Abstract
Background
During intrauterine infection, amniochorionic membranes represent a mechanical and immunological barrier against dissemination of infection. Human beta defensins (HBD)-1, HBD-2, and HBD-3 are key elements of innate immunity that represent the first line of defense against different pathogen microorganisms associated with preterm labor. The aim of this work was to characterize the individual contribution of the amnion (AMN) and choriodecidua (CHD) regions to the secretion of HBD-1, HBD-2 and HBD-3, after stimulation with Candida albicans.
Methods
Full-thickness human amniochorionic membranes were obtained after delivery by elective cesarean section from women at 37-40 wk of gestation with no evidence of active labor. The membranes were cultured in a two-compartment experimental model in which the upper compartment is delimited by the amnion and the lower chamber by the choriodecidual membrane. One million of Candida albicans were added to either the AMN or the CHD face or to both and compartmentalized secretion profiles of HBD-1, HBD-2, and HBD-3 were quantified by ELISA. Tissue immunolocalization was performed to detect the presence of HBD-1, -2, -3 in tissue sections stimulated with Candida albicans.
Results
HBD-1 secretion level by the CHD compartment increased 2.6 times (27.30 [20.9-38.25] pg/micrograms protein) when the stimulus with Candida albicans was applied only on this side of the membrane and 2.4 times (26.55 [19.4-42.5] pg/micrograms protein) when applied to both compartments simultaneously. HBD-1 in the amniotic compartment remained without significant changes. HBD-2 secretion level increased significantly in the CHD when the stimulus was applied only to this region (2.49 [1.49-2.95] pg/micrograms protein) and simultaneously to both compartments (2.14 [1.67- 2.91] pg/micrograms protein). When the stimulus was done in the amniotic compartment HBD-2 remained without significant changes in both compartments. HBD-3 remained without significant changes in both compartments regardless of the stimulation modality. Localization of immune-reactive forms of HBD-1, HBD-2, and HBD-3 was carried out by immunohistochemistry confirming the cellular origin of these peptides.
Conclusion
Selective stimulation of amniochorionic membranes with Candida albicans resulted in tissue-specific secretion of HBD-1 and HBD-2, mainly in the CHD, which is the first region to become infected during an ascending infection.
Background
During normal pregnancy, the human fetus must adapt to a potentially hostile environment in which both maternal and fetal tissues develop different and very complex adaptation strategies to avoid a potentially harmful response that can induce an alloimmune reaction between the mother and the fetus [1].
A growing body of clinical and experimental evidence supports the hypothesis that intrauterine infection during gestation is the principal causal factor in 25 to 40% of cases of preterm delivery; this condition jeopardizes the immunologic privilege of the fetus in the maternal-fetal interface, inducing an uncontrolled secretion of pro-inflammatory modulators that alter the immunologic/endocrinologic equilibrium of the maternal-placental unit [2,3].
The amniotic cavity is a space delimited by amniochorionic membranes, that represent a physical as well as a very active immunologic barrier. These capacities are required to guarantee the effective response against any immunological challenge, including an effective prevention and eventual contention of microorganisms that reach the uterine cavity through an ascending pathway from the cervical-vaginal region [4].
Amniochorionic membranes are a complex bi-laminated tissue constituted by an amniotic epithelium whose cells are in contact with the amniotic fluid and by a chorion zone rich in chorion laeve trophoblasts attached to the maternal decidual lining of the uterine wall; all these cellular populations are embedded in an extracellular matrix rich in collagen types I, III, IV, V, and VI, as well as nidogen and proteoglycans [5,6].
The amniochorionic membranes are minimally vascularized; hence their immunologic capacities have been attributed –in part- to the innate immune system [7]. There is experimental evidence suggesting that the innate immune system plays a key role in the defense mechanisms against any immune and infectious challenge in the fetal-placental interface. Different studies support that amniochorionic membranes have intrinsic antibacterial properties [8-10].
Natural antimicrobial peptides (AMPs) are effector molecules of the innate immune system that have anti-bacterial (gram negative and gram positive bacteria), anti-viral, and anti-fungal actions; generally, they are constituted by 12-50 amino acid residues, and most of them are positively charged and have amphipathic properties [11,12]. These properties are important for their microbial killing mechanism: the cationic character of AMPs results in electrostatic attraction to the negatively-charged phospholipids of microbial membranes and their hydrophobicity aids in integrating them into the microbial cell membrane, leading to membrane disruption [11,13].
Defensins are an evolutionarily ancient class of AMPs present in animals, plants, and fungi [14]. Mammalian defensins can be subdivided into three main classes according to their structural differences: alpha-defensin, beta-defensin, and theta-defensin. Human beta defensins (HBD)-1,-2, -3, and -4 represent the main group of antimicrobials expressed at mucosal surfaces by epithelial cells [15].
