Abstract
The Sonic hedgehog (Shh) signaling pathway carries out a wide range of biological functions such as patterning of the embryonic neural tube and expansion of cerebellar granule cell precursors. We previously have found that the Shh signaling receptors, Patched1 (Ptch1) and Smoothened (Smo), are expressed in hippocampal neurons of developing and adult rats, suggesting the continued presence of Shh signaling in postmitotic, differentiated neurons. Here, we report that Ptch1 and Smo are present in the processes and growth cones of immature neurons in the developing cerebellum, and that, in the mature cerebellum, Ptch1 and Smo are expressed by several types of neurons including Purkinje cells, granule cells, and interneurons. Within these neurons, Ptch1 and Smo are predominantly localized in the postsynaptic side of the synapses, a distribution pattern similar to that found in hippocampal neurons. Our findings provide morphological evidence that Shh signaling events are not confined to neuronal precursors and are likely to have ongoing roles within the postmitotic neurons of the developing and adult cerebellum.
Keywords: Sonic hedgehog, Patched, Smoothened, Cerebellar neuron, Synapse
Introduction
During its development, the growth of the cerebellum depends on several growth factors; one of which is Sonic hedgehog (Shh) [1–4]. Shh stimulates the proliferation of granule cell precursors (GCPs) [5–10] and this stimulatory effect is stronger than other growth factors [7]. The current dogma regarding the source and the target of Shh—in the developing cerebellum—is that Shh is released by Purkinje cells and acts on the GCPs in the external germinal layer before they migrate to the internal granular layer [1, 4–8].
Little is known, however, about why Shh and its signaling receptor components, Patched1 (Ptch1) and Smoothened (Smo) [2, 3], continue to exist in the cerebellum even after the GCP proliferation and migration phase [1, 8, 11, 12; Allen Brain Atlas]. As a first step towards uncovering the functional significance of Shh in the mature cerebellum, we used immunoelectron microscopy to examine the expression and subcellular distribution of Ptch1 and Smo in the cerebellar neurons. The analysis was performed in postnatal day 2 (P2) and adult (P35–37) cerebella; the ages for which we have previously characterized Ptch1 and Smo in hippocampal neurons [13]. We found that Ptch1 and Smo are present in both young and mature cerebellar neurons, and that both Ptch1 and Smo are enriched postsynaptically.
Materials and Methods
Animals
All animal procedures were approved by the NIDCD Animal Care and Use Committee and complied with the NIH Guide for Care and Use of Laboratory Animals.
Antibody Characterization
The Smo and Ptch1 antibodies used in this study have been characterized and described in detail in a previous study [13]. The specificity of the Smo and Ptch1 antibodies has been demonstrated by immunoblot analysis of endogenously and exogenously expressed Smo or Ptch and analysis of Smo or Ptch1 −/− MEFs [13].
Immunoelectron Microscopy
Preembedding electron microscope (EM) immunoperoxidase/diaminobenzidine (DAB) labeling and postembedding immunogold labeling were performed exactly as described in a previously study [13]. For both ages (P2 and P35–37 adult; male, Sprague–Dawley rats) with either immunoperoxidase/DAB or immunogold, two to three animals were examined using each method.
Parasagittal thick sections were taken from the cerebellum in all cases. For EM immunoperoxidase/DAB labeling, thin sections were taken from the thick sections but perpendicular to the thick sections. For EM immunogold labeling, thin sections were taken from the thick sections in the same parasagittal plane. Adult sections for EM immunogold were taken from lobules III–V. The number of images examined was: Smo/immunogold P2, 143; Ptch1/immunogold P2, 89; Smo/DAB adult, 121; Ptch1/DAB adult, 105; Smo/immunogold adult, 146; and Ptch1/immunogold adult, 94.
For all electron micrographs acquired, the images were stored in their original formats and final images for figures were prepared in Adobe Photoshop and exported to Adobe Illustrator for labels. Control sections for immunogold or immunoperoxidase methods omitting the primary antibody showed only rare gold particles or DAB reaction product (Supplemental Figs. S1 and S2).
Identification of specific cerebellar structures was based on multiple published studies on the ultrastructure of the cerebellum [14–18]. Climbing and parallel fiber synapses on Purkinje cell dendrite spines were distinguished by the nature and arrangement of their synaptic vesicles (Supplemental Fig. S1). Climbing fiber terminals have large round vesicles that are densely packed and extend to the cell membrane in all directions. Parallel fiber terminals have slightly smaller and slightly less rounded vesicles that concentrate only near the synaptic active zone and usually do not extend to the opposite side of the cell membrane. In addition, climbing fiber terminals are very large and extensive, and typically the spine forms a synapse on the side opposite of the side of the terminal that contacts the Purkinje dendrite.
