Abstract
Human non-integrin laminin receptor is a multifunctional protein acting as an integral component of the ribosome and a cell surface receptor for laminin-1. Laminin receptor is overexpressed in several human cancers and is also the cell surface receptor for several viruses and pathogenic prion protein, making it a pathologically significant protein. This study focused on the proteomic characterization of laminin receptor interacting proteins from mus musculus. The use of affinity chromatography with immobilized recombinant laminin receptor coupled with mass spectrometry analysis identified 45 proteins with high confidence. Following validation through co-immunoprecipitation, the proteins were classified based on predicted function into ribosomal, RNA processing, signal transduction/ metabolism, protein processing, cytoskeleton/ cell anchorage, DNA/ chromatin and unknown functions. A significant portion of the identified proteins is related to functions or localizations previously described for laminin receptor. This work represents a comprehensive proteomic approach to studying laminin receptor, and provides an essential stepping-stone to a better mechanistic understanding of this protein’s diverse functions.
Keywords: laminin receptor, RPSA, ribosome, cancer, extracellular matrix, mass spectrometry, chromatography
INTRODUCTION
Non-integrin laminin receptor (LamR), also referred to as p40 and ribosomal protein SA (RPSA), was originally identified as a 67 kDa cell surface protein with the ability to interact with laminin-1 in the extracellular matrix 1–3. Since its discovery, multiple functions and subcellular localizations have been described. LamR is important for cellular translation as an integral component of the 40S ribosomal subunit and it is conserved across species from plants to mammals 4–8. LamR also plays a role in ribosomal RNA processing and is critical for maturation of the 40S subunit 4, 9, 10. Additionally, LamR has been observed in the nucleus bound to histone proteins and it is hypothesized to play a role in chromatin regulation 11.
LamR has several functions at the cell membrane. It acts as the cell surface receptor for several viruses including Sindbis virus 12, Venezuelan equine encephalitis virus 13, dengue virus 14, 15, adeno-associated virus serotypes 2, 3, 8 and 9 16, as well as for prion proteins 17, 18, and cytotoxic necrotizing factor type 1 19. Several studies also show that LamR plays a role in cell motility 20–26. More specifically, a recent study from our laboratory indicated that LamR co-localizes with actin at lamellipodia structures and could be a component of focal contacts 27.
Overexpression of LamR is prevalent in a number of human cancers 28–35 and correlates with poor patient prognosis 31, 36, 37. LamR plays a role in several functions related to cellular transformation. There is a direct relationship between tissue vascularity and LamR expression 31, 38. Ribosomal association enables LamR to play a role in maintaining the increased metabolic needs of tumor cells 6, 7. Additionally, LamR plays a central role in tumor invasion and metastasis 39–42.
Even with its multi-functional nature and many observed localizations relatively little is known about LamR-specific interacting proteins. This study used recombinant LamR protein coupled with affinity chromatography and mass spectrometry analysis to isolate novel binding partners. The new knowledge of LamR interacting partners will hopefully shed some light onto previous observations and foster a better understanding of this complex protein.
MATERIALS AND METHODS
Protein Purification
Recombinant LamR (rLamR) was purified as described previously 43. Briefly, a construct expressing human full length LamR was transformed into E.coli strain BL21 (DE3*) and grown in Luria broth to OD600 of 0.6 at 37°C with constant agitation. Protein expression was induced by the addition of 0.1 mM, isopropyl-thiogalactopyranoside, at 20°C for 18 hours. Cells were harvested and lysed by French press. The lysate was cleared by centrifugation at 16,000 RPM for 30 minutes and filtered through a 0.45 μm filter. The protein was purified using the AKTA purification system (General Electric) in two steps: by Ni-NTA chromatography (General Electric) followed by gel filtration chromatography (Superdex 75) (Amersham). Purified protein sample was run on a polyacrylamide gel and stained with Imperial protein stain (Pierce) to assess quality and purity of the purified protein sample.
Cell Line
NIH 3T3 cells were obtained from the American Type Culture Collection. Cells were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal calf serum, 100 μg/ml of penicillin-streptomycin and 0.5 μg/ml amphotericin B (all from Mediatech).
Cell Lysate Preparation
Cell lysates were collected using Mammalian Protein Extraction Reagent (Pierce) with the addition of 150 mM NaCl and supplemented with EDTA-free complete protease inhibitor (Roche) according to the manufacturer’s instructions. For chromatography samples, 20 mM imidazole was also added. Lysates were cleared by centrifugation at 16,000 RPM for 30 minutes and filtered through a 0.45 μm filter. Protein content of the resulting supernatant was measured using BioRad Dc Protein Reagent according to manufacturer instructions (BioRad). For chromatography, lysates containing 15 mg total protein were precleared with Ni-NTA beads (Qiagen) by incubation for 2 hours at 4°C with end-over-end shaking. Precleared supernatant was collected by gravity flow separation from the Ni-NTA beads.
Chromatography
Purified full length LamR was concentrated in spin concentrators (Millipore) and 1 mg protein was rebound to the Ni-NTA column. Five column volumes of buffer were flowed over the column to remove unbound protein. Ultraviolet readout was monitored throughout. Fifteen mg of whole cell lysate was collected from NIH 3T3 cells (as described above) and injected onto the column at a flow rate of 0.5 ml/min. Column was washed with 10 column volumes of buffer to remove unbound protein. Bound proteins were eluted from the column with the addition of imidazole. For LamR only control sample, purified LamR was bound and eluted under the same conditions without the addition of cell lysate. For the lysate only sample, whole cell lysate was flowed over an empty column and then eluted under the same conditions as above.
Mass Spectrometry
Samples corresponding to peak elution fractions from the chromatography experiment (described above) were run on a 10–20% polyacrylamide gel and stained with Imperial protein stain (Pierce). Each lane was excised and cut into 16 slices. Each slice was analyzed using LC/MS/MS at the Rockefeller University Proteomics Facility. Gel samples were reduced, alkylated and then subjected to in-gel proteolytic digestion with trypsin. Peptides were extracted with 50% acetonitrile + 0.1% trifluoroacetic acid. Peptides were resuspended in water and subjected to liquid chromatography/ mass spectrometry (LC/MS/MS) analysis. LC: Ultimate 3000 system (Dionex), with in-house made C18 analytical column (75 μm diameter beads), C18 5 μm trap column from LC Packings, 60 min gradient mixture of water + 0.1% formic acid and acetonitrile + 0.1% formic acid. Flow rate through trap column was 30 μl/min, flow rate through analytical column was 0.2 μl/min. MS/MS: LTQ Orbitrap XL (Thermo Scientific), mass range 400–1600 m/z, ion trap used for MS/MS, 5 μl injections. The precursor scan was carried out at a mass resolution of 30,000. Data was recorded in profile mode. Seven precursors from each scan were selected for fragmentation. Dynamic exclusion was used to resolve the less intense components of the sample with the following parameters: exclusion list size 500, duration, 60 seconds, exclusion by mass with both high and low exlusion mass widths of 1.5. The normalized collision energy of the ion trap was 35. Raw data was used to create .dta files. These files were used by Mascot (Matrix Science) to search nr.fasta (selected for Mus musculus, 2.2.25, 139163 entries) assuming the digestion enzyme trypsin. Mascot was searched with a fragment ion mass tolerance of 0.80 Da and a parent ion tolerance of 25 PPM. Iodoacetamide derivative of cysteine was specified in Mascot as a fixed modification. Oxidation of methionine was specified in Mascot as a variable modification. Mascot files were loaded into Scaffold for further analysis. X! Tandem (version 2007.01.01.1) (The GPM) was run in subset mode during the Scaffold analysis using the same parameters as those used for the Mascot search (listed above). Scaffold (version Scaffold_3.2.0, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm 44. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm 45. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Co-Immunoprecipitation
Whole cell lysate was collected from NIH 3T3 cells (as described above). For co-immunoprecipitation 250 μg cell lysate was incubated with 10 μg of anti-LamR antibody (Santa Cruz) for 2 hours at 4°C with agitation. Dyna beads (Invitrogen) were washed with phosphate-citrate buffer and added to the lysate-antibody complex and incubated overnight at 4°C with agitation. Beads were washed three times with phosphate buffered saline containing 0.05% Tween20. Proteins were eluted with protein sample buffer and heated at 95°C for 10 minutes. Immunoprecipitation samples were run on polyacrylamide gels (BioRad) under reducing conditions. Protein was transferred to polyvinylidine fluoride membrane (Millipore). Membranes were blocked with non-fat dry milk and probed with anti-myosin light polypeptide 6 (MyL-6), anti-erbb2 interacting protein (ERBIN), anti-p21 activated kinase interacting exchange factor (β-PIX), anti-ribosomal protein, small subunit (RPS) 17, anti-cortactin, anti-SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily c, member 1 (SMARCC1)/BRG1 associated factor 155 (BAF155) or anti-zona occludens protein 2 (ZO-2) (all from Santa Cruz). Proteins were detected using horseradish peroxidase conjugated secondary antibodies (Santa Cruz) and then exposed by chemiluminescence (Pierce).
