Abstract
Reported widespread declines of wild and managed insect pollinators have serious consequences for global ecosystem services and agricultural production1-3. Bees contribute around 80% of insect pollination, so it is imperative we understand and mitigate the causes of current declines4-6. Recent studies have implicated the role of pesticides as exposure to these chemicals has been associated with changes in bee behaviour7-11 and reductions in colony queen production12. However the key link between changes in individual behaviour and consequent impact at the colony level has not been shown. Social bee colonies depend on the collective performance of numerous individual workers. So whilst field-level pesticide concentrations can have a subtle/sublethal effect at the individual level8, it is not known whether bee societies can buffer such effects or if it results in a severe cumulative effect at the colony level. Furthermore, widespread agricultural intensification means bees are exposed to numerous pesticides when foraging13-15, yet the possible combinatorial effects of pesticide exposure have rarely been investigated16,17. Here we show that chronic exposure of bumblebees to two pesticides (neonicotinoid and pyrethroid) at concentrations that could approximate field-level exposure impairs natural foraging behaviour and increases worker mortality leading to significant reductions in brood development and colony success. We found worker foraging performance, particularly pollen collecting efficiency, was significantly reduced with observed knock-on effects for forager recruitment, worker losses and overall worker productivity. Moreover, we provide evidence that combinatorial exposure to pesticides increases the propensity of colonies to fail.
The majority of studies to date have focused on pesticide exposure in honeybees, but bumblebees are also crucial pollinators and have smaller colonies making them ideally suited to investigate effects at both the individual (worker) and colony level. This study mimicked a realistic scenario in which 40 early stage bumblebee, Bombus terrestris, colonies received long-term (4-week) exposure to two widely used pesticides frequently encountered when foraging on flowering crops, the neonicotinoid Imidacloprid and the pyrethroid λ-cyhalothrin. Imidacloprid is a systemic pesticide found in all plant tissues including the pollen and nectar consumed by bees (oral exposure18-20). In contrast, λ-cyhalothrin is sprayed directly on to crops, including their flowers, to which bees will be topically exposed (details in Supplementary Information). Foraging bees are thus simultaneously exposed to both chemicals in the field, making them excellent candidates to investigate the potential for combinatorial effects of pesticide exposure. Using a split block design (see Methods), we monitored colonies exposed to each pesticide independently and in combination (ten Control colonies: ten exposed to Imidacloprid (I), ten exposed to λ-cyhalothrin (LC) and ten exposed to I and LC (Mix = M)). Imidacloprid (dissolved in 40% sucrose solution) was provided at a concentration (10 ppb) within the range found in crop nectar and pollen in the field9,21. λ-cyhalothrin was administered following label guidance for field spray application (see Supplementary Information). Bees were able to forage in the field providing a realistic and demanding behavioural setting, and the foraging behaviour of individual workers was recorded using radio frequency identification (RFID) tagging technology10,11,22 (Supplementary Figs 1 and 2). Colonies were motivated to forage because we provided them with no pollen and limited amounts of sucrose solution.
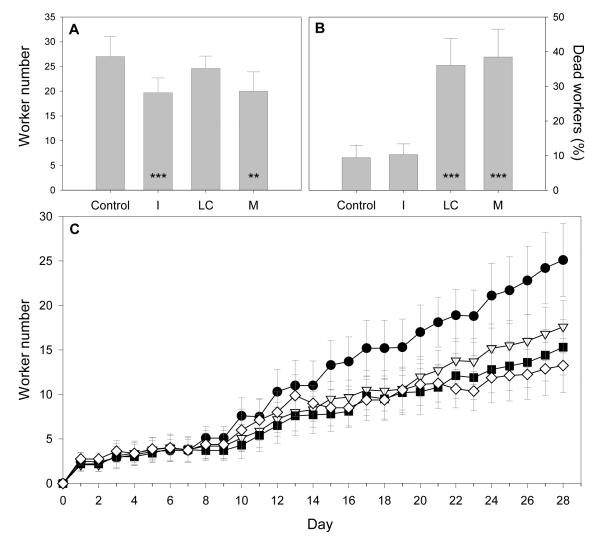
During colony development the production of workers (and their survival) is vital to colony success because workers provide the labour (e.g. brood care, foraging) for the colony. Total worker production at the end of the experiment was significantly lower in Imidacloprid treated colonies (reduced by 27% in I and 9% in M colonies) compared to Control colonies (mean (±s.e.m.) workers per colony: I=19.7±3.0, M=24.4±3.2 vs Control=27.0±4.0; Linear Mixed Effects model (LMER): I: Z=−3.71, P<0.001; M: Z=−2.62, P=0.009; Fig. 1A). Two of the 40 colonies, both M colonies, did not survive the experiment (they ‘failed’ after 3 & 8 days; see Supplementary Information), a colony failure rate significantly higher than other treatments (Fisher’s Exact test: mid-p correction=0.029). These two colonies were excluded from the analyses to provide a conservative assessment of worker production in M colonies (when included in analysis=20.0±3.9 workers). During