Abstract
The mammalian intestine harbors trillions of beneficial commensal bacteria that are essential for the development of the immune system and for maintenance of physiologic processes in multiple organs. However, numerous chronic infectious, inflammatory and metabolic diseases in humans have been associated with alterations in the composition or localization of commensal bacteria that results in dysregulated host-commensal bacteria relationships. The mammalian immune system plays an essential role in regulating the acquisition, composition and localization of commensal bacterial communities in the intestine. Emerging research has implicated innate lymphoid cells (ILCs) as a critical immune cell population that orchestrates some of these host-commensal relationships that can impact immunity, inflammation and tissue homeostasis in the intestine. This review will discuss reciprocal interactions between intestinal commensal bacteria and ILCs in the context of health and disease.
Introduction
The mammalian gastrointestinal tract is colonized by an estimated 100 trillion bacteria composed of thousands of different species (Clemente et al., 2012; Dethlefsen et al., 2007; Ley et al., 2008). In the healthy intestine, these bacterial communities reside in defined anatomical locations and exist in a symbiotic relationship with their hosts, promoting normal physiologic processes and limiting colonization with potentially pathogenic microbes (Hill and Artis, 2010; Honda and Littman, 2012; Hooper et al., 2012; Ley et al., 2006a; Littman and Pamer, 2011). In contrast, the pathogenesis and progression of numerous chronic infectious, inflammatory or metabolic diseases in humans, including viral hepatitis, HIV-AIDS, inflammatory bowel disease (IBD), cancer, diabetes, obesity and cardiovascular disease, have been associated with alterations in either the composition and/or anatomical location of intestinal commensal bacteria (Brenchley and Douek, 2012; Chin et al., 2007; Hill and Artis, 2010; Ley et al., 2006b; McGuckin et al., 2009; Ott et al., 2006; Sandler et al., 2011). Therefore, understanding the mechanisms that regulate healthy host-commensal bacteria relationships could aid in the development of novel therapeutics to prevent or limit chronic human diseases.
Commensal bacteria that reside in the intestinal lumen are separated from the underlying connective tissues of the body by a single layer of intestinal epithelial cells. The immune system is a critical regulator of this epithelial barrier and associated commensal bacteria (Hill and Artis, 2010; Honda and Littman, 2012; Hooper et al., 2012; Littman and Pamer, 2011). For example, cytokines derived from CD4+ T helper cells can profoundly influence the biology of intestinal epithelial cells through regulating epithelial permeability, proliferation, repair and expression of critical factors including tight junction and anti-microbial proteins that control host interactions with intestinal commensal bacteria (Hill and Artis, 2010; Hooper et al., 2012; Littman and Pamer, 2011; Maloy and Powrie, 2011). Conversely, commensal bacteria also have a profound influence on the development and homeostasis of the mammalian immune system. In the absence of commensal bacteria, development of the innate and adaptive immune system is impaired (Hill and Artis, 2010; Honda and Littman, 2012; Hooper et al., 2012; Littman and Pamer, 2011), and more recent studies reported selective regulation of CD4+ T cell subsets by specific species of commensal bacteria (Atarashi et al., 2011; Ivanov et al., 2009; Littman and Pamer, 2011; Round and Mazmanian, 2010). These findings indicate that regulatory pathways through which commensal bacteria influence the mammalian immune system are sophisticated and perhaps highly selective.
Innate lymphoid cells (ILCs) are an emerging population of innate immune cells that share numerous developmental and functional characteristics with CD4+ T cell populations, and recent reports suggest ILCs also play a critical role in regulating epithelial cell responses and maintaining intestinal homeostasis (Colonna, 2009; Sonnenberg et al., 2011a; Spits and Cupedo, 2012; Spits and Di Santo, 2010; Veldhoen and Withers, 2010). While recent comprehensive reviews have focused on the development, heterogeneity and functions of ILCs in the context of inflammation and infection (Colonna, 2009; Cua and Tato, 2010; Monticelli et al., 2012; Sonnenberg et al., 2011a; Spits and Cupedo, 2012; Spits and Di Santo, 2010; Veldhoen and Withers, 2010), this review will provide a broad overview of ILC populations and focus on mechanisms by which commensal bacteria may directly and indirectly influence ILC development and function. Next, the review will explore the reciprocal impact of ILCs on the diversity and anatomical containment of intestinal commensal bacteria. Lastly, ILC-commensal bacteria interactions will be discussed in the context of human health and disease.
The innate lymphoid cell family
The term `innate lymphoid cell' refers to both well-established and recently identified populations of innate immune cells that appear to share a common origin, being derived from an Id2-dependent lymphoid progenitor cell population (Cherrier et al., 2012; Hoyler et al., 2012; Spits and Cupedo, 2012; Spits and Di Santo, 2010; Veldhoen and Withers, 2010; Wong et al., 2012; Yang et al., 2011). ILCs share numerous similarities with CD4+ and CD8+ T cells; however, ILC differentiation occurs independently of somatic recombination, indicating that these cells represent an innate immune cell population that can respond to various stimuli independent of major histocompatibility-dependent interactions (Maloy and Powrie, 2011; Spits and Cupedo, 2012; Spits and Di Santo, 2010; Veldhoen and Withers, 2010). ILCs can be grouped based on their selective dependence on specific transcription factors for their development and function, and currently there are three major groups including T-bet+ ILCs (termed group 1 ILCs), GATA3+ ILCs (group 2 ILCs) and RORγt+ ILCs (group 3 ILCs) (Figure 1).