Results
The mRNA of Smo and Ptch1 is expressed at a detectable level in young and mature cerebella [6, 11, 12, 19; Allen Brain Atlas]. Consistently, Smo and Ptch1 proteins are expressed in the cerebellum of P2 and adult rats [13]. In this study, we used immunoelectron microscopy to examine the subcellular distribution of Smo and Ptch1 in developing and mature cerebellar neurons.
Smo and Ptch1 in the Early Postnatal Cerebellum
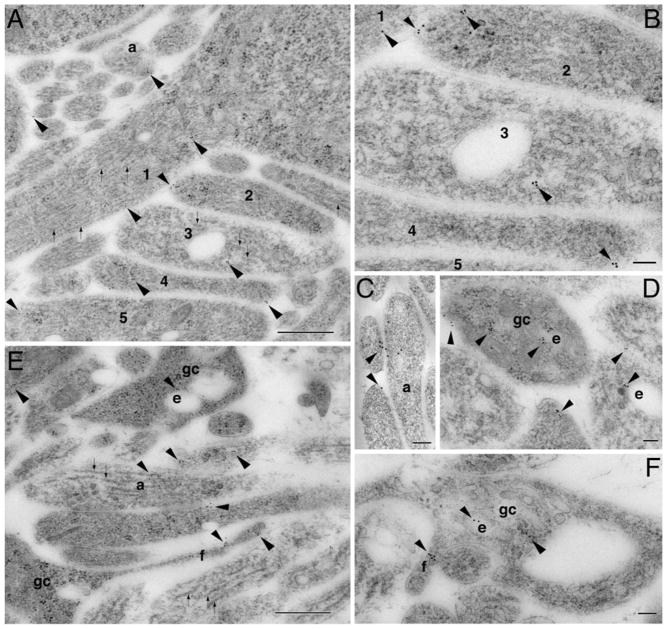
The cerebellum at P2 [15, 16] is largely composed of nascent processes and growth cones. Early synaptic contacts are occasionally observed. By examining the inner (or differentiating) zone of the external germinal layer, we found Smoimmunogold particles in young developing processes (Fig. 1a–c; Supplemental Fig. S3). These young processes were variable in structural characteristics: many were filled with filaments or microtubules (Fig. 1a, b) and some had vesicles (Fig. 1c). In deep neuropil below the external germinal layer, Smo labeling was commonly seen in growth cones and filopodia (Fig. 1d–f). Growth cones were evident at the ultrastructural level in vivo as large irregular structures containing various sized ovoid endosomal vesicles but lacking the organized central microtubule array of axon and dendrite shafts; portions of filopodia were sometimes seen along the edge of the growth cone profile [13, 20, 21]. Within the growth cones, Smo labeling was often seen near endosomes or directly associated with them (Fig. 1d–f).
Fig. 1.
Subcellular localization of Smo in immature processes and growth cones of the developing cerebellum (P2). a In the inner zone of the external germinal layer, Smo immunogold-labeled processes (arrowheads) vary in morphology and structure; processes 1 and 5 have microtubules (~25 nm diameter, linear structures marked by small arrows) and filaments; process 2 has mainly filaments; process 3 has mostly microtubules; and process 4 has only filaments. Profiles of filaments are less than half the diameter of microtubules and probably include intermediate filaments and actin. Smo labeling is also seen near vesicles of a young axonal terminal (a). A lower magnification of this area is shown in supplemental Fig. S2. b Higher magnification of a part of a. c is another example of Smo labeling in young axonal terminals that are filled with vesicles. d–f In deep neuropil of the cerebellar cortex, Smo labeling is common in growth cones (gc) where it is associated with endosomes (e) and filopodia (f). Scale bar is 500 nm in a and e and 100 nm in b, c, d, f
In our examination of Ptch1 immunogold labeling in the same cerebellar areas, we observed Ptch1 labeling in similar types of young processes and growth cones (Fig. 2). Within these processes or growth cones, Ptch1 labeling was also seen on or within endosomes (Fig. 2b, e). Thus, in the early postnatal cerebellum, Smo and Ptch1 are present in growing young processes and growth cones.
Fig. 2.