RESULTS
Affinity Purification of LamR Binding Proteins from NIH 3T3 Cell Extracts
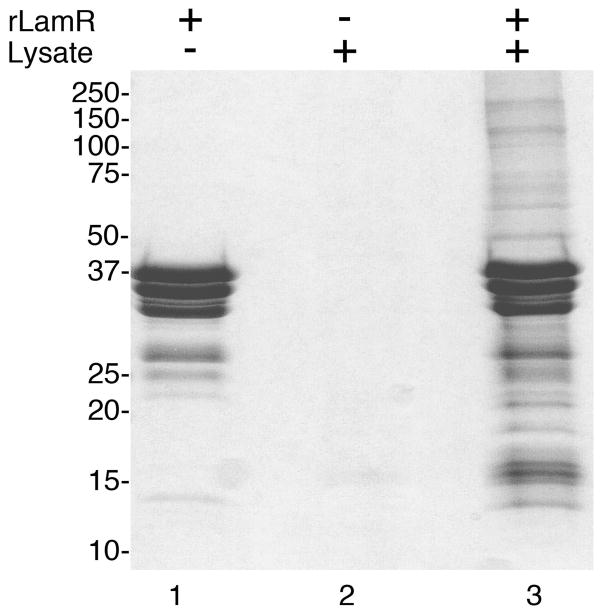
Full length LamR was expressed and purified from E.coli. In previous studies this methodology has proven effective in producing a highly pure sample of functional protein 43. The purified protein contains an N-terminal Histidine tag, which was used to rebind purified recombinant LamR to the Ni-NTA column. Whole cell lysate, extracted from NIH 3T3 cells, was used as a source of cellular proteins. Lysate was injected onto the column containing immobilized rLamR at a flow rate of 0.5 ml/min to facilitate binding. Proteins and rLamR were eluted with the addition of imidazole. To control for background LamR protein and non-specific binding to the column matrix, protein was eluted from a column with only immobilized rLamR and another with lysate only respectively. Elutions were analyzed by SDS-PAGE and stained with Imperial protein stain (Figure 1). Numerous proteins bound to the column containing both immobilized LamR and lysate. The control sample, lysate only, had relatively few proteins bound and the LamR only control column had predominantly three bands, which correspond to the full length LamR protein, and two C-terminal degradation products.
Figure 1. Affinity purification of LamR binding proteins.
Stained elution fractions from affinity chromatography using immobilized recombinant LamR and NIH 3T3 whole cell extract. LamR only and Lysate only (lanes 1 and 2 respectively) served as controls for proteins related to rLamR protein and non-specific binding respectively. Lane 3 represents NIH 3T3 proteins that specially bound to immobilized rLamR protein. The molecular weight in kDa is indicated at the left.
This methodology generated a reproducible staining pattern with Imperial stain and, therefore, these samples were used for mass spectrometry analysis. Each gel lane was divided into 16 slices and following digestion, tryptic peptides were extracted from the gel and analyzed by LC/MS/MS. Data was then analyzed using the Scaffold program for protein identification and to enable inter-sample comparison. Proteins present in the control samples were treated as non-specific and excluded from further analysis. An average of 8 peptides were identified for each protein, with a range from 2–34 peptides. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. These parameters yielded a false discovery rate for peptides of 0.4% and for proteins of less than 0.01% as calculated by Scaffold. This study identified 45 unique proteins that met these stringent criteria. The predicted functions and localization of these proteins are summarized in Figure 2 and tables I–VII. Additionally, lists of the individual peptides identified and the non-specific proteins are available in the supplemental material.
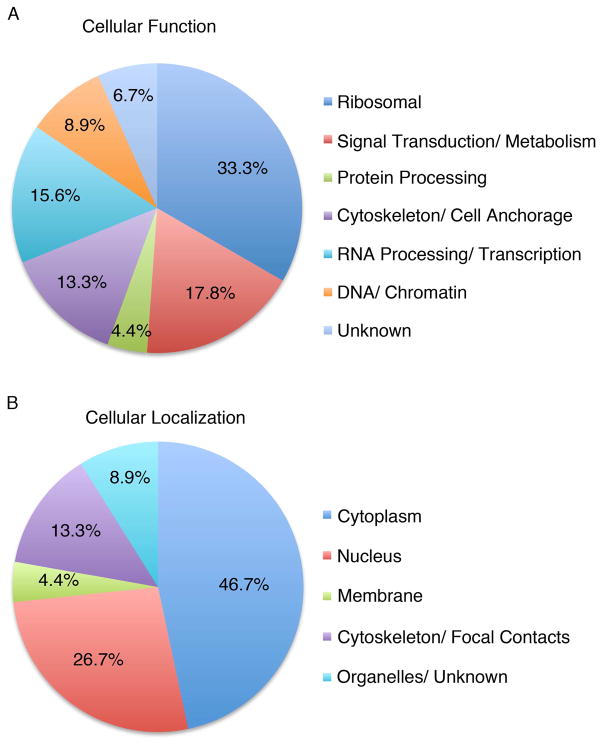
Figure 2. Distribution of LamR binding proteins.
(A) Chart depicting the distribution of LamR binding proteins based on predicted function. (B) Distribution of LamR binding proteins grouped by theoretical cellular localization.
Table I.
Ribosomal proteins that were identified in the LamR-binding fraction. Listed peptides represent those identified with ≥ 95% confidence by Scaffold
| Protein | Accession Number | Number of Peptides | Observed Molecular Weight | Calculated Molecular Weight | References |
|---|---|---|---|---|---|
| Laminin Receptor (RPSA) | gi|171948782 | 30 | 33 kDa | 33 kDa | 4–8 |
| Ribosomal Protein S2 | gi|148705022 | 2 | 25 kDa | 25 kDa | 4–8 |
| Ribosomal Protein S17 | gi|148675005 | 12 | 14 kDa | 14 kDa | 4–8 |
| Ribosomal Protein S15a | gi|30109302 | 7 | 17 kDa | 15 kDa | 4–8 |
| Ribosomal Protein S27 | gi|4506711 | 5 | 10 kDa | 9 kDa | 4–8 |
| Ribosomal Protein L15 | gi|13385036 | 4 | 24 kDa | 24 kDa | 4–8 |
| Ribosomal Protein S29 | gi|4506717 | 5 | 7 kDa | 7 kDa | 4–8 |
| Ribosomal Protein S16 | gi|200796 | 10 | 17 kDa | 16 kDa | 4–8 |
| Ribosomal Protein L18 | gi|51980472 | 8 | 22 kDa | 22 kDa | 4–8 |
| Ribosomal Protein S23 | gi|12846275 | 11 | 16 kDa | 16 kDa | 4–8 |
| Ribosomal Protein L36 | gi|149249185 | 3 | 12 kDa | 12 kDa | 4–8 |
| Ribosomal Protein L23a | gi|20071865 | 16 | 18 kDa | 18 kDa | 4–8 |
| Ribosomal Protein L24 | gi|94386657 | 6 | 18 kDa | 18 kDa | 4–8 |
| Ribosomal Protein S25 | gi|4506707 | 3 | 14 kDa | 14 kDa | 4–8 |
| Ribosomal Protein L27 | gi|148702098 | 2 | 17 kDa | 17 kDa | 4–8 |
Table VII.