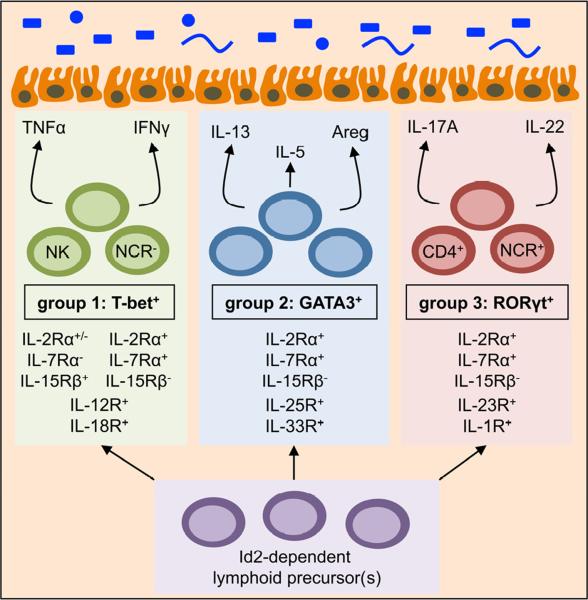
Figure 1. Emerging subsets of innate lymphoid cells.
Innate lymphoid cells can be broadly placed into three groups consisting of T-bet+ ILCs (group 1), GATA3+ ILCs (group 2) and RORγt+ ILCs (group 3). These subsets develop from lymphoid progenitor(s), require the transcription factor Id2 and are independent of somatic recombination. Group 1 T-bet+ ILCs include Natural Killer (NK) cells and natural cytotoxicity receptor (NCR)− ILCs that are differentially regulated by IL-15 and IL-7 respectively, but respond to IL-12 and IL-18, and produce TNFα and IFNγ. Group 2 GATA3+ ILCs are responsive to IL-2, IL-7, IL-25 and IL-33, and produce IL-5, IL-13 and Amphiregulin (Areg). Group 3 RORγt+ ILCs are heterogeneous in expression of CD4 and NCRs, responsive to IL-2, IL-7, IL-23 and IL-1β, and produce IL-17A and IL-22. With the exception of IL-5, these ILC-derived effector cytokines can directly influence epithelial cell responses in the intestine.
Group 1 T-bet+ ILCs include classical NK cells which have been well characterized since their discovery over four decades ago (Biron et al., 1999; Di Santo, 2008; Kiessling et al., 1975; Orange and Ballas, 2006; Yokoyama et al., 2004). NK cells critically depend on T-bet expression and the cytokine interleukin (IL)-15 for optimal differentiation, homeostasis and function (Gordon et al., 2012; Lodolce et al., 1998; Townsend et al., 2004). NK cells are also stimulated through IL-12, IL-18 and a number of activating receptors, such as natural cytotoxicity receptors (NCRs). Stimulation through these pathways can result in the production of pro-inflammatory cytokines including IFNγ or TNFα or degranulation and release of perforin and granzymes to induce lysis of target cells, both of which are critical for tumor suppression and immunity to some intracellular pathogens (Biron et al., 1999; Di Santo, 2008; Ganal et al., 2012; Orange and Ballas, 2006; Schulthess et al., 2012; Yokoyama et al., 2004). Therefore, NK cells share homeostatic and functional similarities with CD8+ T cells. In addition, there may be heterogeneity within T-bet+ ILCs to include cell populations more similar to CD4+ T helper (Th)1 cells that have yet to be well characterized (Figure 1). In support of this, two reports have identified non-NK cell, T-bet+ ILCs that express IFNγ (Buonocore et al., 2010; Powell et al., 2012).
Group 2 GATA3+ ILCs and Group 3 RORγt+ ILCs express CD25 and CD127 in the steady state and IL-2 and IL-7, but not IL-15, are important for their development, homeostasis and function (Moro et al., 2010; Saenz et al., 2010; Satoh-Takayama et al., 2008; Spits and Cupedo, 2012; Spits and Di Santo, 2010). Group 2 GATA3+ ILCs critically depend on GATA3 and RORα for development, respond to IL-25 and IL-33, are potent sources of IL-5, IL-9, IL-13 and amphiregulin, and have recently been associated with immunity to helminth parasites, airway hyper-responsiveness and tissue repair (Hoyler et al., 2012; Mjosberg et al., 2012; Monticelli et al., 2012; Moro et al., 2010; Neill et al., 2010; Saenz et al., 2010; Sandler et al., 2011; Spits and Cupedo, 2012; Wong et al., 2012) (Figure 1). Importantly, two recent reports demonstrated an essential role for GATA3 in the development and maintenance of both human and mouse GATA3+ ILCs (Hoyler et al., 2012; Mjosberg et al., 2012). Group 3 RORγt+ ILCs develop independent of GATA3 but require the orphan nuclear receptor RORγt, respond to IL-23 and IL-1β stimulation and produce IL-17A and IL-22 (Buonocore et al., 2010; Colonna, 2009; Hoyler et al., 2012; Sonnenberg et al., 2011a; Spits and Cupedo, 2012) (Figure 1). Through production of these cytokines, RORγt+ ILCs have been implicated in immunity to extracellular bacteria and promotion of inflammation or tissue repair in the intestine (Buonocore et al., 2010; Cella et al., 2009; Sawa et al., 2011; Sonnenberg et al., 2011a; Sonnenberg et al., 2011b; Spits and Cupedo, 2012). The most well characterized RORγt+ ILC population includes lymphoid tissue inducer (LTi) cells, which initiate lymphoid organogenesis at pre- and post-natal periods (Mebius et al., 1997; van de Pavert and Mebius, 2010). RORγt+ ILCs can be heterogeneous in expression of a number of surface markers including CD4 and NCRs such as NKp46 and NKp44 (Sonnenberg et al., 2011a; Spits and Cupedo, 2012), however the potential lineage relationships and functional significance of these subpopulations requires further study. Collectively, the functional heterogeneity observed in the ILC family shares striking similarities to that observed in T cells with parallels drawn between T-bet+ ILCs with CD8+ T cells and CD4+ T helper (Th)1 cells, GATA3+ ILCs with Th2 cells, and RORγt+ ILCs with Th17 cells.
