Abstract
Introduction
Nicotinic acetylcholine receptors (nAChRs), pentameric ligand-gated cation channels, are potential targets for the development of therapeutics for a variety of disease states.
Areas covered
This article is reviewing recent advances in the development of small molecule ligands for diverse nAChR subtypes and is a continuation of an earlier review in this journal.
Expert opinion
The development of nAChR ligands with preference for α4β2 or α7 subtypes for the treatment of CNS disorders are in the most advanced developmental stage. In addition, there is a fast growing interest to generate so-called PAMs, positive allosteric modulators, to influence the channels’ functionalities.
Keywords: nicotinic acetylcholine receptor, nicotine, choline, cytisine, epibatidine, neurodegenerative disease, positive allosteric modulator
1. Introduction
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated cation channels of the Cys-loop receptor super-family assembled like a rosette as homo- or heteropentamers and permeable to Na+, K+, and Ca2+ [1,2]. They are present in the peripheral nervous system (PNS), central nervous system (CNS), and in non-neuronal cells (e.g. bronchial epithelial cells, aortic endothelial cells, skin keratinocytes, immune tissue) [3]. Seventeen subunits are known for the architecture of diverse subtypes (α1 - α10, β1 - β4, δ, γ (replaced by ε in later stages of development)) [3, 4]. The α7 to α9 subunits can form homopentamers whereas other subunits create heteropentamers. The α8 subunit is known in chicken. Interestingly, the α10 subunit normally forms heteromeric receptors with α7 and α9, respectively [5]. Each subunit is composed of a large amino-terminal extracellular domain, a 4TM (transmembrane) part, and a cytoplasmic domain. The TM2 helix of each subunit is oriented towards the inner channel side. The ACh-binding sites are at the interface between an α-subunit and a non-α-subunit for the heteromeric subtypes [4, 6, 7]. For homopentamers up to five binding sites are possible. In addition, various non-ACh interaction sites are observed and described as sites for so-called allosteric modulators [8]. To make the situation even more complex, discrete functional states are described for the channel protein (e.g. resting closed states, open states, desensitized states) [9, 10].
Six α-subunits (α2 to α7) and three β-subunits are expressed in different parts of the brain forming multifarious combinations and therefore displaying diverse pharmacological and kinetic properties [11]. Important heteromeric nAChR subtypes in the brain are α4β2 receptors recently described to be present as so-called “low-sensitivity” ((α4)3(β2)2) and “high-sensitivity” ((α4)2(β2)3) receptors depending on their ratio of α4 and β2 subunits. Their functionalities are likely to be important related to physiological, pathophysiological and therapeutical aspects [12, 13]. Activation of brain nAChRs results in release of neurotransmitters: dopamine, serotonin, glutamate, and γ-aminobutyric acid (GABA) [14]. Based on these facts it is not surprising that nAChRs are involved in a variety of complex cognitive processes like learning and memory, and therefore in central nervous system disorders e.g. Alzheimer’s and Parkinson’s diseases, attention deficit hyperactivity disorder, depression, schizophrenia, Gilles de la Tourette syndrome, epilepsy, anxiety, pain, obesity, and tobacco dependence. Aside that, there is growing interest to explore the role of nAChRs and their potential as therapeutic targets in inflammation, sepsis, diabetes, respiratory diseases, colitis ulcerosa, skin diseases, arteriosclerosis, and cancer.
Some pathological conditions in which nAChRs are involved are described below.
The most prominent pathological condition associated with nicotinic receptors is nicotine addiction related to the consumption of tobacco products. In β2-knockout mice self-administration of nicotine is abolished, and it is reduced by administration of dihydro-β-erythroidine (DHβE), a selective α4β2 antagonist, in rodents [15, 16]. Furthermore, in addition to β2*, α6* and α7 subtypes are discussed to be involved in increased dopamine (DA) release by nicotine, contributing to the reward process [17, 18]. FDA approved drugs for smoking cessation are nicotine, the antidepressant drug bupropion (non-competitive antagonist at α3β4), and varenicline (partial agonist at α4β2) [19]. Unfortunately, these drugs are suffering from several drawbacks like limited efficacy for nicotine and bupropion, and psychiatric symptoms for varenicline [20]. Therefore, the development of more effective and safer drugs is needed.
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, is characterized by memory impairment accompanied by a loss of cholinergic innervations in the basal forebrain, neocortex, and hippocampus [21, 22]. The reduction of nAChRs in the cerebral cortex is predominantly related to the α4β2 subtype, whereas α7 receptors are affected in the hippocampus [23]. Both subtypes are discussed to interact with Aβ1-42. In AD β-Amyloid peptides’ (Aβ) are accumulating early in the neocortex and hippocampus. Their concentration increases in AD in contrast to non-demented patients. However the understanding and relevance of the interaction of nAChRs and Aβ is still in its early stages. Under physiological conditions the interaction seems to be important since trophic signals are generated [21]. When Aβ levels are becoming pathological, an interruption of these interactions might be useful. α7 nAChR (partial) agonists or positive allosteric modulators (PAMs) have already shown to improve cognitive deficits, and the development of such compounds is currently a very active field in research and in the pharmaceutical industry [22]. The development of α4β2 ligands related to the treatment of Alzheimer’s disease seems to be more challenging. Up to now, full agonists (e.g. nicotine, ABT-418, TC-1734/AZD-3480) failed in chronic settings in clinical trails, but showed mostly positive results in acute settings.
There is a high percentage (ca. 90%) of smokers found in patients suffering from schizophrenia, and tobacco’s nicotine can improve performance related to focus attention [24]. Impaired attention in patients with schizophrenia is caused by a sensory gating deficit linked to a dinucleotide polymorphism at chromosome 15 q13-14, which is also the locus for the α7 subunit gene [25]. In addition, radioligand binding studies with post-mortem tissue of patients suffered from schizophrenia showed a reduction of α7 subtype density in the hippocampus [26]. Interestingly, clozapine, a so-called atypical antipsychotic drug, improves auditory gating in a DBA/2 mouse model which involves an α7 receptor mechanism [27]. In contrast, most typical antipsychotic drugs do no show any effect on P50 auditory gating. The natural product galantamine, an acetylcholine esterase inhibitor and a nAChR PAM, used in AD treatment and the anti-nausea drug tropisetron, a 5-HT3 receptor antagonist and α7 agonist, normalize P50 auditory gating in schizophrenic patients [28, 29].
Like in patients suffering from schizophrenia, there are also a higher percentage of smokers in depressed patients (50-60%) [30]. Nicotine is able to alter affective states, and its dependence shows connection with diverse mood disorders, especially depression [30, 31]. Many antidepressants can block nAChRs, and the most prominent example is bupropion which is on the market as an antidepressant and as a smoking cessation aid. Also mecamylamine, a non-competitive nAChR channel blocker, the α4β2 antagonist DhβE (dihyro-β-erythroidine), or the natural product cytisine, a partial agonist at α4β2, demonstrated antidepressant effects in diverse in vivo models [31, 32].
The treatment of cognitive deficits in attention deficit hyperactivity disorder (ADHD) is also an emerging field for the use of nAChR compounds. Nicotine itself demonstrated positive effects in an adult ADHD trial, but it suffers from too many adverse effects [33]. Therefore, novel drugs with better safety profile are in development. Recently, identical or similar nAChR ligands like developed for AD treatment came into clinical trials in addition to α4β2 partial agonists.
Nicotine is known to possess antinociceptive effects. The α4β2 nAChR subtype is believed to be involved in analgesia and it is distributed throughout multiple locations of the pain pathway [34]. In the past, α4β2 agonists have demonstrated their efficacy in acute, chronic, inflammatory and neuropathic pain. Abbott’s ABT-594 (tebanicline; 5-([(2R)-azetidin-2-yl]methoxy)-2-chloropyridine) displaying the chloropyridine moiety, also found in the very potent frog alkaloid epibatidine, was the first nAChR compound which underwent advanced clinical trials [35]. Unfortunately, this compound suffered from emesis and nausea related to its potent α3β4 interaction. Compounds with probably higher subtype selectivity, but also better safety profile are needed. Another approach would be to combine these compounds with an α4β2 PAM to enhance efficacy, and to reduce possible adverse effects by reducing the dose of the agonist.
Nicotine can also interact via nAChRs on non-neuronal cells, and has proliferative and anti-apoptotic activities [36, 37]. Chronic exposure to nicotine or its derived nitrosamines can negatively influence the balance between cancer-stimulatory (e.g. α7) and cancer-inhibitory (e.g. α4β2) nAChRs [36, 37]. Via the α7 subtype, synthesis and release of autocrine growth factors in e.g. lung cancer could occur along with release of stress neurotransmitters, which bind to beta-adrenergic receptors activating cancer development and progression [36]. Activation of the α4β2 subtype on the other side stimulates the release of dopamine and also GABA, which then block stimulating effects of beta-adrenergic receptors via cyclic AMP inhibition. This could inhibit cancer development and progression [36]. These pathways need also to be considered when developing nAChR drugs for chronic treatment. The heteromeric α3α5β4 nAChR could be another important target, since genetic studies show a possible role of the CHRNA5/A3/B4 gene cluster in the development of lung cancer which could lead to altered gene expression, but also to changed receptor function [38].
The first pharmacophore model for the interaction of nicotine and a few other compounds with nAChRs was developed by Beers and Reich, and it simply described a two-point model, the cationic center (“onium”) and a hydrogen bond acceptor (HBA) part [39, 40]. The cationic part, a positively charged nitrogen, which is able to interact with aromatic amino acids of the receptor peptide via cation-π forces, an hydrogen bond between this nitrogen and the backbone carbonyl of the aromatic amino acid, and an hydrogen bond between the HBA (e.g. a nitrogen or a carbonyl oxygen) and a backbone NH group of an amino acid are still important features for the interaction with the nAChR protein [9, 41-43]. However, the simplest nAChR agonist is tetramethylammonium (TMA), consisting of only a quaternary ammonium group, and together with choline, both missing the HBA part of the Beer and Reich model.
The use of structural complex cationic parts, mostly azabicyclic systems, in the development of nAChR compounds continues. There are only few patents with an acyclic amine component for the cation-π interaction, also monocyclic amine containing ligands are rare. (Di)azabicyclic octanes and diazabicyclic nonanes are the majority representing the nAChR pharmacopeia. The classical HBA part is mostly represented by certain N-heterocyclic systems, often with fused ring systems. Additionally, so-called positive allosteric modulators (PAMs) for α4β2 and mostly for α7 subtypes are being developed [44]. Figure 1 shows the endogenous ligands choline (Ch) and acetylcholine (ACh), natural lead compounds nicotine, cytisine, epibatidine, ferruginine, and anatoxin-a, and the approved drug varenicline.
Figure 1.
The endogenous ligands choline (Ch) and acetylcholine (ACh), natural lead compounds nicotine, epibatidine, ferruginine, anatoxin-a, and cytisine, and the approved drug varenicline.
2. Patents
This patent review of nAChR ligands (2006 – June 2011) describes small molecule compounds grouped by structural templates or scaffolds and like in the previous patent review [45] most are sorted by their major pharmacophoric element – the “cationic” part, starting with the simple acyclic amine moiety as cationic center and finishing with adamantanes. Positive allosteric modulators (PAMs) are in a separate section divided by their main nAChR target, α4β2 or α7, and only those compounds which are explicitly stated as PAMs by the inventors can be found in this section (2.4), other compounds with sometimes claimed modulatory effect can be found in the other sections. Often inventors are describing or claiming a variety of bicyclic systems in the same patent, but only representative or/and important compounds are highlighted here. In most patents and in general very common, Markush structures are used to increase the number of possible derivatives related to the proposed template. The majority of the patents are related to α7 ligands, an observation which has been consistent for several years. The list for the use of nAChR compounds is now impressive long. CNS disorders, especially related to memory dysfunction remain the primary targets of nAChR-based therapeutics. In addition the listing continues with e.g. inflammatory disorders, a variety of pain types, eating disorders, addiction, depression, posttraumatic syndrome, chronic fatigue syndrome, autism, premenstrual syndrome, conditions associated with smooth muscle contractions, angina pectoris, diabetes, cancer, AIDS, age-related macular degeneration, glaucoma, diarrhoea, asthma, pheochromocytoma, hypertension and arrhythmias.
