Abstract
Microspore-derived embryos of Brassica napus cv Reston were used to examine the effects of exogenous (+)-abscisic acid (ABA) and related compounds on the accumulation of very-long-chain monounsaturated fatty acids (VLCMFAs), VLCMFA elongase complex activity, and induction of the 3-ketoacyl-coenzyme A synthase (KCS) gene encoding the condensing enzyme of the VLCMFA elongation system. Of the concentrations tested, (+)-ABA at 10 μm showed the strongest effect. Maximum activity of the elongase complex, observed 6 h after 10 μm (+)-ABA treatment, was 60% higher than that of the untreated embryos at 24 h. The transcript of the KCS gene was induced by 10 μm (+)-ABA within 1 h and further increased up to 6 h. The VLCMFAs eicosenoic acid (20:1) and erucoic acid (22:1) increased by 1.5- to 2-fold in embryos treated with (+)-ABA for 72 h. Also, (+)-8′-methylene ABA, which is metabolized more slowly than ABA, had a stronger ABA-like effect on the KCS gene transcription, elongase complex activity (28% higher), and level of VLCMFAs (25–30% higher) than ABA. After 24 h approximately 60% of the added (+)-[3H]ABA (10 μm) was metabolized, yielding labeled phaseic and dihydrophaseic acid. This study demonstrates that (+)-ABA promotes VLCMFA biosynthesis via increased expression of the KCS gene and that reducing ABA catabolism would increase VLCMFAs in microspore-derived embryos.
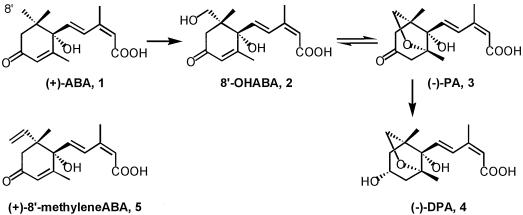
The phytohormone (+)-ABA (Fig. 1, structure 1) plays regulatory roles in many physiological processes, such as control of stomatal aperture, seed dormancy, seed development and germination, synthesis of seed storage protein and lipid, and stress tolerance (Zeevaart and Creelman, 1988; Davies and Jones, 1991; Hetherington and Quatrano, 1991; Thomas, 1993). ABA regulates the expression of many genes, including those encoding oil-body proteins in microspore-derived and zygotic embryos of Brassica napus (Vance and Huang, 1988; Wilen et al., 1990; Holbrook et al., 1991, 1992; Taylor and Weber, 1994), and (±)-ABA promotes VLCMFA accumulation in zygotic embryos of B. napus (Finkelstein and Somerville, 1989). Recently, Zou et al. (1995) demonstrated that exogenously applied (+)-ABA and its metabolite 8′-OH ABA (Fig. 1, structure 2) induced the transcripts of oleosin and Δ15 desaturase genes, as well as the accumulation of VLCMFAs in microspore-derived embryos of B. napus cv Reston, whereas (−)-PA (Fig. 1, structure 3) had little effect. These results suggested that ABA and/or 8′-OH ABA may regulate the accumulation of VLCMFAs in developing oilseed embryos.
Figure 1.
Chemical structures of (+)-ABA (1), 8′-OH ABA (2), (−)-PA (3), (−)-DPA (4), and (+)-8′-methylene ABA (5).
The biosynthesis of VLCMFAs occurs by successive condensations of malonyl-CoA with 18:1-CoA to give eicosenoyl (20:1) and erucoyl (22:1) moieties. The synthesis is catalyzed by a microsomal fatty acid “elongase complex” composed of four enzymes, beginning with the condensing enzyme (von Wettstein-Knowles, 1982; Fehling and Mukherjee, 1991). The Arabidopsis Fatty Acid Elongation1 (FAE1) gene was shown to share homology with three condensing enzymes: chalcone synthase, stilbene synthase, and β-ketoacyl carrier protein synthase III. Based on this homology and the functional studies of this gene (Millar and Kunst, 1997), it was proposed that the gene identified by the FAE1 mutation encodes KCS, the seed-specific condensing enzyme, which catalyzes the first reaction of the microsomal fatty acid elongation system involved in the biosynthesis of VLCMFAs (James et al., 1995). Studies in Arabidopsis (Millar and Kunst, 1997) and in other Brassicaceae spp. (Lassner et al., 1996) have shown that the condensing enzyme is limiting for the accumulation of VLCMFAs. It was also demonstrated that in the elongase complex, it is the specificity of the condensing enzyme that determines which VLCMFAs accumulate (Lassner et al., 1996; Millar and Kunst, 1997). In this study we expect to use the FAE1 gene as a probe to detect the transcript levels of KCS genes in microspore-derived embryos of B. napus.
Since completing much of the experimental work described here, the B. napus KCS homolog of the Arabidopsis KCS gene (identified by the lesion at the FAE1 locus) has been reported (Clemens and Kunst, 1997). The Arabidopsis and B. napus KCS genes are 85% homologous at the DNA level and 84 to 86% similar at the protein level, as assessed by the DNAStar suite of programs. This high homology explains the very strong hybridization signal observed in our studies, in which B. napus KCS transcript levels were monitored by probing with the Arabidopsis KCS (FAE1) homolog.
The major pathway of metabolism of natural (+)-(S)-ABA (Fig. 1, structure 1) involves oxidation at the 8′-methyl group to produce 8′-OH ABA (Fig. 1, structure 2), which cyclizes to form (−)-PA (Fig. 1, structure 3). (−)-PA may then be reduced to DPA (Fig. 1, structure 4) (Gillard and Walton, 1976; Loveys and Milborrow, 1984; Balsevich et al., 1994; Sorce et al., 1996). PA in either the natural or racemic form has been found to possess ABA-like activity in a few bioassays (Dashek et al., 1979; Robertson et al., 1994; Hill et al., 1995), but in most cases PA and DPA have minimal biological activity (Dashek et al., 1979; Ho, 1983; Balsevich et al., 1994; Robertson et al., 1994; Hill et al., 1995; Zou et al., 1995; Sorce et al., 1996).