Regulation of ILC development, maintenance and function by commensal bacteria
ILCs have been found to be resident immune cell populations at barrier surfaces of the mammalian body including the skin, airway and intestinal tract (Spits and Cupedo, 2012; Spits and Di Santo, 2010; Veldhoen and Withers, 2010). Through production of soluble effector molecules including IFNγ, TNFα, IL-13, IL-17A, IL-22 and amphiregulin, ILCs can have a profound impact on epithelial cells that are in direct contact with commensal bacteria (Maloy and Powrie, 2011; Sonnenberg et al., 2011a; Spits and Cupedo, 2012; Spits and Di Santo, 2010; Veldhoen and Withers, 2010) (Figure 1). Given the spatial proximity of commensal bacteria, epithelial cells and ILCs, numerous groups have investigated the impact of commensal bacteria on the development of ILCs. Studies employing germ-free mice that lack live commensal bacteria identified that NK cells and GATA3+ ILCs can develop in the absence of commensal colonization (Ganal et al., 2012; Monticelli et al., 2011). However, there have been conflicting reports on the requirement of commensal bacteria on the development of RORγt+ ILCs. Subsets of RORγt+ ILCs can develop independently of commensal bacteria, as evident by the presence of LTi cells and the generation of secondary lymphoid structures in the sterile environment of the fetus prior to birth (Mebius et al., 1997; van de Pavert and Mebius, 2010). However, following birth, the maturation of intestinal cyptopatches into isolated lymphoid follicles is compromised in germ-free mice, suggesting impairment in the function of some populations of LTi-like RORγt+ ILCs (Bouskra et al., 2008; Tsuji et al., 2008). Direct examination of RORγt+ ILCs by several groups identified normal development of all RORγt+ ILC subsets in the absence of live commensal bacteria in both germ-free and antibiotic-treated mice (Reynders et al., 2011; Sawa et al., 2010; Sawa et al., 2011; Sonnenberg et al., 2012). In contrast, three reports identified a substantial reduction in NCR+ RORγt+ ILCs and a lack of expression of Rorc or Il22 in the small intestine lamina propria of germ-free or antibiotic-treated mice (Sanos et al., 2009; Satoh-Takayama et al., 2008; Vonarbourg et al., 2010). Whereas some of these differences may relate to host genetics or differential exposure to non-live bacterial- or diet-derived signals, it is clear that further investigation will be necessary to clarify the influence of commensal bacteria on the development of NCR+ RORγt+ ILCs.
Although commensal bacteria do not appear to be essential for the development of most groups of ILCs, signals derived from commensal bacteria may substantially impact the function of ILCs. For example, mice devoid of live commensal bacteria exhibit impaired NK cell cytolytic and IFNγ responses to poly-IC stimulation or following infection with mouse cytomegalovirus (Ganal et al., 2012), which is consistent with additional reports demonstrating impaired antiviral immune responses in the absence of commensal bacteria (Abt et al., 2012; Ganal et al., 2012; Ichinohe et al., 2011). These data indicate that commensal bacteria may be essential to promote optimal NK cell responses. In contrast, Eberl and colleagues reported that commensal bacteria can suppress intestinal RORγt+ ILC production of IL-22 in healthy mice, a process that could be reversed following experimental damage to the intestinal epithelium (Sawa et al., 2011). Further, although it is clear that the absence of commensal bacteria or dysbiosis is associated with altered NK T cell or basophil function and exaggerated allergic inflammation (Hill and Artis, 2010; Hill et al., 2012; Olszak et al., 2012), whether commensal bacteria influence the function of GATA3+ ILCs that express Th2 cell-associated cytokines remains to be tested. It is clear that additional studies will be required to comprehensively define the impact of commensal bacteria on the development, homeostasis and function of ILCs. The following section will discuss how commensal bacteria may influence the homeostasis and function of ILC populations through direct and indirect mechanisms.
Commensal bacteria directly regulate ILCs
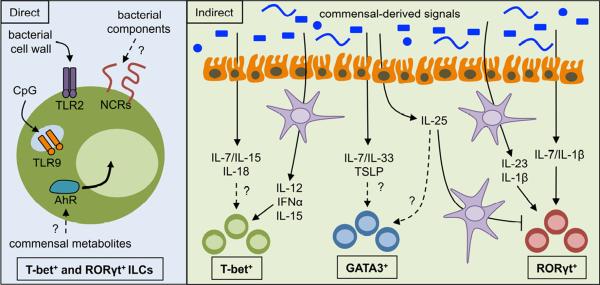
Signals derived from commensal bacteria can be directly recognized by a number of immune cell receptors, including the toll-like receptor (TLR) family that can be activated by components of both pathogenic and commensal bacteria (Palm and Medzhitov, 2009; Philpott and Girardin, 2004). Although few studies have demonstrated the presence of TLR expression in murine ILC populations, the presence of functional TLR2 and TLR9 has been reported on human peripheral blood NK cells (Martinez et al., 2010; Sivori et al., 2004). Further, Spits and colleagues reported that human RORγt+ ILCs express functional TLR2 (Crellin et al., 2010), and stimulation with TLR2 agonists induced IL-2 that acted in an autocrine manner to enhance IL-22 expression (Crellin et al., 2010) (Figure 2, left). In addition to TLRs, NK cells and NCR+ RORγt+ ILCs might directly sense commensal bacteria through NCRs such as NKp44 and NKp46, which have recently been found to be activated by a number of components derived from commensal bacteria (Chaushu et al., 2012; Esin et al., 2008; Vankayalapati et al., 2002) (Figure 2, left). RORγt+ ILCs also express the aryl hydrocarbon receptor (AhR), which is critical for ILC development, IL-22 production, maturation of intestinal lymphoid follicles and immunity to the murine enteric pathogen Citrobacter rodentium (Kiss et al., 2011; Lee et al., 2012; Qiu et al., 2012). In addition to environmental toxins and endogenous factors, the AhR can be activated by ligands generated from tryptophan metabolism by intestinal commensal bacteria (Perdew and Babbs, 1991; Stockinger et al., 2011), suggesting that commensal bacteria and their metabolic products may directly regulate RORγt+ ILCs (Figure 2, left). The presence of TLRs or other receptors that can directly sense commensal bacteria on GATA3+ ILCs has yet to be identified. One study found that direct stimulation of purified GATA3+ ILCs with TLR ligands did not induce IL-9 expression (Wilhelm et al., 2011), suggesting that GATA3+ ILCs are not directly activated by TLR ligands. Additional studies are required to clarify the ability of commensal bacteria or products derived from commensal bacteria to directly influence ILC responses.
