Abstract
BACKGROUND & AIMS
The Study of Biologic and Immunomodulator-Naïve Patients With Crohn’s Disease (SONIC) showed that combination therapy with infliximab and azathioprine (IFX/AZA) is more effective than treatment with IFX alone. Numbers and types of adverse events were roughly equivalent among groups, although enrollment was limited, so it was not clear how rare adverse events might affect overall outcomes in practice. We sought to define the frequency at which a rare adverse event would have to occur for the risks of combination therapy to outweigh the benefits of treatment.
METHODS
We constructed a decision model to compare the risks and benefits of IFX/AZA with IFX monotherapy. Model parameters were taken from SONIC and other published literature. The base-case analysis was patients with active Crohn’s disease who are naïve to both medications (similar to those in SONIC) who were treated for 1 year. We used sensitivity analyses to determine the thresholds at which the risks of side effects from IFX/AZA outweigh its benefits.
RESULTS
During 1 year, the benefits of IFX/AZA would outweigh the risks, unless serious infections occurred in 20% or more of the population or lymphoma in 3.9% or more. These thresholds are 5-fold and 65-fold higher than base-case estimates, respectively.
CONCLUSIONS
On the basis of data from 1 year of SONIC, the combination of IFX/AZA was more effective than IFX alone in patients with Crohn’s disease who are naïve to either drug. For the risks of combination therapy to outweigh the benefits in this time frame, the incidence of serious adverse events would have to be higher than seems clinically realistic.
Keywords: Inflammation, Anti-Tumor Necrosis Factor (TNF) Agent, Side Effect, Intestine, IBD, Complication
Crohn’s disease is a chronic inflammatory bowel disease affecting more than half a million people in the United States.1 If not treated appropriately, Crohn’s disease can lead to substantial morbidity because of persistent symptoms and multiple surgeries. The goal of medical therapy is to induce and maintain a clinical remission without the need for long-term steroid use. The 2 classes of medications used most effectively to achieve this goal include immunomodulators (6-mercaptopurine, azathioprine [AZA]) and anti–tumor necrosis factor (TNF) agents (infliximab [IFX], adalimumab, and certolizumab pegol). Anti-TNF agents were approved for use in the treatment of Crohn’s disease in 1998 and have proved to be effective when immunomodulators have failed.2 During the past decade since anti-TNF agents have been available, gastroenterologists have been working to optimize use of these agents. Similar to a treatment approach in rheumatology,3 one important question has been whether anti-TNF agents are most effective if used alone or together with immunomodulators.
To answer the question of whether combination therapy is more effective in Crohn’s disease, a recent randomized controlled trial studied the efficacy of AZA versus IFX versus combination therapy (AZA/IFX).4 This study (Study of Biologic and Immunomodulator-Naïve Patients With Crohn’s Disease [SONIC]) showed a clear benefit for combination therapy versus either drug alone. However, concerns that 2 immunosuppressant drugs taken together will lead to a higher rate of adverse events has dampened enthusiasm for these findings. Specifically, the rate of non-Hodgkin lymphoma (NHL) and serious infections might be higher in patients treated with combination therapy.5,6 Despite the superiority of combination therapy for the treatment of Crohn’s disease, physicians might be reluctant to use this approach unless they are comfortable with the tradeoff of benefits and risks.
Because severe adverse events (SAEs) are rare, it is unlikely that a clinical trial will ever be adequately powered to compare the safety of anti-TNF monotherapy versus combination therapy. The purpose of this study was to use decision analytic techniques to evaluate the benefits and risks of IFX monotherapy versus combination AZA/IFX therapy and to determine how high the risk of combination therapy would have to be for this regimen to no longer be the favored approach.