ABA 8′ hydroxylase (the enzyme that converts ABA to PA via 8′-OH ABA) has been shown to be induced by ABA in several experimental systems (e.g. Uknes and Ho, 1984; Cutler et al., 1997), providing a homeostatic mechanism to reduce high ABA levels. The content of ABA in seeds changes markedly during development (Hetherington and Quatrano, 1991), but the role of ABA 8′ hydroxylase as a controlling factor is unknown. Increasing ABA degradation is potentially important for modulating endogenous concentrations (Zeevaart and Creelman, 1988; Kende and Zeevaart, 1997). If changes in the rate of ABA turnover are a major determinant of ABA concentration in B. napus embryos, then genetically reducing ABA degradation has the potential to enhance ABA-like effects on VLCMFA production and lipid accumulation.
(+)-8′-Methylene ABA (Fig. 1, structure 5), a new ABA analog, is metabolized more slowly than ABA in corn cells, resulting in enhanced biological activity relative to ABA (Abrams et al., 1997). In fact, 8′-methylene ABA has been shown to be more effective than (+)-ABA in several biological assays because it provides the equivalent of an extended pulse of ABA (Abrams et al., 1997). The consequences of reduced ABA degradation can therefore be inferred by comparing the hormonal activity of 8′-methylene ABA with that of ABA itself. The present study was undertaken to determine if it will be feasible in future transgenic experiments to elevate VLCMFA production by blocking ABA catabolism. To this end we have extended previous studies (Zou et al., 1995) by measuring ABA metabolism and examining the effects of ABA and related substances on the expression of the B. napus KCS condensing enzyme and the resulting VLCMFA production in microspore-derived embryos of B. napus cv Reston. 8′-Methylene ABA has been used to provide an indication of how VLCMFA biosynthesis is affected by reduced ABA catabolism.
MATERIALS AND METHODS
Chemical Reagents
(+)-ABA (Fig. 1) was obtained by preparative HPLC resolution of (±)-methyl abscisate followed by hydrolysis of the resolved esters, as described previously (Dunstan et al., 1992). (−)-PA, the naturally occurring enantiomer (Fig. 1), was obtained from the medium of suspension cultures of corn (Zea mays L. cv Black Mexican Sweet) that had been supplied with (+)-ABA, according to the procedure of Balsevich et al. (1994). DPA (Fig. 1) was prepared from the isolated PA as described by Zeevaart and Milborrow (1976). (+)-[3H]ABA was synthesized according to a reported procedure (Balsevich et al., 1994). (+)-8′-Methylene ABA was synthesized as described previously by Abrams et al. (1997).
[1-14C]Oleic acid (58 mCi/mmol) was purchased from Amersham and converted to [1-14C]oleoyl-CoA by an enzymatic method described previously (Taylor et al., 1990). Neutral lipid standards were obtained from NuChek Prep., Inc. (Elysian, MN), and polar lipids were purchased from Sigma. HPLC-grade solvents (Omni-Solv, BDH Chemicals, Poole, UK) were used throughout these studies. All other chemicals were of reagent grade.
Plant Material, Microspore Culture, and Hormone Treatments
Brassica napus L. cv Reston, a high-erucic acid variety accumulating both C20 and C22 fatty acids in developing seeds, was obtained from the University of Manitoba (Winnipeg, Canada). Plants were grown in controlled-environment growth chambers as described by Zou et al. (1995). Microspores were isolated and cultured according to the methods described previously (Taylor et al., 1990, 1992; Holbrook et al., 1992; Zou et al., 1995). At 16 to 19 d in culture, microspore-derived embryo preparations enriched in early- to mid-cotyledonary stages were obtained by filtration through a sterile, 0.2-μm filter and replated at a density of 0.25 to 0.3 g fresh weight in 10 mL of medium in 100- × 10-mm Petri plates. After a 24-h equilibration period, embryo cultures were supplemented with either 10 μm (+)-ABA, (+)-8′-methylene ABA, (−)-PA, or (−)-DPA in 0.1% (v/v) ethanol (hormone treatment) or 0.1% ethanol only as a control treatment, and maintained in the dark at 25°C on a rotary shaker at 50 rpm. After 0, 1, 2, 4, 6, 12, 24, 48, or 72 h of treatment, individual plates of embryos for each treatment (three to six Petri plates) were harvested by suction filtration and rinsed thoroughly with distilled water, and the fresh weight was recorded. The medium was stored at −70°C for subsequent analysis. A portion of the harvested samples was removed for dry-weight determination after desiccation at 100°C for 48 h. The remainder was used to prepare homogenates as described below for determination of total lipid content, fatty acid composition, total protein, and VLCMFA elongase complex activity studies. Total homogenate protein was estimated by the method of Bradford (1976) using BSA as a standard.
Because there were not enough microspore-derived embryos supplied at one time for all experiments in the study, different batches of early- to mid-cotyledonary embryos were used in individual experiments. All experiments were performed at least twice. Data shown are from representative experiments.
Metabolism Studies Using (+)-[3H]ABA
(+)-[3H]ABA at the initial concentration of 10 μm was supplied to the equilibrated B. napus embryo cultures. The mass of embryos in the medium at the time of hormone addition was about 300 mg fresh weight per plate in 10 mL of medium. Each treatment was replicated four to six times. In time-course experiments microspore-derived embryos were harvested by vacuum filtration at the following time intervals after the addition of 10 μm (+)-[3H]ABA: 0, 2, 6, 24, 48, or 72 h; they were then rinsed thoroughly with distilled water and the fresh weight recorded. Aliquots of harvested embryos intended for metabolite analysis were immediately frozen in liquid N2 and stored at −70°C. The medium at each time point was also stored at −70°C for further analysis.
Frozen embryos (300 mg fresh weight) were ground with a mortar and pestle under liquid N2 for extraction and analysis of [3H]ABA and its metabolites. The ground sample was extracted overnight with 30 mL of solution containing 95% isopropanol and 5% glacial acetic acid, filtered, and further extracted twice with 15 mL of the same solution for 10 min each time. The bulked filtrate was concentrated by a rotary evaporator. The residue was dissolved in 2 mL of 1 n NaOH solution and then washed three times with 3 mL of methylene chloride to remove lipophilic materials. The aqueous fraction was brought to approximately pH 3.5 with 1 n HCl and partitioned three times into EtOAc; the EtOAc extracts were then combined and evaporated under N2. The dried residue was then dissolved in 100 μL of methanol for analysis. An aliquot (25 μL) of sample was applied to a Silica Gel GF254 TLC plate for separation of ABA and its metabolites. The TLC plate was developed with toluene:EtOAc:acetic acid (25:15:2, v/v). Sample radioactivity was located by autoradiography and identified by co-chromatography with known standards. The bands corresponding to ABA, PA, and DPA standards were excised and their radioactivity measured by liquid-scintillation counting.