Different natural AMPs play an active role during an infection process and the amniochorionic membranes are especially active in their production. There is clinical and experimental evidence indicating that: 1) primary amnion epithelial cells express messenger RNA for HBD-1 to 3, as well as secretory leukocyte protease inhibitor (SLPI) and elafin. Treatment with IL-β –a pro-inflammatory cytokine associated with labor in normal and pathological conditions– increases significantly HBD-2 [16]; 2). Stimulation of FL cells (ATCC CCL-62), a human amnion-derived line with LPS induces up-regulation of mRNA of HBD-3 [17]; 3) human amniotic epithelial cells (HAEC), isolated from human fetal membranes with chorioamnionitis, secrete significantly more HBD-3 after stimulation with LPS than cells isolated from normal pregnancy membranes [18]; 4) HBD-2 concentrations are increased in the amniotic fluid of women with microbial invasion of the amniotic cavity (MIAC) [19]; 5) the concentrations of immunoreactive α-defensins, human neutrophil peptides (HNP)-1-3, bactericidal/permeability increasing protein (BPI), and calprotectin are significantly higher in patients with MIAC, preterm parturition, and preterm premature rupture of membranes (PPROM) [20]; 6) mRNA expression of alpha-defensin-1 and calgranulin B is significantly higher in fetal membranes of patients with preterm labor and histologic chorioamnionitis than in membranes of those without chorioamnionitis [21]; 7) elafin and HBD-3 increase in chorioamnionitis, and levels of HNP1-3 increase in plasma and amniotic fluid in women affected by microbial invasion of the uterus [22].
During pregnancy, HBD-1-3 plays a key role in the mechanisms that protect the maternal and fetal tissues and there is experimental evidence that they are expressed by the amnion’s epithelium, decidual, placental, and chorion trophoblasts from term pregnancies [10]. Evidence from our laboratory indicates that chorioamniotic membranes have tissue-specific capacities to synthesize and secrete HBDs. Using a two-compartment model of culture, we demonstrated that regardless of the primary site of stimulation with Streptococcus agalactiae, this bacterium induces secretion of HBD-2 and HBD-3 by both choriodecidual and amniotic regions; in contrast, HBD-1 remains without significant changes [23].
On the other hand, using the same experimental model, we demonstrated that Gardnerella vaginalis induces secretion of HBD-1 mainly in the amniotic compartment, and HBD-2 and HBD-3 were secreted by the choriodecidual region [24]. Stimulation with Eshcerichia coli elicits a similar secretion HBD-2 profile in both choriodecidual and amniotic regions, however, HBD-3 was secreted mainly by the choriodecidual region but only if the stimulus was applied on the amniotic side [25].
Candida albicans is considered a microorganism associated with bacterial vaginosis, a condition that has been identified as a previous condition for the development of intrauterine and/or intra-amniotic infections [4,26]. Chorioamnionitis cases due to yeast colonization remain rare despite 10-20% rate of vaginal yeast colonize during pregnancy. A growing body of clinical evidence indicates, however, that C. albicans can be related with chorioamnionitis [27-29] and neonatal infections [26,30,31].
Epidemiological data indicate that 69-94% of neonates with a birth weight less than 1500 g die because of C. albicans-induced infections. Moreover, there are reports of fetal death during midgestation (18-21 weeks) associated to sepsis by C. albicans[31,32].
HBDs exhibit anti-fungal activity against Candidas spp, including C. albicans[33-35], therefore, the rationale for the present study was to test the individual contribution of the amnion and the choriodecidual regions to the secretion of HBD-1, -2, and -3 after stimulation with Candida albicans. Our findings suggest that HBD-1-3 may provide protection against penetration by microorganisms into the immune-privileged amniotic cavity.
Methods
Biological samples
The Internal Review Board of the Instituto Nacional de Perinatolgía “Isidro Espinosa de los Reyes” (INPer IER) in Mexico City approved this study (#06151) and written informed consent was obtained from all participants.
Ten intact/whole term fetal membranes were collected from women who underwent elective cesarean section at term (37-39 weeks). All women were from an urban area of Mexico City, 23-34 years old, previously normotensive, with no history of diabetes mellitus, thyroid, liver, or chronic renal disease. They were cared for at the obstetrics out-patient service of the INPer IER. All women had uneventful pregnancies, with no evidence of active labor, cervical dilation or loss of the mucus plug. In addition, none had any clinical or microbiological signs of chorioamnionitis or of lower genital tract infection. Multi-fetal pregnancies were excluded from this study.
To confirm lack of infection, general microbiological analyses, including aerobic and anaerobic microorganisms, were conducted on the placenta and fetal membranes. Immediately after delivery, a sterile swab was rolled across randomly selected areas of the fetal membrane. The swabs were rolled onto Columbia agar with 5% sheep blood, which was used as a primary isolation medium for fastidious and non-fastidious aerobic microorganisms.
Appropriate selective media were included for the detection of specific pathogens, e.g. MacConkey II agar (E. coli). Gardnerella selective agar with 5% human blood (G. vaginalis), potato dextrose agar (C. albicans), agar with 5% human blood (group B streptococci), and chocolate II agar (N. gonorrhoeae) were included.
A CDC anaerobic 5% sheep blood agar plate was streaked to isolate obligate and facultative anaerobes, as well as microaerophilic bacteria, using a Gas Pak EZ anaerobic system. All culture media were purchased from BD (Germany) and were incubated following the manufacturer’s instruction. An additional swab was inoculated into Urea- Arginine LYO 2 broth (BioMérieux, Switzerland) to detect infection due to mycoplasma species.
Only membranes negative for aerobic and anaerobic microorganisms were used for this study.
Amniochorionic membranes explants culture
This model has been previoously validated and published by our group [36] and reproduced by others [37,38]. Briefly, all specimens were collected under aseptic conditions in the operating room and transported to the laboratory within 10 min of delivery in sterile Dulbecco’s Modified Eagle Medium (DMEM) (Gibco BRL, Bethesda, MD) supplemented with 1X antibiotic-antimycotic solution (100 U/ml penicillin, 100 μg/ml streptomycin) (Gibco) and rinsed in sterile saline solution (0.9% NaCl) to remove adherent blood clots.