Figure 2. Direct and indirect regulation of ILC responses by commensal bacteria.
Commensal bacteria can influence ILC populations through direct recognition (left) of commensal bacteria or commensal bacteria-derived products by toll-like receptors (TLRs), natural cytotoxicity receptors (NCRs) or the aryl hydrocarbon receptor (AhR). Commensal bacteria can also promote or inhibit ILC populations though indirect recognition (right) of commensal bacteria or commensal bacteria-derived products by resident myeloid or epithelial cells and subsequent cytokine production.
Commensal bacteria indirectly regulate ILCs through myeloid cells and epithelial cells
In addition to direct regulation, commensal bacteria can indirectly regulate ILCs through modulation of myeloid cell or epithelial cell responses. Recent evidence has demonstrated that commensal bacteria are essential for optimal antiviral immunity, in part through promoting optimal pro-inflammatory cytokine responses in mononuclear phagocytes (Abt et al., 2012; Ganal et al., 2012; Ichinohe et al., 2011). In one report, commensal bacteria modulated mononuclear phagocytes through Myd88, Trif and epigenetic pathways to promote IL-6, IL-12, IL-15, TNFα and type 1 interferon production which was essential to promote optimal NK cell responses (Ganal et al., 2012). Additional reports have implicated that commensal bacteria within the Lactobacillus genus can induce IFNγ and cytolytic responses in intestinal NK cells through engagement of TLR2 and TLR4 on dendritic cells (DCs) and subsequent induction of IL-12 (Fink et al., 2007; Koizumi et al., 2008) (Figure 2B, right).
Commensal bacteria can also influence RORγt+ ILC responses through regulation of IL-1β and IL-23 production by myeloid cells. Commensal bacteria were recently found to promote steady state expression of IL-1β in intestinal macrophages (Shaw et al., 2012), a cytokine that can induce IL-22 production from RORγt+ ILCs (Hughes et al., 2010). CX3CR1+ phagocytes are also elicited in the intestine following colonization with commensal bacteria and are important for promoting IL-22 production from RORγt+ ILCs (Manta et al., 2012; Niess and Adler, 2010). Further, systemic administration of flagellin was found to stimulate CD103+ CD11b+ intestinal dendritic cells via TLR5 to promote expression of IL-23 and subsequent IL-22 responses from RORγt+ ILCs (Kinnebrew et al., 2012; Van Maele et al., 2010) (Figure 2, right). Although the flagellin used in these studies was derived from Salmonella, which is generally considered to be an enteric pathogen, it is possible that components of flagellin derived from commensal bacteria could elicit a similar effect. Finally, GATA3+ ILC responses can be influenced by myeloid cell expression of IL-33. Following influenza infection, alveolar macrophages were found to be a dominant source of IL-33 (Chang et al., 2011), however the influence of commensal bacteria on myeloid-derived IL-33 remains to be explored.
Commensal bacteria colonize barrier surfaces of the mammalian body and can directly interact with epithelial cells lining this barrier. These interactions modulate expression of epithelial cell-derived cytokines that influence resident ILC populations. For example, MyD88-deficient mice exhibit substantially decreased expression of IL-15 in intestinal epithelial cells (Yu et al., 2006), suggesting that epithelial cell recognition of commensal bacteria is critical for NK cell homeostasis. Similarly, germ-free or antibiotic administered mice exhibit decreased expression of intestinal epithelial cell derived IL-7 (Shalapour et al., 2010; Vonarbourg et al., 2010), a factor critical for homeostasis and function of GATA3+ and RORγt+ ILCs. Diefenbach and colleagues suggested that commensal bacteria-dependent induction of IL-7 in epithelial cells is required to maintain expression of RORγt in intestinal NCR+ RORγt+ ILCs (Vonarbourg et al., 2010). Of note, blockade of IFNγ signaling in intestinal epithelial cells substantially reduces IL-7 production (Shalapour et al., 2010), suggesting a potential sequential engagement or cross-regulation of IFNγ-producing NK cells and IL-7-responsive ILCs by intestinal commensal bacteria. Intestinal epithelial cell expression of IL-1 family members IL-1β, IL-18 and IL-33 can also elicit responses from RORγt+, T-bet+ and GATA3+ ILCs respectively (Hughes et al., 2010; Moro et al., 2010; Schulthess et al., 2012); however the influence of commensal bacteria on epithelial cell expression of these cytokines is poorly understood (Figure 2, right). Finally, an additional study by Eberl and colleagues found that commensal bacteria can suppress RORγt+ ILC responses through induction of intestinal epithelial cell expression of IL-25 in the steady state (Sawa et al., 2011). Intestinal epithelial cell-derived IL-25 was substantially increased in conventional versus germ free or antibiotic administered mice (Sawa et al., 2011; Zaph et al., 2008) and acted through dendritic cells to suppress RORγt+ ILC responses (Sawa et al., 2011) (Figure 2, right). Intestinal epithelial cell-derived IL-25 can also influence GATA3+ ILCs (Neill et al., 2010), suggesting that commensal bacteria could potentially promote GATA3+ ILC responses (Figure 2, right), however this pathway has not yet been investigated. Collectively, these studies indicate that commensal bacteria can influence ILC development and functional potential indirectly through myeloid or epithelial cell populations, highlighting the complexity and sophistication of interactions between the ILCs, epithelial cells and commensal bacteria.