Aliquots of the medium (10 mL) were acidified to pH 3.5 with 1 n HCl and extracted three times with 20 mL of EtOAc. The combined EtOAc fractions were washed once with 30 mL of saturated NaCl, and then anhydrous Na2SO4 was added to remove water. The filtrate was evaporated to dryness. The residue was dissolved in 100 μL of methanol and analyzed via TLC/autoradiography as described above. In an initial experiment, it was determined that (+)-[3H]ABA was stable in both embryo-free and spent media.
Identification of ABA and Metabolites by MS
B. napus microspore-derived embryos from cultures supplemented with 100 μm (+)-ABA were harvested and extracts prepared as described above. Samples were treated with diazomethane and analyzed by GC-MS as described previously (Sorce et al., 1996), with the exception that samples were analyzed by ammonia chemical ionization and detection of DPA was facilitated through further derivatization by co-injection with N,O-bis-(trimethylsilyl)-acetamide. GC retention times and MS data were consistent with those of standard samples of ABA, PA, and DPA.
Extraction of Lipids from Microspore-Derived Embryos and Analysis of Fatty Acyl Composition
Embryo homogenates were prepared as described previously (Holbrook et al., 1992; Zou et al., 1995) and adjusted to give a fresh weight equivalent of 100 mg/mL homogenate. The filtered homogenates (cell-free extract) were used directly for isolation and analysis of total lipid and fatty acid composition, and for in vitro assays of VLCMFA elongase complex activity.
Total acyl lipids were extracted immediately from fresh microspore-derived embryo homogenates prepared as described above (0.5 mL, equivalent to 50 mg fresh weight), according to the method described previously (Zou et al., 1995). A portion of the TLE was transmethylated directly for assay of total acyl composition. An internal standard of 17:0 free fatty acid was added to the TLE to permit quantitative fatty acid analysis. The acyl composition of the fatty acid methyl esters was determined on a gas chromatograph (model 5880, Hewlett-Packard) fitted with a DB-23 column (30 m × 0.25 mm; film thickness 0.25 μm; J&W Scientific, Folsom, CA). The GC conditions were as described by Holbrook et al. (1992). The remaining portion of the TLE was further separated into its polar and neutral lipid components by TLC on Silica Gel H as described previously (Taylor et al., 1992), and individual lipid classes were transmethylated and analyzed for acyl composition by GC.
Assay for VLCMFA Elongase Complex Activity
The elongation assay method was adapted from that originally described by Agrawal and Stumpf (1985). This assay measures the cumulative activity of all four enzymes of the elongase complex, including KCS, the condensing enzyme. A portion of embryo homogenate (equivalent to 0.2–0.25 mg of protein) was used as a source of protein in elongase assays. The reaction mixture components and conditions and 14C-fatty acid extraction and methylation were as described by Zou et al. (1995). The 14C-fatty acid methyl esters were separated and quantified by radio HPLC as described by Holbrook et al. (1992). The VLCMFA elongase activity was calculated on the basis of the known specific activity (10 nCi/nmol) of the [14C]oleoyl-CoA substrate and expressed in milligrams of protein.
Northern Analysis
A plasmid construct, pNAPIN-FAE1, carrying a cDNA clone encoding the FAE1 gene (encoding KCS or the condensing enzyme of the elongase complex) from Arabidopsis was generously provided by Dr. Ljerka Kunst (Department of Botany, University of British Columbia, Vancouver, Canada). cDNA inserts were excised by treatment with SacI and XbaI restriction enzymes, the DNA fragments were purified with the Sephaglas Band Prep Kit (Pharmacia), labeled with [32P]dCTP using the High Prime Kit (Boehringer Mannheim) as described by the manufacturer, and used as a probe in northern analyses. Total RNA was prepared using Trizol Reagent (GIBCO-BRL). Total RNAs (about 6–7 μg) were subjected to electrophoresis according to the method described by Pelle and Murphy (1993) and blotted onto Hybond-N+ nylon membranes (Amersham). Ethidium bromide-stained RNA gel was used as a control for loading and transfer. The blot was hybridized to the randomly primed FAE1 probe using QuikHyb hybridization solution (Stratagene) at 68°C, and washed at high stringency (0.1× SSC and 0.1% SDS at 60°C).
RESULTS AND DISCUSSION
(+)-ABA Metabolism
The metabolism of (+)-ABA in many plants or cell-culture systems has been well documented, and the major oxidized metabolites have been identified as PA (Uknes and Ho, 1984; Dunstan et al., 1992; Balsevich et al., 1994) or DPA (Lehmann et al., 1983; Kubik et al., 1992; Aneja et al., 1996; Sorce et al., 1996). However, little is known about ABA metabolism in the microspore-derived embryo system. A previous study has demonstrated that upon treatment with exogenously applied ABA, the level of ABA significantly increased in microspore-derived embryos of B. napus within 8 h (Zou et al., 1995), showing that ABA is readily taken up by the embryos. To investigate the relationship between ABA metabolism, the regulation of KCS gene expression, and accumulation of gene products during VLCMFA synthesis, the major ABA metabolites were identified and a time-course study using (+)-[3H]ABA was performed.
Initial GC-MS analysis of microspore-derived embryos treated with 100 μm (+)-ABA for 24 h confirmed PA and DPA as the major products of metabolism. Microspore-derived embryos were then treated with 10 μm (+)-[3H]ABA and both embryo and culture medium samples were extracted and analyzed via TLC/autoradiography over the course of 72 h. Radiolabeled PA and DPA were observed as the two major metabolites.