Ptch1 is also found in immature processes and growth cones of the developing cerebellum (P2). a In the inner zone of the external germinal layer, Ptch1 immunogold labeling (arrowhead) is seen on young processes. b–e In deep neuropil of the cerebellar cortex, Ptch1 labeling is seen in and on developing processes including growth cones (gc). e, endosome. Scale bar, 100 nm
Smo and Ptch1 in the Adult Cerebellum
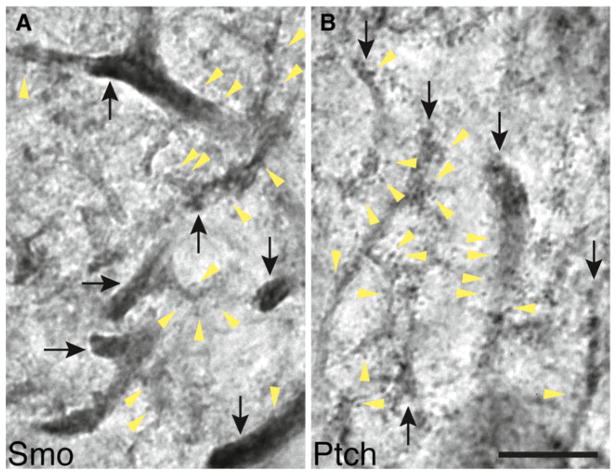
We next examined the adult cerebellum. Light microscopic analysis of immunoperoxidase/DAB labeling revealed that both Smo and Ptch1 labeling were pronounced in the dendrites of Purkinje cells (Fig. 3). Upon a closer examination, we noticed a subtle difference in the pattern of their dendritic distribution. Smo labeling was more intense in dendritic shafts than it was in dendritic spines (Fig. 3a), whereas Ptch1 labeling in dendritic shafts was similar to or even less intense than it was in dendritic spines (Fig. 3b).
Fig. 3.
Distribution of Smo and Ptch1 in the dendrites of Purkinje cells in the adult cerebellum revealed by immunoperoxidase/DAB light microscopy. a Smo labels more intensely in dendrites (black arrows) than it does in the dendritic spines (yellow arrowheads). b In contrast, Ptch1 antibody labels the dendritic spines (yellow arrowheads) equally to or more intensely than it labels in dendrites (black arrows). Note that a part of the Purkinje cell body is visible in the lower right. Scale bar, 10 μm
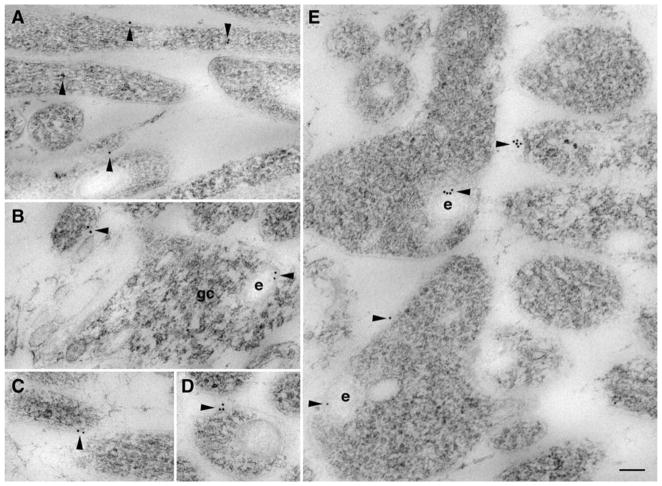
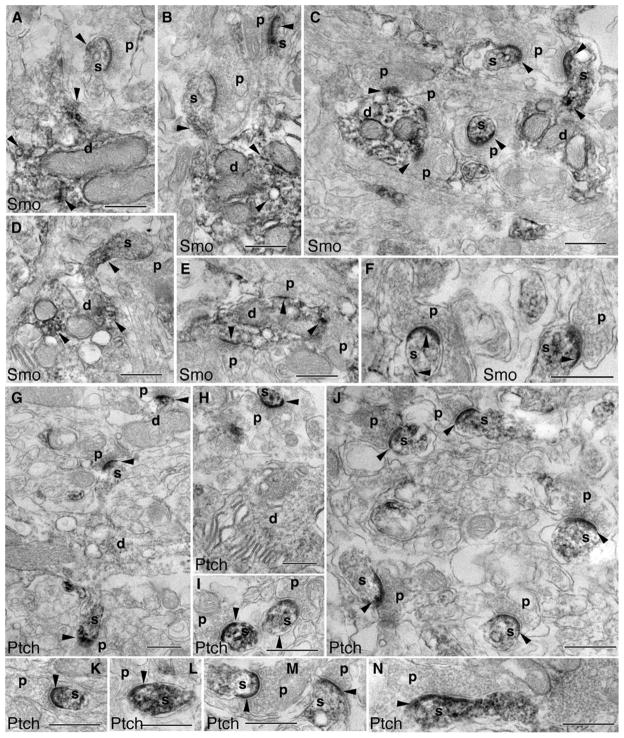
To further focus on the synaptic distribution of Smo and Ptch1, we used electron microscopy to examine the immunoperoxidase/DAB-labeled cerebellar tissues. Analysis of the molecular layer of the cerebellum showed conspicuous Smo labeling in dendritic shafts and spines of Purkinje cells (Fig. 4a–d, f) and in the dendritic shafts of interneurons (Fig. 4c, e). The dendrites of interneurons were distinguished from Purkinje cell dendrites by the presence of excitatory synapses (asymmetric synapse with thick postsynaptic density; round presynaptic vesicles) formed directly on the dendrite shaft of the former. In contrast to the prevalent postsynaptic labeling, the presynaptic terminals of parallel fibers opposed to the Smo-labeled postsynaptic terminals did not show visible Smo labeling. Similar to the Smo-labeling pattern, Ptch1-labeling products were also prominent in the postsynaptic terminals resulting in a great contrast to the opposed—but unlabeled—presynaptic terminals (Fig. 4g–n). Comparing the dendritic patterns, Smo labeling was seen in spines as well as dendrites, whereas Ptch1 labeling in general was strong in the spines but weak in the dendrites (Fig. 4g–n), consistent with the dendritic patterns revealed by the light microscopic analysis (Fig. 3). To confirm these results, we examined the adult molecular layer with immunogold labeling (Fig. 5). For both Smo and Ptch1, immunogold labeling was found in neurons, including labeling associated with the postsynaptic and extrasynaptic membranes of the spines of Purkinje cells.