the experiment 223 (21% of total) workers were found dead inside nest-boxes. On average, 36±7.3% and 39±7.5% of workers from LC and M colonies respectively died in the nest-box: a figure four times higher than Control (9±3.4%) colonies (LMER): LC: t=4.31, P<0.001; M: t=4.23, P<0.001; Fig. 1B). Moreover, 43% of the workers found dead in LC and M colonies lived fewer than four days after eclosion – an apparent waste of resources required for future colony growth given that such young members are unlikely to have contributed any work (e.g. foraging) to offset the resources invested to produce them. Queen loss occurred in 14 colonies, although loss rate did not differ significantly among treatments (Control=4; I=5; LC=2; M=3; Fisher’s exact test: mid-P-correction=0.40) and we accounted for queen loss in our analyses (see Supplementary Information).
Fig. 1.
Worker production and mortality. A) Mean (±s.e.m.) number of workers per colony that eclosed by the end of the experiment. B) Mean percentage of workers per colony found dead inside the next-box by the end of the experiment. C) Colony growth shown by daily counts of the cumulative number of workers eclosed minus the cumulative number of workers found dead (mean (±s.e.m.) per colony). Control=filled circle (n=10), I=open triangle (n=10), LC=filled square (n=10), M=open diamond (n=10). M treatment includes the two collapsed colonies. Asterisks indicate a significant treatment difference from Control (p-value: 0.1■, 0.05, * 0.01, ** 0.001, ***).
Daily counts of newly eclosed bees showed worker production in I colonies did not become significantly lower than Control colonies until the end of week-2, and for M colonies until the end of week-4 (Fig. 1C; see Supplementary Information and Supplementary Table 1). Daily counts of dead bees also revealed that worker mortality in LC colonies did not become significantly higher than Control colonies until the end of week-3, but for M colonies as early as the end of week-1. The delayed effect of Imidacloprid exposure on worker productivity in I and M colonies coincides with the time taken by workers to develop from egg to adult (~22 days) suggesting the observed effect is a result of Imidacloprid on brood development. Indeed, the total number of larvae and pupae combined found in colonies at the end of the experiment (‘brood number’) was significantly lower in I and M colonies compared to Control colonies (LMER: I: Z=−6.23, P<0.001; M: Z=−5.60, P<0.001). Overall, this represented a 22% reduction in brood production in I colonies and a 7% reduction in M colonies (mean (±s.e.m.) brood number: I=36±8.0, M=43±11.7 [including failed colonies: M=39±9.6] vs Control=46±9.7). Despite this, there was no significant difference in the mass of nest-structure across treatments at the end of the experiment (LMER: I: t=−1.12, P=0.27; M: t=−1.22, P=0.23; Supplementary Fig. 3) indicating that I and M colonies attempted to raise similar brood numbers but a lower proportion of larvae/pupae survived to eclosion.
Whilst Imidacloprid could be directly affecting brood (physiological) development, it could also indirectly affect the brood by causing changes to colony behaviour and/or structure: for example, changes to foraging behaviour leading to food limitation23,24. We tested the latter hypothesis by studying worker foraging performance using RFID technology to automatically record the exact time workers left or entered each colony (Supplementary Figs 1 and 2). Overall, we collected data from 259 recognised foragers from 32 colonies (n colonies: Control=7; I=10; LC=8; M=7) making 8751 foraging bouts (median (inter-quartile range) per worker=23(10-44); for criteria used to classify foragers and foraging bouts see Methods). We examined whether pesticide treatment affected foraging activity and forager recruitment. We found that foragers from M colonies performed fewer foraging bouts compared to Control colonies (LMER: t=−2.55, P=0.011; Fig. 2A), and that there were significantly more foragers in both I and M colonies compared to Control colonies over the 4-weeks (LMER: I: Z=4.20, P<0.001; M: Z=3.49, P<0.001; Fig. 2A). The higher number of foragers in I and M colonies (compared to Control) is unlikely to be due to either pesticide causing a significant repellent or anti-feedant effect (this corroborates the lack of published evidence for pyrethroid repellency in bumblebees despite reports of pyrethroids being repellent to honeybees25). Although workers did not have to visit the feeder, as they could forage for nectar outside, we found no difference among treatments in the amount of sucrose collected from feeders (LMER: t≤1.63, P≥0.11; Supplementary Fig. 6).