2.1 Acyclic cationic centres (Figure 2)
Figure 2.
Compounds with an acyclic cationic center.
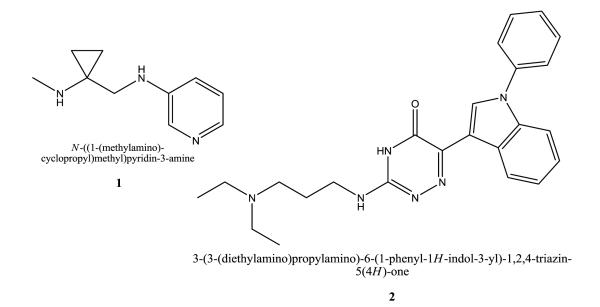
Cyclopropanamines with substituted pyridine moiety were developed by Les Laboratoires Servier [46, 47]. These compounds are mimicking to a certain extent Abbott’s famous pyridylether scaffold (e.g. see A-85380 (3)), but with a cyclopropane ring reducing conformational flexibility. The compounds were highly selective for binding to α4β2 over muscle, ganglionic, and α7 subtypes, and also over muscarinic receptors (M2/M4). The pyridinyl-amino-cyclopropanamine derivative 1 (S 38232) has a Ki value of 2.2 × 10−8 M for α4β2 tested with [3H]cytisine in rat brain homogenates and is a full agonist at rat α4β2 nAChR expressed in Xenopus laevis oocytes (EC50 = 3.4 × 10−6 M). Compound 1 is a very week partial agonist at rat α7 (EC50 = 1.3 × 10−4 M). In addition, 1 showed enhanced attentional performance in Wistar rats [48, 49].
A 4H-1,2,4-triazin-5-one scaffold with e.g. an acyclic cationic center (e.g. 2) were developed by Aventis Pharma S.A. [50]. Compound 2 interacts with the α7 subtype (Ki = 737 nM) evaluated by [3H]MLA (methyllycaconitine) binding assays in rat hippocampus membranes.
2.2 Azacyclic cationic centre (Figures 3, 4, and 5)
Figure 3.
Azetidine based ligands.
Figure 4.
Pyrrolidine based compounds.
Figure 5.
Compounds bearing diverse N-containing six- or seven-cyclic systems as cationic centers.
2.2.1 Monocyclic amine moiety
Azetidine, pyrrolidine, piperidine, piperazine, (di)azepane containing N-cyclic systems, and even pyridiniums as permanently protonated systems can be found in this category. Interestingly, these structural templates are attached to reiterating heteroaryls like pyridine, pyrazole, triazine, and oxadiazole. The monocyclic amine moieties can also be connected to spacers (e.g. ethenyl) or fused to other cyclic systems.
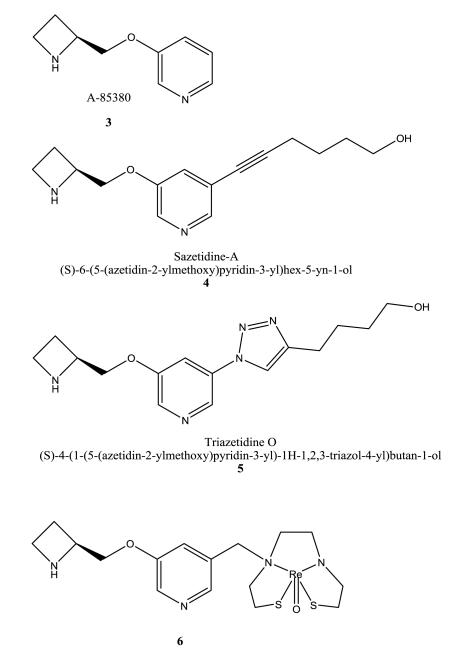
Nicotinic desensitizers based on the former Abbott template A-85380 3 are described to be useful for the treatment of a wide range of pathologic conditions (pain, addiction, etc.) [51]. The synthesis and characterization of the important prototype, sazetidine-A 4, and its analogs are described. These compounds are selective for α4β2 nAChRs. Parameters to identify suitable nicotinic desensitizers are mentioned by the inventors: a) the affinity should be less than 1 μM to the receptor; b) agonist activity (Emax) less than 10% of the Emax of an agonist; c) the inhibiting receptor activation by an agonist via desensitization (IC50(D)) should be less than 10 μM explored by a 10 min pretreatment; d) the inhibiting receptor activation by an agonist (IC50(A)) should be more than 1 μM when applied simultaneously; and e) the IC50(D) < the IC50(A). The developed compounds were tested for α4β2 and α3β4 subtypes using SH-EP1 cells expressing human α4β2 (hα4β2), HEK cells expressing rat α4β2 or rat α3β4 nAChRs in [3H]epibatidine based assays. Functional properties were explored by stimulation of 86Rb+ efflux. Sazetidine-A 4 does not show any effects in these ion efflux assays, only when there was a 10 min pretreatment, then it inhibited nicotine-stimulated 86Rb+ efflux. Sazetidine-A 4 has high affinity (Ki = 0.41 nM) for rα4β2 and a Ki value of 10,000 nM for rα3β4, respectively, which therefore display high subtype selectivity for α4β2 over α3β4. Low α3β4 nAChR agonist activity is preferable to avoid gastrointestinal adverse effects. Sazetidine-A’s 4 potency in desensitizing α4β2 is higher than its antagonistic effect (IC50(D, 10 min) = 26 nM; IC50(A) = > 10,000 nM). It also shows analgesic effects and a decrease in nicotine-self-administration in animal studies.
A further development of sazetidine-A 4 is triazetidine-O 5 [52]. It has improved crystallinity over sazetidine-A 4 by replacing the alkynyl group in 4 by the small heteroaryl group triazole. Its Ki values for α4β2 and α3β4 receptors are 3.51 nM, and 62,000 nM, respectively, reflecting a very high selectivity for the α4β2 receptor. Triazetidine-O 5 is also a potent desensitizer of α4β2 with an IC50 of 100 nM. The relative affinity of triazetidine-O 5 for α4β2 is lower than the affinity observed for sazetidine-A 4 (Ki = 0.41), but still in the low nanomolar range. But both compounds (4, 5) suffer from non-ideal logP (partition coefficient) and PSA (polar surface area) values regarding their efficacy of CNS drugs in vivo. Their logP values are relatively low (1.12 and 0.80, respectively) and the PSA values seems too high for suitable CNS drugs (113.5 Å2, and 156.6 Å2, respectively). Recently, further sazetidine-A based compound series were developed with cyclopropane, isoxazole, phenyl or amino side chains in position 5 of the A-85380 template. Some evaluated compounds of these series show high affinity for α4β2 subtypes, good selectivity over α3β4, and positive effects in the forced swim test in mice observing a reduction in immobility time [53].
Abbott’s famous pyridyl ether templates were also used by several groups for the development of α4β2 nAChR in vivo tracers [54, 55]. Chelates containing rhenium (Re) or technetium (Tc) radionuclides were attached to azetidinylmethoxypyridine based ligands for the diagnosis of e.g. Alzheimer’s disease. In radioligand binding assay using Wistar rat cerebral cortex homogenates, 6 inhibited [3H]cytisine binding with a Ki value of 22.0 nM. 18F-radiolabeled nifrolidine 7 and azetidinylmethoxypyridine analogs are claimed for in vivo imaging. The in vitro binding affinity of nifrolidine 7 was measured in rat brain slices using [125I]epibatidine (Ki = 2.89 nmol/L) or [125I]α-bungaratoxin. It showed in its F-18 labeled form binding to α4β2 receptors in the thalamus, presubiculum and brain cortex.
Abbott’s pyridylether derivative ABT-089 8 (pozanicline), which is a partial agonist at α4β2 with high affinity (Ki = 14 nM), and related compounds demonstrating selective α4β2 neuronal nAChR activity and weak stimulating effects on α4β2, α3β4 or α3β2 subtypes were used to study the efficacy in ADHD human clinical studies [56]. ABT-089 8 was well tolerated and successfully assessed using the Connor’s Adult ADHD Rating Scale (CAARS).
Targacept Inc. reported about a series of pyrrolidinylethenyl derivatives as nicotinic acetylcholine receptor modulators/agonists [57-59]. A prototype of this invention is 9 displaying high affinity for hα4β2 (SH-EP1 cells) and for rat cortices α4β2 (Ki values: 2-4 nM) whereas it showed a Ki value of higher than 10,000 nM in [3H]MLA experiments with rat brain homogenates. Affinity values for α3β4, using SH-SY5Y membranes, and for human native muscle-type receptors (TE-671 membranes) were obtained in [3H]epibatidine radioligand binding assays presenting Ki values of 3,400 nM and of 25,000 nM, respectively. Its functionality profile displays an EC50 value of 0.1 μM (Emax: 76%) in a calcium ion flux assay for SH-EP1/human α4β2 cells, and EC50 values of 11 μM (Emax: 13%), and 13 μM (Emax: 37%) for SH-SY5Y/human α3β4 and TE-671/human muscle cells, respectively. The IC50 for hERG channels (human HEK-239 cells) was determined to be 84 μM. Also, multiple receptor screening (65 receptors) for 9 provided a good off-target profile. After p.o. administration of compound 9, normal rats showed improved long-term visual episodic/declarative memory in the novel object recognition (NOR) task. The minimum effect dose (MED) level was determined to be 0.004 μmol/kg. The recognition index at this dose was 70% at 8h. In a second cognitive test, the radial arm maze (RAM) test, 9 attenuated the cognitive deficits induced by scopolamine. Finally, a screening at five major CYP450 isoenzymes showed no evidence of inhibition (IC50 > 20 μM).
Different series of 7-azaindoles with partial agonism on the α4β2 nAChR and dopamine reuptake inhibition properties were developed by Solvay Pharmaceuticals B.V., Netherlands [60]. The compounds were designed as conformationally restricted analogs of trans-meta-nicotine (TC-2403) and evaluated for rat brain nAChR activity in [3H]cytisine binding assays. Compounds 10 and 11 exhibited Ki values of 125 nM and 10 nM, respectively. [3H]Dopamine (DA) reuptake inhibitory activities measured in rat synaptosomes showed pIC50 values of 5.1 and 5.8 for 10 and 11, respectively. [3H]Dopamine release was determined to be 151% and 187%, respectively. 10 has a partial agonist profile displaying 62% of the in vitro efficacy of nicotine also blocking nicotine’s response in vitro (70%). Further evaluation of 10 revealed good metabolic stability in human liver homogenates (t1/2: 266 min) and brain penetration. However, i.v./p.o. studies in rats showed a low bioavailability (11.3%). In contrast to 10, compound 11 is a full agonist with similar efficacy profile like nicotine.
Diverse oxadiazoles, more precisely 5-aryl-1,3,4-oxadiazole-2-amines, are claimed by the Glaxo Group, which bear e.g. pyrrolidine or piperidine rings as the amine part (12) [61-64]. These compounds are described as α7 receptor agonists. They were assessed by a FLIPR-Ca2+ (Fluorometric Imaging Plate Reader) assay using GH4C1 cells stably transfected with human α7 nAChR. The compounds exhibited pEC50 values ≥ 5-7.
Novel anabaseine derivatives are disclosed as ligands for α7 nAChRs. Example compound 13 displayed a modest Ki value of 200 nM for the α7 subtype [65, 66].