Fig. 4.
Subcellular distribution of Smo and Ptch1 in the molecular layer of the adult cerebellum, revealed by immunoperoxidase/DAB electron microscopy. a–f Prominent Smo labeling (arrowheads) in dendrites (d) and postsynaptic spines (s) of Purkinje cells. Smo-labeled spines are commonly opposed to unlabeled presynaptic terminals (p) of parallel fibers. Postsynaptic Smo labeling is also seen in synapses on the dendrite shaft of interneurons (c, e). g–n Examples of Ptch1 labeling. Compared to Smo, Ptch1 labeling (arrowheads) is usually not prominent in dendrites (d) but is dense in postsynaptic Purkinje spines (s). Dendrites (d) shown in g (bottom) and h are from Purkinje cells, and another in g (top) is from an interneuron. Ptch1-labeled Purkinje cell spines are opposed to unlabeled presynaptic terminals (p) from parallel fibers (g–m); postsynaptic labeling also is seen in some dendrite shaft synapses (g, top). n A climbing fiber synapse with prominent postsynaptic Ptch1 labeling. Scale bars, 500 nm
Fig. 5.
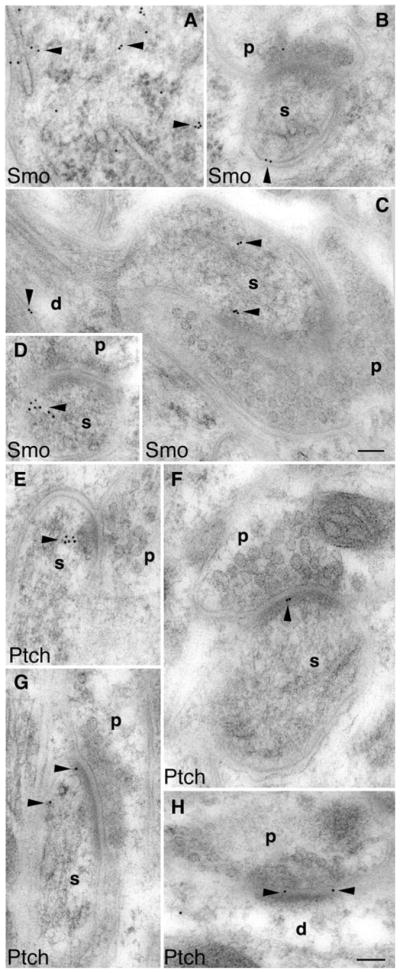
Immunogold electron microscopy also shows postsynaptic localization of Smo and Ptch1 in the molecular layer of the adult cerebellum. a Smo labeling (arrowheads) in the cell body of a Purkinje cell. b and d Smo labeling in postsynaptic Purkinje spines (s) opposed to the presynaptic terminals (p) of parallel fibers. c Smo labeling in a Purkinje dendrite (d) as well as its spine (s) that is opposed to a presynaptic terminal (p) of a climbing fiber. e–g Ptch1 labeling (arrowheads) in postsynaptic Purkinje spines (s) opposed to the presynaptic terminals (p) of parallel fibers. h Postsynaptic Ptch1 labeling at a dendritic (d) synapse of an interneuron. Scale bars, 100 nm
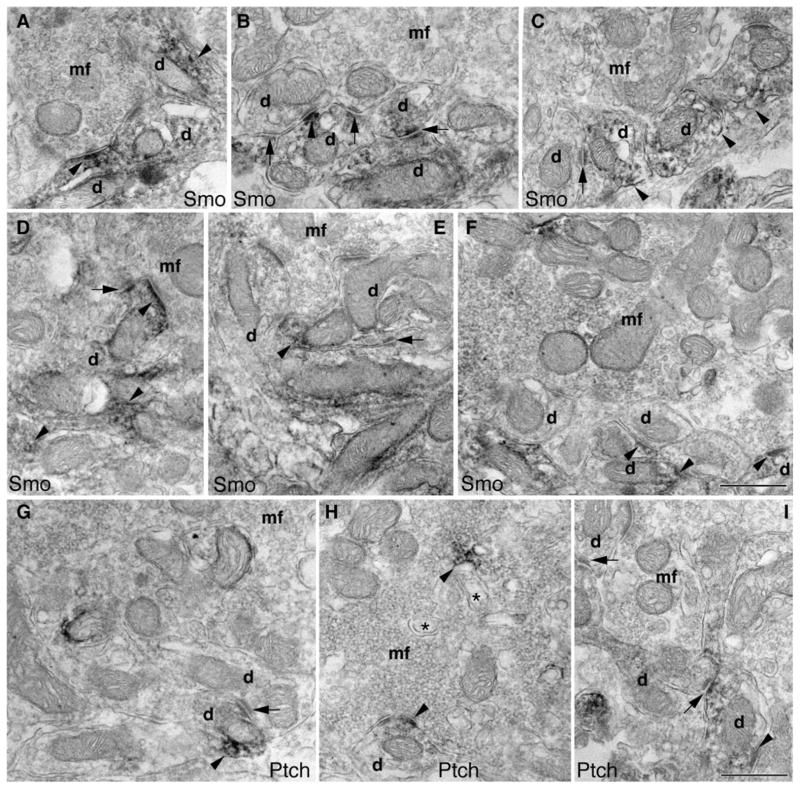