Fig. 2.
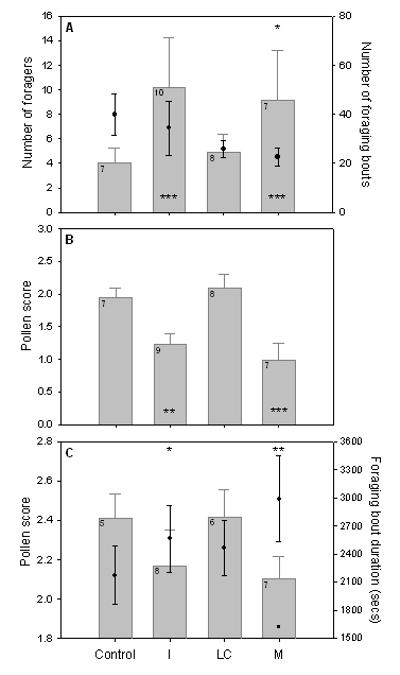
Foraging performance. A) Mean (±s.e.m.) number of foragers per colony (column), and foraging bouts per worker per colony (filled circles: n=259 foragers). B) Mean pollen score per worker per colony for all observed foraging bouts (n=228 foragers). C) Mean pollen score per successful foraging bout for each worker per colony (column), and mean duration of successful foraging bouts per worker per colony (filled circles) (n=147 foragers). Significant differences from Control treatment for column data shown at base of columns, and for filled circle data shown above columns (panels A and C). n colonies shown in top left corner of columns.
Given that I and M colonies recruited higher numbers of workers to forage we evaluated whether this was a response to reduced individual foraging efficiency by monitoring pollen foraging performance and observing the size of pollen loads (load size scored as: small=1, medium=2, large=3; see Methods) brought back by foragers (n=20 hours of observation per colony). Crucially, Imidacloprid exposed foragers returned with significantly smaller pollen loads per foraging bout compared to Control colonies (LMER: I: t=−3.31, P=0.0011; M: t=−3.38, P<0.001; Fig. 2B). Imidacloprid exposed foragers collected pollen successfully in a significantly lower percentage of their foraging bouts (mean (±s.e.m.): I=59±7.3%, M=55±8.6% vs Control=82±5.8%; LMER: I: t=−3.16, P=0.0018; M: t=−3.05, P=0.0026; Supplementary Fig. 4) and we also found that the average duration of successful foraging bouts (during which pollen was collected) was significantly longer for Imidacloprid exposed foragers than for Control foragers (LMER: I: t=2.10, P=0.037; M: t=2.87, P=0.005; Fig. 2C). Together, these data show that Imidacloprid exposed workers were significantly less efficient at collecting pollen in the field.
A consequence of recruiting a greater number of workers to forage is that it increases the proportion of colony workforce going outside to undertake a potentially hazardous task22. Indeed, our RFID data show the number of foragers per colony was significantly correlated with the number of workers leaving the colony and getting ‘lost’ outside (i.e. workers that did not return: Spearman’s Rank: ρ=0.801, P<0.001; Supplementary Fig. 5). Consequently, we found that on average the percentage of workers getting lost in I and M colonies was 50% and 55% higher than Control colonies (I=30±3.1%, M=31±5.3% vs Control=20±2.9%; LMER: I: t=2.83, P=0.008; M: t=2.26, P=0.03). Furthermore, when considering worker mortality and losses combined over the 4-weeks (mean (±s.e.m): I=41±4.2%, LC=51±6.8%, M=69±7.1% vs Control=30±5.0%, LMER: I: t=1.79, P=0.08; LC: t=3.25, P=0.0026; M: t=5.24, P<0.001; Table 1, Fig. 3), we found that colonies treated with both pesticides (M) suffered most severely. Moreover, M colonies had significantly higher overall worker losses than either I colonies (LMER: t=−3.69, P<0.001) or LC colonies (LMER: t=−2.31, P=0.027).
