Abstract
Introduction
Preeclampsia is a disorder of pregnancy, characterized by hypertension and proteinuria after 20 weeks of gestation. Here, we evaluated the role of aspirin triggered-lipoxin A4 (ATL, 15-epi-LXA4) on the modulation of the adhesion of human polymorphonuclear neutrophils (PMN) to endothelial cells initiated by preeclamptic plasma.
Materials and methods
Plasma from preeclamptic, normotensive pregnant, and non-pregnant women were analysed for factors involved in regulating angiogenesis, inflammation and lipid peroxidation. Plasma from preeclamptic women was added to human umbilical vein endothelial cells, and the adhesion of PMN (incubated with or without ATL) to cells was evaluated.
Results
Preeclampsia was associated with some augmented anti-angiogenic, oxidative and pro-inflammatory markers, as well as increasing human PMN-endothelial cell adhesion. This cell adhesion was reduced when human PMN were incubated with ATL prior to addition to endothelial monolayers.
Discussions and Conclusions
Our results are the starting point for further research on the efficacy and rational use of aspirin in preeclampsia.
Keywords: Preeclampsia, anti-angiogenesis, inflammation, oxidative stress, endothelial dysfunction, human polymorphonuclear neutrophils, 15-epi-lipoxin A4
Introduction
Preeclampsia is a multisystem disorder of pregnancy defined by the onset of hypertension and proteinuria and is a significant cause of maternal and perinatal morbidity and mortality worldwide [1]. The majority of the annual maternal deaths take place in developing countries, whereas Western Europe and the United States probably have preventable cases [2]. To date, delivery is the only definitive intervention for preeclampsia and may be required even at the expense of fetal prematurity, which may lead to severe medical complications, death in the neonatal period or sequelae in child and adulthood. The limitation in treatment modalities reflects our incomplete understanding of the molecular and cellular mechanisms of this disease. It is accepted that preeclampsia is a vascular disorder with key pathogenic features of increased inflammation, oxidative stress and endothelial dysfunction [3–5]. Conceptually, these are thought of as individual entities, however these components are closely interconnected because inflammatory and oxidized mediators cause endothelial damage, which exacerbates and potentiates further endothelial impairment and dysfunction, resulting in clinical complications.
Low-dose aspirin is used in the prevention and treatment of diverse alterations of gestation, such as miscarriage and preeclampsia [6, 7]. Systematic review of 59 clinical trials (37,560 women) investigating anti-platelet therapy for prevention of preeclampsia has demonstrated that low-dose aspirin reduces the risk of preeclampsia by approximately 17% [8]. Although there is controversy regarding the efficiency and empirical administration of this medicine in pregnancy, it is safe, low cost and easily accessible to all, which is highly advantageous especially within less developed countries.
Aspirin’s traditional mechanism of action is to inhibit the cyclooxygenase-1 (COX-1) enzyme irreversibly, which converts arachidonic acid (AA, 20:4n-6) to prostaglandin endoperoxides, and thus reduces the biosynthesis of prostaglandins and thromboxanes, but this by itself does not explain the repertoire of anti-inflammatory actions of this drug [9]. Recently, a leukocyte-directed mechanism was described, which involves aspirin-triggered lipoxins (ATL) from arachidonic acid through a process known as transcellular biosynthesis during cell–cell communication between cells bearing COX-2 enzyme (vascular endothelial cells or epithelial cells) and leukocytes [10]. Aspirin acetylates COX-2 and re-directs the catalytic activity of COX-2, to producing another intermediate, 15(R)-hydroxyeicosatetraenoic acid. The COX-2 enzyme with this modification remains catalytically active. Thus, this intermediate is converted to ATL by 5-lipoxygenase in activated leukocytes and then rapidly released [11].
15-epi-LXA4 (ATL) shares the actions of LXA4 but is longer acting [11]. ATL leads to the resolution of inflammation and is an angiogenic and immune modulator, which could be promising in the treatment of preeclampsia. For example, ATL blocks the generation of reactive oxygen species in endothelial cells [12–14] and is a potent anti-inflammatory, inhibiting cell chemotaxis of polymorphonuclear neutrophil cells, leukocyte–endothelial interaction [15, 16], nuclear factor kappa B activation [13, 17], and tumor necrosis factor-alpha (TNF-α) secretion in activated T cells [18]. In addition, ATL increases nitric oxide synthesis via constitutive and inducible nitric oxide synthases [19]. Accordingly, ATL shows promise for treating a number of inflammatory diseases, as possesses potent protective actions in a variety of experimental animal models of disease, including peritonitis [20], colitis [21], dermal inflammation [22], ischemia-reperfusion injury [23], angiogenesis [24], and periodontitis [25]. Nevertheless, the potential applications for ATL in the area of reproductive biology remain unexplored.