Methods
Patient Population
The population of interest was 35-year-old patients with moderately to severely active Crohn’s disease who are naïve to both immunomodulators and anti-TNF agents. This population represents both patients who would receive these treatments in clinical practice and the study population of the SONIC trial.4
Model Structure
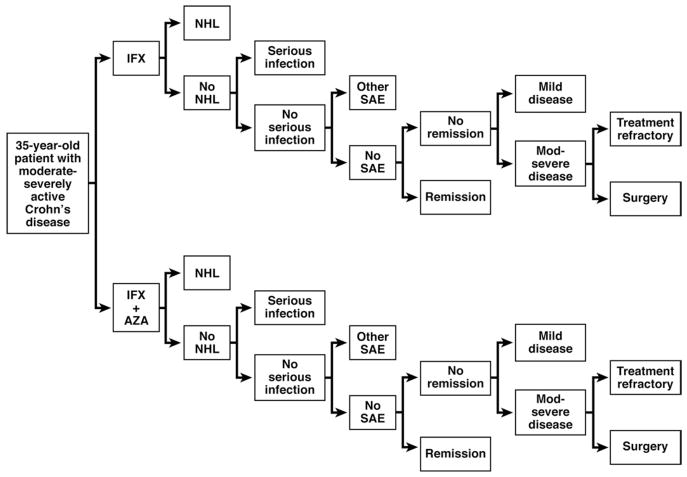
A decision tree model was constructed to compare IFX monotherapy with combination treatment with AZA and IFX over a 1-year time horizon (Figure 1). The model assesses the expected health utility measured by quality-adjusted life-years (QALYs).
Figure 1.
Simplified model schematic showing the 2 treatment arms of IFX monotherapy and IFX + AZA combination therapy. When treatment is withdrawn because of a serious infection, other SAE, or because a patient is treatment refractory, then they remain in the moderate-severe disease activity state.
In each treatment arm, patients can develop lymphoma, a serious life-threatening infection, another SAE, or have no adverse events and either respond or not respond to treatment (Figure 1). If patients do respond, they either go into clinical remission or improve to mildly active disease. When treatment is withdrawn because of a serious infection or other SAE, patients remain in the moderate-severe disease activity state. If they do not respond to treatment, they either remain in moderate-severe active disease or undergo surgery for Crohn’s disease. In both groups, death could result from lymphoma, a severe infection, a Crohn’s disease flare, a surgical complication, or some other age-specific cause of mortality. The model was constructed by using a decision analysis software program (TreeAge Pro Suite 2009, Williamstown, MA).
Assumptions
The model includes several important assumptions. First, other than lymphoma and serious infections, there are other SAEs that are non–life-threatening but lead to cessation of therapy and continued disease in the moderate-severely active state (“other SAE”). Second, combination therapy is associated with a higher risk of NHL than monotherapy with IFX, and IFX monotherapy has the same risk of NHL as AZA monotherapy. Although there are meta-analysis data to estimate the risk of NHL with combination therapy5 and immunomodulator monotherapy,7 this assumption is required because there are not enough patients treated with anti-TNF monotherapy without prior exposure to immunomodulators to provide a baseline estimate.
Model Inputs
Benefits and risks of therapy
The base-case estimates for the chance of remission after treatment with AZA/IFX combination therapy or IFX monotherapy come from the SONIC trial.4 To use the most conservative estimate of efficacy, week 50 data are used for all randomized patients, assuming that patients who did not enter the trial extension did not achieve the end point through week 50. The percentage of serious infections for each treatment arm comes from SONIC week 54 data, and SAEs other than serious infections were calculated by subtracting the rate of serious infections from the overall SAE rate (which included serious infections) to yield the “other SAE” rate. Death related to serious infection was calculated on the basis of the proportion of patients who died of serious infection as identified in a previous systematic review.8 The lymphoma rates were based on 2 recent meta-analyses, one on the rate of NHL associated with immunomodulator exposure and the other with the use of combination anti-TNF and immunomodulator treatment.5,7 The base-case estimates for these and other variables are shown in Table 1. These point estimates all have associated uncertainty based on the sample size and design of the study from which they were derived, and this uncertainty is addressed in the sensitivity analyses.
Table 1.
