of the CYP2D6-mediated O-demethylation of dextromethorphan [20, 21]. This is supported by our recent study that demonstrated that methadone significantly inhibited the CYP2D6-mediated O-demethylation of codeine to morphine and its glucuronides [22]. Clinically, this has the potential to alter tramadol analgesia in a similar manner as paroxetine (a potent CYP2D6 inhibitor) that reversed tramadol analgesia to cold pressor pain [23]. In contrast, while buprenorphine and its major metabolite norbuprenorphine have been reported to possess in vitro CYP2D6 inhibitor activity (microsomes expressing CYP2D6 c-DNA Ki= 1.8 and 42 µm, respectively) [24], this is not expected to be of clinical importance at the plasma concentrations reached following maintenance dosing (∼4 to 12 nm after 4 to 24 mg dose [25]).
Therefore, although tramadol has the potential to be used as an alternative analgesic in patients on methadone or buprenorphine maintenance therapy, there are no reports regarding the impact of either methadone or buprenorphine on tramadol metabolism, and hence safety and efficacy in this population. Consequently, the aim of the current study was to compare the impact of co-administration of methadone and buprenorphine on the metabolism of tramadol to its major metabolites, M1 and M2 in CYP2D6 genotypic extensive metabolizers.
Methods
The study was conducted as an open label, parallel group investigation. The participants were patients maintained on methadone or buprenorphine recruited from the Warinilla Clinic (Drug and Alcohol Services SA, Adelaide, Australia). The study was approved by the Royal Adelaide Hospital Research Ethics Committee (RAH Protocol no. 070525) and signed informed consent was obtained from each participant.
Both males and females, between the ages of 18–55 years, who had been on their current maintenance medication for at least 4 weeks and were on a stable dose for at least the past week, were eligible for inclusion into the study. Exclusion criteria included taking any known CYP2D6 inhibitor medication in the week prior to the study or having a positive urine drug screen for opioids (excluding their maintenance medication, see below; Microcheck Multidrug Screening Test, Thermo Fisher Scientific, Scoresby, Australia: limit of detection 300 ng ml−1). Genotypic CYP2D6 poor metabolizers and those who had liver function test results [alanine aminotransferase (ALT), alkaline phosphatase (ALP) and gamma glutamyl transferase (GGT)] greater than three times the upper normal limit were also excluded from analysis after the completion of the study.
Subjects received their prescribed daily dose of methadone or buprenorphine as per normal clinic protocol. Upon submitting a urine sample for testing and it being confirmed to be negative for opioids other than methadone or buprenorphine, they received a single, orally administered 100 mg dose of tramadol as two 50 mg tramadol hydrochloride capsules (Zydol™, Grünenthal, Germany distributed by Arrow Pharmaceuticals Limited, NSW, Australia) with 200 ml water. Blood pressure and self-reported adverse effects (including nausea, vomiting and dizziness) were monitored for the duration of the study.
Biological sample collection
A 15 ml venous blood sample was taken by venepuncture at 4 h for CYP2D6 genotype and liver function tests (LFTs), and a plasma aliquot retained for quantification of tramadol, methadone, buprenorphine and norbuprenorphine (for methadone and buprenorphine maintenance participants, respectively). All urine passed for 4 h after tramadol dosing was collected, volume and pH/[H+] recorded and an aliquot retained for analysis of tramadol and its M1 and M2 metabolites. Samples were stored at −20°C until required for analysis.
Genotyping
Extraction of genomic DNA, PCR reactions, DNA sequencing and the subsequent CYP2D6 genotype (identifiable alleles *1-*10,*16,*33,*41,*45A/*45B/*46) was determined as previously described by us [26].
Drug quantification analysis
O-desmethyltramadol (M1) and N-desmethyltramadol (M2) were a kind donation from Grünenthal GmbH (Stolberg, Germany). Acetonitrile, ethyl acetate, di-potassium hydrogen phosphate and sodium hydroxide were from BDH Chemicals (Poole, UK) and hydrochloric acid and 85% orthophosphoric acid were from Ajax Chemicals (Auburn, Australia). All chemicals and reagents were of the highest analytical grade.