Proteins of unknown function that were identified in the LamR-binding fraction. Listed peptides represent those identified with ≥ 95% confidence by Scaffold.
Classification of LamR Binding Protein Functions and Cellular Localizations
LamR is a multifunctional protein that plays a role in translation, as a component of the ribosome, cell motility, as a cell surface receptor, and nuclear functions through its ability to bind to histone proteins. Results of this study support and extend the current literature. Functional classification of the identified proteins indicated interacting partners involved in ribosomal functions, RNA processing, nuclear functions and cytoskeletal functions as well as some new classifications such as protein processing and signal transduction (Figure 2A). Additionally, the ability to perform these diverse functions is supported by LamR’s multiple cellular localizations, which were mirrored in the identified interacting partners (Figure 2B).
Validation of Mass Spectrometry Results
This study identified proteins from diverse cellular processes many of which were not previously known to be interacting partners of LamR. To determine the validity of these newly identified proteins co-immunoprecipitation from whole cell extracts was used. The use of co-immunoprecipitation also evaluates the ability of these proteins to interact in the context of the cellular environment rather than the screening method, which used recombinant LamR. In total 7 proteins, approximately 15% of those identified were chosen for validation. The selected proteins are from different functional classifications and localizations as not to bias the validation process. Co-immunoprecipitations were performed from whole cell extracts using LamR-specific antibodies and matched normal IgG as a negative control. Each of the 7 selected proteins interacted with LamR over three separate experiments (Figure 3). These data serve to validate the proteins identified from the mass spectrometry screen.
Figure 3. Validation of a subset of LamR binding proteins.
Co-immunoprecipitation was employed to validate the protein-protein interactions predicted by chromatography/ mass spectrometry analysis. LamR-specific antibodies were used to capture LamR-protein complexes. Co-immunoprecipitation with normal rabbit IgG served as a negative control. Interactions between LamR and MyL-6, ERBIN, β-PIX, RPS17, Cortactin, BAF 155 and ZO-2 were successfully validated.
Specific LamR Binding Proteins Identified by Mass Spectrometry
Tables I–VII summarize the LamR binding proteins identified by mass spectrometry. Tables are functionally grouped, each listing the protein, accession number and related references. The number of peptides identified with ≥ 95% confidence by Scaffold is also listed. Additionally, two molecular weight assignments have been listed for each protein, the observed molecular weight, which is based on the gel migration and the calculated molecular weight, which is the predicted molecular weight value for each protein.
Table I lists the identified proteins involved in translation and ribosomal functions. LamR is an integral component of the small ribosomal subunit 6. As expected, several other components of the 40S ribosomal subunit were also identified including RPS2, RPS17, RPS15a, RPS27, RPS29, RPS16, RPS23, and RPS25. Additionally, proteins from the large ribosomal subunit were also identified ribosomal protein, large subunit (RPL) 15, RPL18, RPL36 and RPL23a. Presumably these proteins were detected in the LamR binding fractions because they were in complex with the ribosome.
Table II describes proteins related to RNA processing and transcription that were identified from the LamR binding fraction. The Bms1-putative endonuclease protein, a GTPase that is part of the U3 RNA processing complex 46 and small subunit processome component 20, another component of the U3 complex 47, were identified. The U3 complex is critical for rRNA processing and ribosome biogenesis 48. Aquarius, and splicing factor 3B (subunit 2), two intron-binding proteins, required for small nucleolar ribonucleoprotein (snoRNP) assembly were also identified 49, 50. In addition to being a component of the ribosome, LamR plays a role in rRNA processing 9, 10. It is possible that interactions with spliceosome components are related to the processing of ribosomal RNA. Large subunit GTPase 1 homolog, which is required for nuclear export of the formed 60S subunit was also identified. Again, this interaction is most likely related to LamR’s role in ribosome biogenesis. U2-associated protein (SR140) and pre-mRNA processing factor 40 both associate with pre-mRNAs in the nucleus 51, 52. B cell lymphoma 2 (bcl-2) associated transcription factor 1, a ribonucleoprotein involved in mRNA splicing was also isolated 53.
Table II.
Proteins involved in RNA processing that were identified in the LamR-binding fraction. Listed peptides represent those identified with ≥ 95% confidence by Scaffold.
| Protein | Accession Number | Number of Peptides | Observed Molecular Weight | Calculated Molecular Weight | References |
|---|---|---|---|---|---|
| Bms1- putative endonuclease | gi|39930555 | 5 | 146 kDa | 146 kDa | 9, 10, 46 |
| small subunit processome component 20 homolog | gi|62510597 | 3 | 320 kDa | 318 kDa | 9, 10, 47 |
| aquarius | gi|123123505 | 3 | 171 kDa | 171 kDa | 9, 10, 49 |
| splicing factor 3B, subunit 2 | gi|30794206 | 13 | 98 kDa | 98 kDa | 50 |
| U2-associated protein SR140 | gi|171460908 | 9 | 119 kDa | 118 kDa | 51 |
| pre-mRNA processing factor 40 | gi|148694968 | 5 | 94 kDa | 94 kDa | 52 |
| bcl-2- associated transcription factor 1 | gi|24496776 | 7 | 106 kDa | 106 kDa | 53 |
| large subunit GTPase 1 homolog | gi|30017373 | 2 | 74 kDa | 73 kDa | 9 |
Table III lists proteins functioning in signal transduction and metabolism that were identified from the LamR binding fraction. β-PIX is an exchange factor for Rac/ cell division control protein 42 (Cdc42). It promotes lamellipodia formation and the turnover of focal adhesions both of which stimulate cell migration 54, 55. Studies show that LamR plays a role in cell migration 40, 56, 57 and localizes to lamellipodia structures in migrating cells 27. The LamR-β-PIX interaction may be important for regulating the dynamics of focal contacts and facilitating cell movement. ERBIN, a cytoskeleton-bound protein 58, regulates the erbb2 protein and disrupts the ras/raf interaction 59. Literature also indicates that ERBIN plays a role in the maintenance of cell junctions 60, which coincides well with tight junction protein 2 (Table V), also identified in this study. These interactions suggest that LamR plays a role in signal transduction for cell motility.
Table III.
Proteins involved in signal transduction or metabolism that were identified in the LamR-binding fraction. Listed peptides represent those identified with ≥ 95% confidence by Scaffold.
Table V.