Segments representing all zones of the membranes were manually cut into 14-18 mm diameter discs and held together with silicone rubber and placed on the upper chamber of a Transwell device, which were placed in the 12-well culture plates (Costar, New York, NY).
One milliliter of DMEM supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, and 1X antibiotic-antimycotic solution (100 U/ml penicillin, 100 μg/ml streptomycin) (DMEM-FBS) (Gibco BRL) was added to each side of the chamber. The mounted explants were incubated in 5% CO2 at 37°C.
Explants stimulations
The first 24 hours in culture were used to stablize the membranes after manipulation. On the second day the membranes in both compartments are washed with saline solution (0.9% NaCl) to remove FBS. After washing, the explants were cultured with 1 ml of DMEM/compartment suplemented with 0.2% of lactalbumin hydrolysate. Stimulation with the bacteria started immeditely upon addition of the culture medium.
Each experiment included the following set of chambers in triplicate: BASAL, control membranes in which 100 μl of saline solution (vehicle) was added to both culture medium in both compartments of the chamber; CHORIODECIDUA (CHD), 1 × 106 CFU of Candida albicans was added only to the chorion compartment; AMNION (AMN), the yeast was added only to the amnion compartment; BOTH, the yeast stimulus was applied to both chorioamniotic and amniotic compartments simultaneously.
Co-incubation with C. albicans was done for 24 h, and the medium from both compartments of the chambers was collected and filtered (0.22 um); samples were aliquoted and stored at -70°C until assayed. All tissues were weighed and protein concentrations in all culture media were assessed with the Bradford method [39].
The inoculum size of 1 × 106 CFU has been previoulsly standarized/published by our group to induce the secretion of a pro-inflammatory reaction, as well as the secretion of prostaglandin (PG)-E2 and matrix metalloprotease (MMP)-9 [40].
Measurement of HBD1, HBD2 and HBD3 secretion
Concentrations of HBD1, HBD2, and HBD3 were determined by enzyme-linked immunosorbent sandwich assays (ELISA) kits (Pepro Tech, Rock Hill, NJ). Standard curves used were as follows: HBD-1, 15 to 1000 pg/ml with sensitivity of 4 pg/ml; HBD-2, 2 to 200 pg/ml with a detection limit of 1 pg/ml; and HBD-3, 10 to 2000 pg/ml with a sensitivity of 2 pg/ml.
Given that culture medium samples recovered from both compartments after stimulation did not contain fetal bovine serum and that these were filtered to remove any possible contamination with C. albicans and because HBD’s are secreted to the medium, the final concentration of each HBD was expressed per microgram of the total protein concentration of each sample.
Immunohistochemistry
After stimulation with Candida albicans, the membranes were fixed, embedded in paraffin wax and 10-15 μm sections were processed for immunohistochemical staining using the following polyclonal antibodies; rabbit anti-hHBD-1 and anti-hHBD-3, and a goat anti-hHBD-2 (Pepro Tech). The antibodies were used at a 10ug/ml dilution. The same membranes without stimulation were used as controls.
Binding of primary antibodies was detected using the avidin-biotinylated peroxidase technique and biotinylated horse anti-rabbit IgG and horse anti-goat IgG antibodies (Vector, Burlingame, CA). Tissue sections were counterstained with Mayer’s hematoxylin and cover-slipped for evaluation by light microscopy.
Statistical analyses
Descriptive statistics (mean, standard deviation, standard error, median, and range) were obtained for each variable. Data distribution was tested for normality using Kolmogorov-Smirnoff and Shapiro-Wilk tests. When distribution was normal, Student’s t test was used to analyze differences between treatments (i.e., control chorion vs stimulated chorion). Mann-Whitney’s U test was used when data were not normally distributed. In every case, a P value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 17 (IBM Corp., USA). Bars in the graphs represent median values and 25-75 interquartile range.
Results
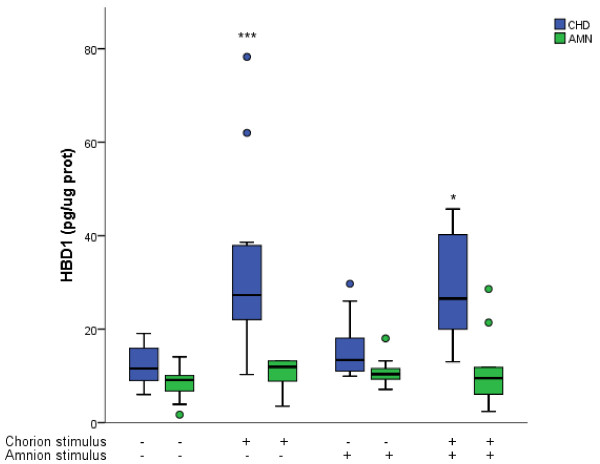
We evaluated HBD-1 secretion pattern in the culture medium after selective stimulation with C. albicans for 24 h. In comparison with basal secretion (11.57 [8.51-16.29] pg/μg protein) in the choriodecidual compartment, stimulation of the choriodecidual side of the membrane induced, at least, a 2.5-fold increase (27.3 [20.9-38.25] pg/μg) of HBD-1 in this compartment. A similar pattern was induced when the stimulus was applied to both AMN and CHD simultaneously (26.55 [19.4-42.5] pg/μg of protein) Figure 1.
Figure 1.
Compartamentalized in vitro secretion of HBD-1 after selective stimulation with Candida albicans. Antimicrobian peptide HBD-1 was measured in the medium from comparments delimited by the amnion (AMN) and the choriodecidua (CHD) under basal conditions and after the three different modalities of infection with 1 × 106 CFU of Candida albicans. Boxes represent median concentration of HBD-1 with 25-75 interquartile range; outlier values are represented by ( · ); (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, n = 10). *Statistically different with respect to control value of choriodecidual compartment.