Plasma tramadol concentrations and urine tramadol, M1 and M2 concentrations were determined directly by high performance liquid chromatography (HPLC) with fluorescence detection modified from Paar et al. [27]. Plasma sample preparation was as follows: plasma (500 µl) was alkalinized with 1 m (100 µl) sodium hydroxide prior to extraction in hexane : ethyl acetate (80 : 20, 3 ml). The organic layer (2.7 ml) was then back extracted into 0.05 m (150 µl) hydrochloric acid, the organic phase was aspirated and 100 µl of the remaining acidic phase was injected on to the HPLC system. Urine sample preparation was as follows: samples were centrifuged (6 min, 400 ×g), the supernatant was diluted 1 in 100 in mobile phase (details below) and injected (100 µl) on to the HPLC system.
The HPLC system comprised a LC Workstation Class LC10 (Shimadzu, Kyoto, Japan) consisting of a SIL-10ADvp autoinjector and LC-10ADvp liquid chromatograph (pump), with fluorescence detection (excitation and emission wavelengths of 210 and 305 nm, respectively; LC-240 Perkin Elmer, Buckinghamshire, UK) and a C-R6A Chromatopac integrator (Shimadzu, Kyoto, Japan). Tramadol, M1 and M2 were separated on a C18 LUNA analytical column (150 × 4.6 mm, Phenomenex, Lane Cove, Australia). The mobile phase for urine samples consisted of acetonitrile : 25 mm dipotassium hydrogen orthophosphate (39 : 61, v/v) adjusted to pH 8.9 with 85% orthophosphoric acid at a flow rate of 1.0 ml min−1 and the mobile phase for plasma samples consisted of acetonitrile : 20 mm potassium dihydrogen orthophosphate (15 : 85, v/v) adjusted to pH 3.0 with 85% orthophosphoric acid at a flow rate of 1.5 ml min−1.
Calibration curves for tramadol quantification from plasma samples were constructed with six final concentrations ranging from 25–1000 ng ml−1. Calibration samples were prepared identically in blank human plasma. Low, medium and high quality control (QC) samples of tramadol were also prepared with final concentrations of 75 200 and 350 ng ml−1, respectively. The extraction procedure was externally standardized as opposed to the use of an internal standard and calculated extraction recovery of tramadol was 60%. The inter- and intra-assay precision and inaccuracy data of the assay were as follows based on quality control sample analysis (all n= 8, for 75, 200 and 350 ng ml−1, respectively): precision, intra-assay 11.6, 5.9 and 4.9%, inter-assay 5.2, 7.2 and 3.6%; inaccuracy, intra-assay 1.6, 9.7 and 3.3%, inter-assay 7.5, 7.1 and 5.1%.
Calibration curves for tramadol, M1 and M2 quantification from urine samples were constructed with six final concentrations ranging from 100–1000, 10–100 and 10–100 ng ml−1, respectively. Calibration samples were prepared identically in blank human urine. Low, medium and high QC samples of tramadol, M1 and M2, respectively were also prepared with final concentrations of: low 150, 15 and 15 ng ml−1, respectively, medium 500, 50 and 50 ng ml−1, respectively and high 950, 95 and 95 ng ml−1, respectively. The inter- and intra-assay precision and inaccuracy of the assay were as follows based on quality control sample analysis: tramadol, 150, 500 and 950, respectively, precision, intra-assay 2.5, 1.9 and 2.8%, inter-assay 3.3, 1.3 and 1.5% and inaccuracy, intra-assay 12.6, 6.4 and 2.9%, inter-assay 12.6, 5.8 and 5.1%; M1, 15, 50 and 95, respectively, precision, intra-assay 5.6, 3.0 and 2.6%, inter-assay 7.2, 1.9 and 3.1% and inaccuracy, intra-assay 9.8, 7.0 and 4.6%, inter-assay 12.0, 9.5 and 3.2%; M2, 15, 50 and 95, respectively, precision, intra-assay 1.5, 3.1 and 1.6%, inter-assay 2.1, 3.7 and 0.1% and inaccuracy, intra-assay 8.1, 1.9 and 0.9%, inter-assay 8.9, 0.7 and 0.4%.
Plasma R-, and S-methadone concentrations in methadone maintenance therapy (MMT) participants were determined by HPLC-mass spectrometry as previously described [28].