We next examined the distribution of Smo and Ptch1 in a different cerebellar region, the glomeruli in the granular layer. Figure 6 shows immunoperoxidase/DAB electron microscopy. Smo labeling was visible in the dendritic claws of granule cells that encircle mostly unlabeled mossy fiber terminals (Fig. 6a–e). Occasionally, we saw some Smo labeling in mossy terminals (Fig. 6f). Within the claws, the Smo labeling sometimes was seen at the synapses, but more often was enriched on the extrasynaptic membrane, including attachment plaques (Fig. 6b–e) [17]. Similarly, Ptch1 labeling was also more prominent in the dendritic claws of granule cells than it was in the mossy terminals (Fig. 6g, h) and was associated with attachment plaques (Fig. 6g, i) and spinules (Fig. 6h; for a description of spinules, see [13]). Immunogold electron microscopy (Fig. 7) confirmed the observations of the immunoperoxidase/DAB electron microscopy for both Smo and Ptch1 in the dendritic claws of granule cells, including associations with attachment plaques and spinules (Fig. 7).
Fig. 6.
Subcellular distribution of Smo and Ptch1 in the granular layer (glomeruli) of the adult cerebellum revealed by immunoperoxidase/DAB electron microscopy. a–f Smo labeling (arrowheads) in the dendritic claws (d) of granule cells that surround the unlabeled mossy fiber terminal (mf; in b–e). f A mossy fiber terminal with some Smo labeling. Smo labeling of the claws is sometimes seen at the synapse (a, d, and f; lower right), but is often concentrated on the extrasynaptic membrane including the attachment plaques (arrows). In i, the post-synaptic density of the upper dendrite profile is longer than 0.3 μm and this may indicate that this is a dendrite from a unipolar brush cell [18], although otherwise this profile resembles that of a typical granule cell dendritic claw. g–i Similar to Smo, Ptch1 labeling (arrowheads) is prevalent in the dendritic claws (d) that surround the mossy fiber terminal (mf), which shows only occasional labeling (g, h). Note that the Ptch1 labeling in h is associated with adjacent spinules (asterisks). The Ptch1 labeling of the claws is seen at the synapse (h), but is more common on the extrasynaptic membrane area including attachment plaques (arrows). Scale bars, 500 nm
Fig. 7.