Stimulation at the amniotic side of the membrane did not induce significant changes of HBD-1 secretion profile (P = 0.107) in either AMN (10.36 [9.06-12.12] pg/μg of protein) or CHD (13.40 [10.82-20.07]) regions. In comparison with control conditions, CHD was the most active in the secretion of HBD-1 and the amniotic side did not respond in a significant way under any of the three infection modalities Figure 1.
Immunohistochemistry revealed an increase of immunoreactive forms of HBD-1 after simultaneous stimulation of amnion and choriodecidua regions with C. albicans. The strongest HBD-1 signal was localized in the trophoblasts of the chorion. Distribution of HBD-1 signal was similar in all stimulation conditions Figure 2.
Figure 2.
Immunoreactivity of HBD-1 in human chorioamniotic membranes stimulated in both regions with C. albicans. The first panel shows a non-stimulated control membrane (a. 20X). Panel b shows HBD-1 immunoreactive forms in membranes stimulated simultaneously with C. albicans.
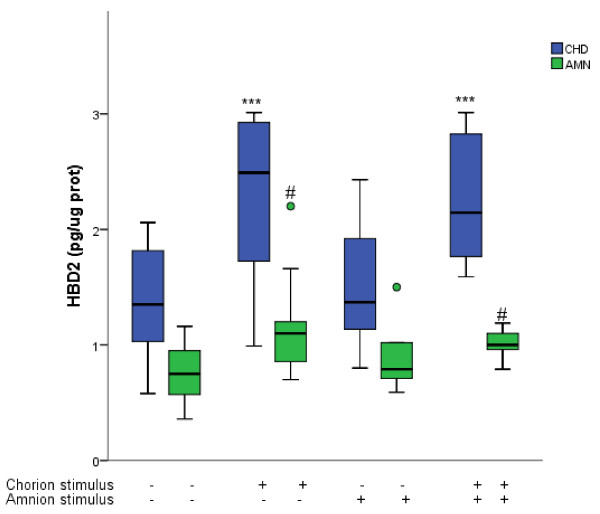
In comparison with basal levels, stimulation with C. albicans in the CHD region induced a significant increase of HBD-2 in both choriodecidual (2.49 [1.49-2.95] pg/μg of protein) and amniotic compartments (1.10 [0.85-1.20] pg/μg of protein). Yeast stimulation at the AMN side of the membrane did not induce any significant change (P = 0.25) in the pattern of HBD-2 secretion in either AMN or CHD Figure 3.
Figure 3.
Compartamentalized in vitro secretion of HBD-2 after selective stimulation with Candida albicans. Antimicrobian peptide HBD-2 was measured in the medium from comparments delimited by the amnion (AMN) and the choriodecidua (CHD) under basal conditions and after the three different modalities of infection with 1 × 106 CFU of Candida albicans. Boxes represent median concentration of HBD-2 with 25-75 interquartile range; outlier values are represented by ( · ) ; (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, n =10). *Statistically different with respect to control values of the choriodecidual compartment. #Statistically different with respect to control values of the amniotic compartment.
In comparison with basal levels in CHD (1.35 [0.99-1.92] pg/μg protein) and AMN (0.75 [ 0.53-0.96] pg/μg protein), simultaneous stimulation induced a significant increase of HBD-2 in chorionic and amniotic compartment (2.14 [1.67-2.91]) and (1.00-[0.9-1.13]) respectively Figure 3.
Control tissues expressed immunoreactive forms of HBD-2 in the amniotic epithelium and trophoblast cells. Stimulation with C. albicans induced the increase of HBD-2 immunoreactive forms in both of these cells populations, especially within the choriodecidua region Figure 4.
Figure 4.
Immunoreactivity of HBD-2 in human chorioamniotic membranes stimulated in both regions with C. albicans. The first panel shows a non-stimulated control membrane (a. 20X). Panel b shows HBD-2 immunoreactive forms in membranes stimulated simultaneously with C.albicans.
ELISA assays indicated that HBD-3 concentration in the membranes stimulated with C. albicans were not significantly different from those found in basal conditions regardless of stimulation conditions Figure 5. Immunolocalization of HBD-3 produced similar results Figure 6.
Figure 5.
Compartamentalized in vitro secretion of HBD-3 after selective stimulation with Candida albicans. Antimicrobian peptide HBD-3 was measured in the medium from comparments delimited by the amnion (AMN) and the choriodecidua (CHD) under basal conditions and after the three differente modalities of infection with 1 × 106 CFU of Candida albicans . Boxes represent median concentration of HBD-3with 25-75 interquartile range; outlier values are represented by ( · ) ; (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, n = 10).
Figure 6.
Immunoreactivity of HBD-3 in human chorioamniotic membranes stimulated in both regions with C. albicans. The first panel shows a non-stimulated control membrane (a. 20X). Panel b shows HBD-3 membranes stimulated simultaneously with C.albicans.
Discussion
The immune innate system enables human amniochorionic membranes and other tissues of the fetal-maternal interface to recognize potentially infectious threats [41] and secrete AMP’s that act as the first defense line to delimit and control an infectious process [10,15]. The key role of fetal membranes as an effective barrier against C. albicans has been demonstrated in different animal experimental models [42,43].