The objective of the present study was to evaluate the inflammatory and oxidative responses initiated by plasma from women with preeclampsia, and to determine the role of ATL on the modulation of the adhesion of PMN to endothelial cells initiated by preeclamptic plasma. Here, we report that ATL reduces the PMN- endothelial cell adhesion initiated by preeclamptic plasma when human PMN were incubated with ATL prior to addition to endothelial monolayers.
Materials & Methods
Patient criteria and plasma sample collection
Blood samples were obtained from forty-five women: fifteen women with preeclampsia as defined by hypertension (systolic and diastolic blood pressures higher than 140/90 mm Hg and proteinuria >0.3 g/day) [26], were recruited from University Hospital San Vicente de Paúl and from the General Hospital, Medellín, Colombia. As comparative groups, seventeen normotensive pregnant women and thirteen non-pregnant women were originally selected to have similar characteristics to those presented by preeclamptic patients, such as age, body mass index (BMI), ethnicity and gestational age +/− 2 weeks (of pregnant women). After delivery of normotensive pregnant women, we verify that no complications were filed including preterm delivery, intrauterine growth restriction and hypertensive disorders. Preeclamptic patients with preexisting arterial hypertension, diabetes mellitus, or renal disease were excluded from the study. Approvals by the appropriate Ethics Review Committee of Universidad de Antioquia and informed consent from all women were obtained. It is important to clarify that not all tests were made with the same number of patients.
Patients had not taken aspirin or non-steroidal anti-inflammatory drugs a few days before the collection of blood. Blood was drawn in anticoagulant citrate dextrose solution and first spin at 500 g for 10 min to obtain platelet rich plasma, and a second spin at 700 g for 15 min to obtain platelet poor plasma. Samples were stored at −80°C until use.
Plasma cytokine, chemokine and lipid mediator analyses
Plasma from preeclamptic, normotensive pregnant, and non-pregnant women were analysed for the following protein mediators: soluble fms-like tyrosine kinase-1 (Flt-1), tumor necrosis factor-alpha (TNF-α), transforming growth factor beta 1 (TGF-β1), interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-4, monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF) and interferon-gamma (IFN-γ) using a Milliplex Map Human Cytokine/Chemokine Panel according to the manufacturer’s instructions (detection limit 3.2 pg/mL; Millipore, Billerica, MA). We performed a quantification of plasma protein by BCA™ Protein Assay (Novagen, San Diego, CA) to normalize the values obtained by Luminex, and thus to confirm whether or not there might be differences in plasma protein content between the study groups. Lipid mediators such as prostaglandin E2, lipoxin A4, thromboxane B2 and leukotriene B4 were determined by ELISA assay (Neogen, Lexington, KY). Identification of LXA4 was confirmed by LC-MS/MS in accordance with published criteria [27].
Measurement of lipid peroxidation
Lipid peroxidation (oxidative degradation of lipids) was measured by production of 8-isoprostane by 15-Isoprostane F2t ELISA Kit® following the manufacturer’s recommendations (Neogen, Lexington, KY), and production of thiobarbituric acid–reactive substance (TBARS) by TBARS assay with some modifications [28]. Briefly in the TBARS assay, 200 μl of plasma was incubated for 1 hour at 37°C, 5% CO2, placed in ice cold water for 15 minutes, and mixed with 1 mL 0.6% thiobarbituric acid reagent and 40% trichloroacetic acid. The solution was incubated at 95°C in a dry bath for 15 min and immediately cooled by placing on ice; extraction was performed by adding 3 mL of butanol and centrifuging at 1600g for 7 min. The absorbance was read on a spectrofluorometer (SpectraMax Gemini XS; Molecular Devices, Downingtown, PA) at an excitation wavelength of 505 nm and an emission wavelength of 546 nm against a blank containing butanol. Results are expressed as nmol/mL plasma.
Human polymorphonuclear neutrophils (PMN) - endothelial cell adhesion assay
Primary human umbilical vein endothelial cells (HUVEC) (2 × 106/well) (passage 1–3; Lonza, Walkersville, MD) were plated on 24-well plates and incubated with 5% of preeclamptic plasma for 18 hours at 37°C. Human PMN were isolated from healthy female blood donors by Ficoll gradient centrifugation and dextran sedimentation as described in [29] (10771, Sigma-Aldrich Company, St. Louis, MO). PMN were labeled with fluorescein-derived dye CFDA-SE [(carboxyfluorescein diacetate, succinimidyl ester; 1:2000) (Invitrogen, Eugene, OR)] in PBS with 1% BSA (15 min, room temperature). PMN were washed and incubated with or without 15-epi-LXA4 (10ng/mL; ATL, Calbiochem, La Jolla, CA) for 15 min at 37°C prior to addition to HUVEC monolayers for 15 min, 37°C, washed twice to remove non-adherent PMN. The number of adherent PMN were calculated based on fluorescence values using a microplate spectrofluorometer (VICTOR3 V™ Multilabel Counter, Perkin Elmer – Wellesley, MA) at an excitation wavelength of 492 nm and an emission wavelength of 517 nm, after hydrolysis. Additional tests were carried out with 8-isoprostane (100 nM; Neogen, Lexington, KY) and TBARS from 1,1,3,3-tetraetoxipropano (10000 nM, Sigma-Aldrich Company, St. Louis, MO) for 18 hours at 37°C.