ILCs regulate the composition and localization of commensal bacteria
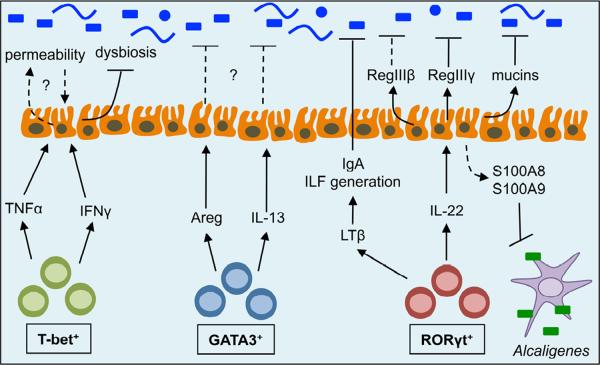
In addition to direct and indirect effects of commensal bacteria on ILC populations, ILCs can reciprocally influence commensal bacterial communities through a number of distinct mechanisms. Cytokines produced by ILCs can dynamically regulate the composition or anatomical location of commensal bacteria. For example, T-bet+ ILCs are critical sources of IFNγ and TNFα, which have been shown to increase the permeability and translocation of commensal bacteria across monolayers of human intestinal epithelial cells (Clark et al., 2005; Mullin and Snock, 1990). Additionally, a recent report by Lord and colleagues identified that T-bet expression was critical for maintenance of IFNγ expression in ILCs and that mice deficient in T-bet developed colitis dependent upon IL-17A-producing RORγt+ ILCs and dysbiosis of Helicobacter typhlonius (Powell et al., 2012). This is consistent with previous reports linking dysbiosis of commensal bacteria and development of transmissible colitis in mice that lack T-bet in the innate immune system (Garrett et al., 2010; Garrett et al., 2007). Collectively, these results suggest that T-bet+ ILCs may play a critical role in regulating the composition of intestinal commensal bacteria and maintaining intestinal tissue homeostasis (Figure 3). In addition, GATA3+ ILCs might be critical regulators of the anatomical containment of commensal bacteria when the epithelial barrier is impaired. This is supported by findings that GATA3+ ILCs and their production of amphiregulin are critical in maintaining epithelial barrier function and restoration of the epithelium in the lung following influenza-induced airway damage (Monticelli et al., 2011), and that IL-33 administration following DSS-induced intestinal damage substantially reduces translocation of intestinal commensal bacteria to peripheral organs and induction of inflammation (Grobeta et al., 2012) (Figure 3). However, further direct evidence of NK cell- or GATA3+ ILC-mediated regulation of commensal bacteria is currently lacking and is an area that will require further investigation.
Figure 3. Regulation of commensal bacteria by ILCs.
ILCs can regulate both the composition and anatomical location of commensal bacteria through the production of cytokines that influence numerous pathways at the intestinal epithelial cell barrier. T-bet+ ILCs produce TNFα and IFNγ which can directly influence intestinal epithelial cell permeability and limit dysbiosis of commensal bacteria. GATA3+ ILCs produce IL-13 and amphiregulin (Areg), however a role for these cytokines in regulating commensal bacteria has not yet been identified. RORγt+ ILCs produce lymphotoxin (LT)β to promote the generation of isolated lymphoid follicles (ILFs) and support intestinal IgA production. RORγt+ ILCs also produce IL-22 to promote epithelial cell production of mucins and anti-microbial proteins (RegIIIβ, RegIIIγ, S100A8 and S100A9), which are critical for maintaining spatial segregation and anatomical containment of commensal bacteria.
In contrast, RORγt+ ILCs have been found to play several important roles in directly regulating commensal bacterial communities in the intestine (Figure 3). RORγt+ ILCs are resident in intestinal tissues of healthy mammals, and are a dominant source of IL-22 in the steady state (Sawa et al., 2010; Sonnenberg et al., 2012). Eberl and colleagues identified that mice deficient in RORγt exhibited elevated titers of serum IgG specific for intestinal commensal bacteria in the steady state (Lochner et al., 2011), indicative of impaired intestinal barrier function and dissemination of commensal bacteria to peripheral tissues. Following induction of epithelial damage with DSS administration, RORγt-deficient mice developed hyperactive B cells that promoted commensal bacteria-dependent intestinal pathology and wasting disease (Lochner et al., 2011). Supporting this, mice deficient in CX3CR1+ phagocytes, which are critical for optimal IL-22-responses from RORγt+ ILCs, exhibit increased translocation of commensal bacteria to the mesenteric LN and susceptibility to DSS-induced inflammation (Manta et al., 2012; Medina-Contreras et al., 2011). Further, we recently demonstrated that administration of IL-22-neutralizing or ILC-depleting monoclonal antibodies to Rag1−/− mice resulted in the dissemination of live commensal bacteria to the spleen and liver and the induction of low-grade systemic inflammation (Sonnenberg et al., 2012). The disseminating commensal bacteria were members of the Alcaligenes genus, previously shown to reside in lymphoid tissues of the intestine such as the Peyer's patches or mesenteric lymph nodes (Obata et al., 2010). These data suggest a critical role for RORγt+ ILCs in promoting the anatomical containment of lymphoid-resident commensal bacteria (Sonnenberg et al., 2012) (Figure 3).