Model Input Estimates (Base Case) for Event Probabilities
| Event | Monotherapy | Combination therapy | Source |
|---|---|---|---|
| Remission | 0.38 | 0.47 | SONIC week 50a,4 |
| Responseb | 0.036 | 0.030 | SONIC week 50a,4 |
| SAEc | 0.24 | 0.15 | SONIC week 544 |
| Serious infectiond | 0.049 | 0.039 | SONIC week 544 |
| Lymphomad | 0.0004 | 0.0006 | Kandiel et al,7 2005; Siegel et al,5 2009 |
| Surgery for flare | 0.10 | 0.10 | Lewis et al,10 2000 |
| Die of lymphoma (1 y) | 0.22 | 0.22 | SEER14 |
| Die of serious infection | 0.05 | 0.05 | Siegel et al,e,8 2006 |
| Die of surgery | 0.0008 | 0.0008 | Lewis et al,10 2000; Gregor et al,9 1997 |
| Die of Crohn’s disease | 0.0018 | 0.0018 | Lewis et al,13 2008 |
| Die all-cause mortality | 0.0012 | 0.0012 | US Life Table12 |
SONIC week 50 for all randomized patients, assuming that patients who did not enter trial extension did not achieve the end point through week 50.
Crohn’s disease activity index of 100 points drop.
SAE is an event other than serious infection.
Ranges for uniform sampling distributions used in probabilistic sensitivity analyses for outcomes of interest were serious infection with monotherapy, 0.03–0.07; serious infection with combination therapy, 0.02–0.06; lymphoma with monotherapy, 0.0001–0.0007; lymphoma with combination therapy, 0.0002–0.001.
Derived from Siegel et al, 2005 analysis.
Quality of life estimates
Quality of life health utility weights for patients with Crohn’s disease were derived from previous work including Gregor et al9 and Lewis et al.10 Utilities for patients with lymphoma were taken from work by Uyl de-Groot et al.11 Table 2 shows these estimates. Patients with treatment refractory disease who do not have surgery or die continue to have the health utility of moderate-severe active disease.
Table 2.
Model Input Estimates for Quality-of-Life Utilities
Analysis
We report expected values of 1-year outcomes for each treatment strategy by using base-case estimates (Table 1). Sensitivity analyses were performed for all pertinent variables to determine the thresholds over which the risks of combination therapy outweigh the benefits, thereby making IFX monotherapy the favored treatment approach. In addition, a one-stage probabilistic sensitivity analysis with Monte Carlo simulation of 100,000 patients in each treatment arm was undertaken. We report the mean absolute number of patients in each disease state (with the standard deviation) at the end of 1 year. For this simulation, uniform sampling distributions were specified for the probability of infection and lymphoma with both monotherapy and combination therapy (distribution of variables reported in footnote of Table 1).
To explore how overall health utility might be affected during a time period beyond 1 year, a sensitivity analysis was performed to evaluate how life expectancy might influence the results. We estimated the life expectancy of patients with Crohn’s disease by using the US Life Table12 and the article by Lewis et al13 that describes the relative risk of dying of mild or moderate-severely active Crohn’s disease. To calculate a Crohn’s disease–specific life expectancy for our model, the relative risk from the study by Lewis et al of 1.27 was applied to the baseline mortality rate for a 35-year-old. With this approach, the life expectancy for a 35-year-old Crohn’s disease patient with mildly active disease is estimated to be 42 years, as opposed to 44.5 years for the average 35-year-old person. The life expectancy for patients who survive NHL was derived from the Surveillance, Epidemiology and End Results (SEER) registry by applying the 10-year survival rate to patients with moderate-severe active disease (greater disease severity is assumed because NHL patients presumably would have stopped immunosuppressive therapy).14 The SEER registry data suggest a 7-year decrement in life expectancy in patients with NHL from a baseline of 35 years with moderate-severely active disease, yielding a 28-year life expectancy for Crohn’s disease patients who have survived lymphoma treatment.