Candida albicans has been identified as a microorganism isolated from the vagina, lower urinary tract, fetus-placental unit, and amniotic fluid during pregnancy and has been associated with an increased risk of premature rupture of membranes (PROM) and PPROM, which are associated with an increased risk of intra-amniotic infection; these are all conditions that negatively affect perinatal morbidity and mortality of newborns [44]. Infants with a birth weight of less than 1250 g comprise the group with the highest risk of developing pulmonary candidiasis [45].
In a study addressed to know whether Candida species can penetrate intact fetal membranes under ex vivo conditions, it was possible to demonstrate that C. albicans was the only one that, once inoculated into the maternal side, penetrated and passed to the fetal side, and caused degeneration of the structure of the membrane epithelium [46].
In the present study, we addressed the question of whether the amniochorionic membranes are able to respond to Candida albicans infection with the production of HBD-1, HBD-2, HBD-3, peptides that directly combat bacterial proliferation and survival. The experimental model used in this study allowed us to reproduce the different contact points between Candida albicans, ascending from the lower genital tract, and the uterine cavity; besides, we reproduced in vivo two compartments separated by a fully functional fetal membrane and analyzed the compartmentalized secretion in the compartment delimited by the amnion and that delimited by the choriodecidua [36].
The present work demonstrates that HBD-1 was the main defensin secreted by the membranes after stimulation with C. albicans. The ELISA and immunohistochemistry results support the hypothesis that the choriodecidua is the region more active in the synthesis and secretion of this defensin.
These results are supported by previous evidence indicating that mRNA and protein of HBD-1 are present in the amnion, decidua, and chorion trophoblast layers of amniochorionic membranes, being the trophoblast region the main source of endogenous antimicrobial molecules during pregnancy [10].
Wiechula et al. [47] demonstrated that the mRNA of HBD-1 is increased in the lavage of female genital tract collected from women with candidiasis, which is in agreement with studies done with human normal vaginal epithelia co-cultured with C. albicans that induce a significant secretion of HBD-1 and HBD-2 [48].
The abundant secretion of HBD-1 in our model could be supported by a possible relationship between pregnancy tissues and the genitourinary tract, which expresses high levels of these defensins [14].
There are reports indicating that under pathological conditions, such as chorioamnionitis and/or PROM, which have been associated with uncontrolled production of harmful pro-inflammatory cytokines, the pattern of HBD-1 synthesis and secretion, as well as its immuno-localization profile, does not change [10]. However in a recent work in our laboratory, using the same experimental model of two independent compartments, we demonstrated that stimulation with G. vaginalis induces upregulation of HBD-1 [24].
In contrast, a growing body of evidence indicates that HBD-2 is up-regulated by inflammatory cytokines in cells derived from reproductive tissues, including fetal membranes [25] and in other organs, such as skin [49], human endometrial epithelial cells [50], oral epithelial cells [51,52], respiratory epithelia [53], and different colonic epithelial cell lines [54].
In the present work, we confirmed that HBD-2 is present at basal level in the membranes, which agrees with previous evidence reported by King et al. [10]. On the other hand, stimulation with C. albicans induced the increase of secreted levels and immunoreactive forms of HBD-2 in both amniotic and choriodecidual compartments.
During pregnancy, HBD-2 is part of the immune machinery associated with the antimicrobial properties of the amniotic fluid; however, under pathological conditions such as MIAC, HBD-2 is up-regulated in the amniotic fluid regardless of the fetal membrane status (intact membranes or PROM) [19]. These results concur with evidence of our lab indicating that the infection of fetal membranes with E. coli –a microorganism associated with chorioamnionitis– elicited high secretion of HBD-2 by both amnion and choriodecidua regions [25].
Additional evidence indicates that transcription of the HBD-2 gene is induced by IL-1β [54,55], a pro-inflammatory cytokine that is produced by the choriodecidual region of human fetal membranes in response to a selective stimulation with C. albicans[40].
Additional experimental evidence indicate that HBD-2 elicits in vitro antifungal activity against Candida albicans, which is killed by a dose of 25 μg/ml of HBD-2 that is lethal for 90% of strains tested (LD90) [54,56] and in an energy-dependent and salt-sensitive manner without causing membrane disruption [57].
HBD-3 is a 4-kDa antimicrobial peptide originally isolated from human psoriatic lesion scales [12] whose strong expression has been demonstrated in keratinocytes and in tonsil tissue, whereas low HBD-3 expression was found in epithelia of the respiratory, gastrointestinal, and genitourinary tracts [58].
In the present study, we found that, independently from whether the yeast was added only to the amnion or to the choriodecidua or to both, stimulation with C. albicans did not induce any significant change in the HBD-3 secretion pattern, which was confirmed by immunohistochemistry.
It has been reported that HBD3 is up regulated by TNFα [59], however, previous evidence from our laboratory indicates that the stimulation of chorioamniotic membranes with C. albicans does not up-regulate the synthesis and secretion of TNFα [40].
Additionally, esophageal cell line OE21 stimulated with supernatants or directly co-cultured with C. albicans induces up-regulation of HBD-2 and HBD-3 expression, and the infection process involves divergent signaling events that differentially govern HBD-2 and HBD-3 [59]; these findings support our results indicating that the stimulation with C. albicans differentially up-regulates HBD-2 but not HBD-3, both defensins have been previously reported with strain-specific activity against Candida sp. including C. albicans[59,60].
Amniotic and choriodecidual regions are constituted by completely different cellular populations, which can explain –in part– the existence of tissue-specific HBD-1, HBD-2, and HBD-3 secretion patterns as part of the response to Candida albicans, this includes the basal protection of the three defensins and the up-regulation of HBD-1 and HBD-2 after stimulation with the yeast.
