Abstract
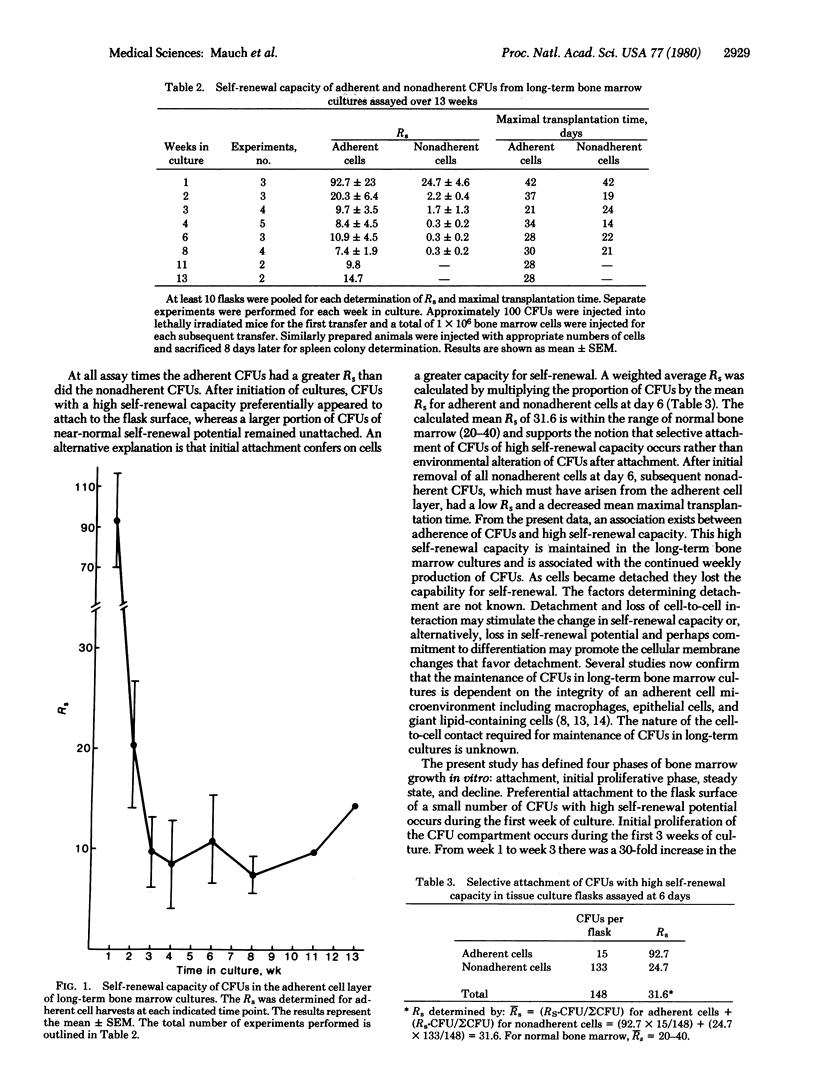
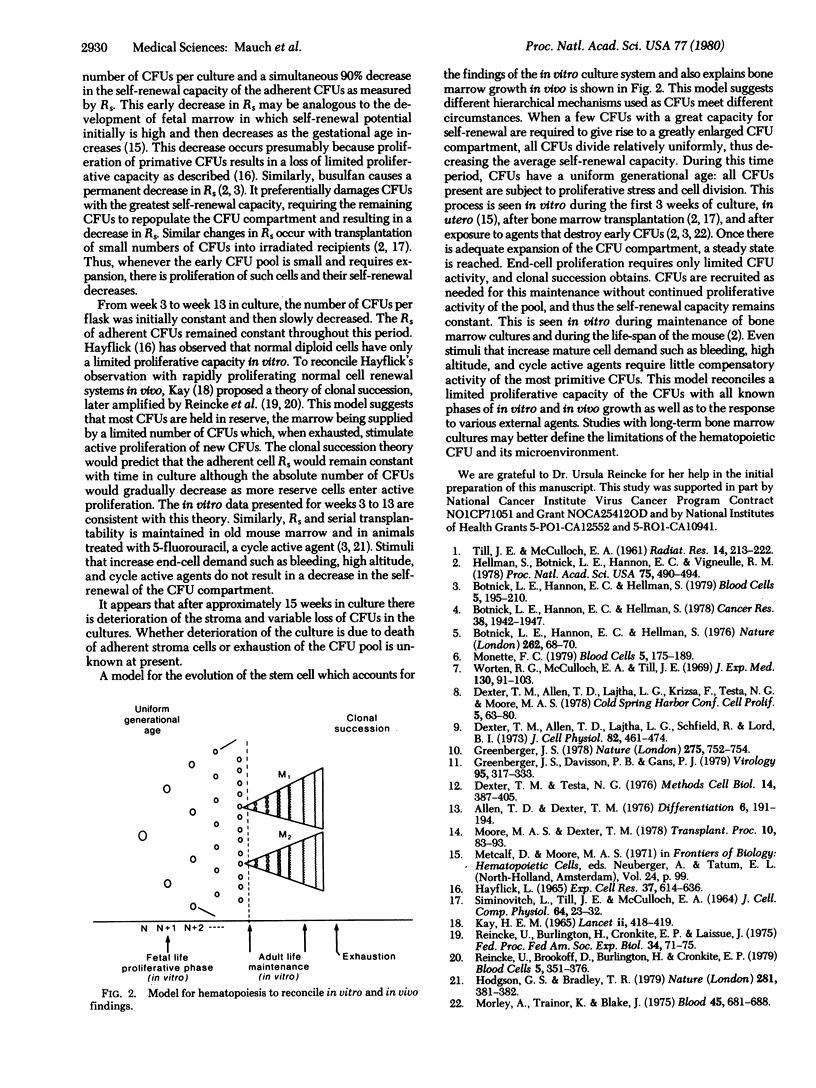
Bone marrow pluripotent stem cells (CFUs) demonstrate capacity for both proliferation and differentiation. The proliferative capacity of CFUs has been measured by serial transplantability and by the Rs, a measurement of CFU production in a single 14-day transfer. In the present study, the self-renewal capacity fo both adherent and nonadherent CFUs from long-term bone marrow cultures was measured. Culture conditions were established such that nonadherent cells were derived from the adherent cell layer. Both adherent and non-adherent cells produced spleen colonies, demonstrating that significant proliferative potential was present in both locations; however, at all times in culture, the CFUs within the adherent stromal cell layer had a significantly greater self-renewal capacity than did the nonadherent CFUs. During the initial establishment of the cultures, the self-renewal capacity of the adherent CFUs decreased as the total number of CFUs per flask increased. After 3 weeks in culture, the self-renewal potential of the adherent CFUs stabilized and was maintained. These results suggest two different mechanisms of stem cell proliferation. In order to increase the most primitive stem cell pool size, there was initial proliferation of early stem cells with a concomitant decrease in self renewal capacity. Once this pool was established, the self-renewal capacity of the adherent CFUs maintained for 13 weeks in culture suggests that CFU production and cell maintenance were achieved by clonal succession.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. D., Dexter T. M. Cellular interrelationships during in vitro granulopoiesis. Differentiation. 1976 Oct 7;6(3):191–194. doi: 10.1111/j.1432-0436.1976.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Botnick L. E., Hannon E. C., Hellman S. Limited proliferation of stem cells surviving alkylating agents. Nature. 1976 Jul 1;262(5563):68–70. doi: 10.1038/262068a0. [DOI] [PubMed] [Google Scholar]
- Botnick L. E., Hannon E. C., Hellman S. Multisystem stem cell failure after apparent recovery from alkylating agents. Cancer Res. 1978 Jul;38(7):1942–1947. [PubMed] [Google Scholar]