Statistics
Data are mean ± s.e.m. Multiple group comparisons were made using one-way or two-way analysis of variance (ANOVA or Kruskal-Wallis test) followed by Dunnett’s or Bonferroni post tests where appropriate, and direct comparisons made using a two-tailed unpaired Mann Whitney test. In all cases, a P value <0.05 was considered significant.
Results
Clinical characteristics of population
The pertinent clinical features of the three comparative groups: non-pregnant women, pregnant women and preeclamptic patients are shown in Table 1. There were no statistically significant differences in maternal age, gestational age at sample collection and BMI between preeclamptic and normal groups. As expected, we observed statistically significant differences in blood pressure between normotensive pregnant women and preeclamptic women.
Table 1.
Clinical characteristics of healthy non-pregnant women, healthy pregnant women and preeclamptic patients.
| Variable | Non-pregnant women (n = 13) | Normotensive pregnant women (n = 17) | Preeclamptic women (n = 15) |
|---|---|---|---|
| Age at enrollment (years) | 30 (16–42) | 28 (15–43) | 25 (16–39) |
| Body mass index (kg/m2) | 25.8 (18.7–43.5) | 23.2 (19.6–30.8) | 26.3 (20.1–32.3) |
| Gestational age at enrollment (weeks) | N/A | 31 (26–40) | 30 (21–34) |
| Systolic blood pressure (mmHg) | ND | 108 (100–114) | 156 (137–170)a |
| Diastolic blood pressure (mmHg) | ND | 71 (60–80) | 101 (89–118)a |
| Proteinuria (g/24 hours urine) | ND | ND | 7.7 (2.5–22.3) |
Data are presented as mean (data range). N/A: Not applicable. ND: Not determined
P< 0.001 between normotensive pregnant women and preeclamptic women.
Preeclamptic patients have elevated mediators of inflammation
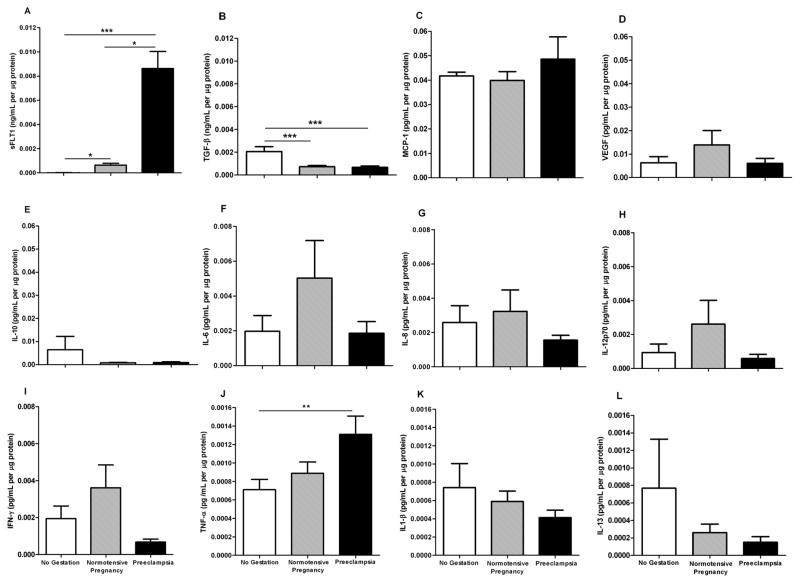
Although the etiology of preeclampsia is unknown, a major characteristic factor is an exacerbated inflammatory response, which contributes to vascular dysfunction. We first analyzed the plasma levels of anti-angiogenic factors, and pro- and anti-inflammatory mediators. The markers such as sFlt-1 and TNF-α were augmented in women with preeclampsia (Figure 1). The levels of TGF-β1 were significantly higher in non-pregnant than normotensive pregnant women and preeclamptic women (P<0.001). There were no statistically significant differences in IL-1β, IL-6, IL-8, IL-10, IL-12p70, IL-13, MCP-1, VEGF and IFN-γ between women with preeclampsia, normotensive pregnant women, and non-pregnant women. IL-4 was not detected in either group (Figure 1).
Figure 1. Plasma levels of pro-angiogenic and pro-inflammatory mediators are elevated in preeclamptic women.
Plasma was obtained from non-pregnant (n=11), normotensive pregnant (n=15) and preeclamptic pregnant (n=14) women and inflammatory markers and angiogenic factors were analyzed by Luminex. Data were normalized by quantification of plasma protein. *P<0.05, **P<0.01,***P<0.001.