The present work and previous experimental evidence [23-25] support the concept that the human chorioamniotic membranes have immune capacities that include innate immune factors, such as HBDs, that are secreted in a tissue specific pattern as part of a complex mechanism of defense/protection against different pathogen microorganisms associated to preterm labor complicated with chorioamnionitis. The action of these molecules may limit the spread of pathogens between maternal and fetal compartments preventing the infection of the amniotic cavity.
Innate immune capacities in pregnancy tissues are key in the recognition, delimitation, and eventual destruction of pathogens that can damage the immunologic equilibrium at the maternal-placental interface. Differential capacities to recognize the pathogen are directly associated with receptors of the innate immune system, Toll like receptors (TLRs) are a family of pattern recognition receptors that respond to the presence of pathogen-derived products and are responsible in part for the control of antimicrobial expression [61,62].
There is evidence supporting the active role of the innate immune system, TLR-4 and TLR-2 are two key receptors in the recognition of linear structure of O-linked mannosyl residues [63] and β-(1,3)-mannosides, which are present in the acid-stable and acid-labile component of mannoproteins and phospholipomannans (PLM) [64] on the Candida albicans cell wall, respectively. Additionally fungal DNA is poorly methylated in contrast to mammalian DNA, and experimental evidence indicates that TLR-9 is involved in the recognition of C. albicans[62,65].
At the maternal-placental interface, TLRs play a key role as sentinels to detect different pathogens, these receptors are expressed not only in the immune cells but also in non-immune cells, including amniotic epithelium, villous and extra-villous trophoblasts, and decidual cells, and their expression pattern can vary according to the stage of pregnancy [61,62].
The expression and activity of TLR-2 and TLR-4 have been associated with spontaneous labor at term, as well as with preterm delivery with histological chorioamnionitis, indicating that these receptors play a key role in the innate immune mechanisms required to respond effectively against an immunologic-infectious process [61].
Conclusions
Our results demonstrate that amniochorionic membranes respond differentially to Candida albicans infection. Amnion and choriodecidua secrete HBD-1 and HBD-2 as part of the innate immune defenses of this tissue.
The choriodecidua is the most responsive region to the infection, as it is the first tissue to be colonized by the microbial pathogen during an ascending intrauterine infection and it is the main barrier to progression of the infection into the amniotic cavity and eventually the fetus.
Tissue-specific capacities of these membranes to secret HBD-1 and HBD-2 represent the first line of defense in amniochorionic membranes and represent part of a very complex mechanism of antimicrobial protection that ensures an initial protection at times when infection may jeopardize the continuity of pregnancy.
Abbreviations
AMN: Amnion; AMP: Natural antimicrobial peptides; BPI: Bactericidal permeability increasing protein; CHD: Choriodecidua; CFU: Colony forming unit; DMEM: Dulbecco modified eagle medium; ELISA: Enzyme-linked immunosorbent assay; FBS: Fetal bovine serum; HBD: Human beta defensins; HEC: Human amniotic epithelial cells; HNP: Human neutrophil peptide; LPS: Lipopolysacharide; MIAC: Microbial invasion of the amniotic cavity; PROM: Premature rupture of membranes; SLPI: Secretory leukocyte protease inhibitor; TLR: Toll like receptors.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VZC, MR, PFE, MOC, and AFP collected the samples, culture membranes, performed stimulation with the bacterium and ELISA assays. GEG and RVS performed the statistical analyses. ISG and IMM performed the microbiological control. VZC and MR participated in the design of the study, data collection, and analysis, as well as manuscript preparation. All authors have read and approved the final manuscript.
Contributor Information
Veronica Zaga-Clavellina, Email: sciencefeedback@gmail.com.
Martha Ruiz, Email: marthalicias@yahoo.com.
Pilar Flores-Espinosa, Email: kipa_kin@hotmail.com.
Rodrigo Vega-Sanchez, Email: vegarodrigo@hotmail.com.
Arturo Flores-Pliego, Email: arturo_fpliego@yahoo.com.mx.
Guadalupe Estrada-Gutierrez, Email: gpestrad@yahoo.es.
Irma Sosa-Gonzalez, Email: irmasosa77@yahoo.com.mx.
Iyari Morales-Méndez, Email: iyari_mm@yahoo.com.mx.
Mauricio Osorio-Caballero, Email: dr_osorio@yahoo.com.
Acknowledgments
These studies were supported by Instituto Nacional de Perinatologia “Isidro Espinosa de los Reyes”, project # 06151 to VZC. We thank Ingrid Mascher for editorial assistance and Dr. Daudi Langat and David Wheaton for language corrections.