Plasma buprenorphine and norbuprenorphine concentrations in buprenorphine maintenance therapy (BMT) participants were determined by HPLC-mass spectrometry as previously described [29].
The percentage dose excreted as tramadol and its two metabolites was calculated after taking into account molecular weight and drug base differences. A urinary metabolic ratio (MR) was calculated as the concentration of metabolites formed via each pathway divided by tramadol: O-demethylation – M1/tramadol and N-demethylation – M2/tramadol.
Data analysis
Urinary recovery, plasma concentrations and urinary metabolic ratios data were statistically compared through the use of method 5 from [30] to provide an indication of the size of the effect of methadone on the metabolism of tramadol. A probability score, U/mn was calculated, where U is the Mann Whitney U-statistic and mn is the product of the two population sample sizes with the possible score ranging between 0 and 1. A probability score of 0 or 1 indicated complete separation of the two maintenance populations' distributions and thus an effect of the methadone maintenance medication on the metabolism of tramadol when compared with buprenorphine, whilst a score of 0.5, the null hypothesis value, indicated overlapping distributions and thus no effect of methadone.
The 95% confidence intervals (CIs) of the probability scores were calculated through the use of an Excel spreadsheet as previously described [22, 31].
Spearman rank correlations were used to investigate associations between: urinary H+ concentration and the percentage recovered dose values, urinary H+ concentration and urinary MR, urinary MR and plasma methadone concentrations, urinary MR and plasma buprenorphine concentrations, urinary MR and plasma norbuprenorphine concentrations, urinary MR and plasma tramadol concentrations, urinary MR and methadone daily doses, and urinary MR and buprenorphine daily doses. These were all performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, USA). Data are reported as median and range.
Results
Participants
Sixteen opioid dependent patients receiving a stable dose of either methadone (eight males, one female; age mean [range]: 43.3 [33–52] years) or buprenorphine (three males, four females; age mean [range]: 26.6 [18–38] years) were recruited from the Warinilla Clinic of the Drug and Alcohol Services of South Australia (Adelaide, Australia). All participants had normal liver and renal function (liver function test and plasma creatinine concentrations less than three times above upper limit of normal ranges) and no history of other medical conditions other than opioid dependence. All were CYP2D6 genotypic extensive metabolizers.
Urinary metabolic ratio and dose recovery as tramadol, M1 and M2
The urinary MR to M1 was significantly lower in the MMT compared with the BMT group [median (range)] 0.071 (0.012–0.103) vs. 0.192 (0.108–0.392), P= 0.0002, probability score 1.0; 95% CI 0.75, 1.00 (Figure 1A). However, there was no difference in the urinary MR to M2 between the MMT and BMT groups [median (range)] 0.059 (0.032–0.153) vs. 0.052 (0.027–0.069), P= 0.2, probability score 0.69; 95% CI 0.41, 0.88 (Figure 1B).
Figure 1.
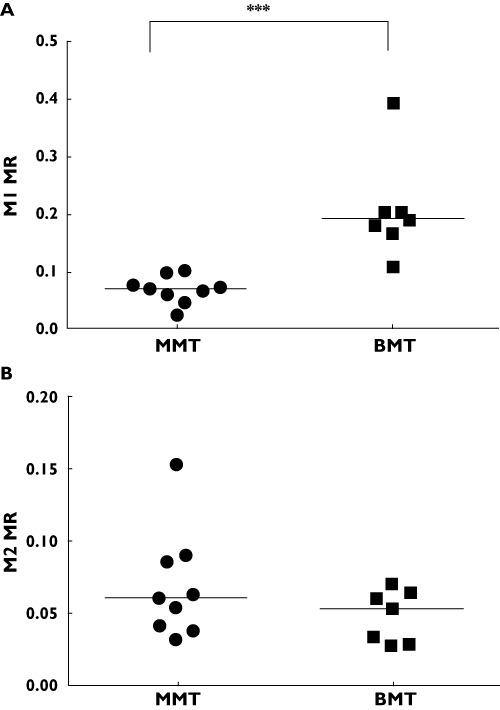
Tramadol urinary metabolic ratios (MR) of M1 (A) and M2 (B) in methadone (MMT, •) and buprenorphine (BMT,  ) maintenance populations, ***P= 0.0002. The line represents the median values
) maintenance populations, ***P= 0.0002. The line represents the median values
There was a significant difference between the percentage of the recovered dose in 4 h urine samples as M1 (1.8-fold lower) and M2 (1.5-fold higher) between the MMT and BMT groups (Table 1, both P= 0.04), but no difference in tramadol urinary recovery (P= 0.35). In addition, there was no difference in the total urinary recovery (mg, MMT 0.97 (0.45–1.46); BMT 0.74 (0.06–1.35), P= 0.47). There was no association between urinary [H+] and the percentages of the recovered dose as tramadol, M1 or M2 (rs= 0.41, 0.08 and 0.21, respectively, P≥ 0.1) or urinary M1 or M2 MR (rs=−0.35 and −0.13, respectively, P≥ 0.2).