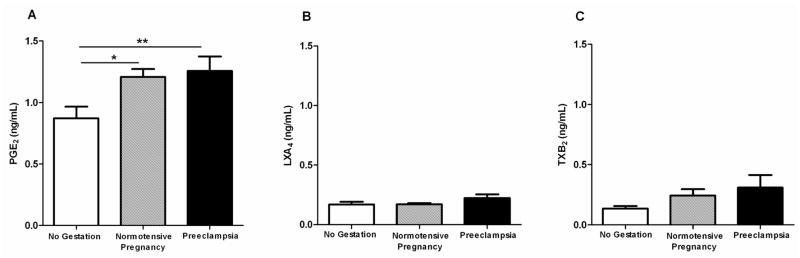
Prostaglandin E2 was augmented in pregnant women (P<0.05) and preeclampsia patients (P<0.01). Lipoxin A4 and thromboxane B2 levels were not statistically significant compared to the normotensive control groups (Figure 2), and leukotriene B4 was not detected in either group. Additionally, total protein content in plasma measured by BCA™ Protein Assay from women with preeclampsia, normotensive pregnant women, and non-pregnant women was similar between groups (data not shown).
Figure 2. Lipid mediator levels are modulated in women with preeclampsia.
Plasma was obtained from non-pregnant (n=13), normotensive pregnant (n=17) and preeclampitc pregnant (n=14) women and analyzed by enzyme–linked immunosorbent assay. *P<0.05, **P<0.01,***P<0.001.
Lipid peroxidation is elevated in Preeclampsia
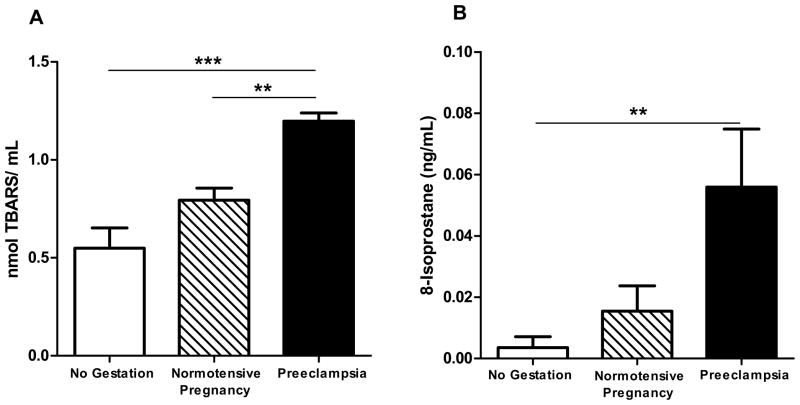
It is known that pregnancy itself is associated with increased inflammation and oxidative stress, however uncontrolled or imbalanced levels of oxidative damage contribute to endothelial dysfunction and are related to pregnancy complications such as preeclampsia [30, 31]. Herein, we assessed the level of oxidative damage by monitoring plasma lipid peroxidation. Indeed, we found that TBARS and 8-isoprostane were augmented in women with preeclampsia (P<0.001) and (P<0.01), respectively (Figure 3).
Figure 3. Systemic oxidative stress is elevated in preeclamptic patients.
Lipid peroxidation levels as measured by TBARS (A), and 8-isoprostane (B) were measured in plasma of women with preeclampsia (n=12) as compared with normotensive pregnant women (n=15) and non-pregnant women (n=8). *P<0.05, **P<0.01,***P<0.001.
Preeclamptic plasma induces leukocyte adhesion
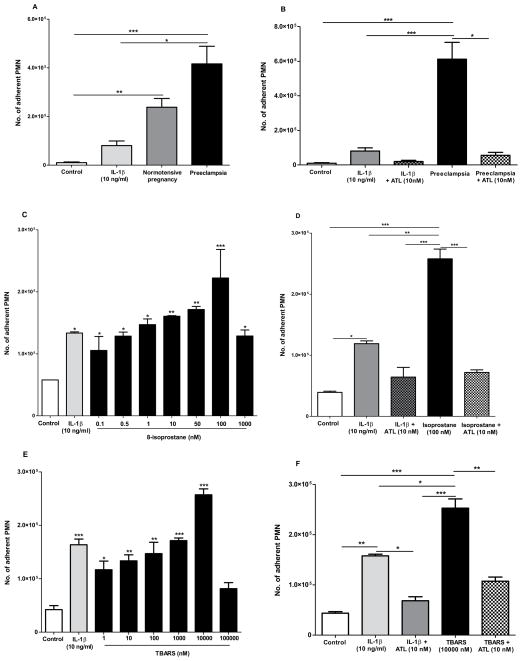
Taking into account that the plasma of women with preeclampsia displayed higher levels of pro-inflammatory and oxidative stress markers such as cytokines and pro-inflammatory lipid mediators, we assessed the effect of the plasma from women with preeclampsia on human endothelial cells and PMN adhesion. We selected normotensive pregnant women, and patients with preeclampsia (with higher 8-isoprostane production). When HUVEC cells were stimulated overnight with plasma from pregnant women, the adhesion of PMN to HUVEC cells significantly increased compared to control (HUVEC without stimulation). This adhesion of PMN to vascular endothelial cells was reduced by ninety-one percent when PMN were incubated with ATL (10 nM) prior to addition to endothelial cells (Figure 4A,B). PMN were incubated with different concentrations of ATL (1, 10 and 100 nM), and a dose response effect was observed (data not shown).