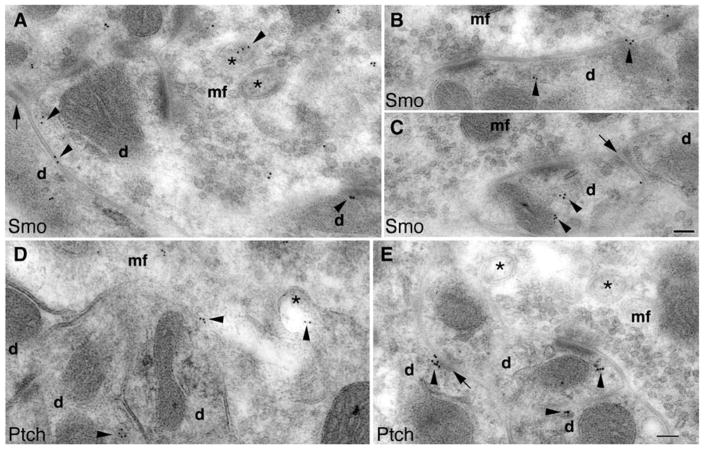
Immunogold electron microscopy also shows dendritic and postsynaptic Smo and Ptch1 in glomeruli of the adult cerebellar granular layer. a–c Smo labeling (arrowheads) is seen in the dendritic claws (d) of granule cells that surround the mossy fiber terminal (mf). Some Smo labeling is associated with spinules (asterisks). d, e Similarly, Ptch1 labeling is also seen in the dendritic claws and spinules. Arrows indicate attachment plaques. Scale bars, 100 nm
Discussion
The consensus with respect to Shh signaling transduction—in the developing cerebellum—is that Shh is produced by Purkinje cells and that it functions as a potent stimulator for the proliferation of GCPs [1, 4–10]. It is also known that Shh and its downstream signaling components continue to exist in the late postnatal and even in the adult cerebellum [1, 6, 8, 11, 12, personal communication with Dr. M. Kengaku (see ref. 19); Allen Brain Atlas], but it remains unclear in what cells of the mature cerebellum that Shh signaling takes place.
Through the results presented here, we have revealed the expression and subcellular distribution of the Shh receptor Ptch1 and the transducer Smo in the neurons of the mature cerebellum. The Ptch1- and Smo-expressing neurons include Purkinje cells, granule cells, and interneurons. The Purkinje cells have been long believed to be the Shh-producing cells in the developing cerebellum [1, 5–7, 22] as well as in the mature cerebellum [8]. Our finding of Ptch1 and Smo in the Purkinje cells indicates that these Purkinje cells—at least in the mature cerebellum—may also receive Shh signaling. Furthermore, although it is well established that GCPs proliferate in response to Shh [5–10], the continued presence of Ptch1 and Smo in the postmitotic granule cells suggests that Shh signaling may actually have ongoing functions in differentiated granule cells—functions beyond its role as a mitogen in the developing neurons.
The distribution patterns of Ptch1 and Smo within the cerebellar neurons seen in this study are virtually identical to what has been observed in the hippocampal neurons [13]. In the neurons of both these brain regions, Ptch1 and Smo are concentrated postsynaptically. And, in both the cerebellar and the hippocampal neurons, postsynaptic Ptch1 is found more frequently in the spines than it is in the dendrites, whereas Smo is seen in many dendrites and only in some spines. Additionally, the labeling for Ptch1 and Smo is often observed in close proximity to, or even associated with, a number of subcellular structures including endosomes, synaptic spinules, attachment plaques, and autophagosomes. Together, this study and the previous study [13] demonstrate not only the existence of Ptch1 and Smo in differentiated neurons but also their distinctive spatial organization at the synapse.
Could the postsynaptic enrichment of Ptch1 and Smo reflect the directionality of Shh signaling transduction across the synapse? One possible scenario is that Shh is released from the presynaptic terminal, crosses the synaptic cleft, and then acts on the postsynaptic terminal. This would resemble the signaling transduction of another morphogen, Wnt, at the synapse [23]. It is not yet known if, like Wnt [23], Shh is released by neurons. The presence of Drosophila Hedgehog in the axons of photoreceptor neurons [24, 25] and of mammalian Shh at or near the presynaptic synaptic contact of hippocampal neurons [26] could be indicative of Shh signaling transduction from the presynaptic to postsynaptic direction. On the other hand, because Shh is also found in the postsynaptic spines and dendrites [26], an alternative but more speculative rationale is that Shh, much like brain-derived neurotrophic factor, is released from the postsynaptic terminals in addition to the presynaptic ones [27]. The postsynaptically produced Shh could activate the pathway in an autocrine manner at the postsynaptic site.
Apart from the source of the Shh ligand, it is noteworthy that, within the postsynaptic site, Ptch1 and Smo do not completely colocate. Rather, Ptch1 is found more frequently in the spines than in the dendrites, whereas Smo is found mostly in the dendrites. We have repeatedly observed this differential pattern in multiple types of neurons including those in the cerebellum (this study) and the hippocampus [13], as well as in cultured hippocampal neurons [13]. We propose a model in which the Ptch1 molecules sit in the spines—ready to interact with the ligand Shh; once binding, the Shh signaling is somehow relayed to Smo, which is placed both geographically and functionally down the pathway. Further understanding of the physiological relevance of the differential arrangement awaits detailed information of the dynamic of Ptch1 and Smo during Shh signaling activation in these neurons.