Results
The results of the Monte Carlo simulation show that with the base-case estimates, more patients in the combination therapy group are in remission, and fewer require surgery (Table 3). Although combination therapy is associated with a higher rate of lymphoma, these events are still very rare, and there are higher overall deaths in the monotherapy group as a result of more patients dying of severe, uncontrolled Crohn’s disease or surgery. Model results also show that combination therapy yields 0.80 QALYs/person versus 0.78 QALYs/person for IFX monotherapy. The higher QALYs for combination therapy are a result of both a higher remission rate and fewer overall Crohn’s disease–related SAEs.
Table 3.
Results of Probabilistic Sensitivity Analysis
| Event | Monotherapy, mean (SD) | Combination, mean (SD) | Difference |
|---|---|---|---|
| Improve | 1610 (20) | 1296 (16) | 314 (monotherapy) |
| Remission | 27,423 (333) | 38,323 (460) | 10,900 (combination) |
| No change | 38,819 (471) | 37,727 (453) | 1092 (monotherapy) |
| Surgery | 4313 (52) | 4192 (50) | 121 (monotherapy) |
| Lymphoma | 40 (17) | 60 (23) | 20 (combination) |
| Serious infection | 4884 (1153) | 3892 (1151) | 992 (monotherapy) |
| Death | 446 (57) | 399 (57) | 47 (monotherapy) |
NOTE. Each treatment arm includes 100,000 patients. Results show the mean (standard deviation) number of patients experiencing each event by treatment. Difference column shows which strategy is favored for each event and net difference for number of patients in that disease state.
Sensitivity Analysis
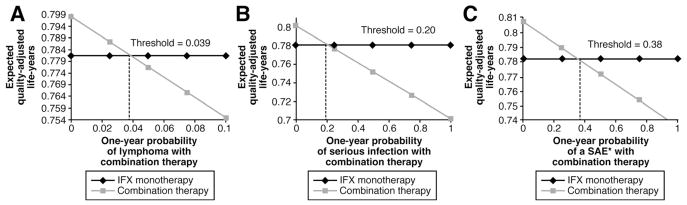
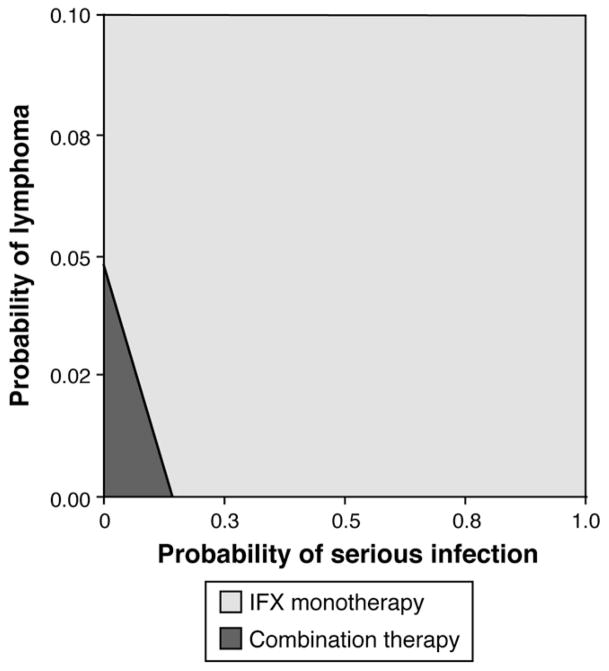
The sensitivity analysis for the rate of lymphoma showed that combination therapy yields higher QALYs as long as the lymphoma rate for combination therapy does not exceed 3.9% (Figure 2A). The sensitivity analysis for the probability of serious infections showed that combination therapy yields higher QALYs as long as the probability of serious infections for combination therapy does not exceed 20% (Figure 2B). The sensitivity analysis for “other SAE” probabilities showed that combination therapy is the favored strategy as long as the “other SAE” probability for combination therapy does not exceed 38% (Figure 2C). Other thresholds from the one-way sensitivity analyses are shown in Table 4. A two-way sensitivity analysis showed that the probability of lymphoma and a serious infection jointly affect the favored treatment, but monotherapy is only favored at relatively high risk levels for both risk outcomes (Figure 3).