Proteins associated with the cytoskeleton or cell anchorage that were identified in the LamR-binding fraction. Listed peptides represent those identified with ≥ 95% confidence by Scaffold.
| Protein | Accession Number | Number of Peptides | Observed Molecular Weight | Calculated Molecular Weight | References |
|---|---|---|---|---|---|
| alpha tubulin | gi|34740335 | 21 | 51 kDa | 50 kDa | 27 |
| Vimentin | gi|2078001 | 20 | 54 kDa | 52 kDa | 22 |
| centrosomal protein (KARP-1 binding protein) | gi|143955299 | 2 | 176 kDa | 175 kDa | 27 |
| dynein cytoplasmic 1 intermediate chain 2 | gi|123207569 | 6 | 69 kDa | 71 kDa | 27, 67 |
| cortactin | gi|75677414 | 16 | 61 kDa | 61 kDa | 27, 68 |
| myosin light polypeptide 6 (MyL-6) | gi|148664460 | 2 | 18 kDa | 15 kDa | 69 |
| tight junction protein 2 | gi|148709667 | 5 | 134 kDa | 138 kDa | 27, 70 |
Table IV describes proteins involved in protein processing. Several components of the T complex protein 1 (TCP-1) chaperone complex were isolated including TCP-1 alpha, TCP-1 theta and TCP-1 zeta. Heat shock protein 65 (hsp65) chaperone protein was also identified. Chaperones bind to nascent polypeptides and facilitate folding 61. These proteins may have bound to LamR due to C-terminal degradation of the recombinant protein, as indicated by lower molecular weight species in Figure 1. This degradation could result in a loss of protein structure, which would likely bind chaperone proteins. Ribophorin II, a component of the N-oligosaccharyl transferase complex, which links mannose to proteins 62 and transmembrane emp24-like trafficking protein 10, a yeast protein with homology to TMP21 63, which is involved in vesicle trafficking, were also identified. Higher molecular weight forms of LamR have been observed, including the originally isolated 67 kDa LamR 64, 65. It is possible that ribophorin II and emp24-like trafficking protein 10 play a role in the maturation of LamR.
Table IV.
Proteins involved in protein processing that were identified in the LamR-binding fraction. Listed peptides represent those identified with ≥ 95% confidence by Scaffold.
| Protein | Accession Number | Number of Peptides | Observed Molecular Weight | Calculated Molecular Weight | References |
|---|---|---|---|---|---|
| TCP-1-alpha | gi|110625624 | 9 | 61 kDa | 60 kDa | |
| TCP-1-theta | gi|126723461 | 11 | 60 kDa | 60 kDa | |
| TCP-1-zeta | gi|62948125 | 6 | 58 kDa | 58 kDa | |
| Hsp65 | gi|183396771 | 2 | 61 kDa | 61 kDa | |
| ribophorin II | gi|123297068 | 5 | 68 kDa | 68 kDa | 62, 64 |
| transmembrane emp24-like trafficking protein 10 | gi|148670919 | 5 | 25 kDa | 26 kDa | 63, 64 |
Table V represents the LamR binding proteins classified as cytoskeleton and cell anchorage. Alpha tubulin, a major component of microtubules and vimentin, an intermediate filament protein, were both identified in this study. Previous studies have shown that ribosomes 20–26 and LamR specifically 27, 66 bind to components of the cytoskeleton. Centrosomal protein KARP-1 binding protein, a component of the centrosome and part of the microtubule organizing center was also identified. Immunofluorescence staining from a previous study indicated that LamR may be present at the microtubule organizing center 27, therefore, it may bind centrosomal protein KARP-1 binding protein in that context. Dynein cytoplasmic 1 intermediate chain 2, a molecular motor protein that converts ATP and transports proteins along microtubules was also isolated 67. Additionally, proteins associated with the actin cytoskeleton, cortactin, which is involved in rearrangement of the actin cytoskeleton and plays a role in cell migration 68, and MyL-6, a structural component of muscle that can signal actin remodeling through integrin signaling 69 were isolated. Tight junction protein 2, also referred to as ZO-2, an integral component of intercellular junctions, which links the extracellular space with the actin cytoskeleton 70 was identified. LamR was shown to associate directly with actin filaments through in vitro binding assays and was also shown to co-localize through immunofluorescence studies 27. Previous work indicated that LamR stabilized lamellipodia structures by bridging the extracellular matrix and actin, however, these interactions could also be important for signaling cytoskeletal rearrangement.
Table VI lists the proteins involved in DNA/ chromatin maintenance. Histone proteins H2A, H3 and H4 were all identified in this study. LamR localizes to the nucleus 71 and has been shown to bind directly to Histones H2 and H4 11. SWI/SNF complex subunit SMARCC1/ BAF155, which is thought to regulate transcription through alteration of chromatin structure 72 was isolated. It has also been shown that the BAF155 homolog in D. melanogaster is associated with nacent preRNPs, which could affect preRNA processing 73. As described above, LamR plays a role in RNA processing and this interaction may relate to this function. Alternatively, its association with both histones and a chromatin modifying protein could indicate a direct role in chromatin maintenance or modification.
Table VI.
Proteins associated with DNA or chromatin maintenance that were identified in the LamR-binding fraction. Listed peptides represent those identified with ≥ 95% confidence by Scaffold.
| Protein | Accession Number | Number of Peptides | Observed Molecular Weight | Calculated Molecular Weight | References |
|---|---|---|---|---|---|
| Histone H2A | gi|56238118 | 11 | 14 kDa | 14 kDa | 11, 71 |
| Histone H3 | gi|78070549 | 5 | 16 kDa | 15 kDa | 71 |
| Histone H4 | gi|117167984 | 15 | 11 kDa | 11 kDa | 11, 71 |
| SMARCC1 (BAF 155) | gi|112421097 | 7 | 121 kDa | 123 kDa | 71–73 |
Table VII describes proteins with unknown functions that were identified in this study. The ubiquitin-associated protein 2-like protein, 74 atlastin GTPase 3, thought to play a role in golgi-ER morphogenesis 75 and sugen kinase protein 269.
DISCUSSION
This study identified 45 LamR-binding proteins from NIH 3T3 whole cell extracts. Several factors indicate that our methodology was effective in isolating authentic LamR-binding proteins. For this study the purity and activity of the recombinant protein was confirmed through SDS-PAGE and in vitro binding assays as described previously 43, (data not shown). The chromatography/ SDS-PAGE analysis showed a reproducible band pattern. Additionally, known LamR binding proteins were isolated from the experiment including alpha tubulin, Histone H2 protein and components of the ribosome.
The identified proteins were grouped based upon predicted function as ribosomal functions, RNA processing, signal transduction/ metabolism, protein processing, cytoskeleton/ cell anchorage, DNA/ chromatin or unknown (Tables I–VII). Several of these categories were expected based on the literature. Previous studies have described the ribosomal function of LamR 6, 7 as well as the related cytoskeletal 27 and RNA processing functions 4, 9, 10. The DNA/ chromatin function has been reported as well 11. There is also some evidence for a role for LamR in signaling after binding and internalization of cytotoxic necrotizing factor-1 76. However within these functional classifications some novel interacting partners have been identified that could shed some light on the specific mechanisms behind LamR functions.
LamR plays a role in intracellular functions, such as translation, through its role as a component of the 40S ribosomal subunit 6, 7. As expected, several components of both the small and large ribosomal subunits were identified. Recently the crystal structure of the eukaryotic 40S ribosomal subunit was published indicating that LamR interacts directly with RPS2, RPS17 and RPS21 77. RPS2 and RPS17 were both identified in this study, however RPS21 was not. RPS21 is a peripheral protein on the ribosome and its binding affinity for LamR may be low. In this study, to reduce non-specific binding, more stringent lysis and wash conditions were used, which could have inhibited the LamR- RPS21 interaction. Presumably, the other ribosomal proteins were identified because they were part of a bound ribosomal complex.