Diverse series of azaaromatic bis-, tris-, or tetrakis-quaternary ammonium salts were prepared and evaluated as modulators at nAChRs [67-72]. The compounds were assayed for [3H]nicotine, and [3H]MLA binding inhibition, in addition to the inhibition of nicotine-evoked [3H]DA release from superfused rat striatal slices. A bis-quaternary ammonium derivative 14 exhibited Ki values of 8.17 μM against [3H]nicotine and >100 μM against [3H]MLA. It inhibited the nicotine-evoked [3H]DA release with an IC50 value of 2 nM. These novel nAChR antagonists are claimed e.g. as smoking cessation agents. Recently, some compounds of these series were further evaluated regarding their activity for e.g. α6 containing subtypes, which mediate some of the reinforcing effects of nicotine, and displayed noncompetitive inhibition at α4/6 chimera expressed in Xenopus oocytes [73, 74].
DMPP 15, a non-selective nAChR agonist, and derived compounds like ASM-002 16 and ASM-003 17 were prepared and tested for anti-inflammatory and smooth muscle relaxing activity [75, 76]. DMPP 15 cannot cross the blood-brain barrier due to its permanently charged nitrogen and its lack of a transporter system. It has anti-inflammatory properties (e.g. decrease of white blood cells number, decrease of cytokine production) and relaxing effect on airway smooth muscle cells after an initial short contractive effect. DMPP 15 and analogs were evaluated for their effects on tumor necrosis factor (TNF) release using human monocytes isolated form the blood of asthmatic patients and stimulated with LPS. Most of them had inhibitory effect on the release. Also DMPP 15 and some analogs were active to down-regulate leukotriene C4 (LTC4) release using blood eosinophils in an enzyme immunoassay. The following results were obtained: 545.00 LTC4 pg/ml for DMPP 15; 246.40 LTC4 pg/ml for ASM-002 16; 1725 LTC4 pg/ml for the control. DMPP 15 and its analogs ASM-002 16 and ASM-003 17 have a dose dependant relaxation effect on tracheal smooth muscles when pre-contracted with metacholine in a mouse experiment. ASM-002 16 was further explored in asthmatic dogs shown to inhibit cellular inflammation in the lungs with similar efficacy as prednisone.
α7 nAChR agonists bearing a piperazine scaffold (e.g. 18) were tested for binding affinities on PC12 cells and exhibited IC50 values between 1 nM and 10 μM [77].
Azepinoquinoxalines were developed by Targacept, Inc., and evaluated for their neuronal nAChR affinity [78]. A compound of this series (19), a simple monocyclic basic N-containing cycloheptane is fused to an aromatic system mimicking a structural part of varenicline – the quinoxaline part. It exhibited preference for α4β2 with a Ki value of 10 nM for the human α4β2 subtype and 17 nM for the rat α4β2 nAChR, an EC50 value of 4.4 μM (Emax: 21%) for human α3β4, and an EC50 value of 75 μM (Emax: 23%) for the human muscle subtype. Using scopolamine in normal rats to impair cognitive performance 19 showed improvement in three tasks (NOR, RAM and MWM (Morris water maze)).
Wyeth and Siena Biotech claimed diverse heterocyclylalkyl amide and urea derivatives with N-biaryl- and N-[(aryl)pyrazolyl] moieties (e.g. 20) as α7 agonists for the treatment of psychotic and neurodegenerative diseases [79-84]. Compounds showed IC50 values between 10 nM and 30 μM against α7 nAChR (GH4C1/rat) in a calcium ion FLIPR assay.
2.2.2 Bicyclo-heptanes (Figure 6)
Figure 6.
Bicyclo-heptanes.
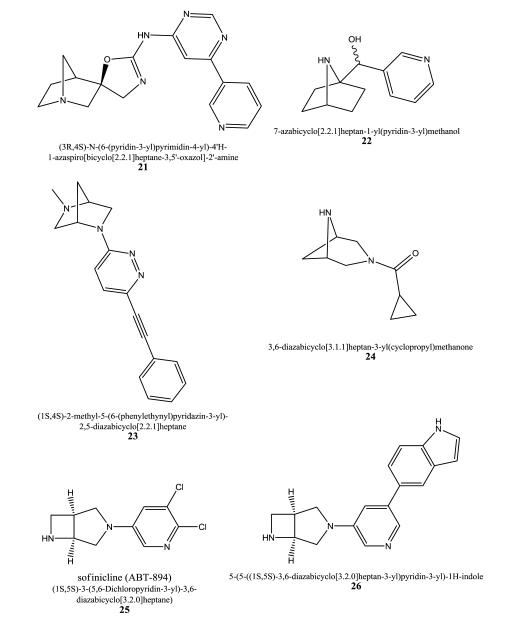
Spiro-cyclic compounds bearing an 1-azabicyclo[2.2.1]heptane and an oxazol-2′-amine component which is further substituted with diverse heteroaryl moieties were developed as α7 ligands by Bristol-Myers Squibb Company [85]. EC50 values derived from FLIPR assays and α7/HEK-293 cells are reported. Compound 21 displayed an EC50 value of 34 nM.
22 is a compound of a 7-azabicyclo[2.2.1]heptane series developed by researchers from the Universiteit Gent, Belgium, for the treatment of e.g. epilepsy, Alzheimer’s disease, Parkinson’s disease, schizophrenia, pain, and nicotine abuse. Unfortunately, no biological data are provided [86].
Diazabicyclic heptanes are developed by several companies with diverse subtype preferences. For example, 23 was synthesized and evaluated for [3H]α-bungarotoxin inhibitory activity using rat brain homogenates by Neurosearch A/S [87]. 23 exhibited an IC50 value of 2.0 μM.
Targacept’s 3,6-diazabicyclo[3.1.1]heptane derivatives are compounds with α4β2 and/or α6-containing subtype modulating activities [88]. When tested at SH-EP1/human α4β2 cell lines they exhibited Ki values in the range of 2-11,000 nM using [3H]nicotine as a radioligand. For the α6β3β4α5 subtype Ki values in a range of 38–100,000 nM were found when tested against [3H]epibatidine. Compound 24 displays Ki values of 140 nM, 3 nM, 2.3 nM for the α6β3β4α5, hα4β2, and rα4β2 subtypes, respectively. Neuroprotective effects were observed on MPP+ (4 μM) injuries for compounds of this invention when a 48h pre-treatment on dopaminergic neurons was used.
Biaryl substituted diazabicycloalkane derivatives using the same structural template displayed in sofinicline (25), 3,6-diazabicyclo[3.2.0]heptane, were developed by Abbott Laboratories [89]. Sofinicline 25 is an important lead compound with high affinity for α4β2 receptors (Ki of 0.10 nM) and shows agonist activity on α4β2 and α3β4 nAChRs expressed in HEK-293 using FLIPR-Ca2+ assays. 25 is in clinical trials for ADHD, but a phase II clinical study for neuropathic pain has been discontinued. The novel derivatives were tested for their affinities in [3H]MLA, [3H]DPPB (tritiated (S,S)-2,2-dimethyl-5-(6-phenyl-pyridazin-3-yl)-5-aza-2-azonia-bicyclo[2.2.1]-heptane iodide), and [3H]cytisine binding assays. They displayed Ki values in the range of 1 nM to 10 μM in the [3H]MLA assay, and Ki values of 50 nM to 100 μM using [3H]cytisine. The preferred compounds (e.g. 26) in this patent had greater potency at α7 which is in contrast to sofinicline 25.
2.2.2 Bicyclo-octanes (Figure 7, 8, 9, and 10)
Figure 7.
Azabicyclo-octanes.
Figure 9.
Quinuclidines.
Figure 10.
Bicyclo[3.3.0]octanes.
Azabicyclic compounds where the nitrogen position is in position 1 of an azabicyclo[3.2.1]octane scaffold were evaluated as α7 ligands [90]. They are homologs of the azabicycloheptane series of the same company, Bristol-Myers Squibb (see e.g. 21), and were also evaluated in FLIPR assays with HEK-293 cells expressing α7 receptors. 27 has an EC50 value of 88 nM in the described assay. According to the patent application the compounds might be able to disrupt the interaction of Aβ1-42 and α7 nAChRs, modify Alzheimer’s disease, and may be beneficial related to inflammatory processes in neurodegenerative diseases, but also in the treatment of RA and OA. Reduction of weight gain and food intake along with reduction of elevated plasma levels of triglycerides, glucose, glycated hemoglobin and TNFα in a mouse model of type II diabetes was also shown for an agonist in this series, but it was not further specified.
28 is a prototype of a 5-(pyridin-3-yl)-1-azabicyclo[3.2.1]octane derivative series developed as α7 receptor ligands [91, 92]. In general, some derivatives were selective for α7, while other analogs were mixed α4β2 and α7 subtype compounds. 28 has an IC50 value of 83 nM for α7 and displayed 36% inhibition at 1 μM of [3H]cytisine.
Series of fused bicycloheterocycle substituted 8-aza-bicyclo[3.2.1]octanes were reported by Abbott Laboratories [93, 94]. Abbott Laboratories are often using [3H]MLA or/and [3H]DPPB binding assay for obtaining α7 affinity values and the [3H]cytisine binding assay for the α4β2 subtype. Ki values were in the range of 1 nM to 10 μM in the [3H]MLA assay, and about 1 nM to 100 μM using [3H]cytisine. Compounds 29 and 30 are examples bearing an oxy-pyridazine-indole or an oxy-thiazole-indole part. The pyridazine-indole or thiazole-indole moiety attached to diverse bicyclic systems can be found very often in novel nAChR ligands claimed by many inventors.
Diaza-bicyclo[3.2.1]octane derivatives were developed by several companies. Neurosearch’s 1,4-diazabicyclo[3.2.1]octane derivatives with heteroaryl and/or fused heterocyclic systems displayed activities in the sub-micromolar range for the binding to the rat brain α7 subtype in [3H]α-BTX assays [95, 96]. Compound 31 has an IC50 value below 0.1 μM.
3,6-diazabicyclo[3.2.1]octanes developed by Pfizer were evaluated for hnAChRα6/α4-β4 receptors with [3H]epibatidine (KD = 0.23 nM). 32 exhibited high affinity on these subtypes (IC50 value of 0.171 nM) [97].
The 3,6-diazabicyclo[3.2.1]octane template was also used by Targacept, Inc. for the development of α4β2 and α7 nicotinic acetylcholine receptor ligands [98, 99]. An example is 33 bearing a carbonyl group for the HBA pharmacophore. It interacts with human α4β2 receptors with high affinity (Ki = 7.3 nM). In addition, certain compounds were tested positive in the NOR task.
3,8-Diazabicyclo[3.2.1]octane aryl derivatives were claimed by Neurosearch A/S as α7 ligands and as modulators of the monoamine receptors and transporters [100-102]. A few compounds were tested for the affinity to α7 with [3H]α-bungarotoxin in cerebral cortices membranes from male Wistar rats and all expressed IC50 values below 1 μM. 34 exhibited an IC50 value of 0.47 μM. The phenylethynylpyridazine moiety found in 34 is also used in other compound series of the same company.
For many years, the quinuclidine (1-aza-bicyclo[2.2.2]octane) scaffold continues to be important for the design of α7 nAChR compounds [45]. Substitutions at position 3 still dominate, mostly with more complex heteroaryl substitutents.
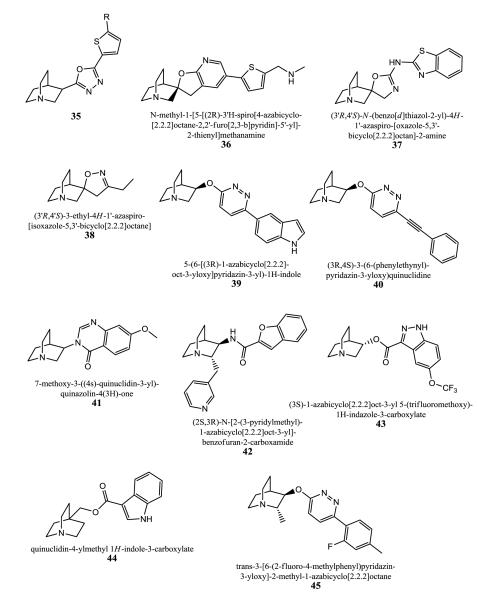
Quinuclidine derivatives with linking 5-membered heteroaryl, such as oxadiazole (35), or triazole were developed as α7 agonists [103]. The prepared quinuclidine derivatives were assayed for α7 ([125I]α-bungarotoxin; rat hippocampal membranes) and also for α4 nAChR binding activity ([3H]nicotine; rat cortical membranes).