Table 1.
Percentage (%) dose recovery as tramadol, M1 and M2 and total recovery (mg) in 4 h urine samples of methadone (MMT, n= 9) and buprenorphine (BMT, n= 7) maintenance populations. Data are median (range)
| MMT | BMT | P value* | Probability score** (95% CI) | |
|---|---|---|---|---|
| Tramadol | 0.90 (0.439–1.50) | 0.68 (0.049–1.28) | 0.35 | 0.65 (0.368, 0.850) |
| M1 | 0.069 (0.057–0.113) | 0.126 (0.021–0.225) | 0.04 | 0.19 (0.061, 0.484) |
| M2 | 0.048 (0.027–0.138) | 0.033 (0.003–0.061) | 0.04 | 0.81 (0.516, 0.939) |
| Total recovery | 0.97 (0.45–1.46) | 0.74 (0.06–1.35) | 0.47 | 0.38 (0.17, 0.66) |
Mann Whitney U-test;
Probability score = U/mn (methadone > buprenorphine).
Associations between methadone dose and urinary MR, plasma methadone, plasma buprenorphine and norbuprenorphine or tramadol
With regard to daily methadone doses, these ranged from 25 to 150 mg (median 88 mg). However, there was no significant association between the dose and the urinary M1 MR (rs=−0.38, P= 0.3) or M2 MR (rs= 0.65, P= 0.07). Similarly over a buprenorphine daily dose range of 8 to 24 mg (median 14.6 mg), there was no significant association between the dose and the urinary M1 MR (rs= 0.29, P= 0.6) or M2 MR (rs= 0.11, P= 0.8).
Plasma R, S -methadone concentrations ranged from 148 to 811 ng ml−1. There was no significant association between these concentrations and the urinary M1 MR (rs=−0.38, P= 0.31) or M2 MR (rS= 0.65, P= 0.07). Plasma buprenorphine concentrations ranged from 0.53 to 8.18 ng ml−1. There was no significant association between these concentrations and the urinary M1 MR (rs=−0.14, P= 0.78) or M2 MR (rs= 0.14, P= 0.78). Plasma norbuprenorphine concentrations ranged from 3.67 to 12.37 ng ml−1. There was no significant association between these concentrations and the urinary M1 MR (rs= 0.54, P= 0.24) or M2 MR (rs= 0.21, P= 0.66). In addition, there was no significant difference in plasma tramadol concentrations between MMT (median [range]: 509 [235−571] ng ml−1) and BMT groups (median [range]: 437 [266−516] ng ml−1): P value = 0.211, probability score (95% CI) = 0.30 (0.12, 0.59).
Although some adverse effects were reported (n= 3 moderate dizziness, nausea, tiredness or flushing, n= 1 visual disturbance for the first half hour following dosing), none was considered serious or of clinical significance and these were reported by both MMT and BMT subjects.
Discussion
This study is the first to demonstrate that co-administration of methadone with tramadol results in significant inhibition of the metabolism of tramadol to O-desmethyltramadol, or M1, the formation of which largely determines the analgesic activity of tramadol. Given this pathway is mediated by CYP2D6, this observation confirms that at clinically relevant concentrations, methadone is an inhibitor of CYP2D6. This is not surprising given that the [I]/Ki for methadone ranges between 0.39 and 0.78, where [I] is the median methadone plasma concentration in this study and Ki is methadone inhibitory constant for dextromethorphan O-demethylation [20] and hence according to FDA guidelines inhibition of CYP2D6 would be predicted [32]. Further, these data agree with previous reports of methadone inhibiting dextromethorphan and codeine O-demethylation [20–22]. In contrast, buprenorphine did not inhibit the formation of M1, confirming previous reports that at clinically relevant concentrations buprenorphine is not a CYP2D6 inhibitor [24, 25].