Table 1.
Summary of observed pesticide effects for each treatment group (I, LC, M) in comparison to the Control group.
| I | LC | M | ||
|---|---|---|---|---|
|
Effects on
individual behaviour: |
Number of foragers: | + | 0 | + |
| Foraging bout frequency: | 0 | 0 | - | |
| Amount of pollen collected: | - | 0 | - | |
| Duration of pollen foraging bouts: | + | 0 | + | |
|
Effects at
colony level: |
Worker production: | - | 0 | - |
| Brood number: | - | 0 | - | |
| Nest-structure weight: | 0 | 0 | 0 | |
| Worker mortality: | 0 | + | + | |
| Worker loss: | + | - | + | |
| Worker mortality & loss: | 0 | + | + | |
| Colony survival (failed/survived): | 0/10 | 0/10 | 2/8 | |
Significant decrease (−), significant increase (+) and no detected effect (0).
Fig. 3.
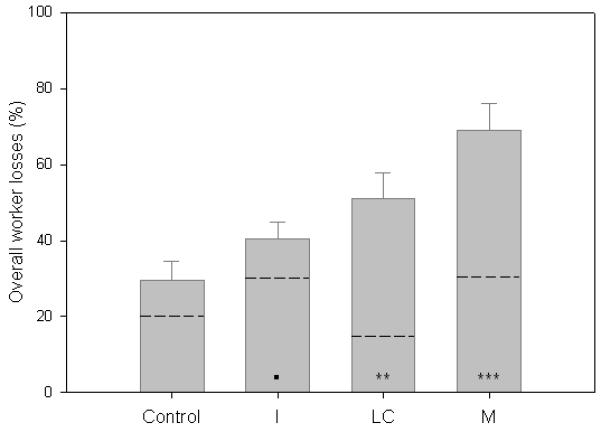
Overall worker losses (n=40 colonies). Mean (±s.e.m.) overall percentage of workers lost per colony, including workers lost outside (below dashed line) and worker mortality (dead workers found in nest-box; above dashed line), during the 4-week experiment.
We have shown that Imidacloprid exposure at concentrations that can be found in the pollen and nectar of flowering crops causes impairment to pollen foraging efficiency, leading to increased colony demand for food as shown by increased worker recruitment to forage. However, Imidacloprid treated colonies (I and M) were still unable to collect as much pollen as Control colonies. Such pollen constraints, coupled with a higher number of workers undertaking foraging rather than brood care, appeared to affect brood development resulting in reduced worker production which can only exacerbate the problem of having an impaired colony workforce. These findings show a mechanistic explanation to link recently reported effects on individual worker behaviour10,11,26-29 and colony queen production12 as a result of neonicotinoid exposure. Moreover, exposure to a second pesticide λ-cyhalothrin (pyrethroid) applied at label guideline concentration for crop use caused additional worker mortality in this study highlighting another potential risk. Bee colonies typically encounter multiple classes of pesticides when foraging in the field13-15 potentially exposing them to a range of combinatorial effects. Indeed, M colonies in our study were consistently negatively affected in all our measures of worker behaviour, suffered the highest overall worker losses (i.e. worker mortality and forager losses) which were twice as great as Control colonies, and two colonies did in fact fail (Table 1).
Pesticide label guidance concentrations and application rates are approved on the basis of ecotoxicological tests using single pesticides and set at a level for field use deemed ‘sublethal’ (i.e. below LD50). However, the risk of exposure to multiple pesticides, or from the same pesticide being applied to different (adjacent) crops, is currently not considered when evaluating the safety of pesticides for (honey) bees. Given the serious impacts on M colonies it is concerning that pesticide products containing mixtures of neonicotinoids and pyrethroids are in current use18. At present there are also no guidelines for testing chronic or sublethal effects of pesticides on bees30, and considering we did not detect significant effects until 2-4 weeks into our study, the current EPPO/OECD guideline of 96-hours (max) exposure (for testing acute effects of pesticides on honeybees) appears insufficient. Our results emphasise the importance of recent recommendations by the EFSA Panel on Plant Protection Products and their Residues (http://www.efsa.europa.eu/en/efsajournal/pub/2668.htm) proposing the need for longer term toxicity testing on both adult bees and larvae, new protocols to detect cumulative toxicity effects, and separate risk assessment schemes for different bee species. Our findings have clear implications for the conservation of insect pollinators in areas of agricultural intensification, especially social bees with their complex social organisation and dependence on a critical threshold of workers performing efficiently to ensure colony survival.