Further similar compounds with a spiro motif (spiroazabicyclooctane-furopyridines) were prepared by the same company, AstraZeneca AB, and display Ki values of <10 μM in α7 nAChR receptor assays with [125I]α-BTX and rat hippocampal membranes and HEK cells expressing human α7 [104-106]. These compounds relate also to a novel method of treatment or prophylaxis of osteoarthritis (OA). Alpha-7 receptors are expressed in human articular cartilage (shown by the inventors). An agonist can reduce the level of MMP13 expression and aggrecanase activity in cartilage in a mouse model of acute articular inflammation using very low dose. The prototype compound 36 reduced MMP13 levels at doses as low as 0.0001 mg/kg.
Bristol-Myers Squibb’s quinuclidines for α7 receptors are spiro compounds and similar to Astra Zeneca’s ligands [107]. The compounds were tested for α7 activity using HEK-293 cells and the Ca2+ sensitive fluorescence-based assay (FLIPR) to evaluate functionality. Compound 37 had an EC50 value below 100 nM.
A third series of quinuclidines with diverse spiro motifs (e.g. 38) are developed as agonists of α7 receptors by the Universita Degli Studi di Milano [108]. Some compounds possess nanomolar affinities for α7 (Ki = 4-80 nM). They were evaluated in [125I]α-BTX and [3H]epibatidine binding assays, also electrophysiologically, and selected compounds were studied in the passive avoidance (PA) task to test the capability to reverse scopolamine-induced amnesia in rats. Data for example compounds were not provided.
Abbott Laboratories claimed the α7 receptor agonist 39 (ABT 107) together with an antipsychotic composition like risperidone for the treatment of schizophrenia [109]. The α7 receptor agonist alone did not display any effect at low dose (0.04 mg/kg), but in combination with a sub-efficacious dose of risperidone (0.1 mg/kg) a maximally efficacious response was observed. Further fused bicycloheterocycle substituted quinuclidine derivatives as α7 ligands were developed by the same company and had Ki values in the range of 1 nM to 10 μM in [3H]MLA assays [110-112].
Quinuclidinyloxypyridazines and azabicyclooctyl-quinazolones are α7-subtype ligands with sub-micromolar affinities. 40 and 41 are examples in these series [113-115].
Quinuclidines substituted at positions 2 and 3, and their heteroaryl-substituted diazatricycloalkane derived compounds express affinity for α7 nAChRs with Ki values below 100 nM in [3H]MLA assays, and possess low affinity for α4β2 subtypes (Ki > 10 μM) tested with [3H]nicotine in rat brain homogenates [116-119]. The compounds’ functional profiles were examined for the human muscle subtype (TE671/RD), for the ganglionic subtype (PC12/rat, SH-SY5Y/human) via 86Rb+ efflux experiments, and for α7 assays in FLIPR-Ca2+ assays. Furthermore dopamine release studies using rat striatal synaptosomes were carried out. Since the quinuclidine template is also often found in muscarinic ligands a possible interaction with M3 receptors was evaluated in [3H]QNB radioligand binding assays with the human clonal cell line TE671/RD. 42 was studied in more details. It has a Ki value of 1nM for the α7 subtype and shows activity for it in contrast to its stereoisomers. Its stereoisomers have the following affinities (Ki values): 2R,3S displays 42 nM, 2R,3R has 1 nM, and 2S,3S shows 25 μM. The active compound has a Ki value of > 2 μM for α4β2. In patch clamp experiments with rat α7 expressed in GH4C1 cells 42 showed an EC50 of 14 nM and Emax = 93% relative to ACh, displaying agonism. In Xenopus oocytes the EC50 value is 33 nM (Emax = 100% of ACh response). There was little or no agonist activity on other nAChR subtypes. Since there is close sequence and structural homology between α7 and 5-HT3 receptors, 42 was evaluated for its affinity and functionality. At a concentration of 10 μM it displayed 59% inhibition at the mouse 5-HT3 and 25% inhibition at the human receptor. Furthermore, 42 did not show any interaction with muscarinic receptors. 42 shows efficacy in two behavioral models of cognition, object recognition paradigm in rats and radial arm maze paradigm. It also has effectiveness in reversing apomorphin-induced locomotor activity and in pre-pulse inhibition models.
1-Azabicyclo[2.2.2]octyl-heterocyclic amide or esters with e.g. indazole, benzothiazole, benzoisothiazole, benzisoxazole, pyrazolopyridine, or isothiazolopyridine as the heterocyclic part were prepared as α7 receptor ligands by Memory Pharmaceuticals Corporation [120, 121]. A prototype 43 is shown in Fig. 9. Affinity values (Ki) are ranging between 1 nM and 10 μM in a [3H]MLA assay with rat brain homogenates.
Quinuclidine derivatives substituted at the position 4 at the azabicyclic skeleton (e.g. 44) were prepared as α7 nAChR compounds and showed Ki values between 20 nM and 1 μM in the [125I]α-BTX assay using rat brain membranes [122].
An example of Novartis’ 3-(heteroaryloxy)-2-alkyl-1-azabicycloalkyl series as selective α7 ligands is 45 [123]. pEC50 values for α7 are given as a range between 5 and 9 using GH3 cells and FLIPR technology. They were also evaluated for other nAChR subtypes and 5-HT3 using the same approach. The compounds induce sensory gating at concentration between 10 and 40 μM in mice with sensory gating deficit (DBA/2-mice). An increase in attention in the 5-choice serial reaction time test (5-CSRTT) with rats was also observed.
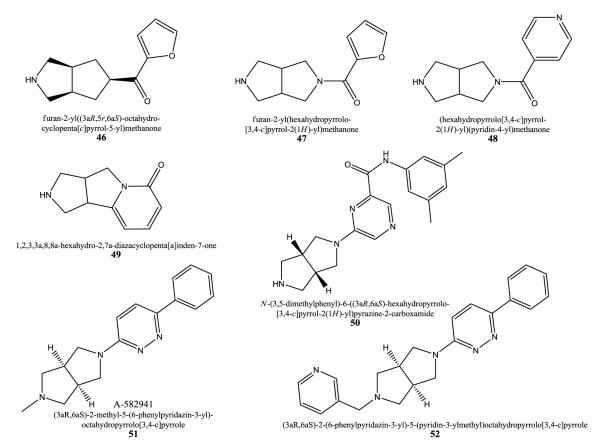
One or two nitrogen containing bicyclo[3.3.0]octanes bearing an amide, ketone, or ester moiety as spacers with HBA qualities were developed as α4β2 ligands [124, 125]. These compounds show preference for the α4β2 subtype with Ki values for many of them less than 100 nM carried out with [3H]nicotine and membranes from rat cortex and from SH-EP1/human cells. [3H]MLA binding was also evaluated and functionality was explored using 86Rb+ efflux. Compound 46 exhibits nanomolar affinity (Ki = 13.2 nM) for hα4β2 receptors, and had no effect on α7. Compound 47 showed Ki values of 20 nM and 33 nM for the rα4β2 and hα4β2, respectively, and 13,000 nM for the α7 subtype. In contrast, 48 displaying a pyridyl moiety failed to show any α7 receptor activity and displayed Ki values of 26 nM and 160 nM for the rα4β2 and hα4β2, respectively.
Keeping the 2-pyridone moiety of cytisine 1,2,3,3a,8,8a-hexahydro-2,7a-diazacyclopenta[a]inden-7-ones were developed in the treatment of addictive disorders [126]. Compounds inhibit the interaction with [3H]nicotine with IC50 values in the range of 1-9 μM in rat brains. 49 bears the 3,7-diazabicyclo[3.3.0]octane scaffold fused with a 2-pyridone.
50 is a 3,7-diazabicyclo[3.3.0]octane derivative with an attached pyrazine moiety connected via an amide bridge with a further 3,5-dimethylphenyl moiety. 50 and analogs interact with the α4β2 subtype and were developed by Abbott Laboratories [127]. Ki values measured in rat brain homogenates against [3H]cytisine are ranging from 0.01 nM to 1.0 μM. In contrast, 51 (A-582941), and 52 have high affinity for α7 (Ki values of 11 nM and 3.6 nM, respectively) in displacement of [3H]A-585539 ((1S,4S)-2,2-dimethyl-5-(6-phenylpyridazin-3-yl)-5-aza-2-azoniabicyclo[2.2.1]heptane) from rat brain. 51 has an EC50 value of 9.36 μM with 84% maximal response in Xenopus oocytes expressing human α7 receptors.
2.2.3 Bicyclo-nonanes (Figures 11 and 12)
Figure 11.
Bicyclononanes I.
Figure 12.
Bicyclononanes II.
(1-Azabicyclo[3.3.1]non-4-yl)-[5-(1H-indol-5-yl)heteroaryl]amines and analogs with aryloxy moiety, [(1H-indol-5-yl)heteroaryloxy]-1-azabicyclo[3.3.1]nonanes, were developed by Novartis Pharma for the treatment of psychotic and neurodegenerative disorders [128, 129]. In a functional assay for the α7 subtype in GH3 cells, they showed pEC50 values of 5-9. The compounds were also studied for sensory gating and are active for concentrations between 10 and 40 μM. 53 is a prototype.
Some 3-azabicyclo[3.3.1]nonanes are developed by Eli Lilly as nAChR agonists [130]. They have Ki values for the α4β2 subtype of less than 50 nM and they express at least 2-fold selectivity for α4β2 over α3β4. Compound 54 shows higher affinity for α4β2 with a Ki value of 5.37 nM comparing with 479 nM for α3β4 examined in a scintillation proximity assay (SPA) with [3H]epibatidine. The EC50 value for dopamine release is 135 nM and its efficacy 41.7%.
55 (SR17026) and 56 (SR16584) are 9-azabicyclo[3.3.1]nonanes and members of a 7- to 11-membered azabicyclo ring compound series claimed by SRI International [131, 132]. SR17026 (55) has affinities (Ki values) of 240 nM for α3β4 and 10 nM for α4β2 receptors measured in [3H]epibatidine radioligand binding assays using membranes derived from HEK cells transfected with rat α4β2 and α3β4 receptors. The indolin-2-one part (SR16584, 56) decreases the affinity for α4β2 receptors dramatically whereas the affinity for α3β4 is quite similar to SR17026 (55).
N-aryl 1,7-diazaspiro[4.4]nonanes (e.g. 57) were disclosed by Targacept, Inc. for the treatment of drug addiction and/or obesity. Evaluated Ki values for α4β2 are less than 1 μM [133].
The bispidine (3,7-diaza[3.3.1]nonane) template, a cytisine’s scaffold, can be found in diverse patents. Targacept, Inc., linked it via a carbonyl functionality with diverse (cyclo)alkyl and (hetero)aryl substituents [134-136]. These amides were tested with the radioligand [3H]nicotine and membranes from rat cortex and from SH-EP1/human for α4β2. [3H]MLA binding for α7 receptors was also evaluated and functionality was explored using 86Rb+ efflux. Ki values range between 1 and 1,000 nM (rα4β2) and 1 and 220 nM (hα4β2), respectively, whereas Ki values for the α7 subtype are in the range of 1,700 to 210,000 nM. Little activity was stated for the ganglionic and muscle subtypes. Certain compounds, but not further specified, were tested in the NOR task and found to be active. The bispidine amide 58 showed α4β2 affinity in the lower nanomolar range (Ki = 18 nM) whereas the Ki value for α7 is much higher (1,700 nM). An example for an oxabispidine is 59. A 5-fluoro-3-pyridyl moiety is attached to the bispidine part and the compound shows nanomolar affinites for α4β2 (Ki values 1.3(r)/1.8(h)) and α7 (Ki values 36(r)/76(h)).