The mechanisms by which RORγt+ ILCs regulate intestinal commensal bacteria may be several-fold. For example, RORγt+ ILCs are essential for the generation of secondary lymphoid tissues and T cell-independent IgA production (Eberl et al., 2004; Tsuji et al., 2008), two components that critically influence intestinal commensal bacteria communities. RORγt+ ILCs also produce IL-22, which can directly act on the intestinal epithelium and induce expression of tissue protective mucin genes and anti-microbial proteins such as RegIIIβ, RegIIIγ, S100A8 and S100A9 (Sonnenberg et al., 2011a). Recent analysis of mice deficient in the mucin Muc2 or the anti-microbial protein RegIIIγ identified a critical function for these proteins in promoting the spatial segregation of intestinal commensal bacteria from the intestinal epithelium and limiting inflammation (Johansson et al., 2008; Vaishnava et al., 2011). Furthermore, S100A8 and S100A9 can heterodimerize to form calprotectin, which inhibits the growth of lymphoid-resident commensal bacteria and contributes to their anatomical containment (Sonnenberg et al., 2012). Collectively, these studies highlight that RORγt+ ILCs play a critical role in regulating intestinal commensal bacteria and limiting local and systemic chronic inflammation. Future studies will be necessary to interrogate the mechanisms by which ILCs regulate commensal bacteria and to identify potential strategies for therapeutic intervention in the face of dysregulated host-commensal interactions.
Interactions between ILCs and commensal bacteria in human health and disease
ILC subsets have been identified in numerous healthy and diseased human tissues, including secondary lymphoid structures, peripheral blood, lungs and intestinal tissues (Monticelli et al., 2012; Sonnenberg et al., 2011a; Spits and Cupedo, 2012). NK cells have been the most well studied population in humans, and the relative importance of NK cells in human health has been highlighted by patients that exhibit mutations that impact NK cell numbers or function (Orange and Ballas, 2006). A great deal of research has identified roles for NK cells in human conditions including viral infections, inflammatory disorders, pregnancy, cancer and bone marrow transplantation (Biron et al., 1999; Caligiuri, 2008; Orange and Ballas, 2006). Similarly, human IL-5- and IL-13-expressing GATA3+ ILCs have recently been characterized in healthy blood, lung and intestine from fetal and adult donors, and in the bronchoalveolar lavage of lung transplant recipients and nasal polyps from patients with chronic rhinosinusitis (Mjosberg et al., 2011; Monticelli et al., 2011). Interesting, a recent report by Spits and colleagues suggests that the accumulation of activation of GATA3+ ILCs in chronic rhinosinusitis patients may be due to increased expression of epithelial cell-derived TSLP, a process that could be induced by administration of TLR ligands such as flagellin (Mjosberg et al., 2012). Despite these observations and the identified role of these cells in murine model systems, the interactions between human T-bet+ and GATA3+ ILCs with commensal bacteria and their relative importance in human health and disease remain unclear.
Human IL-17A- and IL-22-expressing RORγt+ ILCs have been characterized in secondary lymphoid tissues and intestinal tissues from fetal and adult donors (Cella et al., 2009; Cupedo et al., 2009; Sonnenberg et al., 2012). Consistent with murine studies, co-culture experiments revealed that human RORγt+ ILCs could promote expression of adhesion molecules associated with lymphoid organogenesis on mesenchymal stem cells (Cupedo et al., 2009) and proliferation of intestinal epithelial cells (Cella et al., 2009). Several studies have also characterized RORγt+ ILCs in the context of human diseases in which dysregulated host-commensal interactions are thought to substantially contribute to disease pathogenesis and progression. Initial studies identified that active Crohn's disease is associated with an increase in intestinal and systemic levels of IL-22, which were associated with pro-inflammatory gene expression (Brand et al., 2006; Wolk et al., 2007). However, two groups demonstrated that examination at a cellular level revealed a decrease in IL-22-producing NCR+ RORγt+ ILCs and a reciprocal increase in IL-22-producing T cells and IFNγ-producing ILCs in the colon and ileum of Crohn's disease patients relative to healthy control patients (Ciccia et al., 2011; Takayama et al., 2010). Intriguingly, one of the same studies investigated ankylosing spondylitis patients with chronic but subclinical intestinal inflammation and revealed an increase in IL-22-producing NCR+ RORγt+ ILCs (Ciccia et al., 2011), provoking the hypothesis that a balance between IFNγ-expressing and IL-22-expressing ILCs may dictate the disease severity of intestinal inflammation by influencing intestinal permeability and repair. IFNγ expression in ILCs could be induced by co-culture of monocytes stimulated with commensal bacteria (Takayama et al., 2010), suggesting that defects in intestinal barrier function may be critical in determining the balance of IFNγ and IL-22 responses from ILC populations. In addition, Powrie and colleagues recently identified that IL-17A and IL-22 co-expressing NCR− RORγt+ ILCs are considerably increased in the intestinal tissues from Crohn's disease patients (Geremia et al., 2011). The potential importance of changes in IL-22-expressing NCR+ versus NCR− RORγt+ ILC populations in IBD patents might be explained by differential expression of IL-17A in these subsets (Colonna, 2009; Sonnenberg et al., 2011a; Spits and Cupedo, 2012), as IL-17A has previously been found to influence the pro-inflammatory versus tissue protective functions of IL-22 (Sonnenberg et al., 2010). Therefore, the spatial and temporal expression of other cytokines and analysis of multiple subsets of ILCs should be considered in future investigations of the contributions of IL-22-expressing RORγt+ ILCs in human Crohn's disease.