Figure 2.
One-way sensitivity analyses to determine the threshold at which monotherapy is favored over combination therapy for the probability of lymphoma (A), serious infection (B), and other SAE* (C). Expected QALYs are varied for combination therapy, while held constant for IFX monotherapy in these one-way analyses. *SAE other than serious infection.
Table 4.
Thresholds From One-Way Sensitivity Analyses
| Event | Base-case estimate | Threshold at which monotherapy is favored |
|---|---|---|
| Remission combination | 0.47 | 0.33 |
| Remission monotherapy | 0.38 | 0.54 |
| SAE combinationa | 0.15 | 0.38 |
| Serious infection combination | 0.039 | 0.20 |
| Death from infection combination | 0.05 | 0.62 |
| Lymphoma combination | 0.0006 | 0.039 |
| QALY remission | 0.89 | 0.74 |
| QALY moderate-severe disease | 0.74 | 0.90 |
SAE other than serious infection.
Figure 3.
Two-way sensitivity analysis evaluating thresholds for a simultaneous change in lymphoma and serious infection probabilities for combination therapy.
In the exploratory analyses to investigate how life-expectancy assumptions influence model results, one-way sensitivity analyses were performed for all of the derived life-expectancy inputs. The life expectancy of remission of Crohn’s disease was the only value to which the model was sensitive, suggesting that combination therapy is favored unless the life expectancy of a 35-year-old patient in remission is <8 years.
Discussion
The decision to use anti-TNF monotherapy versus combination anti-TNF with an immunomodulator is difficult for providers and patients. Although randomized controlled trial data show a treatment benefit for combination therapy, there is concern that this benefit will not be worth the increased risk of using 2 immunosuppressant medications. This analysis shows that combination AZA/IFX therapy is expected to result in increased QALYs as long as the lymphoma probability is <3.9% and the serious infection probability is <20%. This lymphoma threshold is 65 times the lymphoma rate reported in the literature,5 and the serious infection threshold is 5 times higher than what was seen in SONIC.4 Therefore, for IFX monotherapy to be favored, the risk of these SAEs would have to be higher than might be clinically realistic for most patients.
There might be exceptions, because certain patient populations might be at a higher risk for adverse events. For example, young men appear to be at the highest risk for hepatosplenic T-cell lymphoma,15 and older patients with comorbidities who are taking concomitant corticosteroids are likely to be at a higher risk of serious infections.16,17 Therefore, a decision to use combination therapy should not be universal but should be based on individual patient characteristics.18 However, most patients who are appropriate candidates for immunosuppressive treatment are probably at a much lower risk for infection, lymphoma, or death than the thresholds determined by this analysis, and using combination therapy is therefore likely to be superior in most cases.
We did not explore whether these results can be generalized to other anti-TNF agents used for the treatment of Crohn’s disease. This would depend on whether adalimumab and certolizumab pegol are also more effective when used in combination with immunomodulators, and whether the risk profiles of these medications are different from infliximab.
There are limitations to this analysis. As with any simulation, this model is a simplification of reality. In an attempt to produce a transparent analysis that closely represents the best available data, some relevant components of clinical decisions and potential outcomes were omitted. The 1-year time horizon of this analysis relies on data from SONIC and risk estimates from a systematic review, meta-analyses, and other sources. We chose to maintain this time horizon because the SONIC study has already started to influence practice, despite the lack of outcomes beyond 1 year. To understand how rare adverse events might have impacted the SONIC results (if the study had been large enough), we mirrored the study population and time frame. Although it would be informative to model therapies and treatment decisions over the lifetime of the patients, there are not enough long-term data available to support such an effort. Even with the 1-year time horizon, assumptions had to be made for key inputs including the risks associated with IFX monotherapy. Because the standard of care has been to use anti-TNF agents concomitantly with immunomodulators, data for the risk of anti-TNF monotherapy (eg, risk of lymphoma with anti-TNF therapy without exposure to immunomodulators) are very limited. To address this issue, the risk of lymphoma with combination therapy was varied widely in the sensitivity analysis to consider how combination therapy might compare with monotherapy. Finally, life expectancy in Crohn’s disease has not been previously described. These input estimates were derived from a combination of information available from the US Life Table and previously published literature on the relative risk of mortality associated with different Crohn’s disease health states.13 Although estimates of life expectancy associated with specific health outcomes in the model were based on indirect calculations, these estimates appear clinically reasonable.