Cytisine analogs were claimed by diverse institutions and companies. Some derivatives were described as nicotinic desensitizers [51]. An example of a 10-substituted cytisine derivative is 60 with the following affinity values (Ki): 11 nM for α4β2, 32 nM for α2β2, 3,000 nM for α2β4, 467 nM for α3β2, 10,000 nM for α3β4, and 68 nM for α4β4 subtypes [137].
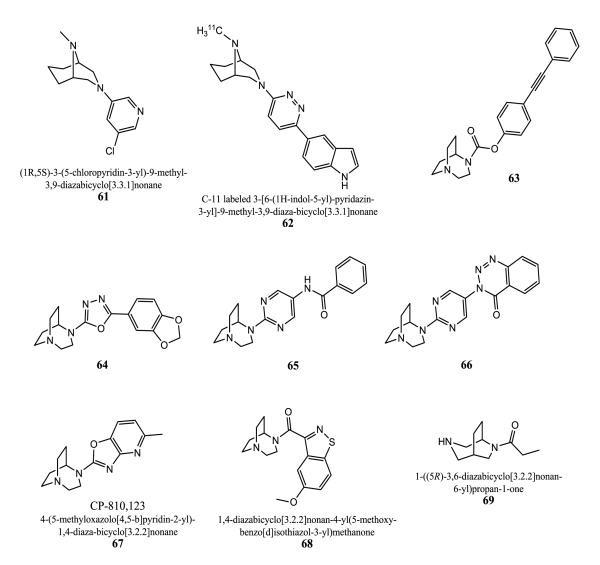
Examples of the 3-heteroaryl-3,9-diazabicyclo[3.3.1]nonane series are 61 and 62 [138-140]. Compound 61 inhibited [3H]α-bungarotoxin binding to rat brain α7 receptors with an IC50 value of 6.3 nM in [3H]cytisine binding assays, and 62 was developed for in vivo imaging (PET) of brain α7 receptors when labeled with C-11.
An enormous variety of 1,4-diazabicyclo[3.2.2]nonane derivatives were developed by Neurosearch A/S and claimed as [3H]α-bungarotoxin inhibitors with IC50 values mostly below 0.4 to 0.01 μM for rat brain [141-166]. The 1,4-diazabicyclo[3.2.2]nonane scaffold can be attached to an ester or (hetero)aryl part like phenyl, pyridine, pyrimidine or oxadiazole, followed by further substitution with mostly (hetero)aryl groups (Fig. 12, 63-66). Corresponding N-oxides are also claimed to be active α7 ligands.
Pfizer developed azabenzoxazoles with the 1,4-diaza-bicyclo[3.2.2]nonane scaffold as α7 agonists for the treatment of CNS disorders [167-170]. They were tested on the chimeric α7-5HT3 ion channel in a FLIPR assay. 67 (CP-810,123) showed a Ki value of 13.5 nM for rat α7 expressed in GH4C1 cells using [125I]α-BTX. Its efficacy is 46% with an EC50 value of 244 nM. 67 has micromolar affinities at other nAChR subtypes: Ki value of 1,350 nM for α4β2 and 5,370 nM for the ganglionic type. At 5-HT3 it displays reasonable affinity (Ki = 269 nM) and an IC50 value of 5 nM in a functional antagonist assay with human skin epithelial cells. There is no effect on hERG channels. It has in vivo efficacy in a P50 gating deficit model using amphetamine and improved performance in the rat NOR task. 67 has good metabolic stability in human liver microsomes and its brain/plasma ratio in rats is favorable with the value of 1.5 determined after 40 min of s.c. application (1 mg/kg).
68 is an example of an α7 ligand series developed by Memory Pharmaceuticals [171, 172]. Biological data were not defined.
Amides of 3,6-diazabicyclo[3.2.2]nonane were prepared as α4β2 ligands by Targacept, Inc. [173]. The propanone derivative 69 has a Ki of 35 nM for α4β2 nAChR.
2.2.4 Bicyclo-decanes (Figure 13)
Figure 13.
Azadecanes and adamantanes.
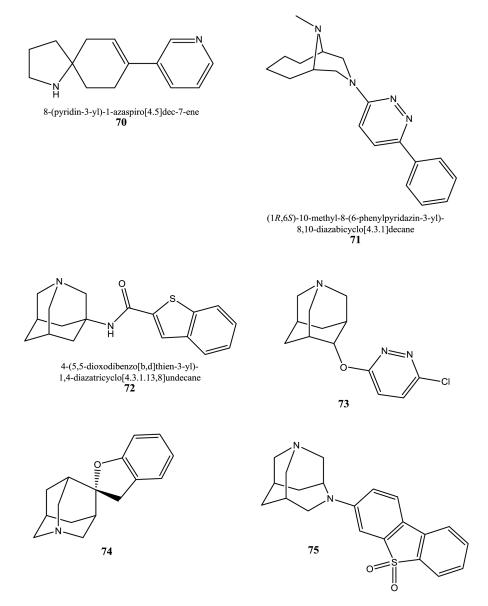
Azaspiroalkenes and azaspiroalkanes developed by Targacept, Inc., were tested for rat α4β2 and α7 using [3H]nicotine and [3H]MLA as radioligands, respectively [174]. They were also evaluated for other nAChR subtypes and dopamine release, but biological data were not provided. Compound 70 displays a prototype.
8,10-Diazabicyclo[4.3.1]decane derivatives possess preference for the α7 subtype [175]. 71 is a homolog of the diazabicyclo-nonane series developed by the same company, Neurosearch A/S.
2.3 Adamantanes (Figure 13)
Abbott Laboratories developed several series of aza-adamantanes interacting with α7 and /or α4β2 receptors [176-184]. Routinely, [3H]MLA or [3H]DPPB were used for the determination of α7 affinity, and [3H]cytisine for α4β2 subtypes. 72-75 are examples of these series. The aza-adamantane part can be attached to e.g. an ester, an amide, a carbamate, an ether, or a spirocyclic system to join further, mostly heteroaryl, substituents. The diazahomoadamantane derivative 72 exhibited a Ki value of 2.3 nM for the α7 subtype in a binding assay using rat brain homogenates and [3H]DPPB.
2.4 Allosteric modulators
The following section will give an overview of so-called positive allosteric modulators (PAMs) stated in the patent applications by the inventors. This area has grown enormously in the last couple of years. PAMs potentiate receptor response to ACh without triggering activation or desensitization themselves.
2.4.1 α4β2 PAMs (Figure 14)
Figure 14.
α4β2 PAMs
Abbott Laboratories developed different series of PAMs. There are 3-benzoyl-3,9-diazaspiro [5.5] undecane and 3,9-diazaspiro[5.5]undecane-3-carboxamide derivatives which increase fluorescence in calcium ion flux assays by 120-500%, and their EC50 values range from 10 nM to 100 μM [185]. Potential adverse effects regarding arrhythmia were screened through the ability to interact with hERG cardiac potassium ion channels. This was measured by radioligand binding assays using the H-3 labeled class III antiarrhythmic drug and potent hERG blocker dofetilide together with HEK-293 cell membranes. 76 had an EC50 value of 0.04 μM in the calcium ion flux assay and displayed a Ki value of 19 μM in the [3H]dofetilide assay. Compounds of the next series are aza-cyclic 1H-indole-2-carboxamides [186]. 77 potentiates the fluorescence response by > 100% in calcium ion flux assays. Compounds displaying bis(hetero)aryl systems with a [1,2,4]oxadiazole core are the largest series of Abbott’s α4β2 PAMs showing an activity range of 200-400% when evaluated in calcium ion flux assays using HEK-293 cells expressing human α4β2 [187-193]. 78 is a prototype of this series and belongs to the activity range of 200-400%. This compound was also used in its tritiated form ([3H]POB) to determine binding to human cortex membranes. It shows a KD value of 60 nM and Bmax value of 2900 fmol/mg protein, respectively. Exemplary compounds, but not further specified, showed efficacies in in vivo models of neuropathic pain and of monoiodoacetate-induced osteoarthritis (MIA-OA). The same structural concept works for isoxazoles. Compound 79 is a dipyridyl analog developed by Abbott Laboratories [194]. The 3,5-diphenyl derivative 80 is commercially available (Sigma Aldrich).
For Neurosearch’s bis(hetero)aryl based α4β2 PAMs diverse core structures are claimed: e.g. oxadiazole, thiadiazole, 1,2,3-triazole, and tetrazole moieties [195-201]. The oxadiazole 81 exhibited an EC50 value of 6.8 μM in calcium ion based FLIPR assays with HEK cells and 97 % of maximal response relative to nicotine. A prototype of the >triazole series is 3-(4-pyridin-3-yl-[1,2,3]triazol-1-yl)-benzonitrile 82 showing a ΔpEC50 value of 1.6 μM which is similar to the other prototype bearing a the tetrazole core 83 (ΔpEC50 = 1.5 μM).
2.4.2 α7 PAMs (Figures 15, 16, and 17)
Figure 15.
α7 PAMs I
Figure 16.
α7 PAMs II
Figure 17.
α7 PAMs III
Pfizer’s PNU-120596 (1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)urea) and Neurosearch’s NS-173810 1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro-5-(trifluoromethyl)phenyl)urea) are substituted bis(hetero)aryl derivatives with an urea core and already known α7 PAM prototypes. Right now, two types of PAMs are discussed. Type I predominately affects the peak current response, whereas type II increases the peak current responses and changes the desensitization profile of the agonist response. Some PAMs have been described to be active in animal models (cognition; gating deficit). Also pharmacophoric elements for some compound series have been reported like an HB-donor moiety (e.g. amide NH moiety) and an HB-acceptor part (e.g. carbonyl group) in the core, and three hydrophobic regions.
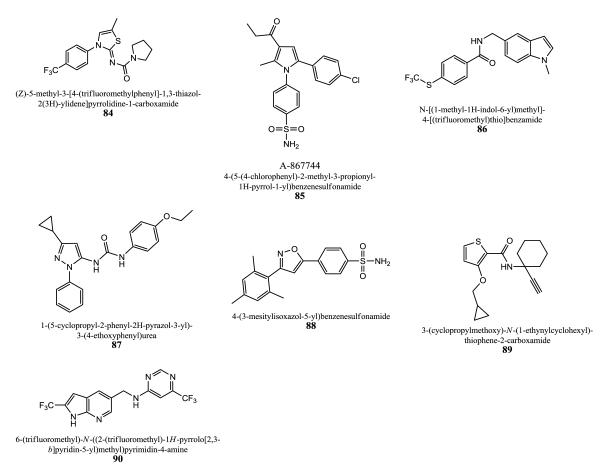
High-throughput calcium ion flux assays were used by Abbott Laboratories to examine thiazoline, oxazoline, and thiazolylidine urea and amide based compounds in IMR-32 cells endogenously expressing α7 receptors [202, 203]. Effects on current responses were also applied to evaluate compounds. A thiazolylidine urea core bearing analog 84 displayed an EC50 value of 4 μM and 300% potentiation regarding 100 μM ACh-evoked α7 currents in oocytes. 85 (A-867744) has an EC50 of 1.0 μM and an efficacy of 52% tested in a FLIPR assay using human IMR-32 neuroblastoma that endogenously express α7 nAChRs. This compound belongs to a pyrrole-sulfonamide series developed by Abbott Laboratories [204]. It does not potentiate Ca2+ transients at human recombinant α4β2 and α3β4 nAChRs expressed in HEK-293 cell lines evoked by nicotine up to a concentration of 30 μM. The bioavailability is high with 83% in rats, lower in dog (55%) and monkey (68%). A terminal elimination half-life was obtained in the range of 1.0-1.7 h. There is no inhibition of major liver metabolic enzymes, and the interaction with hERG using 3H-labeled dofetilide radioligand binding assays did not show any significant activity (IC50. value of > 20 μM). In addition, measurement of phosphor-ERK (extracellular receptor kinase) activity via western blot procedure was used to examine positive allosteric modulators, since an increase of ERK phosphorylation in the presence of selective alpha7 agonists can be observed, but data are not given. The amide moiety is the core moiety of a further compound series from Abbott Laboratories [205]. 86 exhibited an EC50 value of 0.21 μM and its degree of potentiation is 91%.