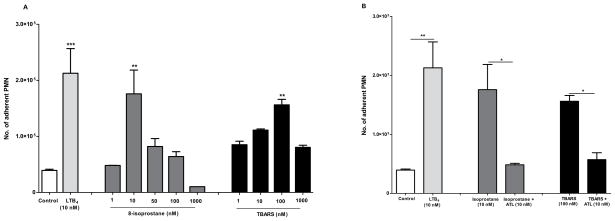
Figure 4. Aspirin-triggered lipoxin (ATL) reduces leukocyte-endothelial interactions initiated by preeclamptic plasma, 8-isoprostane and TBARS: Endothelial and leukocyte directed actions.
HUVEC cells were stimulated overnight with IL-1β (positive control, 10 ng/mL), plasma from normotensive pregnant women (n=4) or preeclamptic women (n=8). Then, PMN labeled with CFDA-SE were added to pre-stimulated HUVEC cells, and cell adhesion was assessed after 15min as described in methods (A). On the other hand, HUVEC were stimulated overnight with 8-isoprostane (0.1–1000 nM) (C) and TBARS (1–100.000 nM) (E). Human PMN were incubated with ATL (10nM; 15min) prior to addition to stimulated HUVEC monolayers with plasma, 8-isoprostane and TBARS, respectively (B,D,F). Results are mean ± s.e.m *P<0.05, **P<0.01,***P<0.001
Since, ATL, isoprostanes and TBARS offered concepts to consider in production of lipid mediators and the actions of non-steroidal anti-inflammatory drugs [32], and because 8-isoprostane and TBARS were increased in patients with preeclampsia, we next determined the direct actions of both components on this adhesion (as we probed above with plasma). We found that both 8-isoprostane and TBARS induced adhesion of PMN to HUVEC cells, when it was added 18 h before to endhotelial cells (Figure 4C,E). The maximum adhesion ocurred at 100 nM and 10000 nM, respectivelly, which was reduced when PMN were pre-incubated with ATL and added to HUVEC cells (Figure 4D,F). In addition, we performed experiments to determine whether 8-isoprostane ant TBARS directly acts on PMN to stimulate cell adhesion. Labeled human PMN were incubated with 8-isoprostane (1–1000 nM; 15min) and TBARS (1–1000 nM; 15 min), before addition to non-stimulated HUVEC monolayers for 15 min. We found that PMN required a concentration 10 times lower of 8-isoprotane (10 nM), and 100 times lower of TBARS (100 nM) than that required by endothelial cells (100 nM) to evoke maximum cell adhesion (Figure 5A,B).
Figure 5. Isoprostane and TBARS stimulate PMN adhesion to HUVEC.
Human PMN were stimulated with Leukotriene B4 (positive control, 10 nM; 30min), 8-isoprostane (1–1000 nM; 30min) or TBARS (1–1000 nM; 30min) and adhesion to non-stimulated HUVEC was assessed after 15min (A). Human PMN were incubated with ATL (10nM; 15min) prior to addition to stimulated HUVEC monolayers (B). *P<0.05, **P<0.01,***P<0.001
Discussion and Conclusions
These results show that preeclampsia was associated with an overall oxidative and pro-inflammatory systemic environment: Augmented amounts of anti-angiogenic factors (sFlt-1), pro-inflammatory (TNF-gamma) mediators and products of lipid peroxidation (TBARS and 8-isoprostane) in the maternal circulation as well an increased PMN-endothelial cell adhesion in preeclamptic patients. Our results are in agreement with the events characteristics of the maternal syndrome of preeclampsia as described by others [30], [33]. However, we found that this cell adhesion was reduced when PMN were incubated with ATL A4 prior to addition to endothelial cells.
Early in normal placental development, extravillous cytotrophoblasts of fetal origin invade the uterine spiral arteries of the decidua and myometrium, and replace the endothelial layer of the maternal spiral arteries, transforming them from small, high-resistance vessels to high-caliber capacitance vessels able to provide sufficient placental perfusion to maintain the growing fetus [34]. In preeclampsia, this vascular remodeling is incomplete and leads to reduced utero-placental arterial flow and episodes of hypoxia followed by reperfusion [5, 35]. These episodes of hypoxia and reoxygenation generate reactive oxygen species that ultimately result in the release of cytokines [5, 36], lipid peroxides [37], and syncytiotrophoblast microfragments [38] from the placenta into the maternal circulation [5, 39]. These molecules can stimulate an inflammatory response in the maternal endothelium that leads to endothelial activation and dysfunction that may set up a fed forward cycle (Figure 6).