What about the primary cilium? Because interfering with the formation of the primary cilium in mammalian cells results in phenotypes starkly similar to those resulting from the loss of Shh signaling activity [28–31], the primary cilium is believed to be crucial for Shh signaling. The primary cilium is generally considered to serve as a hub in which Shh carries out its signaling activity, as several components of the Shh signaling pathway—Ptch1 and Smo included—are found to accumulate on the primary cilium in response to Shh pathway activation [32, 33]. It should be noted that whether endogenous Ptch1 or Smo permanently reside within the primary cilium in vivo has not yet been made explicit. Also, although fully differentiated neurons appear to possess the primary cilium [34–36], the relationship between the primary cilium and the Shh signaling pathway in these neurons is unknown. Our data do not directly address the above questions. The sampling limitations of electron microscopy are exacerbated by the fact that each neuron has only a single primary cilium and the fact that the size of the cilium is substantially smaller in comparison to the neuron and its extensive neurites. Among the 1826 images examined throughout this study, there are fewer than ten neurons in which part of the primary cilium is revealed, and in none of these did the cilia have Ptch1 or Smo immunogold labeling (Fig. S4; data not shown). It is conceivable that the presence of Ptch1 and Smo within the primary cilium is brief and transient, and/or, that the fraction of ciliary Ptch1 and Smo at any given moment in a finite population of neurons is small.
What, then, is the biological significance of the Shh singling pathway in differentiated neurons? The type of morphological evidence we present here cannot definitively address the function of the pathway. Nonetheless, our results offer some hints in light of the unique spatial patterns of Ptch1 and Smo in the neuron and their close vicinity to or coexistence with certain organelles at the synapse. The postsynaptic enrichment and arrangement of Ptch1 and Smo likely represent the location where Shh signaling initiates in these neurons. But the association between Ptch1 or Smo and synaptic spinules, attachment plaques, and autophagosomes suggests that the synapse could also be the site where the Shh signaling pathway exerts its function. Synaptic spinules are small bulges at the synaptic membranes [13, 37–39]. The high incidence of synaptic spinules has been linked to synaptic remodeling [39–41]. Similarly, attachment plaques have been also suggested to have a role in synaptic plasticity [17, 42]. Moreover, as for the autophagosomes, while their role in mammalian synapses is not yet clear, it has been shown that autophagy promotes synapse formation in Drosophila [43]. Therefore, it is possible that the Shh signaling pathway is involved in the synaptic plasticity of differentiated neurons.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of the NIA/NIH and NIDCD/NIH.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12311-012-0374-6) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare no conflict of interest.
Contributor Information
Ronald S. Petralia, Advanced Imaging Core, NIDCD/NIH, Bethesda, MD 20892, USA
Ya-Xian Wang, Advanced Imaging Core, NIDCD/NIH, Bethesda, MD 20892, USA.
Mark P. Mattson, Laboratory of Neurosciences, National Institute on Aging Intramural Research Program, Baltimore, MD 21224, USA
Pamela J. Yao, Email: yaopa@grc.nia.nih.gov, Laboratory of Neurosciences, National Institute on Aging Intramural Research Program, Baltimore, MD 21224, USA. Laboratory of Neurosciences, NIA/NIH Biomedical Research Center, 251 Bayview Boulevard, Baltimore, MD 21224, USA
References
- 1.Vaillant C, Monard D. SHH pathway and cerebellar development. Cerebellum. 2009;8:291–301. doi: 10.1007/s12311-009-0094-8. [DOI] [PubMed] [Google Scholar]
- 2.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–72. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 3.Zheng X, Mann RK, Sever N, Beachy PA. Genetic and biochemical definition of the Hedgehog receptor. Genes Dev. 2010;24:57–71. doi: 10.1101/gad.1870310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatten ME, Roussel MF. Development and cancer of the cerebellum. Trends Neurosci. 2011;34:134–42. doi: 10.1016/j.tins.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahmane N, Ruiz I, Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 6.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–8. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 7.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–14. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 8.Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Corrales JD, Blaess S, Mahoney EM, Joyner AL. The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development. 2006;133:1811–21. doi: 10.1242/dev.02351. [DOI] [PubMed] [Google Scholar]
- 10.Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, et al. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–59. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traiffort E, Charytoniuk DA, Faure H, Ruat M. Regional distribution of Sonic Hedgehog, patched, and smoothened mRNA in the adult rat brain. J Neurochem. 1998;70:1327–30. doi: 10.1046/j.1471-4159.1998.70031327.x. [DOI] [PubMed] [Google Scholar]
- 12.Traiffort E, Charytoniuk D, Watroba L, Faure H, Sales N, Ruat M. Discrete localizations of hedgehog signalling components in the developing and adult rat nervous system. Eur J Neurosci. 1999;11:3199–214. doi: 10.1046/j.1460-9568.1999.00777.x. [DOI] [PubMed] [Google Scholar]