Astrazeneca AB developed diverse series of PAMs: Pyrazole derivatives (e.g. 87), azoles containing an arylsulfonamide moiety (88), and N-propynylthiophene-2-carboxamides (89) [206-208]. Xenopus oocyte current recording and Ca2+ influx-imaging assays were used to evaluate the compounds.
Indoles were explored by Glaxo Group Limited as α7 PAMs [209-212]. An example compound is 90 displaying activity at the GH4C1 cells α7 nAChR and showed pEC50 value of ≥ 6.0. The maximum potentiation of the response area relative to nicotine control was about 1200%.
Janssen Pharmaceutica classified its compounds to PAM types 1-4 related to their kinetic properties in whole-cell voltage-clamp recordings using the agonist choline (Fig. 1) at a concentration of 1 mM. The PAM and choline are simultaneously applied: type 1 enhances the effect size of the current; type 2 enhances the effect size of the current and reduces the rate and/or the extent of desensitization; type 3 enhances the effect size of the current and when applied at higher concentrations (up to 10 uM), it inhibits desensitization; type 4 will show first a desensitization and then a re-opening. Aminothiazoles, trisubstituted triazoles, trisubstituted pyrazoles, and morpholinothiazoles are described by the company [213-219]. Potency pEC50 values and % efficacy are provided for some compounds and are derived from calcium ion assays using α7-wt nAChR in GH4Cl cells. The PAM type was determined from patch clamp current recordings. Efficacy values were found to range from 1.1 to 4.5 relative to choline for some aminothiazole derivatives (e.g. 91). The trisubstituted triazoles with PAM type classification are shown in Figure 16. 92 is a PAM type 1 compound with pEC50 of 6.61 and 490% efficacy. The PAM type 2 compound 93 displays a pEC50 value of 6.7 with 3940% efficacy. Type 3 94 has a value of 6.67 for pEC50 (4660% efficacy), and type 4 95 5.99 with 1392% efficacy. All compounds bear a 1,2,4-triazole core substituted with a substituted aniline moiety, a substituted heteroaryl part, and a more lipophilic alkyl part. 96 is a further development of the triazole series with pEC50 of 6.51 and 1920% efficacy (PAM type 2) which has enhanced aqueous solubility and reduced in vitro affinity for hERG ion channels. An example of the trisubstituted pyrazole series is 97 with a pEC50 value of 7.11 (6268% efficacy) and a PAM type 4 profile. A morpholinothiazole prototype is 98 exhibiting a pEC50 value of 7.70 and an efficacy value of 484 %. 98 is a PAM type 2. It was also tested for kinase activity (225 different kinases) and showed no activity. Some compounds are active in the auditory evoked potential test with DBA/2 mice.
Neurosearch’s compound libraries comprise diphenyl 1,2,3-triazoles, N-acylhydrazone derivatives, further triaryl derivatives, phenyl-pyrimidine-phenylamines, and phenyl-quinoline-carboxylic acid pyridine derivatives [220-228]. Some EC50 values and Imax values for maximal modulation of the control response were reported for α7 receptors heterologously expressed in Xenopus laevis oocytes. A prototype of the 1,2,3-triazole series if 99 with an EC50 value of 1.1 μM and Imax of 277%. Its structure is very similar to some of Abbott’s and Neurosearch’s α4β2 PAMs (Fig. 14). The N-acylhydrazone derivative 100 displays an EC50 value of 4.1 uM and an Imax value of 140%. 101 with a pyrimidine core in its structure has an EC50 value of 0.24 μM (Imax: 631%) in oocytes. A purine core (102) is also tolerated with an EC50 value of 1.2 μM. 103 is a phenyl-quinoline-carboxylic acid pyridine derivative showing an EC50 value of 1.2 μM (Imax: 283%).
104 and 105 are members of a tetrazole-substituted aryl amide and arenecarboxylic acid cyclopropylamide series claimed by Hoffmann-La Roche AG [229, 230]. The two prototypes of each series showed high potency. The tetrazole derivative 104 has an EC50 value of 0.0421 μM (intrinsic activity: 467.65%) using GH4C1 cells and FLIPR technology. An EC50 value of 0.89 μM with an intrinsic activity of 493.6% was obtained for the cyclopropylamide derivative 105.
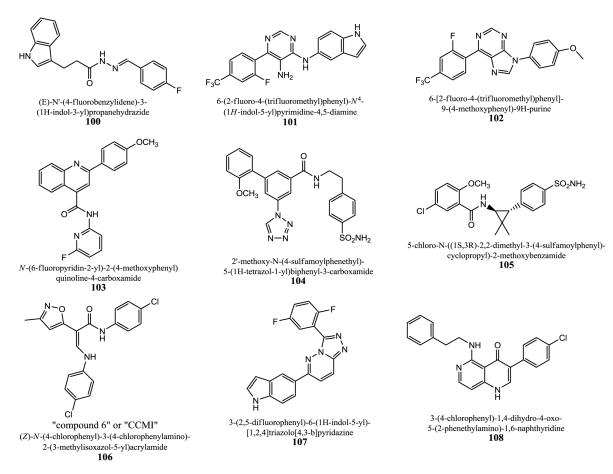
Enaminones substituted by aryl and heteroaryl groups were developed by the University of California. 106 (“compound 6” or “CCMI”) is an important prototype and was tested using oocyte electrophysiology and mice behavioral tests (rotarod performance) to explore possible CNS depressant effects [231]. Like the other indol based series which are also GABAA PAMS from the same inventors, it suffers from lack of being α7 selective. CCMI 106 has an EC50 value of 0.7 μM and a maximal potentiation value of 45% (EC100 ACh response) in X. laevis oocytes expressing human α7 receptors. 107 displays a 1,2,4-triazolo[4,3-b]pyridazine core flanked by the indol moiety and a 2,5-difluorophenyl aromatic part [232].
108 was developed by Anvyl, LLC, among other naphthyridines [233]. These compounds exhibit either at least 100 % positive modulation or a 10 % to 50 % negative modulation of the nicotine EC5 at 10 μM when using oocytes expressing human α7 receptors. Some compounds show 500% positive modulation under these conditions.
3. Expert opinion
For years nicotinic acetylcholine receptors are important targets for drug development especially in cognitive and neurodegenerative disorders therefore the treatment of innumerable CNS diseases are claimed, but there is a growing tendency to involve inflammatory disorders related to α7 subtypes in the listing. Due to their multifarious subunit combinations as pentamers and their various sites of ligand interactions the field of nAChRs is highly complex and challenging. The development of potential drug candidates for α4β2, and especially for α7 receptors is dominating, and only a few patents are considering α3- or α6-containing subtypes as possible targets for drug development. From the compounds’ structural point of view, mostly (di)azabicyclic systems are used for the most important pharmacophoric element, the “cationic center”. The situation for PAMs is quite heterogen and more diverse for the α7 subtype, probably reflecting different interaction sites. The development of PAMs for α4β2 and α7 receptors is just in the beginning, and first pharmacophore models for certain structural templates are appearing. Since the biological data presented in the patents are often insufficient or not present, potential drug candidates are difficult to identify. Often, if any, only ranges of affinity values derived from radioligand binding assays or functional data based on mostly FLIPR technology, seldom on patch clamp methodology are provided for the whole compound series. In only rare cases both, affinity and functional data, are described. Radioligand binding assays do not provide information on the functional character of the compounds, also diverse radioligands along with different receptor preparations are used for the same receptor subtype studied by different inventors. Data derived from ion flux studies and FLIPR technology based assays could be problematic in their interpretation of full or partial agonism related to spare receptor effects. In addition, the use of chimeric receptor constructs makes the situation even more problematic.
Pfizer’s varenicline (Fig. 1; trade names: Chantix (US), Champix), is now the first fully synthetic nAChR compound interacting with the so-called orthosteric site of nicotinic receptors with preference for α4β2 on the market. Varenicline is a therapeutic indicated for smoking cessation, but unfortunately has serious side effects probably associated with the interaction with other nAChR subtypes. Recently, varenicline received warning requirements related to effects like depression and suicidal issues, and later also to increased risk of cardiovascular adverse effects for certain patient populations by diverse drug regulatory agencies.
There is still a long way to go to understand subtype selectivity, the complex aspects regarding the channels’ functionalities, suitable approaches for short and long term therapy or combination therapy with existing therapeutics. But nonetheless, nAChRs as potential drug targets continue to be an exciting area to provide novel approaches for therapeutics for an enormous spectrum of diseases.
Article highlights box.
Nicotinic acetylcholine receptors (nAChRs) are important biological targets for the development of therapeutics for an enormous spectrum of diseases.
Due to multifarious subunit combinations of these pentameric cation channels and various sites of ligand interactions make the development of drugs very challenging.
The development of nAChR ligands with preference for α4β2 or α7 subtypes for the treatment of CNS disorders are in the most advanced developmental stage.
Compounds bearing a (di)azabicyclic moiety are dominating the nAChR pharmacopeia.
The compound arsenal of so-called PAMs, positive allosteric modulators, to influence the channels’ functionalities is growing fast.
Figure 8.
Diazabicyclo-octanes.
Acknowledgments
The authors were supported by a NIH grant (P20RR016467).
Footnotes
Declaration of Interest
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Changeux JP, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med. 2008;14:93–102. doi: 10.1016/j.molmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Tsetlin V, Kuzmin D, Kasheverov I. Assembly of nicotinic and other Cys-loop receptors. J Neurochem. 2011;116:734–741. doi: 10.1111/j.1471-4159.2010.07060.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Lukas RJ. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem Pharmacol. 2011;82:800–807. doi: 10.1016/j.bcp.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- 5.Plazas PV, Katz E, Gomez-Casati ME, et al. Stoichiometry of the alpha9alpha10 nicotinic cholinergic receptor. J Neurosci. 2005;25:10905–10912. doi: 10.1523/JNEUROSCI.3805-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotti C, Clementi F, Fornari A, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Gu R, Zhong YQ, Wei DQ. Structural basis of agonist selectivity for different nAChR subtypes: insights from crystal structures, mutation experiments and molecular simulations. Curr Pharm Design. 2011;17:1652–1662. doi: 10.2174/138161211796355119. [DOI] [PubMed] [Google Scholar]
- 8.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem Pharmacol. 2011;82:915–30. doi: 10.1016/j.bcp.2011.05.001. * This paper reports about α7 PAMs from a functional and chemical point of view.