In addition to its integral ribosomal functions, LamR plays a role in rRNA processing. The LamR homologs in yeast are essential for maturation of the 40S ribosomal subunit 4, 9, 78. In this study several processome components, responsible for RNA processing were identified. Two components of the small subunit processome, which is involved in the production of 18S rRNA 79, bms1 putative endonuclease and small subunit processome component 20 homolog were identified. Bms1 putative endonuclease is the only GTPase required for maturation of the 40S subunit. It forms a complex bound to pre-rRNA and remains bound till the 35S rRNA is cleaved to form 20S rRNA 80. As LamR is involved in the 20S to 18S cleavage step 9, 10, it is possible that bms-1 recruits LamR to the processing complex. Additionally, identification of two components of the U2 spliceosome that bind and remove introns from cellular mRNA, splicing factor 3B and U2 associated protein SR140, could indicate a role for LamR in mRNA processing. The large subunit GTPase 1 homolog was also detected indicating that LamR could play a role in mediating export of the large ribosomal subunit as well.
In addition to its ribosomal functions, LamR has several extra-ribosomal functions, it is a cell surface receptor for several viruses and laminin-1. Although LamR was originally discovered as a 67 kDa protein, its gene encodes a 295 amino acid, 37 kDa precursor protein 81, 82. One study suggests that both isoforms exist within the mouse brain and are capable of binding prion proteins 83. Conflicting data exists about the composition of the higher molecular weight species. Mass spectroscopy analysis indicates that LamR exists as a homo-dimer at the cell surface 64. Another study asserts that LamR hetero-dimerizes with galectin3 84. While a crystallographic dimer interface exists within the 37 kDa LamR 43, LamR does not associate with itself in a yeast two hybrid screen 65. There is evidence that LamR is post-translationally modified by fatty acid acylation 64,84, this modification may be required for formation of a dimeric species and/or membrane association. This study used purified recombinant LamR, which is not post-translationally modified. The unmodified state of the protein could have biased against some interactions. LamR was first identified as a laminin binding protein and characterized based on its cell surface functions. This study did not identify laminin protein and also found relatively few membrane interactions. It is possible that use of LamR in its unmodified state enabled the isolation of proteins involved in the modification process. This study did identify proteins associated with processing functions such as transmembrane emp24 like trafficking protein 10, which is related to vesicle trafficking and membrane delivery. This interaction could be important for transporting LamR to the cell membrane. Additionally, ribophorin II, which is an oligosaccharide linkage protein, was also identified. 120–140 kDa glycoprotein (Gp120/140), another laminin binding protein requires the addition of oligosaccharides to interact with laminin 85. It is possible that these proteins play a role in maturation of LamR and production of the 67 kDa isoform.
As mentioned previously, LamR is a receptor for laminin-1 in the extracellular matrix and therefore plays a role in adhesion 1–3. This study also indicates that LamR binds to ZO-2, an integral component of tight junctions. ZO-2 serves to link the extracellular environment with the actin cytoskeleton; perhaps LamR aids in this process because it also binds extracellular laminin-1 1–3, 43, 86 and the actin cytoskeleton 27. Additionally, ZO-2 has been shown to inhibit cell proliferation through cyclin D 87. LamR also plays a role in the maintenance of cell viability 9, 88–90. Ablation of LamR expression with targeted siRNA results in arrest of the cell cycle 90 and in some cases apoptosis 89. It is possible that interactions with ZO-2 are important for intercellular adhesion as well as cell proliferation.
The cell surface functions of LamR extend beyond just cell adhesion through binding of laminin-1 to include a role in cell motility 20–26. It has been shown that LamR co-localizes with actin at lamellipodia and may be a component of focal contacts 27. This study indicates that LamR binds to βPIX and ERBIN, two proteins that play a role in cell motility signal transduction. Interaction with these proteins indicates a more active role for LamR in cell motility, possibly in signal transduction.
LamR has also been observed in the nucleus bound to histone proteins 11. In addition to histone proteins this study also identified the BAF 155 protein, a component of the SWI/SNF-A chromatin modification complex 72. These interactions indicate a role for LamR in chromosomal maintenance or modification and have interesting implications for LamR in regulating gene expression.
CONCLUSION
LamR is a complex protein with multiple functions and cellular localizations. It is well documented that LamR is upregulated in a number of cancers. Literature also indicates that LamR expression is intimately linked to a variety of processes altered in the tumor environment. Many of these observations lack a mechanistic understanding required to effectively develop anti-cancer therapies. Proteins identified in this study will serve to gain a better mechanistic understanding of LamR. The methodology presented here can also serve as a guide for further study of LamR interactions in different cell types or physiological conditions.
Supplementary Material
Acknowledgments
We thank Dr. Christine Pampeno for the critical reading of this manuscript and Dr. Milica Tesic Mark at the Rockefeller Proteomics Resource Center for help with the mass spectrometry experiment. U.S. Public Health grants CA100687 from the National Cancer Institute, National Institutes of Health, and Department of Health and Human Services supported this study. Funding was also provided by a gift from the Litwin Foundation.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org
The data associated with this manuscript may be downloaded from ProteomeCommons.org Tranche, https://proteomecommons.org/tranche/, using the following hash: Password: LamR2012
1. For specific binding proteins
eJ6IH/CZjr3pZ+l+HbJ3t21AJrv2xy2Z4o240vPPpxtCR7bk7aRhM51HNk0RcUubJM1xMlZAmTiHWnuvq9vl90 KDpTcAAAAAAhcETA==
2. For non-specific binding proteins
Ci5JXnTjDHxWe8HByWK2fRwQUpco+aSEJLD30B2HRlDLL/i4mHAIL+Wdf+pFA/I/imB3S7BVntTmwFVtv jSxira1bVcAAAAAAhc0TA==
References
- 1.Rao NC, Barsky SH, Terranova VP, Liotta LA. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983;111(3):804–8. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- 2.Lesot H, Kuhl U, Mark KV. Isolation of a laminin-binding protein from muscle cell membranes. Embo J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malinoff HL, Wicha MS. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983;96(5):1475–9. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demianova M, Formosa TG, Ellis SR. Yeast proteins related to the p40/laminin receptor precursor are essential components of the 40 S ribosomal subunit. J Biol Chem. 1996;271(19):11383–91. doi: 10.1074/jbc.271.19.11383. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Hernandez M, Davies E, Staswick PE. Arabidopsis p40 homologue. A novel acidic protein associated with the 40 S subunit of ribosomes. J Biol Chem. 1994;269(32):20744–9. [PubMed] [Google Scholar]