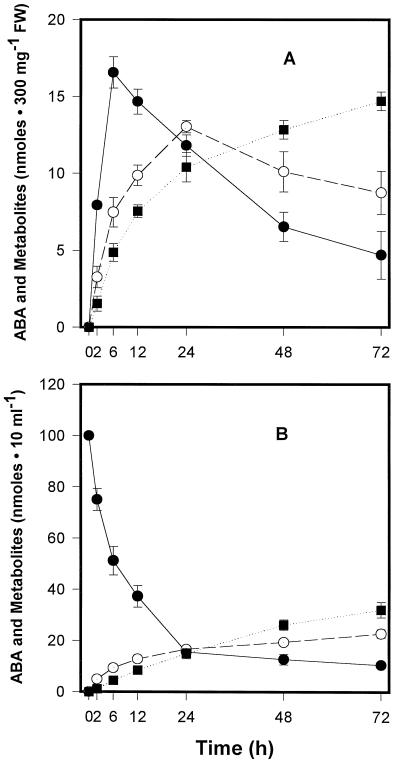
[3H]ABA content in the embryos was shown to increase rapidly, reaching a maximum concentration 6 h after treatment (Fig. 2A), with the medium showing a concurrent decrease in [3H]ABA content during the same period (Fig. 2B). After 24 h of treatment, approximately 85% of the [3H]ABA was depleted from the medium. [3H]PA and [3H]DPA were detected in embryos and media as early as 2 h after the hormone treatment and accumulated with time, with [3H]PA levels decreasing as [3H]DPA levels increased in the embryos, consistent with the expected metabolic pathway in which PA is reduced to DPA. In the medium, [3H]PA levels continued to increase over time, but at a slower rate than levels of [3H]DPA, suggesting that the metabolites were being released from embryos to the medium. These results indicate that microspore-derived embryos have an active system to catabolize (+)-ABA to DPA via PA and therefore suggest that reduced catabolism could greatly increase the time during which a hormonally effective ABA concentration would be maintained in the embryos.
Figure 2.
Time course of metabolism of (+)-[3H]ABA (10 μm) by microspore-derived B. napus embryos. A, Metabolite profiles in embryos. B, Metabolite profiles in the medium. Values shown are means ± se (n = 4). •, [3H]ABA; ○, [3H]PA; and ▪, [3H]DPA. FW, Fresh weight.
The first metabolite in the pathway from ABA to DPA is 8′-OH ABA, which is in equilibrium with PA. Exogenously supplied 8′-OH ABA has been shown to have high activity in the induction of oleosin and Δ15 desaturase genes in microspore-derived embryos of B. napus (Zou et al., 1995) and in the induction of group 3 Late Embryogenesis Abundant mRNA in wheat seedling roots (Walker-Simmons et al., 1997). Due to the transient nature of 8′-OH ABA (typically converted to PA during extraction processes), the levels present in the embryos at the time of sampling could not be determined. Since 8′-OH ABA shows high biological activity, the possibility of its being the active plant hormone could not be discounted. The assays described in this paper cannot separate the effects of exogenously applied (+)-ABA from those of the initially formed metabolite, 8′-OH ABA. This will be the topic of future research; the present discussion will focus on the relationship between ABA turnover and VLCFMA production, including the effects of KCS gene expression and of elongase complex enzyme activity.
The conversion of ABA to PA and then DPA in plants is catalyzed by two enzymes assumed to be sequential, ABA 8′ hydroxylase and PA reductase (Gillard and Walton, 1976). Babiano (1995) demonstrated that PA accumulation in germinating chickpea seeds treated with ABA is correlated with an increase in ABA 8′ hydroxylase activity. Similarly, Uknes and Ho (1984) and Cutler et al. (1997) showed by in vivo studies that ABA 8′ hydroxylase is induced by ABA. Further studies using in vitro assays have shown that induction of the hydroxylase by ABA occurs in corn cells (Krochko et al., 1997).
Induction of KCS Gene Transcripts by (+)-ABA and 8′-Methylene ABA
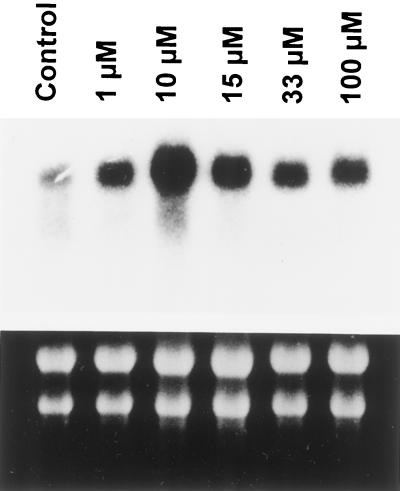
To assess the effects of (+)-ABA and related compounds on VLCMFA synthesis at the gene level, northern analyses were performed to measure the transcript levels of the gene encoding the B. napus KCS using the Arabidopsis FAE1 (KCS) gene as a probe. All concentrations applied, ranging from 1 to 100 μm (+)-ABA, significantly induced the KCS gene transcript compared with the control treatments (Fig. 3). (+)-ABA treatment (10 μm) gave the highest level of transcript.
Figure 3.
Response of KCS gene transcription to various concentrations of (+)-ABA supplied to microspore-derived B. napus embryos in culture for 24 h. The Arabidopsis FAE1 gene was used as a probe. The RNA gel-blot analysis of total RNA (7 μg) was described in Methods. The bottom panel shows ethidium bromide-stained RNA gel as a control for loading.