References
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- Asrat T. Intra-amniotic infection in patients with preterm prelabor rupture of membranes. Pathophysiology, Detection, and Management. Clinic Perinatol. 2001;28:735–751. doi: 10.1016/S0095-5108(03)00074-5. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Parry S, Strauus JF. Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–668. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- Malak TM, Ockleford CD, Bell SC, Dalgleish R, Bright N, Macvicar J. Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta. 1993;14:385–406. doi: 10.1016/S0143-4004(05)80460-6. [DOI] [PubMed] [Google Scholar]
- Klein LL, Gibbs RS. Infection and preterm birth. Obstet Gynecol Clin North Am. 2005;32:397–410. doi: 10.1016/j.ogc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 199;12:285–288. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schønheyder HC, Uldbjerg N, Madsen H. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001;94:224–229. doi: 10.1016/S0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta. 2007;28:161–169. doi: 10.1016/j.placenta.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/S0966-842X(00)01823-0. [DOI] [PubMed] [Google Scholar]
- De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27:1337–1347. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JJ, Unholzer A, Schaller M, Schäfer-Korting M, Korting HC. Human defensins. J Mol Med (Berl) 2005;83:587–595. doi: 10.1007/s00109-005-0657-1. [DOI] [PubMed] [Google Scholar]
- Stock SJ, Kelly RW, Riley SC, Calder AA. Natural antimicrobial production by the amnion. Am J Obstet Gynecol. 2007;196:255.e1–255.e6. doi: 10.1016/j.ajog.2006.10.908. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Jabr M, Buhimschi CS, Petkova AP, Weiner CP, Saed GM. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am J Obstet Gynecol. 2004;191:1678–1687. doi: 10.1016/j.ajog.2004.03.081. [DOI] [PubMed] [Google Scholar]
- Szukiewicz D, Szewczyk G, Pyzlak M, Klimkiewicz J, Maslinska D. Increased production of beta-defensin 3 (hBD-3) by human amniotic epithelial cells (HAEC) after activation of toll-like receptor 4 in chorioamnionitis. Inflamm Res. 2008;57(Suppl 1):S67–S68. doi: 10.1007/s00011-007-0633-z. [DOI] [PubMed] [Google Scholar]
- Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, Santolaya-Forgas J, Romero R. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007;20:15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim YM, Hassan S, Blackwell S, Whitty J, Berman S, Redman M, Yoon BH, Sorokin Y. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- Erez O, Romero R, Tarca AL, Chaiworapongsa T, Kim YM, Than NG, Vaisbuch E, Draghici S, Tromp G. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2009;22:1103–1115. doi: 10.3109/14767050902994796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Innate immune defences in the human uterus during pregnancy. Placenta. 2007;28:1099–1106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Zaga-Clavellina V, Garcia-Lopez G, Flores-Espinosa P. Evidence of in vitro differential secretion of human beta-defensins-1, -2, and -3 after selective exposure to Streptococcus agalactiae in human fetal membranes. J Matern Fetal Neonatal Med. 2012;4:258–263. doi: 10.3109/14767058.2011.578695. [DOI] [PubMed] [Google Scholar]
- Zaga-Clavellina V, Martha RV, Flores-Espinosa P. In vitro secretion profile of pro-inflammatory cytokines IL-1β, TNF-α, IL-6, and of human beta-defensins (HBD)-1, HBD-2, and HBD-3 from human chorioamniotic membranes after selective stimulation with Gardnerella vaginalis. Am J Reprod Immunol. 2012;67:34–43. doi: 10.1111/j.1600-0897.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez G, Flores-Espinosa P, Zaga-Clavellina V. Tissue-specific human beta-defensins (HBD)1, HBD2, and HBD3 secretion from human extra-placental membranes stimulated with Escherichia coli. Reprod Biol Endocrinol. 2010;8:146. doi: 10.1186/1477-7827-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders GG, Moerman P, Caudron J, Van Assche FA. Intra-uterine Candida infection: a report of four infected fetusses from two mothers. Eur J Obstet Gynecol Reprod Biol. 1991;38:233–238. doi: 10.1016/0028-2243(91)90298-Y. [DOI] [PubMed] [Google Scholar]
- Romero R, Reece EA, Duff GW, Coultrip L, Hobbins JC. Prenatal diagnosis of Candida albicans chorioamnionitis. Am J Perinatol. 1985;2:121–122. doi: 10.1055/s-2007-999928. [DOI] [PubMed] [Google Scholar]
- Bruner JP, Elliott JP, Kilbride HW, Garite TJ, Knox GE. Candida chorioamnionitis diagnosed by amniocentesis with subsequent fetal infection. Am J Perinatol. 1986;3:213–218. doi: 10.1055/s-2007-999870. [DOI] [PubMed] [Google Scholar]
- Berry DL, Olson GL, Wen TS, Belfort MA, Moise KJ Jr. Candida chorioamnionitis: a report of two cases. J Matern Fetal Med. 1997;6:151–154. doi: 10.1002/(SICI)1520-6661(199705/06)6:3<151::AID-MFM6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Baley JE. Neonatal candidiasis: the current challenge. Clin Perinatol. 1991;18:263–280. [PubMed] [Google Scholar]
- Ng PC, Siu YK, Lewindon PJ, Wong W, Cheung KL, Dawkins R. Congenital Candida pneumonia in a preterm infant. J Paediatr Child Health. 1994;30:552–554. doi: 10.1111/j.1440-1754.1994.tb00734.x. [DOI] [PubMed] [Google Scholar]
- Engelhart CM, van de Vijver NM, Nienhuis SJ, Hasaart TH. Fetal Candida sepsis at midgestation: a case report. Eur J Obstet Gynecol Reprod Biol. 1998;77:107–109. doi: 10.1016/S0301-2115(97)00222-4. [DOI] [PubMed] [Google Scholar]
- Krishnakumari V, Rangaraj N, Nagaraj R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob Agents Chemother. 2009;53:256–260. doi: 10.1128/AAC.00470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res. 2005;8:445–450. doi: 10.1177/154405910508400509. [DOI] [PubMed] [Google Scholar]