- 6.Auth D, Brawerman G. A 33-kDa polypeptide with homology to the laminin receptor: component of translation machinery. Proc Natl Acad Sci U S A. 1992;89(10):4368–72. doi: 10.1073/pnas.89.10.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheiman J, Tseng JC, Zheng Y, Meruelo D. Multiple functions of the 37/67-kd laminin receptor make it a suitable target for novel cancer gene therapy. Mol Ther. 2010;18(1):63–74. doi: 10.1038/mt.2009.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis SC, Tzagoloff A, Ellis SR. Characterization of a yeast mitochondrial ribosomal protein structurally related to the mammalian 68-kDa high affinity laminin receptor. J Biol Chem. 1992;267(8):5508–14. [PubMed] [Google Scholar]
- 9.Ford CL, Randal-Whitis L, Ellis SR. Yeast proteins related to the p40/laminin receptor precursor are required for 20S ribosomal RNA processing and the maturation of 40S ribosomal subunits. Cancer Res. 1999;59(3):704–10. [PubMed] [Google Scholar]
- 10.O’Donohue MF, Choesmel V, Faubladier M, Fichant G, Gleizes PE. Functional dichotomy of ribosomal proteins during the synthesis of mammalian 40S ribosomal subunits. J Cell Biol. 2010;190(5):853–66. doi: 10.1083/jcb.201005117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinoshita K, Kaneda Y, Sato M, Saeki Y, Wataya-Kaneda M, Hoffmann A. LBP-p40 binds DNA tightly through associations with histones H2A, H2B, and H4. Biochem Biophys Res Commun. 1998;253(2):277–82. doi: 10.1006/bbrc.1998.9699. [DOI] [PubMed] [Google Scholar]
- 12.Wang KS, Kuhn RJ, Strauss EG, Ou S, Strauss JH. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol. 1992;66(8):4992–5001. doi: 10.1128/jvi.66.8.4992-5001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig GV, Kondig JP, Smith JF. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J Virol. 1996;70(8):5592–9. doi: 10.1128/jvi.70.8.5592-5599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thepparit C, Smith DR. Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. J Virol. 2004;78(22):12647–56. doi: 10.1128/JVI.78.22.12647-12656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tio PH, Jong WW, Cardosa MJ. Two dimensional VOPBA reveals laminin receptor (LAMR1) interaction with dengue virus serotypes 1, 2 and 3. Virol J. 2005;2:25. doi: 10.1186/1743-422X-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80(19):9831–6. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauczynski S, Peyrin JM, Haik S, Leucht C, Hundt C, Rieger R, Krasemann S, Deslys JP, Dormont D, Lasmezas CI, Weiss S. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. Embo J. 2001;20(21):5863–75. doi: 10.1093/emboj/20.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauczynski S, Nikles D, El-Gogo S, Papy-Garcia D, Rey C, Alban S, Barritault D, Lasmezas CI, Weiss S. The 37-kDa/67-kDa laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycanes. J Infect Dis. 2006;194(5):702–9. doi: 10.1086/505914. [DOI] [PubMed] [Google Scholar]
- 19.McNichol BA, Rasmussen SB, Carvalho HM, Meysick KC, O’Brien AD. Two domains of cytotoxic necrotizing factor type 1 bind the cellular receptor, laminin receptor precursor protein. Infect Immun. 2007;75(11):5095–104. doi: 10.1128/IAI.00075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Venrooij WJ, Sillekens PT, van Eekelen CA, Reinders RJ. On the association of mRNA with the cytoskeleton in uninfected and adenovirus-infected human KB cells. Exp Cell Res. 1981;135(1):79–91. doi: 10.1016/0014-4827(81)90301-3. [DOI] [PubMed] [Google Scholar]
- 21.Cervera M, Dreyfuss G, Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981;23(1):113–20. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- 22.Traub P, Bauer C, Hartig R, Grub S, Stahl J. Colocalization of single ribosomes with intermediate filaments in puromycin-treated and serum-starved mouse embryo fibroblasts. Biol Cell. 1998;90(4):319–37. [PubMed] [Google Scholar]
- 23.Lenk R, Ransom L, Kaufmann Y, Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- 24.Toh BH, Lolait SJ, Mathy JP, Baum R. Association of mitochondria with intermediate filaments and of polyribosomes with cytoplasmic actin. Cell Tissue Res. 1980;211(1):163–9. doi: 10.1007/BF00233731. [DOI] [PubMed] [Google Scholar]
- 25.Hesketh JE, Horne Z, Campbell GP. Immunohistochemical evidence for an association of ribosomes with microfilaments in 3T3 fibroblasts. Cell Biol Int Rep. 1991;15(2):141–50. doi: 10.1016/0309-1651(91)90105-r. [DOI] [PubMed] [Google Scholar]
- 26.Hamill D, Davis J, Drawbridge J, Suprenant KA. Polyribosome targeting to microtubules: enrichment of specific mRNAs in a reconstituted microtubule preparation from sea urchin embryos. J Cell Biol. 1994;127(4):973–84. doi: 10.1083/jcb.127.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venticinque L, Jamieson KV, Meruelo D. Interactions between laminin receptor and the cytoskeleton during translation and cell motility. PLoS One. 2011;6(1):e15895. doi: 10.1371/journal.pone.0015895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Brule FA, Castronovo V, Menard S, Giavazzi R, Marzola M, Belotti D, Taraboletti G. Expression of the 67 kD laminin receptor in human ovarian carcinomas as defined by a monoclonal antibody, MLuC5. Eur J Cancer. 1996;32A(9):1598–602. doi: 10.1016/0959-8049(96)00119-0. [DOI] [PubMed] [Google Scholar]
- 29.Sanjuan X, Fernandez PL, Miquel R, Munoz J, Castronovo V, Menard S, Palacin A, Cardesa A, Campo E. Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. J Pathol. 1996;179(4):376–80. doi: 10.1002/(SICI)1096-9896(199608)179:4<376::AID-PATH591>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Pelosi G, Pasini F, Bresaola E, Bogina G, Pederzoli P, Biolo S, Menard S, Zamboni G. High-affinity monomeric 67-kD laminin receptors and prognosis in pancreatic endocrine tumours. J Pathol. 1997;183(1):62–9. doi: 10.1002/(SICI)1096-9896(199709)183:1<62::AID-PATH1095>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Menard S, Tagliabue E, Colnaghi MI. The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res Treat. 1998;52(1–3):137–45. doi: 10.1023/a:1006171403765. [DOI] [PubMed] [Google Scholar]
- 32.Basolo F, Pollina L, Pacini F, Fontanini G, Menard S, Castronovo V, Bevilacqua G. Expression of the Mr 67,000 laminin receptor is an adverse prognostic indicator in human thyroid cancer: an immunohistochemical study. Clin Cancer Res. 1996;2(10):1777–80. [PubMed] [Google Scholar]
- 33.Waltregny D, de Leval L, Menard S, de Leval J, Castronovo V. Independent prognostic value of the 67-kd laminin receptor in human prostate cancer. J Natl Cancer Inst. 1997;89(16):1224–7. doi: 10.1093/jnci/89.16.1224. [DOI] [PubMed] [Google Scholar]
- 34.Taraboletti G, Belotti D, Giavazzi R, Sobel ME, Castronovo V. Enhancement of metastatic potential of murine and human melanoma cells by laminin receptor peptide G: attachment of cancer cells to subendothelial matrix as a pathway for hematogenous metastasis. J Natl Cancer Inst. 1993;85(3):235–40. doi: 10.1093/jnci/85.3.235. [DOI] [PubMed] [Google Scholar]
- 35.Vacca A, Ribatti D, Roncali L, Lospalluti M, Serio G, Carrel S, Dammacco F. Melanocyte tumor progression is associated with changes in angiogenesis and expression of the 67-kilodalton laminin receptor. Cancer. 1993;72(2):455–61. doi: 10.1002/1097-0142(19930715)72:2<455::aid-cncr2820720222>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Pellegrini R, Martignone S, Tagliabue E, Belotti D, Bufalino R, Cascinelli N, Menard S, Colnaghi MI. Prognostic significance of laminin production in relation with its receptor expression in human breast carcinomas. Breast Cancer Res Treat. 1995;35(2):195–9. doi: 10.1007/BF00668209. [DOI] [PubMed] [Google Scholar]