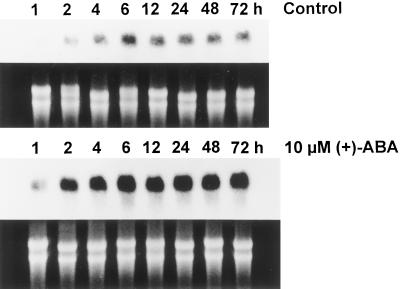
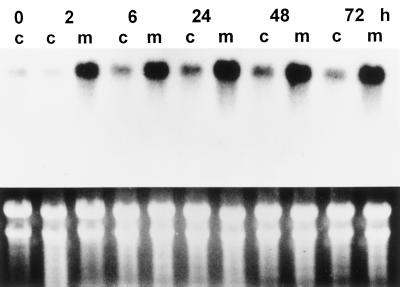
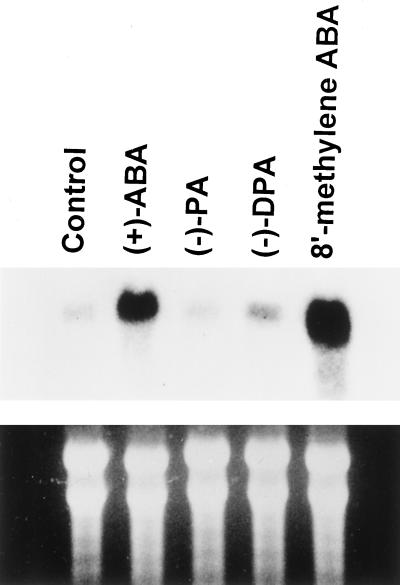
Time-course studies showed that in as little as 1 h after 10 μm (+)-ABA treatment, the transcript of the KCS-condensing enzyme gene was strongly induced and rapidly increased with time of treatment up to 6 h (Fig. 4). This strong induction signal remained throughout the sampling period despite the decrease in embryo ABA content at later time points, as shown in Figure 2. Similarly, in the presence of 10 μm (+)-8′-methylene ABA, strong induction of the KCS message was evident within 2 h and remained at a higher level than in the control throughout the 72-h sampling period (Fig. 5). In the absence of hormone, the transcript of the KCS gene remained relatively low during the 72-h sampling period. In contrast to (+)-ABA and 8′-methylene ABA, PA and DPA had only a slight or no effect on the KCS gene transcript relative to the control (Fig. 6). Similarly, PA had no effect on induction of oleosin and Δ15 desaturase transcripts in microspore-derived embryos (Zou et al., 1995).
Figure 4.
Time course of the effect of 10 μm (+)-ABA treatment (compared with control treatment) on the KCS condensing enzyme gene transcript in microspore-derived B. napus embryos. The Arabidopsis FAE1 gene was used as a probe. Six micrograms of total RNA was loaded onto each lane in this experiment. The top half of the control and ABA panels shows results of northern-blot analysis. The bottom half of these panels shows the corresponding ethidium bromide-stained RNA gel as a control for loading.
Figure 5.
The accumulation of the KCS gene transcript after microspore-derived B. napus embryos were treated with 10 μm (+)-8′-methylene ABA. The Arabidopsis FAE1 gene was used as a probe. Seven micrograms of total RNA from each sample was loaded. The bottom panel shows ethidium bromide-stained RNA gel as a control for loading. c, 0.1% ethanol treatment as controls; m, 10 μm (+)-8′-methylene ABA.
Figure 6.
Comparison of induction of the KCS gene transcript level by (+)-ABA, (−)-PA, (−)-DPA, and (+)-8′-methylene ABA after 24 h. The Arabidopsis FAE1 gene was used as a probe. Seven micrograms of total RNA from each treatment was loaded. The bottom panel shows the ethidium bromide-stained RNA gel as a control for loading.
Fatty Acyl Composition and VLCMFA Elongase Complex Activity
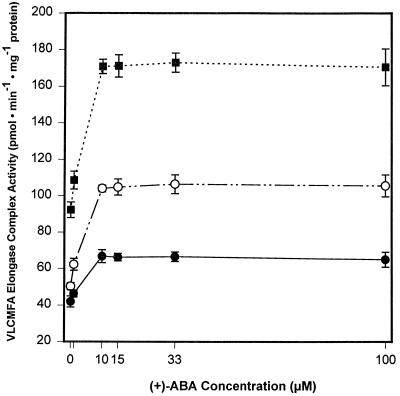
Previous work (Holbrook et al., 1992; Zou et al., 1995) has shown that exogenously supplied (±)- or (+)-ABA altered fatty acid composition and dramatically increased the content of VLCMFAs (20:1 and 22:1) in early- to mid-cotyledonary microspore-derived B. napus embryos in culture. Similar results were obtained in zygotic embryos of B. napus (Finkelstein and Somerville, 1989). In those studies, however, no attempt was made to study the concentration dependence of the ABA effect on VLCMFA accumulation. After a 48-h treatment, all concentrations of (+)-ABA tested in this study caused an increase in the amounts of total fatty acids detected (data not shown), but the strongest and most significant effect was observed on the levels of 20:1 and 22:1, which were generally 40 to 70% higher on a milligrams-of-protein basis and 4 to 8% higher on a mole-percentage basis, respectively, than those of the control (Table I). Of the (+)-ABA concentrations tested, 10 μm gave maximum VLCMFA accumulation. This is consistent with the data for induction of the KCS transcript shown in Figure 3. The specific activity of the elongase increased between 1 and 10 μm (+)-ABA and appeared to saturate thereafter (Fig. 7).
Table I.
Effect of various concentrations of (+)-ABA on VLCMFA accumulation
| (+)-ABA Concentration | VLCMFA Content
|
|||||
|---|---|---|---|---|---|---|
| 20:1 | 22:1 | 20:1 + 22:1 | ||||
| μm | μg mg−1 protein | mol % | μg mg−1 protein | mol % | μg mg−1 protein | mol % |
| 0 | 179 ± 9 | 10 | 220 ± 15 | 11.2 | 399 | 21.2 |
| 1 | 206 ± 15 | 11.6 | 279 ± 8 | 13.7 | 485 | 25.3 |
| 10 | 273 ± 26 | 13.4 | 377 ± 24 | 16.1 | 650 | 29.5 |
| 15 | 277 ± 21 | 12.9 | 345 ± 17 | 15.2 | 622 | 28.1 |
| 33 | 261 ± 19 | 12.2 | 313 ± 20 | 14.3 | 574 | 26.5 |
| 100 | 250 ± 10 | 12.1 | 292 ± 11 | 14.6 | 542 | 26.7 |
Microspore-derived B. napus embryos were incubated with various concentrations of (+)-ABA in culture. After 48 h of treatment, embryos were harvested, total lipids isolated, and fatty acid composition determined as described in Methods. The data shown are means ± se of four replicates.
Figure 7.
Effect of various concentrations of (+)-ABA on VLCMFA elongase complex activity in microspore-derived B. napus embryos. Embryos were subjected to various concentrations of (+)-ABA in culture for 24 h and homogenized to assay for [14C]20:1 and [14C]22:1 biosynthesis from [14C]oleate (18:1)-CoA in vitro as described in Methods. ▪, 20:1 + 22:1; ○, 22:1; and •, 20:1. Values shown are means ± se (n = 4).