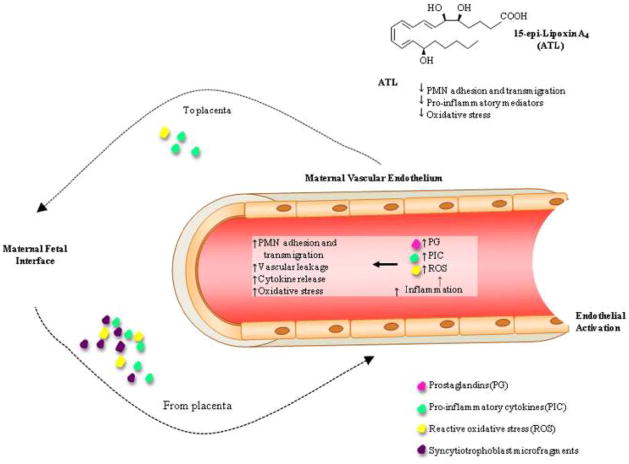
Figure 6. Proposed function of 15-epi-Lipoxin A4 (ATL) on maternal vascular endothelium response to placenta derived substances.
In pregnancy placenta generated pro-inflammatory cytokines (PIC), reactive oxidative stress molecules (ROS) and syncytiotrophoblast microfragments gain access to the maternal systemic circulation. These molecules stimulate inflammation of the maternal vascular endothelium, the production of ROS, PIC, and vasoactive prostaglandins (PG) leading to endothelial activation, polymorphonuclear neutrophil (PMN) adhesion and transmigration and vascular leakage. This sets the stage for a feed forward cyclic process in which mediators from the endothelium stimulate the placenta to produce ROS, PIC and other inflammatory mediators. In preeclampsia this theoretical scenario is increased as compared to normal pregnancies leading to adverse clinical manifestations. ATL may be beneficial in the treatment of preeclampsia by decreasing the oxidative and pro-inflammatory responses and their downstream consequences.
Previous results have shown positive correlations between multiplex bead array and ELISA for most, but not all, of the cytokines tested, but the degree of correlation has varied widely. These variations are likely the result of how these comparisons were made as well as the antibodies used in each of the assays [40]. Although we found no significant differences in the same markers determined by LUMINEX reported in the literature [41], we found augmented amounts of sFlt-1 and TNF-α, and PGE2 by ELISA in the preeclamptic plasma, which might have a central role in the inflammatory response characteristic of the maternal syndrome of preeclampsia [33].
Maintenance of a proliferative phenotype of the trophoblast prevents its differentiation into an invasive, migratory phenotype. These events precede preeclamptic disease, which is promoted by the persistence of low oxygen tensions [42], which subsequently can induce an oxidative stress and lipid peroxidation. We found that TBARS and 8-isoprostane, final products of lipid peroxidation, were augmented in women with preeclampsia. The plasma levels of 8-isoprostane and TBARS could explain the elevated PMN adhesion to HUVEC cells induced by preeclamptic plasma. The cleavage of modified lipids by free-radical-mediated peroxidation gives rise to the 8-isoprostane and TBARS [43], that are released into the maternal circulation and could contribute to maternal endothelial activation and to subsequent leukocyte adhesion and, hence, further PMN activation. Therefore, we directly probed the effect of 8-isoprostane and TBARS on PMN adhesion to endothelial monolayers. Isoprostane and TBARS activated both PMN and HUVEC, enhancing PMN-endothelial adhesion.
When HUVEC cells were stimulated overnight with plasma from pregnant women, the adhesion of PMN to HUVEC cells significantly increased compared to control (P<0.001). However, response induced by plasma from normotensive women was more homogeneous than that induced by plasma from women with preeclampsia. That is, some preeclamptic plasma induced significantly higher cell adhesion compared to that induced by the group from normotensive women. But, we failed to observe statistically significant differences between both groups when we compared the data together. In the future, we will analyze a set of other variables measured in these specific plasma from preeclamptic women to try to determine a common denominator to explain increased cell adhesion and its relationship to preeclampsia.
The above findings, allow us to suggest that these augmented factors in preeclamptic plasma could be involved in the increased PMN adhesion induced on HUVEC cells. However, results derived from this investigation are merely descriptive, and that is how they should be interpreted. Our aim was to evaluate the inflammatory and oxidative responses initiated by plasma of women with preeclampsia, and to determine the role of ATL on the modulation of the adhesion of PMN to endothelial cells initiated by preeclamptic plasma. There are several questions that arise from these findings: although we found increased some anti-angiogenic factors, pro-inflammatory mediators and products of lipid peroxidation in preeclamptic plasma, we have not determined the direct effects of these components by neutralization (e.g. immunodepletetion) on the PMN adhesion yet. The mechanisms that underlie the effects of preeclamptic plasma triggered on endothelial cells, described in this article, will be hypotheses for further research projects – e.g. the differential participation of each potential factor (sFlt-1, TNF-α, TBARS, 8-isoprostane, PGE2) should be addressed.