METHODS
Experimental setup
Each colony contained a queen and an average of four workers (range=0-10), at the start of the experiment, reflecting the development stage of natural colonies when crops tend to flower in Europe31,32, and when most pesticide treatments are applied (March-June)33,34. We used a split block design to account for variation in colony size, developmental stage and potential seasonal variation between replicates (20 colonies in July, and 20 colonies in September: see Supplementary Information). For each replicate, colonies were ranked according to the number of workers and pupae with the four highest ranked (largest) colonies assigned to block-1, the next four highest ranked to block-2, etc. Each replicate consisted of five blocks (n=20 colonies). Within each block the four treatments (Control, I, LC, M) were randomly assigned among the four colonies. There was no significant difference among treatments in either the number of workers or pupae present at the start of the experiment (Supplementary Information). Colonies were provided a two-chambered nest-box; the rear chamber housing the nest (‘brood chamber’) and front chamber used for pesticide exposure (‘food chamber’: Supplementary Figs. 1 & 6). Nest-boxes were kept in the laboratory but connected to the outside environment via an outlet tube leading to an exit hole in the laboratory window, allowing natural foraging (for details see Supplementary Information and Supplementary Fig. 1). Between the outlet tube and nest-box were three sections of transparent tubing allowing observation of bees as they left or entered nest-boxes (Supplementary Fig. 2). Two RFID readers (Maja IV reader modules with optimized antenna for mic3 transponders: Microsensys GmbH) at the nest entrance allowed automatic monitoring of all tagged workers as they entered and left the colony with minimal disturbance to natural foraging patterns22.
Pesticide treatment
Bees were exposed to pesticide treatments in the food chamber using a gravity feeder placed on a petri-dish (90mm diameter) lined with filter paper. The filter paper was sprayed with 0.69±0.046ml of either control solution (Control & I), or 37.5ppm λ-cyhalothrin solution (LC & M) – the maximum label guidance concentration for spray application to oilseed rape in the UK. The gravity feeder contained either a control/sucrose solution (Control & LC) or 10ppb Imidacloprid/sucrose solution (I & M). This concentration falls within the range found in the pollen and nectar of flowering crops visited by bees9,20,21,35-38 (for details on pesticide selection and application see Supplementary Information and Supplementary Box 1). During the experiment the sucrose treatment was applied every two days [three days over weekends] between 13.00-14.00h (Supplementary Table 2). Before refilling feeders we measured the volume of any remaining solution to calculate what the bees had collected (n = 12 feeder replenishments per colony during the 28-day period). We provided 10ml of sucrose treatment per application in week-1, with a 2ml increment at the start of each subsequent week (i.e. week-2 = 12ml, week-3 = 14ml, week-4 = 16ml) to reflect an increase in colony demand as they developed. The amount of sugar provided was less than each colony typically collects by nectar foraging39 ensuring that workers were motivated to forage for nectar and pollen outside.
Spray treatments were applied once at the start of each experimental week (Supplementary Table 2) using a new piece of filter paper for each application. This follows label guidance for maximum application of λ-cyhalothrin to crops recommending at least seven days between spraying events and up to four applications within the flowering season.
Observations and measurements
i) Colony condition and development
Colonies were inspected everyday to assess the number of newly eclosed (callow) workers, the number of dead workers (removed and frozen (−20°C)), and queen condition. Three days before the start of the experiment faecal samples from each queen were checked for the presence of three parasites: the trypanosome Crithidia bombi, the microsporidian Nosema bombi, and the neogregarine Apicystis bombi. This parasite assessment was repeated on the 28th experimental day using faecal samples from the queen (if present) and a subset of workers from each nest-box (for details of parasite assessment see Supplementary Information).
ii) Monitoring foraging performance
All workers present at the start of the experiment (precise age unknown) were individually RFID tagged (for details see Supplementary Information), and during the experiment all newly produced workers were tagged within three days of eclosion (age known). Tagging stopped on the 24th day of the experiment because any workers emerging after this point were unlikely to become foragers40. In total 854 workers were tagged, with each tag providing a unique (16 digit) code for unambiguous identification. We classified a foraging bout as a period of at least five minutes elapsing between a worker leaving and entering a colony. We also specified that workers must perform at least four foraging bouts to be considered a forager (for rationale behind foraging rules see Supplementary Information).