The almost five-fold reduction in the urinary M1 MR observed in MMT patients is in a similar range to the seven-fold reduction observed during our investigation of methadone inhibition of codeine O-demethylation [22] and the five-fold reduction in plasma 0–8 h area under the concentration–time curve M1 : tramadol ratios observed during co-administration of paroxetine, another potent CYP2D6 inhibitor [23]. It is also similar to the five-fold lower 24 h urinary M1 MR reported in CYP2D6 poor metabolizers compared with extensive metabolizers [33], while one study in CYP2D6 poor metabolizers and extensive metabolizers reported a much larger (23-fold) reduction in 8 h urinary M1 MR [34]. Nonetheless, as methadone is a competitive inhibitor of CYP2D6 it would be expected that an increase in plasma methadone concentrations in MMT patients would result in a higher degree of inhibition and lower urinary M1 MR. However, this was not observed in the current study, with the most probable explanations for the lack of association being the small number of MMT patients (n= 9) or the 4 h collection of urine as the mean elimination half-life of tramadol is 5–6 h [11]. It is not unexpected therefore, that there was also no association between urinary M1 MR and daily methadone doses.
Although this study did not assess changes in clinical response, the likely clinical consequence of a reduced formation of M1 for MMT patients is that tramadol will not provide sufficient opioid-mediated analgesia (acknowledging the role of tolerance) for the treatment of pain at normally recommended doses. However, for patients lacking CYP2D6, i.e. CYP2D6 PM, tramadol may still be an effective analgesic with elevated doses [35]. Even though the percentage of the recovered dose as tramadol increased and the amount of the M2 metabolite was increased, the relative potency of tramadol and M2 compared with M1 at the µ opioid receptor (Ki= 2400, 12 000 and 5.4 nm, respectively, [8]) indicates that this is not expected to translate into clinically relevant analgesia with a 100 mg dose. Furthermore, the suppression of M1 formation in methadone maintained patients could be one of the mechanisms by which tramadol failed to produce agonist or antagonist effects in a withdrawal precipitation study [36]. However, it should be noted that urinary recovery data were only investigated for 4 h in the current study and these data may change with a longer collection period. Given the nature of the subjects studied, it was not possible to keep them in the clinic beyond 4 h to collect multiple blood samples for more accurate pharmacokinetic analysis. Nonetheless, this study further indicates that tramadol can be used safely in BMT patients for the treatment of acute and chronic pain. Our observations in MMT patients adds to our previous report that codeine is also unlikely to be an effective analgesic in MMT due to reduced CYP2D6-mediated O-demethylation of codeine to morphine and its glucuronides [22]. Therefore, it can be postulated that any opioid requiring O-demethylation via CYP2D6 to produce an active metabolite will not provide adequate analgesia in MMT patients, so alternative analgesics that do not rely on CYP2D6 mediated metabolism need future consideration in this population. Further, given the increasing use of methadone in persistent pain, the potential for other interactions with co-analgesics (e.g. antidepressants, anti-emetics) that undergo CYP2D6 metabolism requires careful consideration.
Acknowledgments
We would like to thank Martin Hurley (Pharmacist, Warinilla Clinic) for assisting with participant recruitment. J.R. Michalakas was a recipient of a University of Adelaide, School of Medical Sciences Honours Scholarship, J.K. Coller is a FTT Fricker Research Fellow (University of Adelaide Medical Endowment Funds). Research was supported by the National Health and Medical Research Council of Australia.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.UNODC. 2008. World drug report. United nations office on drugs and crime 2008.