- 9.Yakel JL. Gating of nicotinic ACh receptors: latest insights into ligand binding and function. J Physiol. 2010;588:597–602. doi: 10.1113/jphysiol.2009.182691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson G, Karlin A. Acetylcholine receptor channel structure in the resting, open, and desensitized states probed with the substituted-cysteine-accessibility method. Proc Natl Acad Sci USA. 2001;98:1241–1248. doi: 10.1073/pnas.031567798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotti C, Riganti L, Vailati S, Clementi F. Brain neuronal nicotinic receptors as new targets for drug discovery. Curr Pharm Des. 2006;12:407–428. doi: 10.2174/138161206775474486. [DOI] [PubMed] [Google Scholar]
- 12.Anderson D, Malysz J, Grønlien JH, et al. Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity α4β2 subunit combination. Biochem Pharmacol. 2009;78:844–851. doi: 10.1016/j.bcp.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Marks MJ, Meinerz NM, Brown RWB, Collins AC. 86Rb+ Efflux Mediated by α4β2*-Nicotinic Acetylcholine Receptors with High and Low Sensitivity to Stimulation by Acetylcholine Display Similar Agonist-Induced Desensitization. Biochem Pharmacol. 2010;80:1238–1251. doi: 10.1016/j.bcp.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 15.Picciotto MR, Zoli M, Rimondini R, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 16.Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacol. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 17.Grottick AJ, Trube G, Corrigall WA, et al. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- 18.Salminen O, Murphy KL, McIntosh JM, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–35. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 19.Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17:1343–50. doi: 10.2174/138161211796150837. [DOI] [PubMed] [Google Scholar]
- 20.Tonstad S, Davies S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf. 2010;33:289–301. doi: 10.2165/11319180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Picciotto MR, Zoli M. Nicotinic receptors in aging and dementia. J Neurobiol. 2002;53:641–655. doi: 10.1002/neu.10102. [DOI] [PubMed] [Google Scholar]
- 22.Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–43. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- 23.Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. J Neurochem. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- 24.Sharma T, Antonova L. Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr Clin North Am. 2003;26:25–40. doi: 10.1016/s0193-953x(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 25.Freedman R, Leonard S. Genetic linkage to schizophrenia at chromosome 15q14. Am J. Med Genet. 2001;105:655–657. doi: 10.1002/ajmg.1548. [DOI] [PubMed] [Google Scholar]
- 26.Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 27.Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacol. 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- 28.Rosse RB, Deutsch SI. Adjuvant galantamine administration improves negative symptoms in a patient with treatment-refractory schizophrenia. Clin Neuropharmacol. 2002;25:272–275. doi: 10.1097/00002826-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Koike K, et al. Tropisetron improves deficits in auditory P50 suppression in schizophrenia. Schizophr Res. 2005;76:67–72. doi: 10.1016/j.schres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. ** Important aspects of cholinergic hypothesis about depression summarized.
- 31.Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacol. 2007;52:1256–62. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippiello PM, Beaver JS, Gatto GJ, James JW, Jordan KG, Traina VM, Xie J, Bencherif M. TC-5214 (S-(+)-mecamylamine): a neuronal nicotinic receptor modulator with antidepressant activity. CNS Neurosci Ther. 2008;14:266–77. doi: 10.1111/j.1755-5949.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granon S, Changeux JP. Attention-deficit/hyperactivity disorder: a plausible mouse model? Acta Paediatr. 2006;95:645–649. doi: 10.1080/08035250600719747. [DOI] [PubMed] [Google Scholar]
- 34.Decker MW, Meyer MD. Therapeutic potential of neuronal nicotinic acetylcholine receptor agonists as novel analgesics. Biochem Pharmacol. 1999;58:917–923. doi: 10.1016/s0006-2952(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 35.Decker MW, Curzon P, Holladay MW, et al. The role of neuronal nicotinic acetylcholine receptors in antinociception: effects of ABT-594. J Physiol Paris. 1998;92:221–224. doi: 10.1016/s0928-4257(98)80014-4. [DOI] [PubMed] [Google Scholar]
- 36.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. ** Exhaustive review on nAChRs and cancer.
- 37.Improgo MR. Tapper AR, Gardner PD. Nicotinic acetylcholine receptor-mediated mechanisms in lung cancer. Biochem Pharmacol. 2011;82:1015–1021. doi: 10.1016/j.bcp.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Improgo MR, Scofield MD, Tapper AR, Gardner PD. From smoking to lung cancer: the CHRNA5/A3/B4 connection. Oncogene. 2010;29:4874–4884. doi: 10.1038/onc.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beers WH, Reich E. Structure and activity of acetylcholine. Nature. 1970;228:917–922. doi: 10.1038/228917a0. [DOI] [PubMed] [Google Scholar]
- 40.Glennon RA, Dukat M. Central nicotinic receptor ligands and pharmacophores. Pharm Acta Helv. 2000;74:103–114. doi: 10.1016/s0031-6865(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 41.Xiu X, Puskar NL, Shanata JAP, Lester HA, Dougherty DA. Nicotine binding to brain receptors requires a strong cation-π interaction. Nature. 2009;458:534–537. doi: 10.1038/nature07768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum AP, Lester HA, Dougherty DA. Nicotinic pharmacophore: the pyridine N of nicotine and carbonyl of acetylcholine hydrogen bond across a subunit interface to a backbone NH. Proc Natl Acad Sci U S A. 2010;107:13206–11. doi: 10.1073/pnas.1007140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sine SM. The nicotinic receptor ligand binding domain. J Neurobiol. 2002;53:431–446. doi: 10.1002/neu.10139. [DOI] [PubMed] [Google Scholar]
- 44.Faghih R, Gopalakrishnan M, Briggs CA. Allosteric modulators of the α7 nicotinic acetylcholine receptor. J Med Chem. 2008;51:701–712. doi: 10.1021/jm070256g. [DOI] [PubMed] [Google Scholar]
- 45.Gündisch D. Nicotinic acetylcholine receptor ligands as potential therapeutics, Expert Opin. Ther. Patents. 2005;15:1–19. doi: 10.1517/13543776.2011.637919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Les Laboratoires Servier; France: 2007. WO2007012761. [Google Scholar]
- 47.Les Laboratoires Servier; Feance: 2007. WO2007085750. [Google Scholar]
- 48.Lagostena L, Danober L, Challal S, et al. Modulatory effects of S 38232, a non a-7 containing nicotine acetylcholine receptor agonist on network activity in the mouse hippocampus. Neuropharmacol. 2010;58:806–815. doi: 10.1016/j.neuropharm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Howe WM, Ji J, Parikh V, Williams S, et al. Enhancement of Attentional Performance by Selective Stimulation of a4b2* nAChRs: Underlying Cholinergic Mechanisms. Neuropsychopharmacol. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aventis Pharma S.A.; Fr.: 2006. WO2006111662. [Google Scholar]
- 51.Bethesda Pharmacology Associates, LLC; USA: 2008. WO2008011484. [Google Scholar]
- 52.Georgetown University; USA: 2009. WO2009143507. [Google Scholar]
- 53.PsychoGenics Inc.; USA: 2010. US20100152450. [Google Scholar]
- 54.The Regents of the University of California; USA: 2006. WO2006086068. [Google Scholar]
- 55.Kyoto University; Japan: 2011. WO2011040574. [Google Scholar]
- 56.Abbott Laboratories; USA: 2011. WO2007084535. [Google Scholar]
- 57.Targacept Inc.; USA: 2010. WO2010065443. [Google Scholar]
- 58.Targacept Inc.; USA: 2010. WO2010065447. [Google Scholar]
- 59.Targacept Inc.; USA: 2010. WO2010065449. [Google Scholar]
- 60.Solvay Pharmaceuticals B.V.; Netherlands: 2008. WO2008003736. [Google Scholar]
- 61.Glaxo Group 2007. WO2007138033.
- 62.Glaxo Group 2009. WO2009071519.
- 63.Glaxo Group 2009. WO2009071576.
- 64.Glaxo Group 2009. WO2009071577.
- 65.Critical Therapeutics, Inc.; USA: 2007. WO2007089626. [Google Scholar]
- 66.University of Florida Research Foundation, Inc.; USA: 2006. WO2006133303. [Google Scholar]
- 67.University of Kentucky Research Foundation; USA: 2009. US20090143424. [Google Scholar]
- 68.University of Kentucky Research Foundation; USA: 2007. WO2007076112. [Google Scholar]
- 69.University of Kentucky Research Foundation; USA: 2007. WO2007094912. [Google Scholar]
- 70.University of Kentucky Research Foundation; USA: 2007. WO2007133614. [Google Scholar]
- 71.University of Kentucky Research Foundation; USA: 2007. WO2007149163. [Google Scholar]
- 72.University of Kentucky Research Foundation; USA: 2007. WO2007149392. [Google Scholar]
- 73.Papke RL, Dwoskin LP, Crooks PA, et al. Extending the analysis of nicotinic receptor antagonists with the study of a6 nicotinic receptor subunit chimeras. Neuropharmacol. 2008;54:1189–1200. doi: 10.1016/j.neuropharm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahman S, Zhang Z, Papke RL, et al. Region-specific effects of N,N’-dodecane-1,12-diyl-bis-3-picolinium dibromide on nicotine-induced increase in extracellular dopamine in vivo. Br J Pharmacol. 2008;153:792–804. doi: 10.1038/sj.bjp.0707612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Universite Laval; Can.: 2008. US20080221085. [Google Scholar]
- 76.Universite Laval; Can.: 2006. WO2006005195. [Google Scholar]
- 77.Critical Therapeutics, Inc.; 2007. WO2007146066. [Google Scholar]
- 78.Targacept, Inc.; USA: 2010. WO2010096384. [Google Scholar]
- 79.Wyeth, John, Brother Ltd. Siena Biotech S.p.A.; USA: 2007. WO2007098826. [Google Scholar]
- 80.Wyeth, John, Brother Ltd. Siena Biotech S.p.A.; USA: 2008. WO2008087529. [Google Scholar]
- 81.Wyeth, John, Brother Ltd. Siena Biotech S.p.A.; USA: 2009. US20090181952. [Google Scholar]
- 82.Wyeth, John, Brother Ltd. Siena Biotech S.p.A.; USA: 2009. US20090181953. [Google Scholar]
- 83.Siena Biotech S.p.A.; 2006. WO2006008133. [Google Scholar]
- 84.Siena Biotech S.p.A.; 2007. WO2007082731. [Google Scholar]