Pollen foraging was observed in each colony for one hour per day (five days a week) to record pollen foraging activity. Observation periods were always two (approx. 16.00) and 21 hours (approx. 10.00 the following day) after treatment application/renewal (Supplementary Table 2). We recorded the time that each tagged worker entered a colony (observing when it passed through the transparent tubes and under the RFID readers) using a stopwatch synchronised with the RFID (host) data-logger. We scored the amount of pollen in the forager’s corbiculae (pollen baskets) as small (score of 1), medium (score of 2) or large (score of 3) relative to the size of the worker.
iii) End of experiment
Nest-box entrances were closed after dark on the evening of the 28th experimental day. Each nest-box, containing bees and brood, was placed in a freezer (−20°C). Window exits remained open for 18 hours with each outlet tube connected to an individual bottle trap to catch any returning foragers. All tagged workers were identified and recently eclosed (untagged) workers assumed to have developed in the colony they were found in. Worker thorax width was measured using digital callipers. All pupae and larvae were dissected from each nest, counted and weighed to provide final measures of brood development, and the nest-structure was also weighed.
Supplementary Material
Acknowledgements
We thank Mark Brown, Mark Clook, Jim Culverhouse, Adrian Dixon, Matthias Fürst, Dave Garthwaite, Alice Horsell, Vincent Jansen and Inti Pedroso-Rovira for comments and technical assistance, and Syngenta Bioline Bees for supplying colonies. The study was supported by the Insect Pollinator Initiative (funded under auspices of the Living with Environmental Change programme, BBSRC, Wellcome Trust, Scottish Government, DEFRA and NERC: BB/I000178/1).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Information The authors declare no competing financial interests.
References
- 1.Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 2.Klein AM, et al. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. B. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kremen C, et al. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol. Lett. 2007;10:299–314. doi: 10.1111/j.1461-0248.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 4.Potts SG, et al. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Oldroyd BP. What’s killing American honey bees? PLoS Biol. 2007;5:e168. doi: 10.1371/journal.pbio.0050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MJF, Paxton RJ. The conservation of bees: a global perspective. Apidologie. 2009;40:410–416. [Google Scholar]
- 7.Thompson HM. Behavioural effects of pesticides in bees - their potential for use in risk assessment. Ecotoxicology. 2003;12:317–330. doi: 10.1023/a:1022575315413. [DOI] [PubMed] [Google Scholar]
- 8.Desneux N, Decourtye A, Delpuech JM. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007;52:81–106. doi: 10.1146/annurev.ento.52.110405.091440. [DOI] [PubMed] [Google Scholar]
- 9.Cresswell JE. A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (Imidacloprid) on honey bees. Ecotoxicology. 2011;20:149–157. doi: 10.1007/s10646-010-0566-0. [DOI] [PubMed] [Google Scholar]
- 10.Schneider CW, Tautz J, Grünewald B, Fuchs S. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS One. 2012;7:e30023. doi: 10.1371/journal.pone.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry M, et al. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- 12.Whitehorn PR, O’Connor S, Wackers FL, Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science. 2012;336:351–352. doi: 10.1126/science.1215025. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RM, Ellis MD, Mullin CA, Frazier M. Pesticides and honey bee toxicity - USA. Apidologie. 2010;41:312–331. [Google Scholar]
- 14.Mullin CA, et al. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One. 2010;5:e9754. doi: 10.1371/journal.pone.0009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS One. 2012;7:e29268. doi: 10.1371/journal.pone.0029268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RM, Pollock HS, Berenbaum MR. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 2009;102:474–479. doi: 10.1603/029.102.0202. [DOI] [PubMed] [Google Scholar]
- 17.Pilling ED, Jepson PC. Synergism between EBI fungicides and a pyrethroid insecticide in the honeybee (Apis mellifera) Pestic. Sci. 1993;39:293–297. [Google Scholar]