- 2.Doran CM, Shanahan M, Mattick RP, Ali R, White J, Bell J. Buprenorphine versus methadone maintenance: a cost-effectiveness analysis. Drug Alcohol Depend. 2003;71:292–302. doi: 10.1016/s0376-8716(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–8. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Huxtable CA, Roberts LJ, Somogyi AA, Macintyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39:804–23. doi: 10.1177/0310057X1103900505. [DOI] [PubMed] [Google Scholar]
- 5.Hay JL, White JM, Bochner F, Somogyi AA, Semple TJ, Rounsefell B. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain. 2009;10:316–22. doi: 10.1016/j.jpain.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain. 2001;93:155–63. doi: 10.1016/S0304-3959(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 7.Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144:127–34. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:116–21. doi: 10.1007/s002100000266. [DOI] [PubMed] [Google Scholar]
- 9.Paar WD, Frankus P, Dengler HJ. The metabolism of tramadol by human liver microsomes. Clin Investig. 1992;70:708–10. doi: 10.1007/BF00180294. [DOI] [PubMed] [Google Scholar]
- 10.Subrahmanyam V, Renwick AB, Walters DG, Young PJ, Price RJ, Tonelli AP, Lake BG. Identification of cytochrome P-450 isoforms responsible for cis-tramadol metabolism in human liver microsomes. Drug Metab Dispos. 2001;29:1146–55. [PubMed] [Google Scholar]
- 11.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43:879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 12.Coller JK, Christrup LL, Somogyi AA. Role of active metabolites in the use of opioids. Eur J Clin Pharmacol. 2009;65:121–39. doi: 10.1007/s00228-008-0570-y. [DOI] [PubMed] [Google Scholar]
- 13.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–85. [PubMed] [Google Scholar]
- 14.Driessen B, Reimann W, Glertz H. Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Br J Pharmacol. 1993;108:806–11. doi: 10.1111/j.1476-5381.1993.tb12882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulsen L, Arendt-Nielsen L, Brøsen K, Sindrup S. The hypoalgesic effect of tramadol in relation to CYP2D6. Clin Pharmacol Ther. 1996;60:636–44. doi: 10.1016/S0009-9236(96)90211-8. [DOI] [PubMed] [Google Scholar]
- 16.Slanar O, Nobilis M, Kvetina J, Idle J, Perlik F. CYP2D6 polymorphism, tramadol pharmacokinetics and pupillary response. Eur J Clin Pharmacol. 2006;62:75–6. doi: 10.1007/s00228-005-0039-1. [DOI] [PubMed] [Google Scholar]
- 17.Levo A, Koski A, Ojanpera I, Vuori E, Sajantila A. Post-mortem SNP analysis of CYP2D6 gene reveals correlation between genotype and opioid drug (tramadol) metabolite ratios in blood. Forensic Sci Int. 2003;135:9–15. doi: 10.1016/s0379-0738(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 18.Enggaard TP, Poulsen L, Arendt-Nielsen L, Brosen K, Ossig J, Sindrup SH. The analgesic effect of tramadol after intravenous injection in healthy volunteers in relation to CYP2D6. Anesth Analg. 2006;102:146–50. doi: 10.1213/01.ane.0000189613.61910.32. [DOI] [PubMed] [Google Scholar]
- 19.Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F. Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin Pharmacol Ther. 2007;82:41–7. doi: 10.1038/sj.clpt.6100152. [DOI] [PubMed] [Google Scholar]
- 20.Wu D, Otton SV, Sproule BA, Busto U, Inaba T, Kalow W, Sellers EM. Inhibition of human cytochrome P450 2D6 (CYP2D6) by methadone. Br J Clin Pharmacol. 1993;35:30–4. doi: 10.1111/j.1365-2125.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiran MR, Chowdry J, Rostami-Hodjegan A, Ellis SW, Lennard MS, Iqbal MZ, Lagundoye O, Seivewright N, Tucker GT. A discordance between cytochrome P450 2D6 genotype and phenotype in patients undergoing methadone maintenance treatment. Br J Clin Pharmacol. 2003;56:220–4. doi: 10.1046/j.1365-2125.2003.