In conclusion, in this decision analysis, combination therapy with AZA/IFX yielded overall higher expected QALYs than IFX monotherapy over 1 year. This only reverses to favor IFX monotherapy when (1) the rate of lymphoma with combination therapy reaches clinically unrealistic frequencies or (2) the rate of serious infections is nearly 5 times higher than seen in the most rigorous clinical trial comparing these strategies. Although there might be subgroups of patients in which the risks of combination therapy outweigh the benefits, this analysis suggests that for the majority of patients with Crohn’s disease, combination therapy is more likely to provide superior results.
Supplementary Material
Acknowledgments
Funding
Dr Siegel is supported by a CCFA career development award and by grant number K23DK078678 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations used in this paper
- AZA
azathioprine
- IFX
infliximab
- NHL
non-Hodgkin’s lymphoma
- QALY
quality-adjusted life-years
- SAE
serious adverse event
- SEER
Surveillance, Epidemiology and End Results
- SONIC
Study of Biologic and Immunomodulator-Naïve Patients With Crohn’s Disease
- TNF
tumor necrosis factor
Footnotes
To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at doi:10.1016/j.cgh.2011.09.017.
Conflicts of interest
These authors disclose the following: Dr Siegel serves as a consultant to Abbott Laboratories, UCB, and Elan and has delivered CME talks for Merck and Abbott Laboratories. Dr Sands serves as a consultant to Abbott Laboratories, Centocor, and UCB. The remaining authors disclose no conflicts.
References
- 1.Loftus CG, Loftus EV, Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 2.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease: Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 3.Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. Aiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: the BeSt study. Clin Exp Rheumatol. 2006;24:S-77–S-82. [PubMed] [Google Scholar]
- 4.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 5.Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel CA, Hur C, Korzenik JR, et al. Risks and benefits of infliximab for the treatment of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1017–1024. doi: 10.1016/j.cgh.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Gregor J, McDonald JW, Klar N, et al. An evaluation of utility measurement in Crohn’s disease. Inflamm Bowel Dis. 1997;3:265–276. [PubMed] [Google Scholar]
- 10.Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn’s disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000;118:1018–1024. doi: 10.1016/s0016-5085(00)70353-2. [DOI] [PubMed] [Google Scholar]
- 11.Uyl-de Groot CA, Hagenbeek A, Verdonck LF, et al. Cost-effectiveness of ABMT in comparison with CHOP chemotherapy in patients with intermediate- and high-grade malignant non-Hodgkin’s lymphoma (NHL) Bone Marrow Transplant. 1995;16:463–470. [PubMed] [Google Scholar]
- 12.US life tables 2004: National Vital Statistics Report. Center for Health Statistics, CDC; [Accessed June 25, 2010.]. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_09.pdf. [Google Scholar]
- 13.Lewis JD, Gelfand JM, Troxel AB, et al. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1428–1435. doi: 10.1111/j.1572-0241.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed July 28, 2009.];SEER Surveillance, Epidemiology, and End Results database. Available at: http://seer.cancer.gov/
- 15.Data on file. Centocor, Inc; Malvern, PA: 2009. [Google Scholar]
- 16.Colombel JF, Loftus EV, Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn’s disease: the Mayo Clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Melmed GY, Spiegel BM, Bressler B, et al. The appropriateness of concomitant immunomodulators with anti-tumor necrosis factor agents for Crohn’s disease: one size does not fit all. Clin Gastroenterol Hepatol. 2010;8:655–659. doi: 10.1016/j.cgh.2010.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.