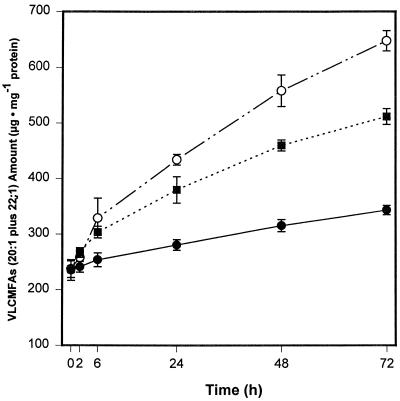
A time-course study was carried out to examine further the effect of ABA and its metabolites on the elongase complex activity and accumulation of VLCMFAs. Microspore-derived embryos were incubated with the optimal concentration (10 μm) of (+)-ABA and harvested at various times. Within 6 h, an increase in the accumulation of 20:1 and 22:1 could be detected, and embryos showed a strong, nearly linear accumulation of VLCMFAs for 72 h on a milligrams-of-protein basis, relative to the control embryos (Fig. 8). The stimulation of VLCMFA accumulation by 10 μm (+)-8′-methylene ABA was approximately 20 to 30% higher than that produced by the (+)-ABA treatment after 24 h. This is in agreement with the higher KCS gene transcript level induced by (+)-8′-methylene ABA compared with that induced by ABA (Fig. 6).
Figure 8.
Time course of effect of (+)-ABA and 8′-methylene ABA on the accumulation of VLCMFAs in microspore-derived B. napus embryos. The early-cotyledonary microspore-derived embryos were incubated with 10 μm (+)-ABA (▪) and 8′-methylene ABA (○), or only 0.1% ethanol (control, •). At various times, embryos were harvested and VLCMFA content was measured as described in Methods. Values shown are averages ± se (n = 4).
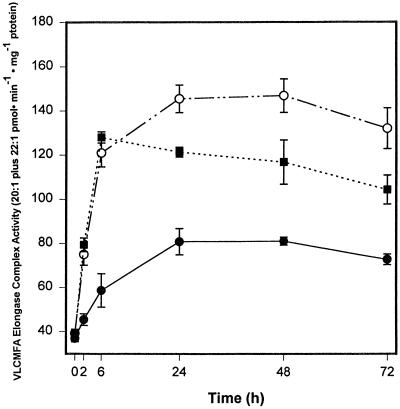
The same trend of increase in VLCMFA accumulation caused by ABA and 8′-methylene ABA was also observed on a dry-weight basis (data not shown). The findings are consistent with the data for the induction of VLCMFA elongase complex activity presented in Figure 9. After as little as 2 h in the presence of (+)-ABA, the activity of VLCMFA elongase complex (expressed as activity per milligram of protein) was significantly stimulated and this activity increased further up to 6 h. After 6 h, the activity declined, but remained about 50% higher than that of corresponding controls (Fig. 9). In this experiment the maximum specific activity of the elongase at 6 h after 10 μm (+)-ABA treatment was 138 pmol min−1 mg−1 protein, 60% higher than that of the control (87 pmol min−1 mg−1 protein) at 24 h. After 48 h of treatment, the specific activities of the elongase complex in 10 μm (+)-ABA- and 8′-methylene ABA-treated embryos were 47 and 75%, respectively, higher than those of the control. A decrease in the elongase activity in the ABA-treated samples after 6 h of treatment (especially if control activities are subtracted) may be related to ABA catabolism, since ABA levels in microspore-derived embryos also decreased after 6 h (Fig. 2), although (as we noted above) levels in KCS transcripts remained high from 6 to 72 h (Fig. 4).
Figure 9.
Time course of the effect of 10 μm (+)-ABA (▪) and 8′-methylene ABA (○) on VLCMFA elongase complex activity in microspore-derived B. napus embryos. Each data point represents the mean ± se of four replicates. •, Control.
The idea that declining embryo ABA content is related to the decrease in elongase activity after 6 h is consistent with the fact that addition of fresh (+)-ABA (10 μm) into the treatment medium at 24 h restored the maximum activity of the elongase enzyme (data not shown). Also, treatment with the more slowly metabolized (+)-8′-methylene ABA resulted in a more prolonged induction of elongase activity (Fig. 9). The ABA metabolites that accumulate in the embryo make no significant contribution, since it has been shown that PA had only a slight effect on the elongase activity (Zou et al., 1995). In the present study, DPA had essentially no effect (data not shown). When the elongase data were expressed in terms of total activity (picomoles per minute), a similar decrease was also observed in (+)-ABA- and (+)-8′-methylene ABA-treated embryos after 6 h of treatment (data not shown). However, it should be noted that an increase (10–14%) in embryo protein content over the course of the experiment may also contribute to the apparent decrease in elongase activity, when expressed per milligram of protein, at later time points in ABA- and (+)-8′-methylene ABA-treated samples.
Accumulation of TAG and Polar Lipids
To test the effects of (+)-ABA and (+)-8′-methylene ABA on storage lipids and the incorporation of VLCMFAs into TAGs in microspore-derived B. napus embryos, the content of TAG and polar lipids and the distribution pattern of VLCMFAs in these lipids were determined in (+)-ABA- and 8′-methylene ABA-treated embryos versus the control embryos. After a 48-h treatment with 10 μm (+)-ABA, 8′-methylene ABA, and DPA, total fatty acids in the TAG fraction in embryos were about 50, 70, and 8%, respectively, higher than those of the control treatment on a milligrams-of-protein basis (Table II). A similar trend was obtained on a dry-weight basis (data not shown). However, there was no significant difference in total fatty acid content in the polar lipid pool between the control and hormone treatments. VLCMFA content in the TLE of embryos treated with (+)-ABA, (+)-8′-methylene ABA, or DPA were about 55, 85, and 10%, respectively, higher than that of the control embryos. As expected, 80 to 85% of the VLCMFAs accumulated during hormone treatments were found in the TAG fraction (Table II). The data presented here demonstrate that 8′-methylene ABA produces a stronger ABA-like effect on the accumulation of TAG and VLCMFAs. DPA had little or no effect on the induction of TAG (8% increase) and VLCMFA (10% increase) accumulation.
Table II.