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelston EA, Coller JK, Lopatko OV, James HM, Schmidt H, White JM, Somogyi AA. Methadone inhibits CYP2D6 and UGT2B7/2B4 in vivo: a study using codeine in methadone and buprenorphine maintained subjects. Br J Clin Pharmacol. 2011 doi: 10.1111/j.1365-2125.2011.04145.x. DOI: 10.1111/j.1365-2125.2011.04145.x. Epub Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laugesen S, Enggaard TP, Pedersen RS, Sindrup SH, Brosen K. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther. 2005;77:312–23. doi: 10.1016/j.clpt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Ramamoorthy Y, Tyndale RF, Sellers EM. Interaction of buprenorphine and its metabolite norbuprenorphine with cytochromes P450 in vitro. Drug Metab Dispos. 2003;31:768–72. doi: 10.1124/dmd.31.6.768. [DOI] [PubMed] [Google Scholar]
- 25.Ciraulo DA, Hitzemann RJ, Somoza E, Knapp CM, Rotrosen J, Sarid-Segal O, Ciraulo AM, Greenblatt DJ, Chiang CN. Pharmacokinetics and pharmacodynamics of multiple sublingual buprenorphine tablets in dose-escalation trials. J Clin Pharmacol. 2006;46:179–92. doi: 10.1177/0091270005284192. [DOI] [PubMed] [Google Scholar]
- 26.James HM, Coller JK, Gillis D, Bahnisch J, Sallustio BC, Somogyi AA. A new simple diagnostic assay for the identification of the major CYP2D6 genotypes by DNA sequencing analysis. Int J Clin Pharmacol Ther. 2004;42:719–23. doi: 10.5414/cpp42719. [DOI] [PubMed] [Google Scholar]
- 27.Paar WD, Frankus P, Dengler HJ. High-performance liquid chromatographic assay for the simultaneous determination of tramadol and its metabolites in microsomal fractions of human liver. J Chromatogr B Biomed Sci Appl. 1996;686:221–7. doi: 10.1016/s0378-4347(96)00236-8. [DOI] [PubMed] [Google Scholar]
- 28.Foster DJ, Morton E, Murtder T, Somogyi A. Stereoselective quantification of methadone and a d(6)-labeled isotopomer using high performance liquid chromatography-atmospheric pressure chemical ionization mass-spectrometry: application to a pharmacokinetic study in a methadone maintained subject. Ther Drug Monit. 2006;28:559–67. doi: 10.1097/00007691-200608000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Jensen ML, Foster D, Upton R, Grant C, Martinez A, Somogyi A. Comparison of cerebral pharmacokinetics of buprenorphine and norbuprenorphine in an in vivo sheep model. Xenobiotica. 2007;37:441–57. doi: 10.1080/00498250701251126. [DOI] [PubMed] [Google Scholar]
- 30.Newcombe RG. Confidence intervals for an effect size measure based on the Mann-Whitney statistic. Part 1: general issues and tail-area-based methods. Stat Med. 2006;25:543–57. doi: 10.1002/sim.2323. [DOI] [PubMed] [Google Scholar]
- 31.Newcombe RG. Confidence intervals for an effect size measure based on the Mann-Whitney statistic. Part 2: asymptotic methods and evaluation. Stat Med. 2006;25:559–73. doi: 10.1002/sim.2324. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Zhang Y, Zhao P, Huang SM. Predicting drug-drug interactions: an FDA perspective. AAPS J. 2009;11:300–6. doi: 10.1208/s12248-009-9106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paar WD, Poche S, Gerloff J, Dengler HJ. Polymorphic CYP2D6 mediates O-demethylation of the opioid analgesic tramadol. Eur J Clin Pharmacol. 1997;53:235–9. doi: 10.1007/s002280050368. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen RS, Damkier P, Brøsen K. Tramadol as a new probe for cytochrome P450 2D6 phenotyping: a population study. Clin Pharmacol Ther. 2005;77:458–67. doi: 10.1016/j.clpt.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Stamer UM, Lehnen K, Hothker F, Bayerer B, Wolf S, Hoeft A, Stuber F. Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain. 2003;105:231–8. doi: 10.1016/s0304-3959(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 36.Carroll CP, Walsh SL, Bigelow GE, Strain EC, Preston KL. Assessment of agonist and antagonist effects of tramadol in opioid-dependent humans. Exp Clin Psychopharmacol. 2006;14:109–20. doi: 10.1037/1064-1297.14.2.109. [DOI] [PubMed] [Google Scholar]