Although the pathophysiology of preeclampsia is becoming more evident, there remains an unmet clinical need for effective therapeutics for the prevention and treatment of this disorder, and pharmacological approaches to counteract the anti-angiogenic, oxidative and pro-inflammatory states in a single medicine could be promising. In a randomized, double-blind, placebo-controlled clinical trial, low-dose of aspirin (81 mg/day), which is used as anti-thrombotic for long-term prevention of cardiovascular events significantly enhanced the biosynthesis of ATL after 8 weeks. This suggests that aspirin at levels used for cardioprotection may be anti-inflammatory because it is able to initiate endogenous ATL biosynthesis [44]. Of note, a lowered incidence of hypertension in pregnancy was suggested after periconception low-dose aspirin treatment [45]. Low-dose aspirin is thought to correct an imbalance in the ratio of thromboxane A2 to prostacyclin that is associated with increased vasoreactivity. A meta-analysis of individual data of patients from 31 randomized trials showed that aspirin was associated with reduction in preeclampsia and prematurity (less than 34 weeks of gestation), and that aspirin seemed to be safe [46]. Hence, in view of these results, the use of low-dose aspirin should be considered on an individual basis, in relation to the maternal risk factors obtained from their obstetric and medical history.
Results from human studies have demonstrated that oral administration of low-dose aspirin diminishes leukocyte recruitment into cantharidin-induced skin blisters and, importantly aspirin’s action was mediated via the local biosynthesis of ATL and elevated lipoxin receptor (ALX/FPR2) expression [16]. The present results indicate that ATL reduces leukocyte adhesion and activation induced by inflammatory mediators during preeclampsia. In our future studies we will assess whether there is a positive correlation between enhanced ATL production and improved clinical measures in preeclamptic women taking low-dose aspirin.
Along these lines, plasma ATL is both age and gender-dependent in healthy subjects taking low-dose aspirin [47]. In women, a positive correlation was identified between age and changes in levels of ATL, which contrasts a negative correlation obtained for men [44]. Thus, low-dose aspirin has a gender-specific impact on endogenous ATL production, which may contribute to the gender-dependent clinical benefits of aspirin, i.e., low-dose aspirin.
Together these results suggest that ATL could explain some of the beneficial actions of aspirin in treating adverse disorders associated with pregnancy. Our results are the starting point for further research on true efficacy and rational use of aspirin in preeclampsia.
Acknowledgments
Financial support: Administrative Department of Science and Technology and Innovation-Colciencias, Colombia [grant number 1115-408-20531]; Research Committee-Universidad de Antioquia [grant number 91196]; and National Institutes of Health [grant (GM-38765]. A.M.G.V. is a fellow of Colciencias. C.N.S and Arthritis Research UK fellowship 18445 to L.V.N.
The authors thank Bernardo Agudelo M.D. (Hospital Universitario San Vicente de Paúl, Medellín-Colombia), and Luis Escobar, M.D. (Hospital General, Medellín-Colombia) for their involvement in the recruitment of the patients.
Abbreviations
- ATL
aspirin-triggered lipoxin or 15-epi-LXA4
- COX-2
cyclooxygenase-2
- TNF-α
tumor necrosis factor-alpha
- BMI
body mass index
- TBARS
thiobarbituric acid–reactive substance
- PMN
human polymorphonuclear neutrophils
- HUVEC
human umbilical vein endothelial cells
- CFDA-SE
carboxyfluorescein diacetate-succinimidyl ester
- sFlt-1
soluble fms-like tyrosine kinase-1
- TNF-α
tumor necrosis factor-alpha
- TGF-β
transforming growth factor
- MCP-1
monocyte chemoattractant protein-1
- VEGF
vascular endothelial growth factor
- IFN-γ
interferon-gamma
- PGE2
prostaglandin E2
- ALX/FPR2
Lipoxin A4 receptor
Footnotes
Conflict of interest disclosure
C.N.S. is the inventor on patents for lipoxin analogs (aspirin triggered-lipoxin A4) that are assigned to Brigham and Women’s Hospital and licensed for clinical development. CNS’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meis PJ, Goldenberg RL, Mercer BM, et al. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol. 1998;178:562–7. doi: 10.1016/s0002-9378(98)70439-9. [DOI] [PubMed] [Google Scholar]
- 2.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–9. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 4.Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy. 2002;21:205–23. doi: 10.1081/PRG-120015848. [DOI] [PubMed] [Google Scholar]
- 5.Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 6.Cadavid A, Pena B, Garcia G, et al. Heparin plus aspirin as a “single” therapy for recurrent spontaneous abortion associated with both allo- and autoimmunity. Am J Reprod Immunol. 1999;41:271–8. doi: 10.1111/j.1600-0897.1999.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 7.Heyborne KD. Preeclampsia prevention: lessons from the low-dose aspirin therapy trials. Am J Obstet Gynecol. 2000;183:523–8. doi: 10.1067/mob.2000.106757. [DOI] [PubMed] [Google Scholar]