- 37.Martignone S, Menard S, Bufalino R, Cascinelli N, Pellegrini R, Tagliabue E, Andreola S, Rilke F, Colnaghi MI. Prognostic significance of the 67-kilodalton laminin receptor expression in human breast carcinomas. J Natl Cancer Inst. 1993;85(5):398–402. doi: 10.1093/jnci/85.5.398. [DOI] [PubMed] [Google Scholar]
- 38.Gasparini G, Barbareschi M, Boracchi P, Bevilacqua P, Verderio P, Dalla Palma P, Menard S. 67-kDa laminin-receptor expression adds prognostic information to intra-tumoral microvessel density in node-negative breast cancer. Int J Cancer. 1995;60(5):604–10. doi: 10.1002/ijc.2910600506. [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238(4830):1132–4. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- 40.Mafune K, Ravikumar TS. Anti-sense RNA of 32-kDa laminin-binding protein inhibits attachment and invasion of a human colon carcinoma cell line. J Surg Res. 1992;52(4):340–6. doi: 10.1016/0022-4804(92)90113-e. [DOI] [PubMed] [Google Scholar]
- 41.Omar A, Reusch U, Knackmuss S, Little M, Weiss SF. Anti-LRP/LR-Specific Antibody IgG1-iS18 Significantly Reduces Adhesion and Invasion of Metastatic Lung, Cervix, Colon and Prostate Cancer Cells. J Mol Biol. 2012;419(1–2):102–9. doi: 10.1016/j.jmb.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 42.Zuber C, Knackmuss S, Zemora G, Reusch U, Vlasova E, Diehl D, Mick V, Hoffmann K, Nikles D, Frohlich T, Arnold GJ, Brenig B, Wolf E, Lahm H, Little M, Weiss S. Invasion of tumorigenic HT1080 cells is impeded by blocking or downregulating the 37-kDa/67-kDa laminin receptor. J Mol Biol. 2008;378(3):530–9. doi: 10.1016/j.jmb.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Jamieson KV, Wu J, Hubbard SR, Meruelo D. Crystal structure of the human laminin receptor precursor. J Biol Chem. 2008;283(6):3002–5. doi: 10.1074/jbc.C700206200. [DOI] [PubMed] [Google Scholar]
- 44.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74(20):5383–92. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 45.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75(17):4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 46.Karbstein K, Jonas S, Doudna JA. An essential GTPase promotes assembly of preribosomal RNA processing complexes. Mol Cell. 2005;20(4):633–43. doi: 10.1016/j.molcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Liu J, Zhao H, Lu W, Zhao J, Yang L, Li N, Du X, Ke Y. Human 1A6/DRIM, the homolog of yeast Utp20, functions in the 18S rRNA processing. Biochim Biophys Acta. 2007;1773(6):863–8. doi: 10.1016/j.bbamcr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Staley JP, Woolford JL., Jr Assembly of ribosomes and spliceosomes: complex ribonucleoprotein machines. Curr Opin Cell Biol. 2009;21(1):109–18. doi: 10.1016/j.ceb.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, Kodzius R, Shimokawa K, Bajic VB, Brenner SE, Batalov S, Forrest AR, Zavolan M, Davis MJ, Wilming LG, Aidinis V, Allen JE, Ambesi-Impiombato A, Apweiler R, Aturaliya RN, Bailey TL, Bansal M, Baxter L, Beisel KW, Bersano T, Bono H, Chalk AM, Chiu KP, Choudhary V, Christoffels A, Clutterbuck DR, Crowe ML, Dalla E, Dalrymple BP, de Bono B, Della Gatta G, di Bernardo D, Down T, Engstrom P, Fagiolini M, Faulkner G, Fletcher CF, Fukushima T, Furuno M, Futaki S, Gariboldi M, Georgii-Hemming P, Gingeras TR, Gojobori T, Green RE, Gustincich S, Harbers M, Hayashi Y, Hensch TK, Hirokawa N, Hill D, Huminiecki L, Iacono M, Ikeo K, Iwama A, Ishikawa T, Jakt M, Kanapin A, Katoh M, Kawasawa Y, Kelso J, Kitamura H, Kitano H, Kollias G, Krishnan SP, Kruger A, Kummerfeld SK, Kurochkin IV, Lareau LF, Lazarevic D, Lipovich L, Liu J, Liuni S, McWilliam S, Madan Babu M, Madera M, Marchionni L, Matsuda H, Matsuzawa S, Miki H, Mignone F, Miyake S, Morris K, Mottagui-Tabar S, Mulder N, Nakano N, Nakauchi H, Ng P, Nilsson R, Nishiguchi S, Nishikawa S, Nori F, Ohara O, Okazaki Y, Orlando V, Pang KC, Pavan WJ, Pavesi G, Pesole G, Petrovsky N, Piazza S, Reed J, Reid JF, Ring BZ, Ringwald M, Rost B, Ruan Y, Salzberg SL, Sandelin A, Schneider C, Schonbach C, Sekiguchi K, Semple CA, Seno S, Sessa L, Sheng Y, Shibata Y, Shimada H, Shimada K, Silva D, Sinclair B, Sperling S, Stupka E, Sugiura K, Sultana R, Takenaka Y, Taki K, Tammoja K, Tan SL, Tang S, Taylor MS, Tegner J, Teichmann SA, Ueda HR, van Nimwegen E, Verardo R, Wei CL, Yagi K, Yamanishi H, Zabarovsky E, Zhu S, Zimmer A, Hide W, Bult C, Grimmond SM, Teasdale RD, Liu ET, Brusic V, Quackenbush J, Wahlestedt C, Mattick JS, Hume DA, Kai C, Sasaki D, Tomaru Y, Fukuda S, Kanamori-Katayama M, Suzuki M, Aoki J, Arakawa T, Iida J, Imamura K, Itoh M, Kato T, Kawaji H, Kawagashira N, Kawashima T, Kojima M, Kondo S, Konno H, Nakano K, Ninomiya N, Nishio T, Okada M, Plessy C, Shibata K, Shiraki T, Suzuki S, Tagami M, Waki K, Watahiki A, Okamura-Oho Y, Suzuki H, Kawai J, Hayashizaki Y. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–63. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 50.Champion-Arnaud P, Reed R. The prespliceosome components SAP 49 and SAP 145 interact in a complex implicated in tethering U2 snRNP to the branch site. Genes Dev. 1994;8(16):1974–83. doi: 10.1101/gad.8.16.1974. [DOI] [PubMed] [Google Scholar]
- 51.Will CL, Urlaub H, Achsel T, Gentzel M, Wilm M, Luhrmann R. Characterization of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein. EMBO J. 2002;21(18):4978–88. doi: 10.1093/emboj/cdf480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faber PW, Barnes GT, Srinidhi J, Chen J, Gusella JF, MacDonald ME. Huntingtin interacts with a family of WW domain proteins. Hum Mol Genet. 1998;7(9):1463–74. doi: 10.1093/hmg/7.9.1463. [DOI] [PubMed] [Google Scholar]
- 53.Merz C, Urlaub H, Will CL, Luhrmann R. Protein composition of human mRNPs spliced in vitro and differential requirements for mRNP protein recruitment. RNA. 2007;13(1):116–28. doi: 10.1261/rna.336807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13(4):383–93. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang F, Lemmon CA, Park D, Romer LH. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX. Mol Biol Cell. 2007;18(1):253–64. doi: 10.1091/mbc.E06-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wewer UM, Taraboletti G, Sobel ME, Albrechtsen R, Liotta LA. Role of laminin receptor in tumor cell migration. Cancer Res. 1987;47(21):5691–8. [PubMed] [Google Scholar]
- 57.Castronovo V, Taraboletti G, Liotta LA, Sobel ME. Modulation of laminin receptor expression by estrogen and progestins in human breast cancer cell lines. J Natl Cancer Inst. 1989;81(10):781–8. doi: 10.1093/jnci/81.10.781. [DOI] [PubMed] [Google Scholar]
- 58.Jaulin-Bastard F, Arsanto JP, Le Bivic A, Navarro C, Vely F, Saito H, Marchetto S, Hatzfeld M, Santoni MJ, Birnbaum D, Borg JP. Interaction between Erbin and a Catenin-related protein in epithelial cells. J Biol Chem. 2002;277(4):2869–75. doi: 10.1074/jbc.M109652200. [DOI] [PubMed] [Google Scholar]
- 59.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127(3):635–48. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 60.Izawa I, Nishizawa M, Tomono Y, Ohtakara K, Takahashi T, Inagaki M. ERBIN associates with p0071, an armadillo protein, at cell-cell junctions of epithelial cells. Genes Cells. 2002;7(5):475–85. doi: 10.1046/j.1365-2443.2002.00533.x. [DOI] [PubMed] [Google Scholar]