Commensal bacteria have also been found to disseminate to systemic tissues in numerous chronic human diseases including HIV-AIDS, viral hepatitis, cancer, diabetes and cardiovascular disease (Amar et al., 2011; Brenchley and Douek, 2012; Lescut et al., 1990; Renko et al., 2008; Sandler et al., 2011). We recently reported that depletion of ILCs resulted in the systemic dissemination of Alcaligenes commensal bacteria in mice and observed a substantial increase in Alcaligenes-specific IgG in serum from pediatric Crohn's disease and cirrhotic HCV-infected individuals (Sonnenberg et al., 2012). These data suggest that loss or impairment of IL-22-expressing RORγt+ ILCs might precede the peripheral dissemination of commensal bacteria and induction of systemic inflammation in several chronic human diseases, and furthermore represent a potentially important therapeutic target. Supporting this, recent studies have identified a loss of IL-22-expressing RORγt+ ILCs in both Crohn's disease in humans (Ciccia et al., 2011; Takayama et al., 2010) and progressive SIV infection in non-human primates (Klatt et al., 2012; Reeves et al., 2011; Xu et al., 2012). Future studies will be needed to further interrogate whether IL-22-expressing RORγt+ ILCs are lost across multiple chronic human diseases, determine the mechanisms by which this occurs and identify novel therapeutic strategies to restore normal ILC homeostasis and prevent or limit pathologic host-commensal interactions.
Conversely, pathogenic ILC responses that have been associated with mouse models of airway hyper-responsiveness, asthma, intestinal inflammation and psoriasis could also be targeted in human disease (Buonocore et al., 2010; Chang et al., 2011; Pantelyushin et al., 2012). This may be accomplished in part with already available humanized monoclonal antibodies that target pathways including IL-23, IL-17 and CD25 (Ding et al., 2008; Leonardi et al., 2012; Perry et al., 2012). As a proof of principle, it was recently found that blockade of CD25 with daclizumab in multiple sclerosis patients shifted the balance of circulating ILC subsets by decreasing RORγt+ ILCs and increasing NK cells, and was also associated with reduced parameters of inflammation (Perry et al., 2012). Additional studies will be necessary to determine the full effects of targeting these pathways on ILC biology, and it is likely that additional immuno-modulatory agents will need to be developed that selectively regulate the balance of ILC responses and restore healthy host-commensal interactions.
Summary and future perspectives
Emerging evidence of the development, function and heterogeneity of the ILC family represents an exciting advance in the field of immunology. These cells appear to play important roles at barrier surfaces of the body such as the intestine and are intimately associated with commensal bacteria that colonize these tissues. There is a need to develop new tools and models to specifically target ILC responses, determine their roles in murine model systems and interrogate potential interactions of ILCs with adaptive immune cell populations. Translational studies examining ILCs in the context of human health and disease with extensive analysis of cytokine and surface marker expression will be valuable to better understand the functional contributions of human ILCs. Given the recent appreciation that dysregulated host-commensal relationships are associated with the pathogenesis and progression of numerous chronic human infectious, inflammatory and metabolic diseases, targeting interactions between ILCs and commensal bacteria may offer new approaches to develop preventative and therapeutic treatments for these diseases.
Acknowledgements
We thank members of the Artis laboratory for discussions and critical reading of the manuscript. This work is supported by the National Institutes of Health (AI061570, AI087990, AI074878, AI083480, AI095466, AI095608 and AI097333 to D.A.; T32-AI055428 and DP5OD012116 to G.F.S.) and the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (D.A.).
Abbreviations used
- AhR
Aryl hydrocarbon receptor
- DC
dendritic cells
- ILC
innate lymphoid cell
- IL
interleukin
- LTi
lymphoid-tissue inducer
- NCR
natural cytotoxicity receptor
- TLR
toll-like receptor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity. 2012 doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, Lepage P, Klopp C, Mariette J, Bouchez O, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011 doi: 10.1007/s00125-011-2329-8. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. Microbial Translocation Across the GI Tract (*) Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaushu S, Wilensky A, Gur C, Shapira L, Elboim M, Halftek G, Polak D, Achdout H, Bachrach G, Mandelboim O. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog. 2012;8:e1002601. doi: 10.1371/journal.ppat.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier M, Sawa S, Eberl G. Notch, Id2, and RORgammat sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J Exp Med. 2012;209:729–740. doi: 10.1084/jem.20111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin KF, Kallam R, O'Boyle C, MacFie J. Bacterial translocation may influence the long-term survival in colorectal cancer patients. Dis Colon Rectum. 2007;50:323–330. doi: 10.1007/s10350-006-0827-4. [DOI] [PubMed] [Google Scholar]
- Ciccia F, Accardo-Palumbo A, Alessandro R, Rizzo A, Principe S, Peralta S, Raiata F, Giardina A, De Leo G, Triolo G. Interleukin-22 and IL-22-producing NKp44(+) NK cells in the subclinical gut inflammation of patients with ankylosing spondylitis. Arthritis Rheum. 2011 doi: 10.1002/art.34355. [DOI] [PubMed] [Google Scholar]
- Clark E, Hoare C, Tanianis-Hughes J, Carlson GL, Warhurst G. Interferon gamma induces translocation of commensal Escherichia coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology. 2005;128:1258–1267. doi: 10.1053/j.gastro.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol. 2008;9:473–475. doi: 10.1038/ni.f.201. [DOI] [PubMed] [Google Scholar]
- Ding C, Xu J, Li J. ABT-874, a fully human monoclonal anti-IL-12/IL-23 antibody for the potential treatment of autoimmune diseases. Current Opinion in Investigational Drugs. 2008;9:515–522. [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, Maisetta G, Florio W, Arancia G, Campa M. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun. 2008;76:1719–1727. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink LN, Zeuthen LH, Christensen HR, Morandi B, Frokiaer H, Ferlazzo G. Distinct gut-derived lactic acid bacteria elicit divergent dendritic cell-mediated NK cell responses. Int Immunol. 2007;19:1319–1327. doi: 10.1093/intimm/dxm103. [DOI] [PubMed] [Google Scholar]
- Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, Diefenbach A. Priming of Natural Killer Cells by Nonmucosal Mononuclear Phagocytes Requires Instructive Signals from Commensal Microbiota. Immunity. 2012 doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, Reiner SL. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobeta P, Doser K, Falk W, Obermeier F, Hofmann C. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.22900. [DOI] [PubMed] [Google Scholar]
- Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, Kambayashi T, Larosa DF, Renner ED, Orange JS, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18:538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyler T, Klose CSN, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. Gata3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012 doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Wakita D, Sato T, Mitamura R, Izumo T, Shibata H, Kiso Y, Chamoto K, Togashi Y, Kitamura H, Nishimura T. Essential role of Toll-like receptors for dendritic cell and NK1.1(+) cell-dependent activation of type 1 immunity by Lactobacillus pentosus strain S-PT84. Immunol Lett. 2008;120:14–19. doi: 10.1016/j.imlet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- Lescut D, Colombel JF, Vincent P, Cortot A, Fournier L, Quandalle P, Vankemmel M, Triboulet JP, Wurtz A, Paris JC, et al. Bacterial translocation in colorectal cancers. Gastroenterol Clin Biol. 1990;14:811–814. [PubMed] [Google Scholar]
- Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–788. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006a;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006b;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, Eberl G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208:125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- Manta C, Heupel E, Radulovic K, Rossini V, Garbi N, Riedel CU, Niess JH. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6:e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3-LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121:4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjosberg J, Bernink J, Golebski K, Julien KJ, Peters C, Blom B, te Velde A, Fokkens W, van Drunen C, Spits H. GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012 doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011 doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Artis D. Innate lymphoid cells: critical regulators of allergic inflammation and tissue repair in the lung. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172–2176. [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, Setoyama H, Matsuki T, Nonaka K, Shibata N, Gohda M, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A. 2010;107:7419–7424. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Ott SJ, El Mokhtari NE, Musfeldt M, Hellmig S, Freitag S, Rehman A, Kuhbacher T, Nikolaus S, Namsolleck P, Blaut M, et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation. 2006;113:929–937. doi: 10.1161/CIRCULATIONAHA.105.579979. [DOI] [PubMed] [Google Scholar]
- Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew GH, Babbs CF. Production of Ah receptor ligands in rat fecal suspensions containing tryptophan or indole-3-carbinol. Nutr Cancer. 1991;16:209–218. doi: 10.1080/01635589109514159. [DOI] [PubMed] [Google Scholar]
- Perry JS, Han S, Xu Q, Herman ML, Kennedy LB, Csako G, Bielekova B. Inhibition of LTi Cell Development by CD25 Blockade Is Associated with Decreased Intrathecal Inflammation in Multiple Sclerosis. Sci Transl Med. 2012;4:145ra106. doi: 10.1126/scitranslmed.3004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol. 2004;41:1099–1108. doi: 10.1016/j.molimm.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Powell N, Walker AW, Stolarcyzk E, Canavan JB, Gokmen MR, Marks E, Jackson I, Hashim A, Curtis MA, Howard JK, et al. T-bet regulates intestinal inflammation mediated by IL-7R+ innate lymphoid cells. Immunity. 2012 doi: 10.1016/j.immuni.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RK, Rajakumar PA, Evans TI, Connole M, Gillis J, Wong FE, Kuzmichev YV, Carville A, Johnson RP. Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection. Blood. 2011;118:3321–3330. doi: 10.1182/blood-2011-04-347260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renko J, Lepp PW, Oksala N, Nikkari S, Nikkari ST. Bacterial signatures in atherosclerotic lesions represent human commensals and pathogens. Atherosclerosis. 2008;201:192–197. doi: 10.1016/j.atherosclerosis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, Nikitas G, Escaliere B, Renauld JC, Dussurget O, et al. Identity, regulation and in vivo function of gut NKp46+RORgammat+ and NKp46+RORgammat− lymphoid cells. EMBO J. 2011;30:2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T, Douek DC. Host Response to Translocated Microbial Products Predicts Outcomes of Patients with HBV or HCV infection. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage Relationship Analysis of ROR{gamma}t+ Innate Lymphoid Cells. Science, science. 2010:1194597. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORgammat(+) innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- Schulthess J, Meresse B, Ramiro-Puig E, Montcuquet N, Darche S, Begue B, Ruemmele F, Combadiere C, Di Santo JP, Buzoni-Gatel D, Cerf-Bensussan N. Interleukin-15-Dependent NKp46(+) Innate Lymphoid Cells Control Intestinal Inflammation by Recruiting Inflammatory Monocytes. Immunity. 2012;37:108–121. doi: 10.1016/j.immuni.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Shalapour S, Deiser K, Sercan O, Tuckermann J, Minnich K, Willimsky G, Blankenstein T, Hammerling GJ, Arnold B, Schuler T. Commensal microflora and interferon-gamma promote steady-state interleukin-7 production in vivo. Eur J Immunol. 2010;40:2391–2400. doi: 10.1002/eji.201040441. [DOI] [PubMed] [Google Scholar]
- Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci U S A. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011a;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011b;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2010 doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Hirota K, Duarte J, Veldhoen M. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23:99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, Matuzaki Y, Suzuki S, Sugita A, Koganei K, et al. Imbalance of NKp44(+)NKp46(−) and NKp44(−)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterology. 2010;139:882–892. 892, e881–883. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, Janot L, Erard F, Bertout J, Leger H, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol. 2010;185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankayalapati R, Wizel B, Weis SE, Safi H, Lakey DL, Mandelboim O, Samten B, Porgador A, Barnes PF. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168:3451–3457. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Withers DR. Immunology. Innate lymphoid cell relations. Science. 2010;330:594–595. doi: 10.1126/science.1198298. [DOI] [PubMed] [Google Scholar]
- Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. Journal of Immunology. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- Yu Q, Tang C, Xun S, Yajima T, Takeda K, Yoshikai Y. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J Immunol. 2006;176:6180–6185. doi: 10.4049/jimmunol.176.10.6180. [DOI] [PubMed] [Google Scholar]
- Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]