- 13.Petralia RS, Schwartz CM, Wang YX, Mattson MP, Yao PJ. Subcellular localization of Patched and Smoothened, the receptors for sonic hedgehog signaling, in the hippocampal neuron. J Comp Neurol. 2011;519:3684–99. doi: 10.1002/cne.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. New York: Springer; 1974. p. 348. [Google Scholar]
- 15.Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. New York: CRC Press; 1997. p. 783. [Google Scholar]
- 16.Zhao H-M, Wenthold RJ, Petralia RS. Glutamate receptor targeting to synaptic populations on Purkinje cells is developmentally regulated. J Neurosci. 1998;18:5517–28. doi: 10.1523/JNEUROSCI.18-14-05517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petralia RS, Wang YX, Wenthold RJ. NMDA receptors and PSD-95 are found in attachment plaques in cerebellar granular layer glomeruli. Eur J Neurosci. 2002;15:583–7. doi: 10.1046/j.1460-9568.2002.01896.x. [DOI] [PubMed] [Google Scholar]
- 18.Mugnaini E, Sekerkova G, Martina M. The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev. 2011;66:220–45. doi: 10.1016/j.brainresrev.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki N, Kurisu J, Kengaku M. Sonic hedgehog signaling regulates actin cytoskeleton via Tiam1-Rac1 cascade during spine formation. Mol Cell Neurosci. 2010;45:335–44. doi: 10.1016/j.mcn.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Rees RP, Bunge MB, Bunge RP. Morphological changes in the neuritic growth cone and target neuron during synaptic junction development in culture. J Cell Biol. 1976;68:240–63. doi: 10.1083/jcb.68.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang PY, Petralia RS, Wang Y-X, Wenthold RJ, Brenowitz SD. Functional NMDA receptors at axonal growth cones of young hippocampal neurons. J Neurosci. 2011;31:9289–97. doi: 10.1523/JNEUROSCI.5639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haldipur P, Bharti U, Govindan S, Sarka C, Iyengar S, Gressens P, et al. Expression of Sonic hedgehog during cell proliferation in the human cerebellum. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0206. in press. [DOI] [PubMed] [Google Scholar]
- 23.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Z, Kunes S, Hedgehog transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–22. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- 25.Chu T, Chiu M, Zhang E, Kunes S. A C-terminal motif targets Hedgehog to axons, coordinating assembly of the Drosophila eye and brain. Dev Cell. 2006;10:635–46. doi: 10.1016/j.devcel.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Petralia RS, Wang YX, Mattson MP, Yao PJ. Sonic hedgehog distribution within mature hippocampal neurons. Commun Integr Biol. 2011;4:775–7. doi: 10.4161/cib.17832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, et al. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185–98. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signaling in the mouse requires intra-flagellar transport proteins. Nature. 2003;426:83–7. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 29.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–11. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 30.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Vierkotten J, Dildrop R, Peters T, Wang B, Rüther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–77. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- 32.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 33.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–6. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 34.Green JA, Mykytyn K. Neuronal ciliary signaling in homeostasis and disease. Cell Mol Life Sci. 2010;67:3287–97. doi: 10.1007/s00018-010-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louvi A, Grove EA. Cilia in the CNS: the quiet organelle claims center stage. Neuron. 2011;69:1046–60. doi: 10.1016/j.neuron.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arellano JI, Guadiana SM, Breunig JJ, Rakic P, Sarkisian MR. Development and distribution of neuronal cilia in mouse neocortex. J Comp Neurol. 2012;520:848–73. doi: 10.1002/cne.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westrum LE, Blackstad TW. An electron microscopic study of the stratum radiatum of the rat hippocampus (regio superior, CA 1) with particular emphasis on synaptology. J Comp Neurol. 1962;119:281–309. doi: 10.1002/cne.901190303. [DOI] [PubMed] [Google Scholar]
- 38.Tarrant SB, Routtenberg A. The synaptic spinule in the dendritic spine: electron microscopic study of the hippocampal dentate gyrus. Tissue Cell. 1977;9:461–73. doi: 10.1016/0040-8166(77)90006-4. [DOI] [PubMed] [Google Scholar]
- 39.Spacek J, Harris KM. Trans-endocytosis via spinules in adult rat hippocampus. J Neurosci. 2004;24:4233–41. doi: 10.1523/JNEUROSCI.0287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner HJ, Djamgoz MB. Spinules: a case for retinal synaptic plasticity. Trends Neurosci. 1993;16:201–6. doi: 10.1016/0166-2236(93)90155-f. [DOI] [PubMed] [Google Scholar]
- 41.Tao-Cheng JH, Dosemeci A, Gallant PE, Miller S, Galbraith JA, Winters CA, et al. Rapid turnover of spinules at synaptic terminals. Neurosci. 2009;160:42–50. doi: 10.1016/j.neuroscience.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petralia RS. Distribution of extrasynaptic NMDA receptors on neurons. The Scientific World J. 2012 doi: 10.1100/2012/267120. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J Cell Biol. 2009;187:71–9. doi: 10.1083/jcb.200907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.