- 8.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007:CD004659. doi: 10.1002/14651858.CD004659.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 10.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thromb Haemost. 2007;97:88–98. [PubMed] [Google Scholar]
- 13.Cezar-de-Mello PF, Vieira AM, Nascimento-Silva V, Villela CG, Barja-Fidalgo C, Fierro IM. ATL-1, an analogue of aspirin-triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br J Pharmacol. 2008;153:956–65. doi: 10.1038/sj.bjp.0707650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorucci S, Distrutti E, Mencarelli A, et al. Evidence that 5-lipoxygenase and acetylated cyclooxygenase 2-derived eicosanoids regulate leukocyte-endothelial adherence in response to aspirin. Br J Pharmacol. 2003;139:1351–9. doi: 10.1038/sj.bjp.0705356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris T, Stables M, Hobbs A, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–96. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 16.Morris T, Stables M, Colville-Nash P, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci U S A. 2010;107:8842–7. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci U S A. 2002;99:13266–71. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–72. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 19.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–9. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 20.Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br J Pharmacol. 2003;139:89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gewirtz AT, Collier-Hyams LS, Young AN, et al. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–7. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- 22.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–26. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang N, Gronert K, Clish CB, O’Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–16. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A(4) and lipoxin A(4) J Pharmacol Exp Ther. 2002;300:385–92. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Jain A, Marleau S, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–65. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JR, de Swiet M. Blood-pressure measurement and classification in pregnancy. Lancet. 2001;357:131–5. doi: 10.1016/S0140-6736(00)03552-2. [DOI] [PubMed] [Google Scholar]
- 27.Serhan CN, Lu Y, Hong S, Yang R. Mediator lipidomics: search algorithms for eicosanoids, resolvins, and protectins. Methods Enzymol. 2007;432:275–317. doi: 10.1016/S0076-6879(07)32012-0. [DOI] [PubMed] [Google Scholar]
- 28.Gutteridge JM, Tickner TR. The characterisation of thiobarbituric acid reactivity in human plasma and urine. Anal Biochem. 1978;91:250–7. doi: 10.1016/0003-2697(78)90838-2. [DOI] [PubMed] [Google Scholar]
- 29.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 30.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 31.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. 2009;215:27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–58. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 33.Sidani M, Siddik-Sayyid SM. Preeclampsia, a new perspective in 2011. Middle East J Anesthesiol. 2011;21:207–14. [PubMed] [Google Scholar]
- 34.De Wolf F, De Wolf-Peeters C, Brosens I, Robertson WB. The human placental bed: electron microscopic study of trophoblastic invasion of spiral arteries. Am J Obstet Gynecol. 1980;137:58–70. doi: 10.1016/0002-9378(80)90387-7. [DOI] [PubMed] [Google Scholar]
- 35.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 36.Hayman R, Brockelsby J, Kenny L, Baker P. Preeclampsia: the endothelium, circulating factor(s) and vascular endothelial growth factor. J Soc Gynecol Investig. 1999;6:3–10. [PubMed] [Google Scholar]
- 37.Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 38.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–40. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 39.Walsh SW. What causes endothelial cell activation in preeclamptic women? Am J Pathol. 2006;169:1104–6. doi: 10.2353/ajpath.2006.060713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–23. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonsson Y, Ruber M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Laresgoiti-Servitje E, Gomez-Lopez N, Olson DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update. 2010 doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 43.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang N, Serhan CN. New mechanism for an old drug: aspirin triggers anti-inflammatory lipid mediators with gender implications. Compr Ther. 2006;32:150–7. doi: 10.1007/s12019-006-0005-6. [DOI] [PubMed] [Google Scholar]
- 45.Lambers MJ, Groeneveld E, Hoozemans DA, et al. Lower incidence of hypertensive complications during pregnancy in patients treated with low-dose aspirin during in vitro fertilization and early pregnancy. Hum Reprod. 2009;24:2447–50. doi: 10.1093/humrep/dep245. [DOI] [PubMed] [Google Scholar]
- 46.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–8. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 47.Chiang N, Hurwitz S, Ridker PM, Serhan CN. Aspirin has a gender-dependent impact on antiinflammatory 15-epi-lipoxin A4 formation: a randomized human trial. Arterioscler Thromb Vasc Biol. 2006;26:e14–7. doi: 10.1161/01.ATV.0000196729.98651.bf. [DOI] [PubMed] [Google Scholar]