- Lupetti A, Danesi R, Wout JW V't, Van Dissel JT, Senesi S, Nibbering PH. Antimicrobial peptides: therapeutic potential for the treatment of Candida infections. Expert Opin Investig Drugs. 2002;11:309–318. doi: 10.1517/13543784.11.2.309. [DOI] [PubMed] [Google Scholar]
- Zaga V, Estrada-Gutierrez G, Beltran-Montoya J, Maida-Claros R, Lopez-Vancell R, Vadillo-Ortega F. Secretions of interleukin-1 beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod. 2004;71:1296–1302. doi: 10.1095/biolreprod.104.028621. [DOI] [PubMed] [Google Scholar]
- Thiex NW, Chames MC, Loch-Caruso RK. Tissue-specific cytokine release from human extra-placental membranes stimulated by lipopolysaccharide in a two-compartment tissue culture system. Reprod Biol Endocrinol. 2009;7:117. doi: 10.1186/1477-7827-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MF, Loch-Caruso R. Comparison of LPS-stimulated release of cytokines in punch versus transwell tissue culture systems of human gestational membranes. Reprod Biol Endocrinol. 2010;8:121. doi: 10.1186/1477-7827-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Zaga-Clavellina V, López GG, Estrada-Gutierrez G, Martinez-Flores A, Maida-Claros R, Beltran-Montoya J, Vadillo-Ortega F. Incubation of human chorioamniotic membranes with Candida albicans induces differential synthesis and secretion of interleukin-1beta, interleukin-6, prostaglandin E, and 92 kDa type IV collagenase. Mycoses. 2006;49:6–13. doi: 10.1111/j.1439-0507.2005.01171.x. [DOI] [PubMed] [Google Scholar]
- Abrahams VM. Pattern recognition at the maternal-fetal interface. Immunol Invest. 2008;37:427–447. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- Gow NA, Knox Y, Munro CA, Thompson WD. Infection of chick chorioallantoic membrane (CAM) as a model for invasive hyphal growth and pathogenesis of Candida albicans. Med Mycol. 2003;41:331–338. doi: 10.1080/13693780310001600859. [DOI] [PubMed] [Google Scholar]
- Jacobsen ID, Grosse K, Berndt A, Hube B. Pathogenesis of Candida albicans infections in the alternative chorio-allantoic membrane chicken embryo model resembles systemic murine infections. PLoS One. 2011;6:e19741. doi: 10.1371/journal.pone.0019741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velemínský M, Tosner J. Relationship of vaginal microflora to PROM, pPROM and the risk of early-onset neonatal sepsis. Neuro Endocrinol Lett. 2008;29:205–221. [PubMed] [Google Scholar]
- Frezza S, Maggio L, De Carolis MP, Gallini F, Puopolo M, Polimeni V, Costa S, Vento G, Tortorolo G. Risk factors for pulmonary candidiasis in preterm infants with a birth weight of less than 1250 g. Eur J Pediatr. 2005;164:88–92. doi: 10.1007/s00431-004-1571-1. [DOI] [PubMed] [Google Scholar]
- Gürgan T, Diker KS, Haziroglu R, Urman B, Akan M. In vitro infection of human fetal membranes with Candida species. Gynecol Obstet Invest. 1994;37:164–167. doi: 10.1159/000292549. [DOI] [PubMed] [Google Scholar]
- Wiechuła B, Cholewa K, Ekiel A, Romanik M, Dolezych H, Martirosian G. HBD-1 and hBD-2 are expressed in cervico-vaginal lavage in female genital tract due to microbial infections. Ginekol Pol. 2010;81:268–271. [PubMed] [Google Scholar]
- Liu Z, Chen L. Congenital anti-candida of human vaginal epithelial cells. Beijing Da Xue Xue Bao. 2008;40:174–177. [PubMed] [Google Scholar]
- Sørensen OE, Thapa DR, Rosenthal A, Liu L, Roberts AA, Ganz T. Differential regulation of beta-defensin expression in human skin by microbial stimuli. J Immunol. 2005;174:4870–4879. doi: 10.4049/jimmunol.174.8.4870. [DOI] [PubMed] [Google Scholar]
- King AE, Fleming DC, Critchley HO, Kelly RW. Regulation of natural antibiotic expression by inflammatory mediators and mimics of infection in human endometrial epithelial cells. Mol Hum Reprod. 2002;8:341–349. doi: 10.1093/molehr/8.4.341. [DOI] [PubMed] [Google Scholar]
- Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–2915. doi: 10.1128/IAI.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M, Jia HP, Guthmiller JM, Losh G, Graham S, Johnson GK, Tack BF, McCray PB Jr. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999;67:2740–2745. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schröder JM. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- Schröder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–651. doi: 10.1016/S1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- Hiemstra PS. Epithelial antimicrobial peptides and proteins: their role in host defence and inflammation. Paediatr Respir Rev. 2001;2:306–310. doi: 10.1053/prrv.2001.0165. [DOI] [PubMed] [Google Scholar]
- Vylkova S, Nayyar N, Li W, Edgerton M. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob Agents Chemother. 2007;51:154–161. doi: 10.1128/AAC.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- Steubesand N, Kiehne K, Brunke G, Pahl R, Reiss K, Herzig KH, Schubert S, Schreiber S, Fölsch UR, Rosenstiel P, Arlt A. The expression of the beta-defensins hBD-2 and hBD-3 is differentially regulated by NF-kappaB and MAPK/AP-1 pathways in an in vitro model of Candida esophagitis. BMC Immunol. 2009;10:36. doi: 10.1186/1471-2172-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Maze C, McCray PB Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams VM, Mor G. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–1355. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG. 2007;114:1326–1334. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouault T, El Abed-El Behi M, Martínez-Esparza M, Breuilh L, Trinel PA, Chamaillard M, Trottein F, Poulain D. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]