- Botnick L. E., Hannon E. C., Hellman S. Nature of the hemopoietic stem cell compartment and its proliferative potential. Blood Cells. 1979 Jun 15;5(2):195–210. [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G., Schofield R., Lord B. I. Stimulation of differentiation and proliferation of haemopoietic cells in vitro. J Cell Physiol. 1973 Dec;82(3):461–473. doi: 10.1002/jcp.1040820315. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Testa N. G. Differentiation and proliferation of hemopoietic cells in culture. Methods Cell Biol. 1976;14:387–405. doi: 10.1016/s0091-679x(08)60498-7. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Davisson P. B., Gans P. J. Murine sarcoma viruses block corticosteroid-induced differentiation of bone marrow preadipocytes associated with long-term in vitro hemopoiesis. Virology. 1979 Jun;95(2):317–333. doi: 10.1016/0042-6822(79)90487-2. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S. Sensitivity of corticosteroid-dependent insulin-resistant lipogenesis in marrow preadipocytes of obese-diabetic (db/db) mice. Nature. 1978 Oct 26;275(5682):752–754. doi: 10.1038/275752a0. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hellman S., Botnick L. E., Hannon E. C., Vigneulle R. M. Proliferative capacity of murine hematopoietic stem cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):490–494. doi: 10.1073/pnas.75.1.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- KAY H. E. HOW MANY CELL-GENERATIONS? Lancet. 1965 Aug 28;2(7409):418–419. doi: 10.1016/s0140-6736(65)90763-4. [DOI] [PubMed] [Google Scholar]
- Monette F. C. Antibodies against pluripotent stem cells: their use in studying stem cell function. Blood Cells. 1979 Jun 15;5(2):175–191. [PubMed] [Google Scholar]
- Moore M. A., Dexter T. M. Stem cell regulation in continuous hematopoietic cell culture. Transplant Proc. 1978 Mar;10(1):83–90. [PubMed] [Google Scholar]
- Morley A., Trainor K., Blake J. A primary stem cell lesion in experimental chronic hypoplastic marrow failure. Blood. 1975 May;45(5):681–688. [PubMed] [Google Scholar]
- Reincke U., Brookoff D., Burlington H., Cronkite E. P. Forced differentiation of CFU-S by Iron-55 erythrocytocide. Blood Cells. 1979 Aug;5(3):351–376. [PubMed] [Google Scholar]
- Reincke U., Burlington H., Cronkite E. P., Laissue J. Hayflick's hypothesis: an approach to in vivo testing. Fed Proc. 1975 Jan;34(1):71–75. [PubMed] [Google Scholar]
- SIMINOVITCH L., TILL J. E., MCCULLOCH E. A. DECLINE IN COLONY-FORMING ABILITY OF MARROW CELLS SUBJECTED TO SERIAL TRANSPLANTATION INTO IRRADIATED MICE. J Cell Physiol. 1964 Aug;64:23–31. doi: 10.1002/jcp.1030640104. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Worton R. G., McCulloch E. A., Till J. E. Physical separation of hemopoietic stem cells differing in their capacity for self-renewal. J Exp Med. 1969 Jul 1;130(1):91–103. doi: 10.1084/jem.130.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]