- 85.Bristol-Myers Squibb Company; USA: 2011. WO2011056503. [Google Scholar]
- 86.Universiteit Gent; Belg.: CA2638573. [Google Scholar]
- 87.Neurosearch A/S; Den.: 2006. WO 2006087305. [Google Scholar]
- 88.Targacept, Inc.; USA: 2011. WO2011071758. [Google Scholar]
- 89.Abbott Laboratories; USA: 2009. WO2009067586. [Google Scholar]
- 90.Bristol-Myers Squibb Company; USA: 2011. WO 2011056573. [Google Scholar]
- 91.Sanofi Aventis; Fr.: FR2889701. [Google Scholar]
- 92.Sanofi Aventis; Fr.: 2007. WO2007020343. [Google Scholar]
- 93.Abbott Laboratories; USA: 2007. WO2007137030. [Google Scholar]
- 94.Abbott Laboratories; USA: 2009. WO2009067579. [Google Scholar]
- 95.Neurosearch A/S; Den.: 2009. WO2009112462. [Google Scholar]
- 96.Neurosearch A/S; Den.: 2010. WO2010130768. [Google Scholar]
- 97.Pfizer Inc.; USA: 2009. WO2009081246. [Google Scholar]
- 98.Targacept, Inc.; USA: 2010. WO2010028033. [Google Scholar]
- 99.Targacept, Inc.; USA: 2008. WO2008112734. [Google Scholar]
- 100.Neurosearch A/S; Den.: 2006. WO2006045716. [Google Scholar]
- 101.Neurosearch A/S; Den.: 2006. WO2006087306. [Google Scholar]
- 102.Neurosearch A/S; Den.: 2006. WO2006058879. [Google Scholar]
- 103.AstraZeneca AB; Swed.: 2006. WO2006065217. [Google Scholar]
- 104.AstraZeneca AB; Swed.: 2007. WO2007133155. [Google Scholar]
- 105.AstraZeneca AB; Swed.: 2011. US2011/0136804. [Google Scholar]
- 106.AstraZeneca AB; Swed.: 2009. WO2009066107. [Google Scholar]
- 107.Bristol-Myers Squibb Company; USA: 2009. US20090270405. [Google Scholar]
- 108.Consiglo Nazionale delle Ricerche. Universita Degli Studi di Milano; Italy: 2008. WO2008000469. [Google Scholar]
- 109.Abbott Laboratories; 2006. US20060211686. [Google Scholar]
- 110.Abbott Laboratories; USA: 2006. WO2006065233. [Google Scholar]
- 111.Abbott Laboratories; USA: 2007. US20070060588. [Google Scholar]
- 112.Abbott Laboratories; USA: 2008. US20080064703. [Google Scholar]
- 113.Neurosearch A/S; Den.: 2006. WO2006040352. [Google Scholar]
- 114.Neurosearch A/S; Den.: 2006. WO2006040352. [Google Scholar]
- 115.Neurosearch A/S; Den.: 2009. WO2009150141. [Google Scholar]
- 116.Targacept, Inc.; USA: 2007. WO2007024814. [Google Scholar]
- 117.Targacept, Inc.; USA: 2009. WO2009018505. [Google Scholar]
- 118.Targacept, Inc.; USA: 2010. WO2010085724. [Google Scholar]
- 119.Targacept, Inc.; USA: 2010. WO2010056622. [Google Scholar]
- 120.Memory Pharmaceuticals Corporation; USA: 2006. WO2006069097. [Google Scholar]
- 121.Memory Pharmaceuticals Corporation; USA: 2007. WO2007038367. [Google Scholar]
- 122.Comentis, Inc.; USA: 2009. US20090088418. [Google Scholar]
- 123.Novartis AG. Novartis Pharma GmbH; Switz.: 2006. WO2006005608. [Google Scholar]
- 124.Targacept, Inc.; USA: 2008. WO2008121686. [Google Scholar]
- 125.Targacept, Inc.; USA: 2008. WO2008057938. [Google Scholar]
- 126.Pfizer Products Inc.; USA: 2006. WO2006061711. [Google Scholar]
- 127.Abbott Laboratories; USA: 2009. WO2009137308. [Google Scholar]
- 128.Novartis A-G. Novartis Pharma G.m.b.H.; Switz.: 2007. WO2007068475. [Google Scholar]
- 129.Novartis A-G. Novartis Pharma G.m.b.H.; Switz.: 2007. WO2007068476. [Google Scholar]
- 130.Eli Lilly and Company; USA: 2006. WO2006096358. [Google Scholar]
- 131.SRI International; USA: 2009. WO2009058120. [Google Scholar]
- 132.SRI International; USA: 2009. US20090118326. [Google Scholar]
- 133.Targacept, Inc.; USA: 2006. WO2006023630. [Google Scholar]
- 134.Targacept, Inc.; USA: 2008. WO2008057938. [Google Scholar]
- 135.Targacept, Inc.; USA: 2009. WO2009111550. [Google Scholar]
- 136.Targacept, Inc.; USA: 2010. WO 2010002971. [Google Scholar]
- 137.Georgetown University; USA: 2007. WO2007115092. [Google Scholar]
- 138.Neurosearch A/S; Den.: 2007. WO2007065892. [Google Scholar]
- 139.Neurosearch A/S; Den.: 2007. WO2007090888. [Google Scholar]
- 140.Neurosearch A/S; Den.: 2009. WO2009150139. [Google Scholar]
- 141.Neurosearch A/S; Den.: 2007. WO2007093600. [Google Scholar]
- 142.Neurosearch A/S; Den.: 2007. WO2007135120. [Google Scholar]
- 143.Neurosearch A/S; Den.: 2007. WO2007135122. [Google Scholar]
- 144.Neurosearch A/S; Den.: 2007. WO2007138037. [Google Scholar]
- 145.Neurosearch A/S; Den.: 2007. WO2007138039. [Google Scholar]
- 146.Neurosearch A/S; Den.: 2007. WO2007138038. [Google Scholar]
- 147.Neurosearch A/S; Den.: 2007. WO2007138040. [Google Scholar]
- 148.Neurosearch A/S; Den.: 2007. WO2007138041. [Google Scholar]
- 149.Neurosearch A/S; Den.: 2009. WO2009024515. [Google Scholar]
- 150.Neurosearch A/S; Den.: 2009. WO2009024516. [Google Scholar]
- 151.Neurosearch A/S; Den.: WO2009024517. [Google Scholar]
- 152.Neurosearch A/S; Den.: 2009. WO2009062987. [Google Scholar]
- 153.Neurosearch A/S; Den.: 2009. WO2009062988. [Google Scholar]
- 154.Neurosearch A/S; Den.: 2009. WO2009062989. [Google Scholar]
- 155.Neurosearch A/S; Den.: 2009. WO2009112460. [Google Scholar]
- 156.Neurosearch A/S; Den.: 2009. WO2009150138. [Google Scholar]
- 157.Neurosearch A/S; Den.: 2010. WO2010086280. [Google Scholar]
- 158.Neurosearch A/S; Den.: 2010. WO2010086279. [Google Scholar]
- 159.Neurosearch A/S; Den.: 2010. WO2010084185. [Google Scholar]
- 160.Neurosearch A/S; Den.: 2010. WO2010084184. [Google Scholar]
- 161.Neurosearch A/S; Den.: 2010. WO2010063784. [Google Scholar]
- 162.Neurosearch A/S; Den.: 2010. WO2010043539. [Google Scholar]
- 163.Neurosearch A/S; Den.: 2010. WO2010026177. [Google Scholar]
- 164.Neurosearch A/S; Den.: 2010. WO2010026176. [Google Scholar]
- 165.Neurosearch A/S; Den.: 2011. WO2011073296. [Google Scholar]
- 166.Neurosearch A/S; Den.: 2011. WO2011058002. [Google Scholar]
- 167.Pfizer Products Inc.; USA: 2006. WO2006051394. [Google Scholar]
- 168.Pfizer Products Inc.; USA: 2006. WO2006051407. [Google Scholar]
- 169.Pfizer Products Inc.; USA: 2006. WO2006051413. [Google Scholar]
- 170.Pfizer Products Inc.; USA: 2007. WO2007017750. [Google Scholar]
- 171.Memory Pharmaceuticals Corp.; USA: 2007. WO2007056582. [Google Scholar]
- 172.Memory Pharmaceuticals Corp.; USA: 2009. WO2009055437. [Google Scholar]
- 173.Targacept, Inc.; USA: 2011. WO2010028011. [Google Scholar]
- 174.Targacept, Inc.; USA: 2006. WO2006034089. [Google Scholar]
- 175.Neurosearch A/S; Den.: 2007. WO2007135121. [Google Scholar]
- 176.Abbott Laboratories; USA: 2007. US20070072892. [Google Scholar]
- 177.Abbott Laboratories; USA: 2008. US20080153860. [Google Scholar]
- 178.Abbott Laboratories; USA: 2008. WO2008079570. [Google Scholar]
- 179.Abbott Laboratories; USA: 2008. WO2008058096. [Google Scholar]
- 180.Abbott Laboratories; USA: 2008. WO2008118742. [Google Scholar]
- 181.Abbott Laboratories; USA: 2008. WO2008118743. [Google Scholar]
- 182.Abbott Laboratories; USA: 2008. WO2008118745. [Google Scholar]
- 183.Abbott Laboratories; USA: 2008. WO2008118747. [Google Scholar]
- 184.Abbott Laboratories; USA: 2010. WO2010145208. [Google Scholar]
- 185.Abbott Laboratories; USA: 2010. WO2010151815. [Google Scholar]
- 186.Abbott Laboratories; USA: 2009. WO2009158375. [Google Scholar]
- 187.Abbott Laboratories; USA: 2008. US20080167286. [Google Scholar]
- 188.Abbott Laboratories; USA: 2008. US20080269236. [Google Scholar]
- 189.Abbott Laboratories; USA: 2008. WO2008073942. [Google Scholar]
- 190.Abbott Laboratories; USA: 2008. US20080255203. [Google Scholar]
- 191.Abbott Laboratories; USA: 2008. WO2008127464. [Google Scholar]
- 192.Abbott Laboratories; USA: 2009. WO2009148452. [Google Scholar]
- 193.Abbott Laboratories; USA: 2010. WO2010138600. [Google Scholar]
- 194.Abbott Laboratories; USA: 2009. WO2009149135. [Google Scholar]
- 195.Neurosearch A/S; Den.: 2006. WO2006114400. [Google Scholar]
- 196.Neurosearch A/S; Den.: 2008. WO2008049864. [Google Scholar]
- 197.Neurosearch A/S; Den.: 2011. WO2011073299. [Google Scholar]
- 198.Neurosearch A/S; Den.: 2010. WO2010026134. [Google Scholar]
- 199.Neurosearch A/S; Den.: 2010. WO2010106106. [Google Scholar]
- 200.Neurosearch A/S; Den.: 2011. WO2011073298. [Google Scholar]
- 201.Neurosearch A/S; Den.: 2011. WO2011073297. [Google Scholar]
- 202.Abbott Laboratories; USA: 2008. WO2008002956. [Google Scholar]
- 203.Abbott Laboratories; USA: 2009. WO2009086163. [Google Scholar]
- 204.Abbott Laboratories; USA: 2008. WO2008002974. [Google Scholar]
- 205.Abbott Laboratories; USA: 2009. WO 2009100294. [Google Scholar]
- 206.AstraZeneca AB. Swed.: 2006. WO2006068591. [Google Scholar]
- 207.AstraZeneca AB. Swed.: 2006. WO2006071184. [Google Scholar]
- 208.AstraZeneca AB. Swed.: 2007. WO2007001225. [Google Scholar]
- 209.Glaxo Group Limited; UK: 2009. WO2009127678. [Google Scholar]
- 210.Glaxo Group Limited; UK: 2009. WO2009127679. [Google Scholar]
- 211.Glaxo Group Limited; UK: 2010. WO2010119078. [Google Scholar]
- 212.Glaxo Group Limited; UK: 2011. WO2011045353. [Google Scholar]
- 213.Janssen Pharmaceutica; 2007. WO2007031440. [Google Scholar]
- 214.Janssen Pharmaceutica; 2007. WO2007118903. [Google Scholar]
- 215.Janssen Pharmaceutica; 2009. WO2009050185. [Google Scholar]
- 216.Janssen Pharmaceutica; 2009. WO2009050186. [Google Scholar]
- 217.Janssen Pharmaceutica; 2009. WO2009115547. [Google Scholar]
- 218.Janssen Pharmaceutica; 2009. WO2009135944. [Google Scholar]
- 219.Janssen Pharmaceutica; 2011. WO2011064288. [Google Scholar]
- 220.Neurosearch A/S; Den.: 2009. WO2009019278. [Google Scholar]
- 221.Neurosearch A/S; Den.: 2009. WO2009112459. [Google Scholar]
- 222.Neurosearch A/S; Den.: 2010. WO2010015583. [Google Scholar]
- 223.Neurosearch A/S; Den.: 2009. WO2009065850. [Google Scholar]
- 224.Neurosearch A/S; Den.: 2009. WO2009065854. [Google Scholar]
- 225.Neurosearch A/S; Den.: 2009. WO2009112461. [Google Scholar]
- 226.Neurosearch A/S; Den.: 2010. WO2010040826. [Google Scholar]
- 227.Neurosearch A/S; Den.: 2010. WO2010040806. [Google Scholar]
- 228.Neurosearch A/S; Den.: 2010. WO2010020672. [Google Scholar]
- 229.F. Hoffmann-La Roche AG; Switz: 2009. WO2009043780. [Google Scholar]
- 230.F. Hoffmann-La Roche AG; Switz: 2009. WO2009043784. [Google Scholar]
- 231.The Regents of the University of California; USA: 2006. WO2006138510. [Google Scholar]
- 232.University of California; USA: 2010. WO2010104843. [Google Scholar]
- 233.Anvyl, LLC; USA: 2010. WO2010083444. [Google Scholar]