- 18.Elbert A, Haas M, Springer B, Thielert W, Nauen R. Applied aspects of neonicotinoid uses in crop protection. Pest Manag. Sci. 2008;64:1099–1105. doi: 10.1002/ps.1616. [DOI] [PubMed] [Google Scholar]
- 19.Rortais A, Arnold G, Halm MP, Touffet-Briens F. Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie. 2005;36:71–83. [Google Scholar]
- 20.Chauzat MP, et al. A survey of pesticide residues in pollen loads collected by honey bees in France. J. Econ. Entomol. 2006;99:253–262. doi: 10.1603/0022-0493-99.2.253. [DOI] [PubMed] [Google Scholar]
- 21.Blacquière T, Smagghe G, van Gestel CAM, Mommaerts V. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology. 2012;21:973–992. doi: 10.1007/s10646-012-0863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molet M, Chittka L, Stelzer RJ, Streit S, Raine NE. Colony nutritional status modulates worker responses to foraging recruitment pheromone in the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 2008;62:1919–1926. [Google Scholar]
- 23.Schmid-Hempel R, Schmid-Hempel P. Colony performance and immunocompetence of a social insect, Bombus terrestris, in poor and variable environments. Funct. Ecol. 1998;12:22–30. [Google Scholar]
- 24.Pelletier L, McNeil JN. The effect of food supplementation on reproductive success in bumblebee field colonies. Oikos. 2003;103:688–694. [Google Scholar]
- 25.Thompson H, Wilkins S. Assessment of the synergy and repellency of pyrethroid/fungicide mixtures. B. Insectol. 2003;56:131–134. [Google Scholar]
- 26.Bortolotti L, et al. Effects of sub-lethal imidacloprid doses on the homing rate and foraging activity of honey bees. B. Insectol. 2003;56:63–67. [Google Scholar]
- 27.Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delègue MH. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotox. Environ. Safe. 2004;57:410–419. doi: 10.1016/j.ecoenv.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Mommaerts V, et al. Risk assessment for side-effects of neonicotinoids against bumblebees with and without impairing foraging behavior. Ecotoxicology. 2010;19:207–215. doi: 10.1007/s10646-009-0406-2. [DOI] [PubMed] [Google Scholar]
- 29.Yang EC, Chuang YC, Chen YL, Chang LH. Abnormal foraging behavior induced by sublethal dosage of Imidacloprid in the Honey bee (Hymenoptera: Apidae) J. Econ. Entomol. 2008;101:1743–1748. doi: 10.1603/0022-0493-101.6.1743. [DOI] [PubMed] [Google Scholar]
- 30.Halm MP, Rortais A, Arnold G, Taséi JN, Rault S. New risk assessment approach for systemic insecticides: the case of honey bees and imidacloprid (Gaucho) Environ. Sci. Technol. 2006;40:2448–2454. doi: 10.1021/es051392i. [DOI] [PubMed] [Google Scholar]
References for Methods section
- 31.Thompson HM. Assessing the exposure and toxicity of pesticides to bumblebees (Bombus sp.) Apidologie. 2001;32:305–321. [Google Scholar]
- 32.Brittain C, Potts SG. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 2011;12:321–331. [Google Scholar]
- 33.Garthwaite D, Thomas MR, Parrish G, Smith L, Barker I. Pesticide Usage Survey Report 224. Arable crops in Great Britain 2008 (including aerial applications 07-08) Food and Environmental Research Agency; York, UK: 2008. [Google Scholar]
- 34.Garthwaite D, et al. Pesticide Usage Survey Report 235. Arable crops in UK 2010 (including aerial applications 2010) Food and Environmental Research Agency; York, UK: 2010. [Google Scholar]
- 35.Bonmatin JM, et al. A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants, and in pollens. Anal. Chem. 2003;75:2027–2033. doi: 10.1021/ac020600b. [DOI] [PubMed] [Google Scholar]
- 36.Bonmatin JM, et al. Quantification of imidacloprid uptake in maize crops. J. Agric. Food Chem. 2005;53:5336–5341. doi: 10.1021/jf0479362. [DOI] [PubMed] [Google Scholar]
- 37.Chauzat MP, et al. Influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environ. Entomol. 2009;38:514–523. doi: 10.1603/022.038.0302. [DOI] [PubMed] [Google Scholar]
- 38.Krischik VA, Landmark AL, Heimpel GE. Soil-applied imidacloprid is translocated to nectar and kills nectar-feeding Anagyrus pseudococci (Girault) (Hymenoptera: Encyrtidae) Environ. Entomol. 2007;36:1238–1245. doi: 10.1603/0046-225x(2007)36[1238:siittn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Raine NE, Chittka L. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. Lond. B. 2008;275:803–808. doi: 10.1098/rspb.2007.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goulson D. Bumblebees: Behaviour, Ecology and Conservation. Oxford Univ. Press; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.