Accumulation of VLCMFAs in TLE from microspore-derived embryos incubated with 10 μm (+)-ABA, DPA, or 8′-methylene ABA for 48 h
| Additions | Total Fatty Acid Content in
TLE
|
TLE
|
TAG Fraction
|
|||
|---|---|---|---|---|---|---|
| TAG | Polar lipid | 20:1 | 22:1 | 20:1 | 22:1 | |
| μg mg−1 protein | ||||||
| Control | 1289 ± 21 | 169 ± 9 | 125 ± 5 | 146 ± 7 | 105 ± 4 | 120 ± 6 |
| ABA | 1933 ± 25 | 182 ± 11 | 208 ± 7 | 213 ± 8 | 168 ± 11 | 181 ± 8 |
| 8′-Methylene ABA | 2191 ± 23 | 172 ± 10 | 236 ± 9 | 267 ± 11 | 188 ± 13 | 220 ± 10 |
| DPA | 1395 ± 18 | 160 ± 13 | 130 ± 8 | 170 ± 11 | 112 ± 7 | 131 ± 8 |
CONCLUSIONS
The results presented here are the first, to our knowledge, to integrate the effects of ABA and its metabolites on the processes involved in VLCMFA accumulation at the transcript, gene-product (enzyme activity), and enzyme-product (VLCMFAs) levels. We have shown that the effect of (+)-ABA on VLCMFA biosynthesis and storage lipid accumulation is maximal at 10 μm, and therefore, compared lipid biosynthesis with ABA degradation at this ABA concentration. The observation that 8′-methylene ABA produces stronger effects than ABA in the experiments reported here implies that catabolic removal of ABA restricts VLCMFA production.
Overall, the experiments described here allow us to propose the working hypothesis that ABA catabolism limits VLCMFA production during embryogenesis, especially when exogenous ABA is greater than 10 μm. To test this hypothesis, future experiments will explore how ABA catabolism, as measured by ABA 8′ hydroxylase activity, affects and is affected by changes in embryo ABA content.
ACKNOWLEDGMENTS
The authors thank Dr. Ljerka Kunst (University of British Columbia) for kindly supplying plasmid pNAPIN-FAE1, Dr. John J. Balsevich (Plant Biotechnology Institute) for supplying tritiated (+)-ABA, and Dr. Alison Ferrie (Transgenic Plant Center, Saskatoon) for supplying microspore-derived B. napus embryos. We also gratefully acknowledge Lawrence Hogge and Doug Olson (Mass Spectrometry Laboratory, Plant Biotechnology Institute) for GC-MS analyses.
Abbreviations:
- DPA
dihydrophaseic acid
- EtOAc
ethyl acetate
- KCS
3-ketoacyl-CoA synthase
- 8′-OH ABA
8′-hydroxy ABA
- PA
phaseic acid
- TAG
triacylglycerol
- TLE
total lipid extract
- VLCMFA
very-long-chain monounsaturated fatty acids
- X:Y
a fatty acyl group containing X carbon atoms and Y cis double bonds
Footnotes
This is National Research Council of Canada publication no. 40727.
LITERATURE CITED
- Abrams SR, Rose PA, Cutler AJ, Balsevich JJ, Lei B, Walker-Simmons MK. 8′-Methylene ABA: an effective and persistent analog of abscisic acid. Plant Physiol. 1997;114:89–97. doi: 10.1104/pp.114.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal VP, Stumpf PK. Elongation systems involved in the biosynthesis of erucic acid from oleic acid in developing Brassica junceaseeds. Lipids. 1985;20:361–366. [Google Scholar]
- Aneja M, Gianfagna T, Ng E. Abscisic acid metabolism and episodic growth in cocoa. Plant Growth Regul. 1996;20:209–219. [Google Scholar]
- Babiano M. Metabolism of [2-14C]abscisic acid by a cell-free system from embryonic axes of Cicer arietinumL. seeds. J Plant Physiol. 1995;145:374–376. [Google Scholar]
- Balsevich JJ, Cutler AJ, Lamb N, Friesen LJ, Kurz EU, Perras MR, Abrams SR. Response of cultured maize cells to (+)-abscisic acid, (−)-abscisic acid, and their metabolites. Plant Physiol. 1994;106:135–142. doi: 10.1104/pp.106.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clemens S, Kunst L. Isolation of a Brassica napus cDNA (accession no. AF009563) encoding 3-ketoacyl-CoA synthase, a condensing enzyme involved in the biosynthesis of very long chain fatty acids in seeds (PGR 97-125) Plant Physiol. 1997;115:313–314. [Google Scholar]
- Cutler AJ, Squires TM, Loewen MK, Balsevich JJ. Induction of abscisic acid 8′ hydroxylase by (+)-abscisic acid in cultured maize cells. J Exp Bot. 1997;48:1787–1795. [Google Scholar]
- Dashek WV, Singh BN, Walton DC. Abscisic acid localization and metabolism in barley aleurone layers. Plant Physiol. 1979;64:43–48. doi: 10.1104/pp.64.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Jones HG. Abscisic Acid: Physiology and Biochemistry. Oxford, UK: Bios Scientific Publishers; 1991. [Google Scholar]
- Dunstan DI, Bock CA, Abrams GD, Abrams SR. Metabolism of (+)- and (−)-abscisic acid by somatic embryo suspension cultures of white spruce. Phytochemistry. 1992;31:1451–1454. [Google Scholar]
- Fehling E, Mukherjee KD. Acyl-CoA elongase from a higher plant (Lunaria annua): metabolic intermediates of very-long-chain acyl-CoA products and substrate specificity. Biochim Biophys Acta. 1991;1082:239–246. doi: 10.1016/0005-2760(91)90198-q. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Somerville C. Abscisic acid or high osmoticum promote accumulation of long-chain fatty acids in developing embryos of Brassica napus. Plant Sci. 1989;61:213–217. [Google Scholar]
- Gillard DF, Walton DC. Abscisic acid metabolism by a cell-free preparation from Echinocystis lobataliquid endosperm. Plant Physiol. 1976;58:790–795. doi: 10.1104/pp.58.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Quatrano RS. Mechanisms of action of abscisic acid at the cellular level. New Phytol. 1991;119:9–32. doi: 10.1111/j.1469-8137.1991.tb01004.x. [DOI] [PubMed] [Google Scholar]