- 61.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–32. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 62.Shibatani T, David LL, McCormack AL, Frueh K, Skach WR. Proteomic analysis of mammalian oligosaccharyltransferase reveals multiple subcomplexes that contain Sec61, TRAP, and two potential new subunits. Biochemistry. 2005;44(16):5982–92. doi: 10.1021/bi047328f. [DOI] [PubMed] [Google Scholar]
- 63.Blum R, Feick P, Puype M, Vandekerckhove J, Klengel R, Nastainczyk W, Schulz I. Tmp21 and p24A, two type I proteins enriched in pancreatic microsomal membranes, are members of a protein family involved in vesicular trafficking. J Biol Chem. 1996;271(29):17183–9. doi: 10.1074/jbc.271.29.17183. [DOI] [PubMed] [Google Scholar]
- 64.Landowski TH, Dratz EA, Starkey JR. Studies of the structure of the metastasis-associated 67 kDa laminin binding protein: fatty acid acylation and evidence supporting dimerization of the 32 kDa gene product to form the mature protein. Biochemistry. 1995;34(35):11276–87. doi: 10.1021/bi00035a037. [DOI] [PubMed] [Google Scholar]
- 65.Hundt C, Peyrin JM, Haik S, Gauczynski S, Leucht C, Rieger R, Riley ML, Deslys JP, Dormont D, Lasmezas CI, Weiss S. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 2001;20(21):5876–86. doi: 10.1093/emboj/20.21.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chuong SD, Good AG, Taylor GJ, Freeman MC, Moorhead GB, Muench DG. Large-scale identification of tubulin-binding proteins provides insight on subcellular trafficking, metabolic channeling, and signaling in plant cells. Mol Cell Proteomics. 2004;3(10):970–83. doi: 10.1074/mcp.M400053-MCP200. [DOI] [PubMed] [Google Scholar]
- 67.Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131(6 Pt 1):1507–16. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20(44):6418–34. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 69.Radel C, Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am J Physiol Heart Circ Physiol. 2005;288(2):H936–45. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- 70.Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem. 1999;274(9):5981–6. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- 71.Sato M, Kinoshita K, Kaneda Y, Saeki Y, Iwamatsu A, Tanaka K. Analysis of nuclear localization of laminin binding protein precursor p40 (LBP/p40) Biochem Biophys Res Commun. 1996;229(3):896–901. doi: 10.1006/bbrc.1996.1899. [DOI] [PubMed] [Google Scholar]
- 72.Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol Cell. 1999;3(2):247–53. doi: 10.1016/s1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 73.Tyagi A, Ryme J, Brodin D, Ostlund Farrants AK, Visa N. SWI/SNF associates with nascent pre-mRNPs and regulates alternative pre-mRNA processing. PLoS Genet. 2009;5(5):e1000470. doi: 10.1371/journal.pgen.1000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagase T, Seki N, Tanaka A, Ishikawa K, Nomura N. Prediction of the coding sequences of unidentified human genes. IV. The coding sequences of 40 new genes (KIAA0121-KIAA0160) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 1995;2(4):167–74. 199–210. doi: 10.1093/dnares/2.4.167. [DOI] [PubMed] [Google Scholar]
- 75.Rismanchi N, Soderblom C, Stadler J, Zhu PP, Blackstone C. Atlastin GTPases are required for Golgi apparatus and ER morphogenesis. Hum Mol Genet. 2008;17(11):1591–604. doi: 10.1093/hmg/ddn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chung JW, Hong SJ, Kim KJ, Goti D, Stins MF, Shin S, Dawson VL, Dawson TM, Kim KS. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J Biol Chem. 2003;278(19):16857–62. doi: 10.1074/jbc.M301028200. [DOI] [PubMed] [Google Scholar]
- 77.Rabl J, Leibundgut M, Ataide SF, Haag A, Ban N. Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1. Science. 2011;331(6018):730–6. doi: 10.1126/science.1198308. [DOI] [PubMed] [Google Scholar]
- 78.Tabb-Massey A, Caffrey JM, Logsden P, Taylor S, Trent JO, Ellis SR. Ribosomal proteins Rps0 and Rps21 of Saccharomyces cerevisiae have overlapping functions in the maturation of the 3′ end of 18S rRNA. Nucleic Acids Res. 2003;31(23):6798–805. doi: 10.1093/nar/gkg899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turner AJ, Knox AA, Prieto JL, McStay B, Watkins NJ. A novel small-subunit processome assembly intermediate that contains the U3 snoRNP, nucleolin, RRP5, and DBP4. Mol Cell Biol. 2009;29(11):3007–17. doi: 10.1128/MCB.00029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim Biophys Acta. 2010;1803(6):673–83. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 81.Rao CN, Castronovo V, Schmitt MC, Wewer UM, Claysmith AP, Liotta LA, Sobel ME. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. 1989;28(18):7476–86. doi: 10.1021/bi00444a047. [DOI] [PubMed] [Google Scholar]
- 82.Castronovo V, Claysmith AP, Barker KT, Cioce V, Krutzsch HC, Sobel ME. Biosynthesis of the 67 kDa high affinity laminin receptor. Biochem Biophys Res Commun. 1991;177(1):177–83. doi: 10.1016/0006-291x(91)91965-f. [DOI] [PubMed] [Google Scholar]
- 83.Simoneau S, Haik S, Leucht C, Dormont D, Deslys JP, Weiss S, Lasmezas C. Different isoforms of the non-integrin laminin receptor are present in mouse brain and bind PrP. Biol Chem. 2003;384(2):243–6. doi: 10.1515/BC.2003.027. [DOI] [PubMed] [Google Scholar]
- 84.Buto S, Tagliabue E, Ardini E, Magnifico A, Ghirelli C, van den Brule F, Castronovo V, Colnaghi MI, Sobel ME, Menard S. Formation of the 67-kDa laminin receptor by acylation of the precursor. J Cell Biochem. 1998;69(3):244–51. doi: 10.1002/(sici)1097-4644(19980601)69:3<244::aid-jcb2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 85.Chammas R, Veiga SS, Line S, Potocnjak P, Brentani RR. Asn-linked oligosaccharide-dependent interaction between laminin and gp120/140. An alpha 6/beta 1 integrin. J Biol Chem. 1991;266(5):3349–55. [PubMed] [Google Scholar]
- 86.Jamieson KV, Hubbard SR, Meruelo D. Structure-guided identification of a laminin binding site on the laminin receptor precursor. J Mol Biol. 2011;405(1):24–32. doi: 10.1016/j.jmb.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tapia R, Huerta M, Islas S, Avila-Flores A, Lopez-Bayghen E, Weiske J, Huber O, Gonzalez-Mariscal L. Zona occludens-2 inhibits cyclin D1 expression and cell proliferation and exhibits changes in localization along the cell cycle. Mol Biol Cell. 2009;20(3):1102–17. doi: 10.1091/mbc.E08-03-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaneda Y, Kinoshita K, Sato M, Saeki Y, Yamada R, Wataya-Kaneda M, Tanaka K. The induction of apoptosis in HeLa cells by the loss of LBP-p40. Cell Death Differ. 1998;5(1):20–8. doi: 10.1038/sj.cdd.4400315. [DOI] [PubMed] [Google Scholar]
- 89.Susantad T, Smith DR. siRNA-mediated silencing of the 37/67-kDa high affinity laminin receptor in Hep3B cells induces apoptosis. Cell Mol Biol Lett. 2008;13(3):452–64. doi: 10.2478/s11658-008-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scheiman J, Jamieson KV, Ziello J, Tseng JC, Meruelo D. Extraribosomal functions associated with the C terminus of the 37/67 kDa laminin receptor are required for maintaining cell viability. Cell Death Dis. 2010;1:e42. doi: 10.1038/cddis.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim KJ, Chung JW, Kim KS. 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J Biol Chem. 2005;280(2):1360–8. doi: 10.1074/jbc.M410176200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.