- Hill RD, Liu JH, Durnin D, Lamb N, Shaw A, Abrams SR. Abscisic acid structure-activity relationships in barley aleurone layers and protoplasts. Plant Physiol. 1995;108:573–579. doi: 10.1104/pp.108.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho THD (1983) Biochemical mode of action of abscisic acid. In FT Addicott, ed, Abscisic Acid. Praeger Publishers, New York, pp 147–170
- Holbrook LA, Magus JR, Taylor DC. Abscisic acid induction of elongase activity, biosynthesis and accumulation of very long chain monounsaturated fatty acids and oil body proteins in microspore-derived embryos of Brassica napusL. cv Reston. Plant Sci. 1992;84:99–115. [Google Scholar]
- Holbrook LA, van Rooijen GJH, Wilen RW, Moloney MM. Oil body proteins in microspore-derived embryos of Brassica napusL. cv Reston. Hormonal, osmotic, and developmental regulation of synthesis. Plant Physiol. 1991;97:1051–1058. doi: 10.1104/pp.97.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DW, Lim E, Keller J, Plooy I, Ralston E, Dooner HK. Directed tagging of the Arabidopsis FATTY ACID ELONGATION (FAE1)gene with the maize transposon activator. Plant Cell. 1995;7:309–319. doi: 10.1105/tpc.7.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H, Zeevaart JAD. The five “classical” plant hormones. Plant Cell. 1997;9:1197–1210. doi: 10.1105/tpc.9.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krochko JE, Abrams GD, Loewen MK, Balsevich JJ, Cutler AJ. An in vitroassay for (+)-abscisic acid 8′ hydroxylase activity (abstract no. 232) Plant Physiol. 1997;114:S-63. [Google Scholar]
- Kubik MP, Buta GJ, Wang CY. Changes in the levels of abscisic acid and its metabolites resulting from chilling of tomato fruits. Plant Growth Regul. 1992;11:429–434. [Google Scholar]
- Lassner MW, Lardizabal K, Metz JG. A jojoa β-ketoacyl-CoA synthase cDNA complements the canola fatty acid elongation mutation in transgenic plants. Plant Cell. 1996;8:281–292. doi: 10.1105/tpc.8.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H, Bohm H, Schutte HR. The metabolism of abscisic acid in cell cultures of various plant species. Z Pflanzenphysiol. 1983;109:423–428. [Google Scholar]
- Loveys BR, Milborrow BV. Metabolism of abscisic acid. In: Crozier A, Hillman JR, editors. The Biosynthesis and Metabolism of Plant Hormone. Cambridge, UK: Cambridge University Press; 1984. pp. 71–104. [Google Scholar]
- Millar AA, Kunst L. Very-long-chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant J. 1997;12:121–131. doi: 10.1046/j.1365-313x.1997.12010121.x. [DOI] [PubMed] [Google Scholar]
- Pelle R, Murphy NB. Northern hybridization: rapid and simple electrophoretic conditions. Nucleic Acids Res. 1993;21:2783–2784. doi: 10.1093/nar/21.11.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AJ, Reaney MJT, Wilen RW, Lamb N, Abrams SR, Gusta LV. Effects of abscisic acid metabolites and analogs on freezing tolerance and gene expression in bromegrass (Bromus inermisLeyss) cell cultures. Plant Physiol. 1994;105:823–830. doi: 10.1104/pp.105.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce C, Plaggesi A, Ceccarelli N, Lorenzi R. Role and metabolism of abscisic acid in potato tuber dormancy and sprouting. J Plant Physiol. 1996;149:548–552. [Google Scholar]
- Taylor DC, Barton DL, Rioux KP, Mackenzie SL, Reed DW, Underhill EW, Pomeroy MK, Weber N. Biosynthesis of acyl lipids containing very-long chain fatty acids in microspore-derived and zygotic embryos of Brassica napusL. cv Reston. Plant Physiol. 1992;99:1609–1618. doi: 10.1104/pp.99.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC, Weber N. Microspore-derived embryos of the Brassicaceae-model systems for studies of storage lipid bioassembly and its regulation. Fat Sci Technol. 1994;96:228–235. [Google Scholar]
- Taylor DC, Weber N, Underhill EW, Pomeroy MK, Keller WA, Scowcroft WR, Wilen RW, Moloney MM, Holkbrook LA. Storage protein regulation and lipid accumulation in microspore embryos of Brassica napusL. Planta. 1990;181:18–26. doi: 10.1007/BF00202320. [DOI] [PubMed] [Google Scholar]
- Thomas TL. Gene expression during plant embryogenesis and germination: an overview. Plant Cell. 1993;5:1401–1410. doi: 10.1105/tpc.5.10.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes SJ, Ho THD. Mode of action of abscisic acid in barley aleurone layers. Plant Physiol. 1984;75:1126–1132. doi: 10.1104/pp.75.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance VB, Huang AHC. Expression of lipid body protein gene during maize seed development: spatial, temporal and hormonal regulation. J Biol Chem. 1988;263:1476–1481. [PubMed] [Google Scholar]
- von Wettstein-Knowles PM. Elongase and epicuticular wax biosynthesis. Physiol Veg. 1982;20:797–809. [Google Scholar]
- Walker-Simmons MK, Holappa LD, Abrams GD, Abrams SR. ABA metabolites induce group 3 LEAmRNA and inhibit germination in wheat. Physiol Plant. 1997;100:474–480. [Google Scholar]
- Wilen RW, Maxdel R, Pharis RP, Holbrook LA, Moloney MM. Effects of abscisic acid and high osmoticum on storage protein gene expression in microspore embryos of Brassica napus. Plant Physiol. 1990;94:875–881. doi: 10.1104/pp.94.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–473. [Google Scholar]
- Zeevaart JAD, Milborrow BV. Metabolism of abscisic acid and the occurrence of epi-dihydrophaseic acid in Phaseolus vulgaris. Phytochemistry. 1976;15:493–500. [Google Scholar]
- Zou J, Abrams GD, Barton DL, Taylor DC, Pomeroy MK, Abrams SR. Induction of lipid and oleosin biosynthesis by (+)-abscisic acid and its metabolites in microspore-derived embryos of Brassica napusL. cv Reston. Plant Physiol. 1995;108:563–571. doi: 10.1104/pp.108.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]